Abstract
Recurrent use of prescription opioid analgesics by chronic pain patients may result in opioid dependence, which involves implicit neurocognitive operations that organize and impel craving states and compulsive drug taking behavior. Prior studies have identified an attentional bias (AB) towards heroin among heroin dependent individuals. The aim of this study was to determine whether opioid-dependent chronic pain patients exhibit an AB towards prescription opioidrelated cues. Opioid-dependent chronic pain patients (n = 32) and a comparison group of non-dependent opioid users with chronic pain (n = 33) completed a dot probe task designed to measure opioid AB. Participants also rated their opioid craving and self-reported arousal associated with opioid-related and neutral images, pain severity, and relief from pain treatments. Repeated-measures ANOVA revealed a significant group (opioid-dependent vs. non-dependent opioid user) × presentation duration (200 ms. vs. 2000 ms.) interaction, such that opioid-dependent individuals evidenced a significant AB towards opioid cues presented for 200 ms but not for cues presented for 2000 ms, whereas non-dependent opioid users did not exhibit a significant mean AB at either stimulus duration. Among opioid-dependent individuals, 200 ms opioid AB was significantly associated with opioid craving, while among non-dependent opioid users, 200 ms opioid AB was significantly associated with relief from pain treatments. Furthermore, dependent and non-dependent opioid users experienced opioid cues as significantly more arousing than neutral cues. Opioid dependence among chronic pain patients appears to involve an automatic AB towards opioid-related cues. When coupled with chronic pain, attentional fixation on opioid cues may promote compulsive drug use and addictive behavior.
Keywords: opioid addiction, opioid dependence, craving, attentional bias, chronic pain, implicit cognition, opiate
Opioid therapy for chronic pain conditions often provides effective analgesia but confers significant risk for developing opioid use disorders in a subset of vulnerable individuals (Denisco, Chandler, & Compton, 2008; Passik, 2009). Prolonged, medically-appropriate opioid use produces physical dependence symptoms via neuroadaptations resulting in tolerance to opioids, withdrawal when opioids are discontinued, and, in some instances, opioid-induced hyperalgesia (Chu, Angst, & Clark, 2008), a condition in which longer exposure to opioids tends to induce hypersensitivity to pain. These physical symptoms of opioid dependence are natural responses to extended use of opioids, and do not signal the presence of an opioid use disorder. In contrast, prescription opioid dependence as defined by DSM-IV involves not only physical dependence symptoms, but behavioral symptoms as well, including: taking higher doses than intended; an inability to reduce or stop taking opioids; spending substantial amounts of time using, obtaining, recovering, or thinking of opioids; and continued use in spite of adverse physical or psychological consequences (Zacny, Bigelow, Compton, Foley, Iguchi, & Sannerud, 2003). However, some believe that these behavioral criteria for dependence are inappropriate for opioid-using chronic pain patients, because patients who take opioids as prescribed may be unable to reduce their opioid use or may continue opioid use in spite of adverse health consequences due to the intractability of their chronic pain condition. As such, many pain medicine and addiction specialists use the criteria for opioid addiction as established by the American Pain Society (2002) to identify the presence of disordered opioid use among chronic pain patients, including symptoms of impaired control over opioid use, compulsive opioid use, continued use despite harm, and craving (Wilson, 2007). Although less serious forms of opioid misuse like unauthorized dose escalation are relatively common and may not indicate disordered use of opioids, addictive tendencies among prescription opioid users may be signified by serious opioid misuse behaviors such as selling medication or injecting oral formulas (Ives et al. 2006; Sullivan et al. 2010). Indeed, such aberrant drug-taking behaviors are believed to mark the transition from sanctioned use of opioids to the development of opioid use disorders and addiction (Butler et al., 2007).
Like other forms of substance dependence, prescription opioid use disorders may involve implicit neurocognitive operations that organize and impel craving states and aberrant drug taking (Stacy & Wiers, 2010). Repeated substance use is thought to establish automatic drug-use action schemas, i.e., memory systems that compel and coordinate consumption of the substance through automatized sequences of stimulus-bound, context-dependent behavior, including the biasing of attention towards substance-relevant stimuli (Garland et al., 2011a; Pierce & Vanderschuren, 2010; Tiffany, 1990). As chronic pain patients engage in recurrent opioid use or misuse, opioid cues (e.g., the sight of a pill bottle or a syringe used for illicit intravenous drug use) may become salient both through the pharmacologic reward induced by opioid consumption, as well as through the analgesic effects of the drug which remove or allay the aversive experience of pain (Fields, 2004). Presumably, the incentive salience of opioid cues may increase over time as opioid dependence becomes more deeply entrenched via the process of sensitization of the mesolimbic dopamine system (Robinson & Berridge, 2008). Insofar as cues associated with past drug use are motivationally salient for habitual drug users, they are able to consequently capture attention, which in turn amplifies their motivational salience (Franken 2003). This phenomenon, known as addiction attentional bias (AB), is associated with craving and increased drug use (Field, Munafo, & Franken, 2009), predicts relapse (e.g., Garland, Franken, & Howard, 2012), and is evidenced on dot probe tasks by shorter reaction times (RT) to probes replacing drug-related images relative to probes replacing neutral images (Field & Cox, 2008). This RT difference is considered an index of addiction AB.
Assessing addiction AB may be performed on multiple attentional processes as a function of the duration that the stimulus is presented. According to basic human perception research, individuals require an average of 50 milliseconds (ms) to orient attention to a simple visual stimulus (Duncan, Ward, & Shapiro, 1994), while requiring at least 150 ms to disengage attention from that stimulus and shift it to another location in space (Theeuwes 2005). On tasks involving simultaneous presentation of visually complex drug-related and neutral cues, AB for a cue presented for ≤ 200 ms is theorized to index automatic, initial orienting toward drug cues, because orienting, disengagement, and re-orienting to a new complex stimulus is not possible within 200 ms (Field & Cox, 2008). In contrast, AB for longer duration stimuli (ranging from 500 to 2000 ms in across studies) is theorized to index higher-order attentional processes, such as delayed disengagement of attention from drug-related cues (Field & Cox, 2008) or elaborative cognitive processing (Andreassi, 200; Filion, Dawson, & Schell, 1993).
AB towards heroin-related stimuli has been observed among methadone-maintained heroin dependent individuals performing dot probe tasks (Constantinou et al., 2010; Lubman, Peters, Mogg, Bradley, & Deakin, 2000; Marissen et al., 2006); heroin AB has also been observed with the flicker change blindness paradigm (Bearre, Sturt, Bruce, & Jones, 2007 and the addiction Stroop task (Marissen et al. 2006). In addition, a particularly sophisticated study employing ecological momentary assessment via handheld computers revealed that heroin AB as measured by the addiction Stroop task was elevated one hour prior to episodes of temptation to use heroin (Waters, Marhe, & Franken, 2011). Although these studies have established the presence of AB towards illicit opiates among opiate dependent individuals, to our knowledge no study has identified an AB towards prescription opioids among opioid-dependent chronic pain patients. In light of the dearth of findings in this potentially important area, the purposes of the present study were a) to establish the existence of a prescription opioid AB and b) to determine whether persons suffering from chronic pain who meet DSM-IV diagnostic criteria for opioid dependence exhibit a significantly greater AB towards prescription opioid-related stimuli than opioid users who do not meet DSM-IV criteria for opioid dependence. If so, the presence of a differential AB toward prescription opioids might be reflective of an addictive process in excess of the physical dependence symptoms of withdrawal and tolerance occasioned by authorized use of prescription opioids. Therefore, in the present study a sample of opioid-dependent and nondependent chronic pain patients taking prescription opioid analgesics completed a dot probe task in which opioid and neutral images were presented for 200 and 2000 ms to assess for the presence of AB during attentional orienting and/or disengagement (c.f., Field & Cox, 2008). We hypothesized that, compared to non-dependent opioid users, opioid-dependent patients would have a greater AB to opioid-related cues at both stimulus durations. We also assessed opioid craving to identify relations between opioid AB and appetitive responding. Given the possibility that opioid cues could also compel attention as a result of reinforcement conditioning via the analgesic effects of opioids, we examined associations between opioid AB, pain severity, and pain relief.
METHODS
Participants
Chronic opioid use is defined in clinical guidelines as use of opioids daily or nearly every day for at least 90 days (Chou et al., 2009; Korff et al., 2008). As such, chronic pain patients who were currently taking prescription opioid analgesics (hereafter referred to as opioids) regularly for >3 months were recruited from primary care clinics, pain clinics, and neurology clinics in Tallahassee, FL through flyers, as well as from online classified ads. Advertisements were focused on recruiting participants who suffer from and are prescribed medicine for chronic pain for a study focused on improving ways to address problems with chronic pain and prescription pain medication. Criteria for opioid dependence and abuse were assessed with section J of the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Diagnostic interviews were conducted by clinical assessors trained in making substance use disorder diagnoses with the MINI. Based on diagnosis, participants were grouped into one of two groups: a group of chronic pain patients who met DSM-IV criteria for prescription opioid dependence (the opioid-dependent group), and a group of chronic pain patients who used prescription opioids but did not meet DSM-IV criteria for prescription opioid dependence (the non-dependent opioid user group) or abuse.
The opioid-dependent group consisted of 32 individuals who had taken prescription opioid medications to treat chronic pain conditions including low back pain (31.3%), cervical pain (6.3%), fibromyalgia (25.0%), osteoarthritis (3.1%), chronic daily headache (3.1%), peripheral neuropathy (3.1%), chest pain (6.3%), and systemic scleroderma (3.1%). The nondependent opioid user group consisted of 33 individuals who had taken prescription opioid medications to treat chronic pain conditions including low back pain (39.4%), cervical pain (15.2%), fibromyalgia (21.2%), osteoarthritis (9.1%), extremity pain (3.1%), peripheral neuropathy (6.1%), and reflex sympathetic dystrophy (3.1%).
The prevalence of opioid analgesic medications (including the opiate morphine, the opioid agonist tramadol, and the mixed agonist-antagonist buprenorphine) used by opioid-dependent and non-dependent participants is reported in Table 1. Across the entire sample, the most frequently reported opioid was hydrocodone, followed by tramadol, oxycodone, methadone, fentanyl, morphine, and suboxone. Participants also reported using a number of nonopioid medications to manage pain: nonsteroidal anti-inflammatory drugs (NSAIDS) (n = 7), cyclobenzaprine (n = 4), metaxalone (n = 1), and methocarbamol (n = 1).
Table 1.
Prescription opioid analgesic medications used by opioid dependent and non-dependent opioid user groups.*
| Opioid Dependents (n=32) |
Non-Dependent Opioid Users (n=33) |
|
|---|---|---|
| Hydrocodone | 23 (71.9%) | 20 (60.6%) |
| Tramadol | 5 (15.6%) | 7 (21.2%) |
| Oxycodone | 4 (12.5%) | 4 (12.1%) |
| Fentanyl | 1 (3.1%) | 4 (12.1%) |
| Morphine | 2 (6.3%) | 2 (6.1%) |
| Methadone | 1 (3.1%) | 3 (9.1%) |
| Buprenorphine | 1 (3.1%) | 0 (0.0%) |
No statistically significant difference in prevalence of opioid medication used per group.
Participants were also assessed for co-morbid alcohol and substance use disorders using Sections I and J of the MINI but were not excluded from the study if they met diagnostic criteria for these conditions. Screening did exclude individuals with a history of current psychotic disorder or who had active suicidal ideation and intent as established by the MINI (Sheehan et al., 1998). All participants provided written, informed consent and were compensated $25 for their participation in the study. The study was approved by the human subjects committee of Florida State University and accords with ethical provisions set in the Declaration of Helsinki.
Procedures
Participants were instructed to take their prescribed opioid medication as usual on the day of participation in the study. In a single session, participants completed several validated questionnaires (as described in the “Measures” section below), followed by the dot probe task. Participants also made arousal ratings for each image used in the dot probe task in a separate picture rating task.
Measures
Dot probe task
A dot probe task was used to measure opioid AB. This task was generated in E-Prime 2.0 (PST Inc., Pittsburgh, PA) and presented on an IBM T60 laptop with a 15” screen. Each trial began with a fixation cross presented for 500 ms. Next, two images matched for visual complexity, composition, and figure-ground relationships appeared side by side on the computer screen. On critical trials, pairs of photos containing one opioid-related image and one neutral image were presented. On filler trials, pairs of neutral photos were presented. A set of 12 opioid images were chosen to represent a wide range of commonly prescribed opioids in a number of forms, including photos of pills (e.g., Oxycontin, Vicodin), pill bottles, crushed and powdered opioids for insufflation, and a syringe next to a vial of injectable morphine. Neutral images included 12 photos culled from the International Affective Picture System (Lang, Bradley, & Cuthbert, 1997) depicting household items such as a rolling pin, rubber bands, a lamp, etc. Each pair of images was presented for either 200 or 2000 ms. Presentation duration and left/right position of the images was randomized and counterbalanced across 12 filler trials and 64 critical trials. Both pictures disappeared, and a target probe (one dot) replaced one of the images after a 50 ms inter-stimulus interval (ISI). Probes appeared for 100 ms, and probe location was counterbalanced. Participants were instructed to indicate the location of the target by responding with a left or right button press on a keypad.
Opioid craving
Opioid craving was measured with a prescription opioid version of the Obsessive Compulsive Drug Use Scale (OCDUS; α = .81 in the present sample) modified by the authors for this study and scored on a 5-point Likert-type scale that assessed how often over the past week the respondent experienced craving for opioids (Franken, Hendriksa, & van den Brink, 2002). OCDUS exhibits convergent validity via significant correlations with AB and psychophysiological responses to opiate cues (Franken, Kroon, Wiers, & Jansen, 2000; Zijlstra, Veltman, Booij, van den Brink, & Franken, 2009). The wording of items was changed slightly to reflect prescription opioid use; e.g., the item “How strong is the drive to take drugs?” was changed to “How strong is the drive to take your pain medicine?”
Opioid arousal
Participants were asked to rate how they felt in response to viewing the opioid cues by rating the perceived arousing quality of opioid photos on a 9 point Likert-type scale. The arousal scale was anchored with 1 = completely unaroused (e.g., calm, sluggish, dull) and 9 = completely aroused (e.g., stimulated, excited, jittery). Such rating scales for stimulus-related arousal have been employed in numerous studies using the International Affective Picture System (Lang et al., 1997).
Pain severity and relief from pain treatments
Pain severity and relief obtained from pain treatments (e.g., medication) was assessed with the Brief Pain Inventory-Short Form, a widely-used, well-validated scale (BPI-SF, α = .88 in the present sample). The BPI-SF pain severity scale is computed by summing responses on four 11-point scales rating level of pain (0 = no pain, 10 = pain as bad as you can imagine) at its worst, best, average, and right now. The BPI-SF assesses pain relief with a single question “In the past 24 hours, how much relief have pain treatments or medications provided?” scored on an 11-point scale ranging from 0 to 100% relief.
Data Analysis
With regard to the analysis of AB data for each participant, trials with extreme RTs, defined as those with RTs 3 SD above or below the individual mean RT (c.f., Field, Mogg, Zetteler, & Bradley, 2004; Ratcliff, 1993), were eliminated as outliers (for opioid-dependent individuals, a mean 0.63 ± 1.31 trials were discarded; for non-dependent opioid users, a mean 0.48 ± 1.31 trials were discarded). Trials on which the probe location was incorrectly identified were also omitted in AB analyses; opioid-dependents incorrectly identified the probe location of 14.3% of trials, whereas non-dependent opioid users incorrectly identified the probe location on 13.6% of trials.
AB scores were calculated by subtracting their mean RT to target probes replacing opioid photos from their mean RT to target probes replacing neutral photos, such that positive bias scores indicate an AB toward opioid cues. One-sample Kolmogorov-Smirnov tests revealed that the distributions of the 200ms AB scores (M = 17.99, SD = 34.31; skewness = .58, SE = .30; kurtosis = 2.11, SE = .59) and 2000 ms AB scores (M = 5.26, SD = 38.78; skewness = 1.92, SE = .30; kurtosis = 6.72, SE = .59) did not significantly differ from a normal distribution, p = .698 and p = .164, respectively. We employed analyses of variance (ANOVA) to identify between-groups (opioid-dependent vs. non-dependent opioid user) and within-groups (presentation duration) differences in AB scores for stimuli presented for 200 and 2000 ms. ANOVA was also used to identify differences in ratings of arousal for opioid-related and neutral photographs on the picture rating task. Pearson correlation coefficients were used to examine associations between AB scores, opioid craving, opioid misuse behaviors, pain severity, relief from pain treatments, and mean arousal ratings for opioid and neutral cues.
Sensitivity analysis
To assure the robustness of our findings, we conducted the following sensitivity analyses. Though the distribution of the RT data did not significantly differ from normal, we first log transformed the RTs on trials where the location of the probe was cued by drug cues and the RTs on trials where the location of the probe was cued by neutral cues to compute log transformed AB scores. To check for distortions due to skew or kurtosis, we then re-ran the ANOVA on the log transformed AB data. Next, to ensure that potential between-groups differences in comorbid alcohol and substance use disorders did not confound AB results, these variables were controlled for in an additional ANOVA model as covariates. Last, because tramadol and suboxone putatively have lower abuse liability than other opioid agents, we ran another ANOVA on AB scores after omitting participants who used these medications.
RESULTS
Group characteristics
Summary data for the opioid-dependent individuals (n = 32) and non-dependent opioid users (n = 33) are shown in Table 2. Opioid-dependent individuals had significantly higher levels of craving than non-dependent opioid users. The opioid-dependent and non-dependent opioid user groups did not significantly differ with regard to pain severity or percent relief from pain treatments, nor did they significantly differ with regard to age or gender composition. Pearson chi-square analysis indicated that the opioid-dependent and non-dependent opioid user groups did not significantly differ with regard to the types of opioids used, nor did they significantly differ with regard to history of comorbid alcohol and substance use disorders. Among the opioid-dependent group, 5 individuals met diagnostic criteria for alcohol use disorders and 4 met criteria for substance use disorders, whereas among the non-dependent opioid user group, 2 individuals met criteria for alcohol use disorders and 2 met criteria for substance use disorders. Cannabis was the most commonly reported illicit drug used by participants (n = 12), followed by cocaine (n = 2) and heroin (n = 1).
Table 2.
Characteristics of opioid dependent and non-dependent opioid user groups.
| Opioid Dependents (n=32) |
Non-Dependent Users (n=33) |
t- or χ2statistic |
p-value | |
|---|---|---|---|---|
| Age (years) | 44.86 (SD = 15.45, range 18 – 65) |
48.59 (SD = 11.07, range 20 – 68) |
1.07 | .27 |
| Gender (men, %) | n = 11 (34.4%) | n = 16 (48.0%) | 1.60 | .31 |
| Opioid Craving | 16.42 (SD = 8.70, range 1 – 29) |
9.18 (SD = 5.53, range 0 – 18) |
3.95 | <.001 |
| Comorbid Alcohol Use Disorder |
n = 5 (15.6%) | n = 2 (6.1%) | 1.55 | .21 |
| Comorbid Substance Use Disorder |
n = 4 (12.4%) | n = 2 (6.1%) | .80 | .43 |
| Pain Severity | 5.26 (SD = 1.78, range 1 – 7.50) |
5.88 (SD = 1.56, range 2.25 – 9.5) |
1.48 | .14 |
| Relief from Pain Treatments |
53.89 (SD = 23.82, range 20 – 90) |
57.67 (SD = 23.79, range 10 – 80) |
.70 | .48 |
Between-groups differences in opioid attentional bias
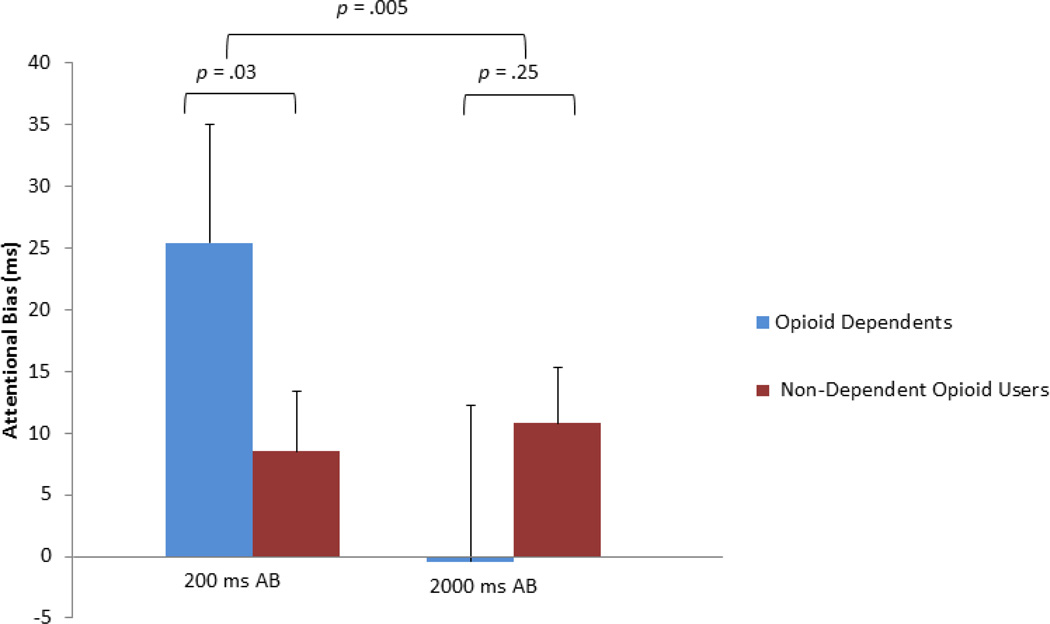
A 2 (Group: opioid-dependents, non-dependent opioid users) × 2 (Presentation duration: 200ms, 2000ms) repeated-measures ANOVA, with photo presentation duration (200 or 2000 ms) as a within-subjects factor and group (opioid-dependent vs. non-dependent opioid user) as a between-subjects factor revealed a significant effect for presentation duration, F(1,62) = 5.97, p = .017, ηρ2 = .09, as well as a significant group × presentation duration interaction, F(1,62) = 8.51, p = .005, ηρ2 = .12. Post-hoc tests indicated that opioid-dependent individuals had significantly greater 200ms AB than opioid non-dependents, F(1,64) = 4.98, p = .029, ηρ2 = .07; at this brief stimulus duration, opioid misusers had shorter reaction times to probes replacing opioid cues relative to probes replacing neutral cues, indicating the presence of an AB toward opioid cues (see Figure 1). However, there was no significant between-groups difference in 2000ms AB, F(1,63) = 1.34, p = .25, ηρ2 = .02. Within-group, one sample t-tests were used to determine whether these AB scores were significantly different than zero. Among opioiddependent individuals, the mean 200ms AB was significantly different from zero, t(31) = 5.27, p < .001, whereas the mean 2000ms AB was not, t (30) = −.08, p = .93. Among non-dependent opioid users, neither the mean 200ms nor the mean 2000ms AB were significantly different from zero, t(32) = 1.32, p = .20, and t(32) = 1.34, p = .19, respectively. The pattern and significance of ANOVA results did not differ substantially when repeated on log reaction time data. Similarly, the pattern and significance of results did not differ when persons taking tramadol or suboxone were omitted from the analyses, or when comorbid alcohol/substance dependence and abuse diagnoses were controlled for in the ANOVA model as covariates.
Figure 1.
Attentional bias 200 and 2000 ms stimulus presentation durations among opioid dependent (n = 32) and non-dependent opioid-using chronic pain patients (n = 33). Positive values denote a bias toward opioid photos. Negative values denote a bias away from opioid photos.
Arousal ratings
Mean arousal ratings were calculated for opioid-related and neutral images for each participant. A 2 × 2 ANOVA was conducted on the arousal ratings, with cue type (opioid vs. neutral) and participant group (opioid-dependent vs. non-dependent opioid user) as independent variables. The arousal analysis revealed a significant main effect for cue type, F(1,63) = 20.76, p < .001, ηρ2 = .25, but no significant group × cue type interaction effect, F(1,63) = 1.42, p = .24, ηρ2 = .02. Both opioid-dependent individuals and non-dependent opioid users rated the opioid photos as more arousing than the neutral photos.
Associations between Opioid AB and Opioid-Related Clinical Factors
Correlations between opioid AB and opioid-related clinical variables were computed within the opioid-dependent and non-dependent opioid user groups separately. Among opioid-dependent individuals, opioid craving as measured by the OCDUS was significantly correlated with opioid 200 ms AB, r = .36, p = .046. In contrast, 200 ms AB was not significantly associated with pain severity or relief from pain treatments among these opioid-dependent individuals. Among this opioid-dependent subsample, 2000 ms AB was not significantly correlated with opioid craving, percent of pain relief provided by treatments, or pain severity.
With regard to the subsample of non-dependent opioid users, 200 ms AB was significantly correlated with relief from pain treatments, r = .37, p = .03, but was not significantly associated with opioid craving or pain severity. Among non-dependent opioid users 2000 ms AB was not significantly associated with craving, relief from pain treatments, or pain severity.
Both within and across the opioid-dependent and non-dependent opioid user groups, the subjective arousal of the opioid photos was not significantly associated opioid AB, nor were the arousal ratings associated with most of the other clinically-relevant variables assessed in this study. However, among opioid-dependent individuals, accuracy on the dot probe task was inversely correlated with arousal ratings of opioid photos, r = −.37, p = .04. In addition, across the entire sample, opioid craving was significantly associated with arousal ratings of opioid photos, r = .34, p = .006.
DISCUSSION
The current study provides the first evidence that opioid-dependent chronic pain patients exhibit an AB towards prescription opioid-related cues beyond that of a comparison group of non-dependent opioid users with chronic pain. This AB was evident for opioid cues presented for brief (200 ms), but not for long (2000 ms) durations. In addition, opioid-dependent individuals reported significantly higher levels of craving than non-dependent opioid users, but did not significantly differ with regard to pain severity or degree of relief from pain treatments. Among opioid-dependent individuals, the 200 ms AB was significantly positively associated with opioid craving, whereas among non-dependent opioid users, the 200 ms AB was significantly positively associated with degree of relief from pain treatments. This differential pattern of association may suggest that the 200 ms AB reflects appetitive responses among opioid-dependent individuals.
The AB towards opioid-related cues observed among this sample of opioid-dependent chronic pain patients occurred for stimuli presented for 200 ms. Some investigators contend that the 200 ms AB indexes automatic attentional orienting to motivationally salient stimuli that arises out of a history of conditioning (Field & Cox, 2008). Indeed, exposure to cues associated with recurrent episodes of substance use results in a conditioned appetitive response (O’Brien et al. 1998). Neural sensitization to the rewarding effects of the substance and to substance-related cues is theorized to impart motivational or incentive salience to these cues coupled with a “wanting” for the substance (Robinson & Berridge, 2001; Robinson & Berridge, 2008). It is possible that the opioid images held greater incentive salience for opioid-dependent chronic pain patients than the neutral images, and thus biased attention towards the location of the probe at an early stage of attentional selection. This interpretation is consistent with our finding that the degree to which an individual’s attention was biased towards these brief exposures to drug cues was positively associated with their self-reported craving for opioids. Although in clinical practice chronic pain patients often deny experiencing a euphoric effect from taking opioids and tend to be more concerned with avoiding the experience of pain between opioid doses than with craving, research suggests that a substantial proportion of chronic pain patients do report craving for prescription opioids (Wasan et al., 2009; Wasan et al., 2012). In the current study, participants who experienced higher levels of craving as indicated by the presence of compulsive and intrusive thoughts and feelings related to opioid use had larger AB towards opioid cues than those who reported less craving. In contrast, opioid-dependent individuals did not evidence a significant AB toward opioid cues presented for 2000 ms. These findings may suggest that among the current opioid-dependent subsample, attention was biased at earlier stages of appetitive orienting, rather than at the later stages of attentional disengagement or elaboration. To test this hypothesis, eye tracking and event-related potential (ERP) analyses are needed to triangulate data from the dot probe task.
Individual differences analyses suggested that the 200 ms AB among non-dependent users was significantly associated with the degree of relief obtained from pain treatments. Thus, among this non-dependent, opioid-using subsample, it is possible that attention to brief opioid cues was influenced by reinforcement conditioning stemming from the analgesic effect of opioids (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Fields, 2004). Indeed, pain relief and reward appear to share common neural substrates (Baliki, Geha, Fields, Apkarian, 2010). However, it should be noted that as a group the non-dependent opioid users did not exhibit a significant mean 200 ms AB, and the observed relation between AB and pain relief among this subsample was only evident in correlational analyses.
Alternatively, the observed results may be explicated by a learning theory explanation (Pearce & Bouton, 2001). From this perspective, over the course of repeated opioid use, the sight of a pill or pill bottle may become a conditioned stimulus (the CS) that is paired with pharmacologic action of opioids in the brain (the UCS). In the typical experience of an opioid-using chronic pain patient, this pairing may remain consistent and intact – the sight of opioid medication is followed by consumption of the opioid and the resultant psychoactive effect. However, in the dot probe task used in the current study, these conditioned stimuli are presented in the absence of the drug - a procedure that is methodologically similar to a Pavlovian extinction trial (Pavlov, 1927). Extinction trials elicit enhanced attention to the CS, putatively due to a prediction error generated by the presentation of the CS without the UCS (Larrauri & Schmajuk, 2008; Pearce & Hall, 1980). As such, the dot probe task itself may evoke heightened attention to opioid cues for persons with a history of opioid use. Because the task was held constant between groups, observed differences in opioid AB may stem from the comparatively greater associative strength of opioid cues for opioid-dependent patients, resulting in a more robust prediction error when the CS is not followed by the UCS on dot probe trials (Pearce & Bouton, 2001).
Notably, both the opioid-dependent and the non-dependent opioid users found the opioid-related images presented in the dot probe task to be more arousing than neutral images, and across the entire sample, opioid arousal ratings were significantly positively correlated with opioid craving. Stimulus-related arousal reflects bottom-up processing via a cortico-limbic loop (Kilpatrick & Cahill, 2003) and drug preoccupation and craving are posited to be subserved via a similar cortico-limbic loop (Koob & Volkow, 2009) - activation of this neural circuitry is known to prime attention (Ledoux, 1995). Thus, one possible neurobiological explanation of these findings is that presentation of opioid cues may have activated cortico-limbic circuits, particularly among opioid-dependent individuals and thereby primed attention for the appearance of the probe in its stead. A primary model of drug addiction more broadly posits that drug dependence is associated with modulation and long-term changes in the neurobiological function of the mesolimbic dopamine reward system (Wolf, 2002; see Koob & Volkow, 2009). Human neuroimaging research has provided preliminary support for this model by finding that self-reported craving predicts the magnitude of dopamine release in the striatum elicited by drug cues (Volkow et al. 2008). Furthermore, aberrant neural function in frontal-executive regions subserving attention and inhibitory control is reported across multiple substance abuse populations including heroin (Yang et al. 2009), cocaine (Garavan, Kaufman, & Hester, 2008), and nicotine (Froeliger, Modlin, Kozink & McClernon, 2011; Froeliger, Modlin, Kozink, Wang & McClernon, 2011) dependent individuals. Among alcohol-dependent persons, degree of AB toward alcohol cues has been significantly associated with increased activation in the ventral striatum, insula, inferior frontal gyrus, and anterior cingulate, insula, and ventral striatum (Vollstädt-Klein, et al., 2011). Speculatively, dysregulation in similar frontal and subcortical regions might partially explain the observed associations between AB and craving response among this sample of individuals exhibiting clinically-disordered, addictive behavior towards prescription opioid medications.
Strengths of the study include the novel application of a dot probe task, a validated behavioral measure that has been broadly employed in cognitive neuroscience research, to help illuminate a clinical issue of considerable significance to public health in the U.S. Yet, the present study was limited in several respects. No biochemical measures were used to corroborate self-reports of opioid use; urine toxicology screens would have helped to establish the presence of inclusion and exclusion criteria. Unfortunately, no resources were available for urine toxicology screens. We did not conduct formal sobriety testing at the time of the study assessment, nor did we measure opioid withdrawal. It is possible that AB effects were influenced by acute opioid intoxication or withdrawal, as many pain patients withdraw even on stable doses and/or between doses of short-acting medications. Relatedly, chronic pain patients tend to experience impaired concentration, and sitting for long periods of time during the testing procedure may have been painful for these patients; thus performance on the dot probe task could have been influenced by these factors. Also, we employed a prescription opioid version of the OCDUS, a measure which was originally designed to assess craving for illicit drugs like heroin, not prescribed opioid analgesics. Although this modified version of the scale evidenced good internal consistency, the measure has not been formally validated in this context. However, given the significant correlation between this modified OCDUS and a behavioral measure of appetitive processes (i.e., 200 ms AB) and arousal elicited by opioid photos, this scale does seem to be capturing the construct of interest in this sample of opioid-dependent chronic pain patients. Lastly, though all participants had been treated for chronic pain with opioids for more than 90 days, we did not have information on the exact duration of opioid use or chronic pain. While we asked an open-ended question about opioid dosing, missing data and the extreme variability in quality of responses made it impossible to quantify this variable for use as a covariate in the current study. It is possible that some of the observed differences in AB stem from variables unaccounted for in the present analysis, such as education level, mood, activity interference, use of non-opioid medications, and past history of pain-related surgery. For example, patients who have taken opioids for a longer period of time are likely to exhibit more severe opioid misuse than those who have taken opioids for less time (Green, Black, Serrano, Budman, & Butler, 2011), and thus chronicity of pain condition and duration of opioid dosing may have contributed to opioid AB. Therefore, study findings should be considered preliminary in nature and will require rigorous validation.
Future studies should redress the foregoing limitations and couple AB measurement with psychophysiological and neuroimaging paradigms to more precisely elucidate mechanisms underlying this phenomenon (e.g., Garland, 2011). In particular, eye tracking and event-related potential (ERP) analyses could be used to isolate the temporal components and dynamics of the opioid AB, and functional magnetic resonance imaging (fMRI) studies might identify the neural substrates of dysregulated bottom-up and top-down neural circuitry function. Furthermore, it would be optimal to include a third comparison group of opioid users without chronic pain to disentangle relationships between opioid AB and chronic pain. In addition, measures of opioid misuse (e.g., the Current Opioid Misuse Measure, Butler et al., 2007) could be used as an alternative to or in conjunction with DSM-IV criteria to determine if patients who are at higher risk for disordered opioid use exhibit greater opioid AB. Lastly, to disentangle the effects of opioid dose-response curves from addictive responses, relative opioid potency estimates and opioid dosing frequency and duration histories should be controlled for in future analyses.
In sum, this study presents the first evidence of an AB towards prescription opioid analgesics among opioid-dependent chronic pain patients. Although many such patients are reluctant to report addictive tendencies towards opioid medication, these individuals may exhibit behaviors reflective of a tendency to preferentially attend to opioid-related stimuli which could indicate the presence of an implicit appetitive process that may operate beneath consciousness. Thus, should opioid AB continue to be observed among opioid-dependent chronic pain patients in future studies, this measure may hold utility as a clinical assessment tool. Hypothetically, when coupled with ever-worsening hyperalgesia and hypervigilance for pain (Garland, in press), attentional fixation on opioid cues may fuel a downward spiral of compulsive drug use that leads to opioid addiction and significant functional impairment. Novel interventions that can facilitate attentional regulation of cue-reactivity (e.g., mindfulness-based therapies; see Garland, 2011; Garland, Gaylord, Boettiger, & Howard, 2010) while reducing chronic pain may be especially efficacious means of addressing this pernicious and prevalent social problem.
Acknowledgements
The first author (E.L.G.) was supported in developing this manuscript by Grant Number DA032517 from the National Institute of Drug Abuse, a grant from the Fahs Beck Fund for Research and Experimentation, and a grant from the Florida State University Council on Research and Creativity. We would like to thank Jaclyn Williams, Olivia Garrison, Susan Howell, and Hillary Gale for their assistance in data collection and data entry.
Contributor Information
Eric L. Garland, Florida State University
Brett Froeliger, Duke University.
Steven D. Passik, Vanderbilt University
Matthew O. Howard, University of North Carolina at Chapel Hill
References
- American Pain Society. Decade of Pain Report. 2002 Retrieved February 2, 2012, from http://www.ampainsoc.org/pub/ bulletin/jul02/pres.html. [Google Scholar]
- Andreassi JL. Psychophysiology human behavior and physiological response. 4th ed. Mahwah, NJ: L. Erlbaum, Publishers; 2000. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeski MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:35–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearre L, Sturt P, Bruce G, Jones BT. Heroin-related attentional bias and monthly frequency of heroin use are positively associated in attenders of a harm reduction service. Addictive Behaviors. 2007;32:784–792. doi: 10.1016/j.addbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fanciullo GJ, Jamison RN. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clinical Journal of Pain. 2010;26:770–776. doi: 10.1097/AJP.0b013e3181f195ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clinical Journal of Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Constantinou N, Morgan CJ, Battistella S, O’Ryan D, Davis P, Curran HV. Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug and Alcohol Dependence. 2010;109:220–225. doi: 10.1016/j.drugalcdep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Denisco RA, Chandler RK, Compton WM. Addressing the intersecting problems of opioid misuse and chronic pain treatment. Experimental and Clinical Psychopharmacology. 2008;16:417–428. doi: 10.1037/a0013636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. European Journal of Pharmacology. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369:313–315. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: The roles of initial orienting and maintained attention. Psychopharmacology. 2004;176:88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological Bulletin. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. State dependent opioid control of pain. Nature Reviews Neuroscience. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink - a tool for investigating early and late attentional processes. Biological Psychology. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: Integrating psychological and neuropsychopharmacological approaches. Progress in Neuropsychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriksa VM, van den Brink W. Initial validation of two opiate craving questionnaires: the Obsessive Compulsive Drug Use Scale and the Desires for Drug Questionnaire. Addictive Behaviors. 2002;27:675–685. doi: 10.1016/s0306-4603(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Franken IH, Kroon LY, Wiers RW, Jansen A. Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology. 2000;14:395. doi: 10.1177/026988110001400408. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin L, Wang L, Kozink RV, McClernon FJ. Nicotine withdrawal modulates frontal brain function during an affective Stroop task. Psychopharmacology. 2012a;220:707–718. doi: 10.1007/s00213-011-2522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, McClernon FJ. Smoking abstinence and depressive symptoms modulate the executive control system during emotional information processing. Addiction Biology. 2012b;17:668–679. doi: 10.1111/j.1369-1600.2011.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL. Trait mindfulness predicts attentional and autonomic regulation of alcohol cue-reactivity. Journal of Psychophysiology. 2011;25:180–189. doi: 10.1027/0269-8803/a000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Primary Care: Clinics in Office Practice. doi: 10.1016/j.pop.2012.06.013. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Howard MO. Targeting cognitive-affective risk mechanisms in stress-precipitated alcohol dependence: An integrated, biopsychosocial model of allostasis, automaticity, and addiction. Medical Hypotheses. 2011;76:745–754. doi: 10.1016/j.mehy.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results of a randomized controlled pilot trial. Journal of Psychoactive Drugs. 2010;42:177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IH, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology. 2012 doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Black R, Grimes Serrano JM, Budman SH, Butler SF. Typologies of prescription opioid use in a large sample of adults assessed for substance abuse treatment. PLOS One. 2011;6:e27244. doi: 10.1371/journal.pone.0027244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago D, Ott U. How does mindfulness meditation Work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, et al. Predictors of opioid misuse in patients with chronic pain: A prospective cohort study. BMC Health Services Research. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff MV, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, et al. De facto long-term opioid therapy for noncancer pain. The Clinical Journal of Pain. 2008;24:521. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4. The Center for Research in Psychophysiology, University of Florida; 1999. International affective picture system (IAPS): Instruction manual and affective ratings. [Google Scholar]
- Laurrauri JA, Schmajuk NA. Attentional, associative, and configural mechanisms in extinction. Psychological Review. 2008;115:640–676. doi: 10.1037/0033-295X.115.3.640. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychological Medicine. 2000;30:169–175. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? Journal of Psychopharmacology. 1998;12:15. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clinic Proceedings. 2009;84:593–601. doi: 10.1016/S0025-6196(11)60748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford: Oxford University Press; 1927. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Pearce JM, Bouton ME. Theories of associative learning in animals. Annual Review of Psychology. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren L. Kicking the habit: The neural basis of ingrained behaviors in cocaine addiction. Neuroscience and Biobehavioral Reviews. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. Methods of dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. Journal of Psychosomatic Research. 2010;68:29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M I N I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and Medicaid insurance plans: The TROUP Study. Pain. 2010;150:332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Neurobiology of attention. London: Elsevier Academic Press; 2005. Irrelevant singletons capture attention; pp. 418–424. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann K, Kiefer F. Validating incentive salience with functional magnetic resonance imaging: Association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction Biology. 2011 doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Marhe R, Franken HA. Attentional bias to drug cues is elevated before and during temptations to use heroin in cocaine. Psychopharmacology. 2012;219:909–921. doi: 10.1007/s00213-011-2424-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clinical Journal of Pain. 2009;25:193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving for prescription opioids in patients with chronic pain: A longitudinal outcomes trial. The Journal of Pain. 2012;13:146–154. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JF. Strategies to stop abuse of prescribed opioid drugs. Annals of Internal Medicine. 2007;146:897–900. doi: 10.7326/0003-4819-146-12-200706190-00017. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: Making the connection between behavioral changes and neuronal plasticity in specific pathways. Molecular Interventions. 2002;2:146–157. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xie J, Shao Y-C, Xie C-M, Fu L-P, Li D-J, Fan M, Ma L, Li S-J. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: An fMRI study. Human Brain Mapping. 2009;30:766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: Position statement. Drug and Alcohol Dependence. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IHA. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug and Alcohol Dependence. 2009;99:183–192. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]