Abstract
Background
The influence of repeated substance use during adolescent neurodevelopment remains unclear as there have been few prospective investigations. The aims of this study were to identify longitudinal changes in fiber tract integrity associated with alcohol and marijuana use severity over the course of 1.5 years.
Method
Adolescents with extensive marijuana and alcohol use histories by mid-adolescence (n = 41) and youth with consistently minimal if any substance use (n = 51) were followed over 18 months. Teens received diffusion tensor imaging and detailed substance use assessments with toxicology screening at baseline and 18-month follow-ups (i.e., 182 scans in all), as well as interim substance use interviews each 6 months.
Results
At 18-month follow-up, substance users showed poorer white matter integrity in seven tracts: (1) right superior longitudinal fasciculus, (2) left superior longitudinal fasciculus, (3) right posterior thalamic radiations, (4) right prefrontal thalamic fibers, (5) right superior temporal gyrus white matter, (6) right inferior longitudinal fasciculus, and (7) left posterior corona radiata (ps< .01). More alcohol use during the interscan interval predicted higher mean diffusivity (i.e., worsened integrity) in right (p<.05) and left (p=.06) superior longitudinal fasciculi, above and beyond baseline values in these bundles. Marijuana use during the interscan interval did not predict change over time. More externalizing behaviors at Time 1 predicted lower fractional anisotropy and higher radial diffusivity (i.e., poorer integrity) of the right prefrontal thalamic fibers (p<.025).
Conclusion
Findings add to previous cross sectional studies reporting white matter disadvantages in youth with substance use histories. In particular, alcohol use during adolescent neurodevelopment may be linked to reductions in white matter quality in association fiber tracts with frontal connections. In contrast, youth who engage in a variety of risk taking behaviors may have unique neurodevelopmental trajectories characterized by truncated development in fronto-thalamic tracts, which could have functional and clinical consequences in young adulthood.
Keywords: Diffusion tensor imaging, Adolescence, Mean Diffusivity, Marijuana, Alcohol
Introduction
Over the past decade, significant progress has been made in understanding the effects of early substance use on adolescent brain development. Chronic use of alcohol and marijuana in youth is associated with poorer neural structure, function, and metabolism, and appears linked to worsened neurocognitive abilities into later adolescence and adulthood (Squeglia et al., 2009). The biological and psychosocial transitions occurring in adolescence result in unique vulnerability to neurotoxic influences. The long-term impact of risky behaviors such as substance use on neurodevelopment is grossly described in retrospective works, but has not been studied prospectively.
The two most widely examined intoxicants for youth and adults continue to be alcohol and marijuana (Johnston et al., 2012). Studies examining the neural effects of alcohol and marijuana, which are often used together, indicate diminutions in neuropsychological performance, particularly attention, visuospatial functioning, and learning and retrieval (Medina et al. 2007b). Heavy alcohol and marijuana use during adolescence is also associated with atypical morphology in the hippocampus (Medina et al., 2007), prefrontal cortex (Medina et al. 2008, 2009), and cerebellum (Medina et al., 2010); alterations in white matter anisotropy and diffusivity (Bava et al., 2010; De Bellis et al. 2008; Jacobus et al., 2009a;McQueeny et al., 2009) and a more distributed functional network and recruitment of alternate brain regions (Schweinsburg et al. 2010; Tapert et al. 2001).
Alterations in white matter integrity are of particular interest considering the ongoing maturation of fiber tracts during adolescence (Giorgio et al., 2008). Alcohol-using teens show anisotropic changes in the genu and isthmus of the corpus callosum (De Bellis et al., 2008) and those with binge-pattern use show dose dependent changes, where higher blood alcohol concentrations are associated with poorer tissue integrity in the corpus callosum, internal and external capsules, and superior corona radiata (McQueeny et al., 2009). White matter integrity is also poorer in marijuana users than non-users (as seen by increases in overall diffusivity and decreases in anisotropic diffusion), particularly in fronto-parietal circuitry and pathways connecting the frontal and temporal lobes (Bava et al., 2009).
The process by which substance use may modulate brain development is best examined within a longitudinal framework. In this study, we prospectively followed 92 adolescents over18 months to assess the influence of substance use on white matter integrity. We used diffusion tensor imaging (DTI), a method of tracking the diffusion of water molecules in cerebral tissue, to assess white matter quality. From this, we examined four scalar variables:fractional anisotropy (FA), an index of the directional coherence of diffusional motion; mean diffusivity (MD), a measure of the overall magnitude of diffusional motion; radial diffusivity (RD), a quantification of the magnitude of diffusion perpendicular to the main fiber axis; and axial diffusivity (AD), the magnitude of diffusion parallel to the fiber axis. High FA suggests strong fiber regularity and organization, but values may also reflect myelination and structural characteristics of the axon. Low MD values reflect greater white matter density (Roberts and Schwartz, 2007). Increases in FA and decreases in MD typically occur in white matter during adolescence (Giorgio et al., 2008; Schmithorst et al., 2002). These changes are often associated with diminutions in RD, possibly attributable to myelination or growth in fiber density, although decreases in RD are not always observed across adolescence (Ashtari et al., 2007; Giorgio et al., 2008). Similarly, both increases and decreases in AD have been reported in adolescence (Ashtari et al., 2007; Eluvathingal et al., 2007).
The literature on effects of heavy alcohol use in adulthood suggests that cellular remodeling, changes in both axon complexity and myelin disruption may be occurring and influencing diffusion parameters. Some evidence supports decreases in FA together with increases in MD may mark fluid accumulation in extracellular space that could be driven by alterations in myelin development, along with possible alterations in axon complexity (Pfefferbaum et al., 2009; Pfefferbaum and Sullivan, 2005). This may be particularly relevant for adolescence, when white matter development is continuing. Therefore, we hypothesized that longitudinal changes related to substance use in these indices of white matter integrity would be greatest in fronto-parietal tracts, given that these regions undergo significant developmental growth during adolescence (Ashtari et al., 2007; Giorgio et al., 2008). Considering the anisotropic and diffusivity abnormalities observed in several of these tracts in adolescent alcohol and marijuana users and adult alcoholics (Bava et al., 2009, 2010b; Pfefferbaum and Sullivan, 2005), we predicted that increased substance use would be associated with diminutions in FA and increases in MD and RD over late adolescence in fronto-parietal fiber bundles, including the superior longitudinal fasciculus, internal capsule, and the anterior and superior portions of the corona radiata.
Materials and Methods
1.1 Participants
Ninety-two adolescents (29 females and 63 males) ages 16 to 20 years (mean 18.1 ± 1.2) were selected from an ongoing longitudinal study of substance use in adolescence. Participants were recruited from local schools in 2005 to 2007. Inclusionary criteria required participants and their parents or legal guardians to provide a comprehensive developmental, medical, and mental health history of the adolescent. Adolescents and their parents were then screened with separate, private interviews to ascertain eligibility. Exclusionary criteria for both Users and Controls were: parental history of bipolar I, psychotic, or antisocial personality disorder; complicated or premature birth (<36 weeks gestation); evidence of maternal drinking or illicit drug use during pregnancy; left handedness; history of neurological disorder or head trauma with loss of consciousness >2 minutes, learning disability or mental retardation, serious medical illness, or DSM-IV Axis I disorder; use of psychoactive medications; MRI contraindications; and clinically abnormal brain anatomy as determined by neuroradiologist review.
Having surpassed these exclusionary criteria, participants were classified as: (1) Users with history of significant lifetime substance use (>100 episodes of marijuana or alcohol use and any range of cigarette and other illicit drug use; < 400 maximum lifetime other drug use episodes, excluding alcohol, marijuana, or nicotine; < 150 cigarettes per month); or (2) Controls with limited substance use history (i.e., lifetime use <10 episodes of marijuana use, <80 episodes of alcohol use, <10 cigarettes, <15 episodes of other drug use). Participants abstained from substance use for at least 24 hours before neuroimaging, to minimize the confound of acute or withdrawal effects during scanning, verified by serial urine toxicology (Smith et al., 2009) and breathalyzer. The most recent marijuana use occurred 3 days prior to imaging, last heavy alcohol use (≥4 or 5 alcoholic beverages in one sitting for females or males, respectively) was 2 days prior, most recent use of tobacco was 1 day prior, and most recent use of other drug use was 3 days prior. Users were excluded if they met criteria for a DSM-IV Axis I disorder other than a marijuana or alcohol use disorder. Participants with complete, valid baseline and follow-up data were 41 Users and 51 demographically similar Controls (see Table 1).
Table 1.
Characteristics of participants, at Time 1 unless otherwise noted.
| Controls (n=51) | Substance Users (n=41) | |
|---|---|---|
| M (SD) or % | M (SD) or % | |
| Age (range 16.26–20.94)* | 17.88 (1.07) | 18.42 (1.20) |
| Age (range 17.41–22.03), Time 2 * | 19.25 (1.08) | 19.81 (1.13) |
| Inter-scan interval (years) | 1.37 (0.17) | 1.38 (0.21) |
| Pubertal Development Scale Category Score (median) | 4 | 4 |
| Pubertal Development Scale Category Score (median), Time 2 | 4 | 5 |
| % Male | 73% | 63% |
| % Caucasian | 55% | 61% |
| Parental history of alcohol or drug abuse or dependence | 24% | 32% |
| Any familial history of psychopathologya | 73% | 81% |
| Hollingshead socioeconomic status index (n = 90) | 32.10 (16.98) | 25.77 (12.28) |
|
| ||
| WASI Vocabulary T-score | 59.04 (9.38) | 58.81 (8.47) |
| WRAT-3 Reading Standard Score | 109.57 (6.79) | 107.39 (7.72) |
|
| ||
| Spielberger State Anxiety total score | 26.61 (7.22) | 29.29 (8.82) |
| Spielberger State Anxiety total score, Time 2 | 25.78 (5.59) | 26.15 (6.02) |
| Beck Depression Inventory total score | 1.98 (2.29) | 3.07 (3.55) |
| Beck Depression Inventory total score, Time 2 | 1.94 (2.65) | 2.85 (3.56) |
| Internalizing Problems T-score (n = 81)b | 43.66 (7.46) | 45.79 (9.48) |
| Internalizing Problems T-score, Time 2b | 41.84 (8.79) | 43.63 (10.77) |
| Externalizing Problems T-score (n = 81)b** | 45.13 (8.95) | 51.03 (8.33) |
| Externalizing Problems T-score, Time 2b* | 48.84 (8.76) | 52.46 (7.69) |
|
| ||
| Baseline lifetime alcohol use episodes** | 17.47 (21.79) | 224.71 (179.75) |
| Alcohol use episodes over 18-month follow-up** | 69.73 (91.28) | 408.07 (312.99) |
| Baseline lifetime marijuana use episodes** | 1.06 (1.90) | 453.07 (309.60) |
| Marijuana use episodes over 18-month follow-up** | 12.92 (41.64) | 651.98 (418.76) |
| Baseline cigarettes smoked, past month* | 0.08 (0.56) | 8.58 (28.23) |
| Cigarettes smoked, past month at Time 2* | 0.51 (2.65) | 21.95 (61.90) |
| Lifetime other drug use episodes* | 0.29 (1.84) | 22.34 (62.57) |
| Other drug use episodes over 18-month follow-up** | 1.76 (6.63) | 36.51 (66.75) |
Includes depression, mania, anxiety, psychosis, or mental health hospitalization.
From the age-appropriate Child Behavior Checklist or Youth Self Report (Achenbach, 2001)
p<.05
p<.001
Abbreviations: WASI, Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999); WRAT-3, Wechsler Wide Range Achievement Test-3 (Wilkinson, 1993)
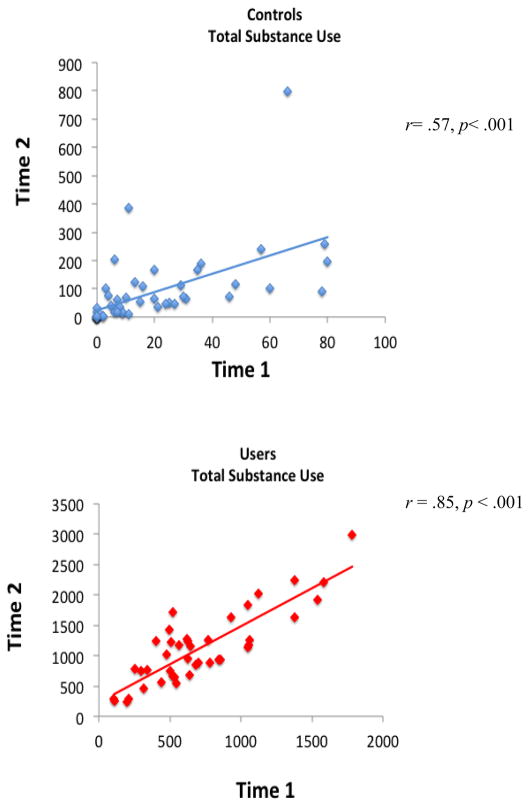
Participants received behavioral and neuropsychological measures after enrollment (Time 1), and at a follow-up (Time 2) ~18 months after the first imaging session (Time 2 mean age 19.5±1.1 years). Users and Controls showed no significant differences in interscan interval. Among Users, measures of depression and psychopathological syndromes remained stable and clinically non-significant from Time 1 to Time 2, except that self-reported anxiety state (STAI) decreased from Time 1 to Time 2 (t = 2.20, p = .03). Among Controls, emotional functioning measures remained stable and clinically non-significant from Time 1 to Time 2. The externalizing problems scale of the CBCL increased over the interscan interval among controls, but also remained clinically non-significant. Both Users and Controls tended to increase insubstance use over time (see Table 1), and for both groups, Time 1 total substance use positively correlated with Time 2 total use (Controls, r = .57; Users, r = .85, ps< .001; i.e., those with more lifetime use at baseline tended to use more over the 18-month follow-up interval (see Figure 1).
Figure 1.
Correlations between lifetime substance use by Time 1 and substance use over the 18-month follow-up reported at Time 2, for Controls and Users (ps < .001).
At enrollment and again at follow-up, for anyone under age 18, informed assent and consent were obtained from participants and legal guardians, respectively; or those age 18 or older, informed consent was obtained from the participant, and separately from the parent/guardian for their participation in completing an interview on their youth’s history and behaviors. Consent and assent procedures were in accordance with UCSD Human Research Protections Program guidelines.
1.2 Measures
1.2.1 Substance use
The Customary Drinking and Drug use Record (Brown et al., 1998) collected detailed information on quantity and frequency of lifetime alcohol, marijuana, cigarette, and other drug use. The Timeline Followback (Sobell and Sobell, 1992) assessed teens’ chronicity and intensity of substance use for each of the 28 days preceding the scan session (see Tapert et al., 2007). Substance use during the 18-month inter-scan interval was quantified as number of days used in each of four classes of substance: alcohol, marijuana, tobacco, and other drugs (e.g. amphetamines, hallucinogens, cocaine, inhalants, opiates, benzodiazepines, ecstasy, ketamine, PCP, and misuse of over-the-counter or prescription medications to getintoxicated).
1.2.2 Mental health
The Beck Depression Inventory (BDI) (Beck, 1978) and Spielberger State Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) assessed mood prior to scanning. The Child Behavior Checklist (CBCL) was completed by the parent at Time 1, and the Youth Self-Report (YSR; ages ≤ 18) or Adult Self-Report (ASR, ages ≥ 19) was completed by the participant at Time 2, to assess internalizing and externalizing psychopathological syndromes (Achenbach and Rescorla, 2001).
1.2.3 Development
The Pubertal Development Scale (PDS) (Petersen, 1988) collected self-reported indicators of participants’ pubertal status. The measure is comprised of 5 questions based on the Tanner stages that pertain to changes in height, body hair, and skin, and additionally menstruation for females and voice changes for males. Responses were summed and transformed into PDS Category Scores (1 = Prepubertal, 2 = Early pubertal, 3 = Midpubertal, 4 = Late pubertal, and 5 = Postpubertal) (Carskadon and Acebo, 1993). Body mass index of each adolescent was calculated as weight (lb)/([height (in)]2 × 703).
1.2.4 Cognition
At Time 1, participants were administered the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) Vocabulary subtest and Wide Range Achievement Test – 3 (Wilkinson, 1993) Reading subtest as estimates of premorbid intellectual functioning.
1.2.5 Family History
History of psychiatric and substance use disorders in biological parents and other first and second degree biological relatives was assessed by interview with biological parent or guardian using the Family History Assessment Module (FHAM) (Rice et al., 1995).
1.3 MR Acquisition
At Time 1 and Time 2, all participants were imaged in the 3T CXK4 short bore Excite-2 MR system (General Electric, Milwaukee, WI) with an 8-channel phase-array head coil (General Electric Medical System, Milwaukee, WI, USA) at the UCSD Keck fMRI Center. Each scan session included a 10-second scout scan to assure good head placement and slice selection covering the whole brain. Participants were placed comfortably on the scanner table and the head was stabilized within the head coil using foam cushions (NoMoCo Pillow, La Jolla, CA). Diffusion-weighted images were collected along 15 noncollinear directions determined by the electrostatic repulsion model which minimizes bias in measurements by sampling with approximately uniform distribution on a sphere (Jones et al., 1999), in addition to a reference image with no diffusion weighting (b=0). The diffusion encoding scheme consisted of a single-shot dual spin echo excitation optimized for minimum TE and reduction of eddy current artifacts (Reese et al., 2003). The following sequence parameters were applied and averaged over four volumes: TE/TR=93/12,000 ms, FOV=240 mm, matrix =128x128, 36 contiguous slices each 3 mm thick, b-value=2000 s/mm2. Two field maps were collected for unwarping (TE/TR=3.8/1,000 ms) to correct for signal loss and geometric distortion due to B0 field in homogeneities (Andersson and Skare, 2002).
1.4 Data Processing
Datasets were visually inspected slice-by-slice for each subject. One Control and two Users were excluded due to motion artifact, and one Control was excluded due to technical problems during scanning. Valid datasets (41 Users and 51 Controls with valid data at both time points) were corrected for head motion, eddy current distortion, and signal loss using FSL tools (FMRIB Software Library, Oxford, United Kingdom; (Smith et al., 2004). Specifically, image acquisitions for each direction were merged into a single 4D file and aligned to the first volume using affine registration with six degrees of freedom and Fourier interpolation to correct for motion (FLIRT-FMRIB’s Linear Image Registration Tool; (Jenkinson et al., 2002). Each of the 15 direction files were then registered to the B0 image using a six-parameter registration in 2D to minimize eddy current distortions (FDT-FMRIB’s Diffusion Toolbox 2.0; (Behrens et al., 2003). Next, phase unwrapping (PRELUDE-Phase Region Expanding Labeler for Unwrapping Discrete Estimates; (Jenkinson, 2003) and regularization (FUGUE-FMRIB’s Utility for Geometrically Unwarping EPIs; (Jenkinson and Smith, 2001) of field maps were conducted for quantifying field distortions. Resulting measurements were translated into voxel shifts, effectively assigning image intensities to correct voxel locations.
Pre-processed images were subjected to tensor decomposition to derive scalar diffusion indices FA, MD, AD, and RD (Le Bihan et al., 2001). This computation was performed in native coordinate space using Analysis of Functional NeuroImages’(Cox, 1996) diffusion plug-in routine, 3dDWItoDT (Cox and Glen, 2006). Diffusion indices were examined with Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006), and involved the following steps. First, to achieve initial alignment, FA maps from both time points were registered to an averaged FA template (FMRIB-58) in MNI-152 standard space using an affine-only registration. This was followed by a non-linear transformation into 1-mm cubic voxel dimensions (FNIRT-FMRIB’s Non-linear Registration Tool). Data were examined for laterality, orientation, and cross-subject anatomical alignment. Next, transformed images were averaged across subjects to create a mean FA image, from which a white matter skeleton was derived, representing tracts common to all subjects. Individual transformed FA images were then projected onto the skeleton. To minimize partial-volume effects and areas of high inter-subject variability, values were thresholded at FA>0.2. MD, AD, and RD data from Time 1 and 2 were processed using the same nonlinear transformation, skeleton, and skeleton-projection vectors derived from the FA analysis. FA, MD, AD, and RD data from each voxel on the skeleton from each subject at each time point formed the basis of statistical comparisons.
1.5 Statistical Analysis
To constrain the number of regions examined, we identify anatomical regions likely influenced by substance use by comparing Users and Controls on independent samples t-tests to voxels in skeleton space FA, MD, RD, and AD data from Time 2 using AFNI (afni.nimh.nih.gov/afni/; Cox et al., 1996). Multiple comparisons were corrected for with a combination of individual voxel probability and cluster size thresholding using Monte Carlo simulations. The typical intrinsic smoothness of skeletonized datasets on the order of 4 mm full width half maximum (Smith et al., 2006) was considered in determining the Type I error control. Only clusters ≥165 μl (165 contiguous 1 × 1 × 1 mm voxels) with an individual voxel effect of α<.01 were interpreted, yielding a brain-wise α<.05 of finding such a cluster under the null hypothesis. Cohen’s d effect sizes (Cohen, 1988) were computed from the average t-value within each significant cluster. Anatomical identification of tract structures was confirmed using a white matter atlas (Mori et al., 2008).
Clusters showing significant group differences in Time 2 FA, MD, RD, or AD were subjected to hierarchical regressions, to assess the influence of follow-up substance use on white matter anisotropy at Time 2 above and beyond the status of the tract at Time 1. The regression model entered Time 1 FA (or MD, RD, or AD) on step 1, covariates on step 2, and substance use during the inter-scan interval on step 3. Covariates included age at Time 1 and months between Time 1 and Time 2 scans. The externalizing problems T-score of the Achenbach behavior scales were found to differ between Users and Controls at Time 1 and Time 2 (p<.05), so were also included as covariates. Predictors of interest were number of days used in each of four classes of substance: alcohol, marijuana, tobacco, and other drugs; externalizing problems were also evaluated as a unique predictor of white matter change.
Results
Between-Group Differences in Participant Characteristics
Groups were statistically similar on gender distribution, ethnic composition, pubertal development, history of familial psychopathology and substance dependence, socioeconomic status, and interscan interval (see Table 1). Estimated premorbid IQ and academic reading achievement were also similar between groups, typically falling in the average to high average range. Measures of emotional functioning did not differ between groups and fell within normal limits at both time points. Users were about 6 months older than Controls on average (p<.05). Users had higher externalizing problems T-scores than Controls on the Achenbach behavior rating scales at Time 1 and Time 2, so these variables along with age, inter-scan interval, and Time 1 DTI indices were included as covariates in regression analyses.
Between-Group Differences in Time1 and 2 DTI Indices
Whole-brain independent samples t-tests revealed 3 clusters (each comprised of at least 165 contiguous voxels each with effect at α<.01) where Users had significantly lower FA than Controls at Time 2 (see Table 2): right prefrontal thalamic fibers, splenium, and posterior corona radiata, and (ps<.001 to .024). There were no regions where Users had higher FA than Controls. MD, RD, and AD were also subjected to whole-brain analysis in independent samples t-tests. Users showed significantly higher MD (42 clusters), RD (23 clusters), and AD (12 clusters) than Controls at Time 2 (each cluster ≥165 μl comprised of contiguous voxels each with effect at α<.01). No clusters were found in which Users showed lower MD, RD, or AD than Controls. Given the large number of significant between-group clusters found for the diffusivity indices, only clusters that exhibited the largest effect sizes in their index and showed anatomical overlap and correlation (α=.05) with clusters in other indices were analyzed. This yielded 7 clusters: (1) right superior longitudinal fasciculus, (2) left superior longitudinal fasciculus, (3) right posterior thalamic radiations, (4) right prefrontal thalamic fibers, (5) right superior temporal gyrus white matter, (6) right inferior longitudinal fasciculus, and (7) left posterior corona radiata (see Table 2, Figure 2).
Table 2.
Clusters showing significant differences between Users (n=41) and Controls (n=51) at Time 2 on fractional anisotropy, and those with strongest effects for mean diffusivity, radial diffusivity, and axial diffusivity (each cluster ≥ 165 contiguous voxels each with effect of α<.01).
| Anatomic Region | Cluster Size (Voxels) | MNI | Effect Size (Cohen’s d)† | Group Difference | ||
|---|---|---|---|---|---|---|
| Coordinates‡ | ||||||
| x | y | z | ||||
|
Fractional Anisotropy
| ||||||
| Splenium of the corpus callosum, right†† | 373 | 16 | −44 | 18 | 0.68 | Controls>Users |
| Prefrontal thalamic fibers, right * | 199 | 11 | −18 | 6 | 0.49 | Controls>Users |
| Posterior corona radiata, right†† | 248 | 27 | −36 | 23 | 0.34 | Controls>Users |
|
Mean Diffusivity (of 42 clusters)
| ||||||
| Posterior thalamic radiations, right *,†† | 1398 | 24 | −74 | 10 | 0.81 | Users>Controls |
| Prefrontal thalamic fibers, right * | 684 | 15 | −17 | 1 | 0.75 | Users>Controls |
| Posterior corona radiata, right | 209 | 27 | −35 | 25 | 0.72 | Users>Controls |
| Superior longitudinal fasciculus, left * | 2965 | 40 | 17 | 33 | 0.71 | Users>Controls |
| Superior longitudinal fasciculus, right * | 2976 | −33 | 19 | 40 | 0.69 | Users>Controls |
|
Radial Diffusivity (of 23 clusters)
| ||||||
| Prefrontal thalamic fibers, right * | 727 | 13 | −17 | 1 | 0.79 | Users>Controls |
| Inferior longitudinal fasciculus, right *,†† | 204 | 37 | −38 | −17 | 0.66 | Users>Controls |
| Superior temporal gyrus white matter, right * | 413 | 50 | −10 | −4 | 0.64 | Users>Controls |
| Posterior thalamic radiations, right * | 180 | 25 | −82 | 3 | 0.62 | Users>Controls |
| Superior longitudinal fasciculus, right * | 708 | −23 | 28 | 48 | 0.55 | Users>Controls |
| Superior longitudinal fasciculus, left * | 197 | 31 | 24 | 39 | 0.39 | Users>Controls |
|
Axial Diffusivity (of 12 clusters)
| ||||||
| Posterior corona radiata, left *, †† | 335 | −18 | −41 | 39 | 0.45 | Users>Controls |
Exhibited the largest effect sizes in their index and showed anatomical overlap and correlation (α=.05) with listed clusters in each diffusion category (e.g., FA, MD)
Coordinates of the center of mass
Effect size computed from the average t-value for each cluster
Exhibited between-group differences at Time 1 (p<.01) in same direction as group differences at Time 2.
Figure 2.
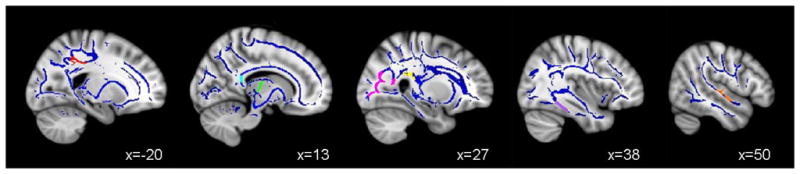
Clusters of significant white matter integrity differences between Controls and Users (ps < .01), in the (A) left posterior corona radiata (AD Users > AD Controls; red); (B) splenium of the corpus callosum (FA Users < FA Controls; cyan), right prefrontal thalamic fibers (FA Users < FA Controls; green and RD Users > RD Controls; green); (C) right posterior corona radiata (FA Users < FA Controls; magenta), right thalamic radiations (MD Users > MD Controls; yellow); (D) right superior longitudinal fasciculus (RD Users > RD Controls; purple); and (E) right superior temporal gyrus (RD Users > RD Controls; orange).
Between groups differences at Time 1 with effect at α<.01were also examined within these seven clusters identified by whole-brain analysis. Clusters within the splenium of the corpus callosum, right and left posterior corona radiata, right posterior thalamic radiations, and the right inferior longitudinal fasciculus showed differences at Time 1 in the same direction as Time 2 differences (see Table 2).
Predictors of Changes in White Matter Integrity over Time
To determine the influence of substance use over the inter-scan interval on white matter integrity at Time 2, hierarchical regression analyses were conducted on FA, MD, RD, and AD in the seven clusters found to show significant robust Time 2 between-group differences. Regression models entered the comparable Time 1 DTI measure on step 1; age at Time 1, months between Time 1 and Time 2 scans, and externalizing problems T-score at Time 1 and Time 2on step 2; and substance use during the inter-scan interval on step 3. Greater alcohol use over the inter-scan interval predicted higher MD in the right superior longitudinal fasciculus (F(9, 71)=3.3, p< .01; β=.30, p =.047, R2Δ=5%) at Time 2, above and beyond variability attributable to the equivalent Time 1 white matter integrity measure (see Figure 3),Time 1 age, inter-scan interval, and externalizing scores. A similar trend was observed for inter-scan alcohol use to predict high MD in the left superior longitudinal fasciculus (F(9,71) = 4.4,p< .01; β=.27, p=.06, R2Δ = 5%). More alcohol use during the inter-scan interval was also associated with higher AD in the left posterior corona radiata (F(9,71) = 13.9, p< .01; β =.32, p < .01, R2Δ = 6%) at Time 2 above and beyond covariates. The regressions were re-run adding family history of a substance use disorder to step 1, as well as examining the user group (n=41) independently. In the former analyses, greater alcohol use over the inter-scan interval continued to predict higher MD in the right (p = .05) and left (p =.06) superior longitudinal fasciculus, along with the AD in the left posterior corona radiata (p< .01 ) controlling for family history. Examining the user group only, days of alcohol use between scan sessions remained a significant predictor of MD in both the right (β =.50, p=.03) and left (β=.49, p<.04) superior longitudinal fasciculus, as well as AD in the left posterior corona radiata (p< .01); findings also remained significant within the user group after controlling for family history (ps< .03).Marijuana use severity over inter-scan interval did not predict diffusion indices at Time 2. (ps>.05).
Figure 3.
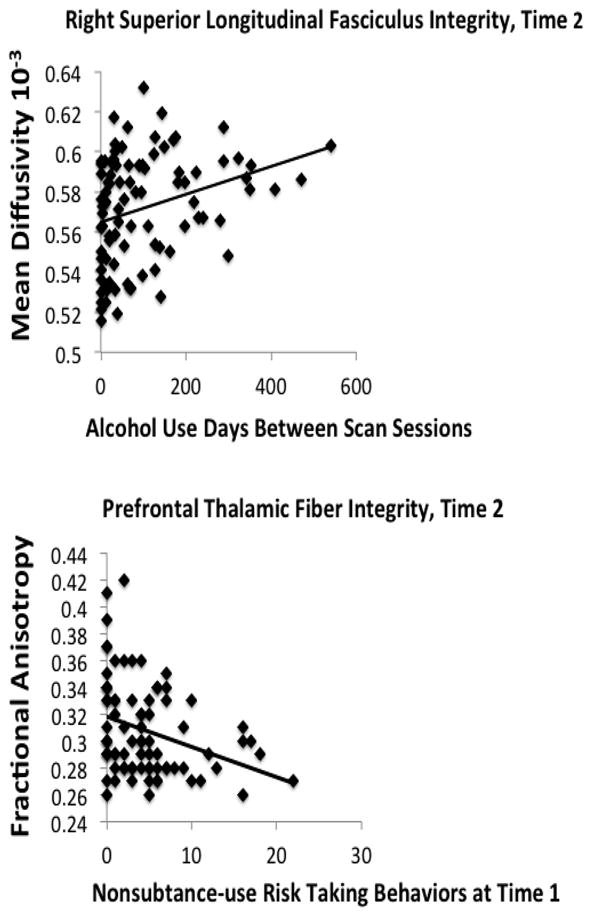
Bivariate correlations between (A) severity of alcohol use over follow-up and white matter integrity in cortical and subcortical fiber tracts (r = .30, p < .01) and (B) risk taking behaviors at baseline and follow-up white matter integrity (r = −.32 p < .01).
Externalizing problems at Time 1 and Time 2 were examined in separate regressions to reduce multicollinearity bias. Higher externalizing problem scores at Time 1 predicted lower Time 2 FA in the right prefrontal thalamic fibers (F(8,72)=2.1, p = .04; β=−.28, p=.016, R2Δ = 3%), and higher externalizing problem scores at Time 2 predicted lower Time 2FA in the splenium (F(8,83)=5.7, p<.01; β=−.22, p=.031, R2Δ=2%), both above and beyond variability accounted for by Time 1 age and duration between scans. To better understand whether white matter at Time 2 was associated with externalizing problems that are distinct from substance use behaviors, we examined a 26-item index of risk-taking generated from the Achenbach scales that does not include any items pertaining to substance use, reflecting non-substance use risk-taking such as aggressive and delinquent risk taking behaviors. The sum of these items yielded an index with good internal consistency (Cronbach’s alpha ≥ .80) (Jacobus et al., 2012). Similar to the findings above, higher risk-taking scores at Time 1 predicted lower FA (F(9,70)=2.1, p = .04;β=−2.7, p=.02, R2Δ =2%) and higher RD (F(8, 71)=4.0, p<.01;β=.23, p =.031, R2Δ=3%) in the prefrontal thalamic fibers at Time 2 (see Figure 3), above and beyond Time 1 values in this tract, age, and inter-scan duration.
Discussion
The current study examined cross-sectional differences and longitudinal changes in white matter anisotropy and diffusivity among adolescents with and without heavy alcohol and marijuana use patterns. As follow-up to our previous studies (e.g., Bava et al., 2010), which found between group differences at an initial baseline evaluation, this investigation continues to show disadvantaged integrity between groups 1.5 years later in numerous cortical and subcortical white matter fiber tracts (i.e., right superior longitudinal fasciculus, left superior longitudinal fasciculus, right posterior thalamic radiations, right prefrontal thalamic fibers, right superior temporal gyrus white matter, right inferior longitudinal fasciculus, and left posterior corona radiata).One important question is whether adolescent substance use is linked to changes over time in white matter quality indicators. We evaluated that greater alcohol use over an interscan interval during mid/late adolescence predicted worsened white matter integrity at follow-up in the right and left superior longitudinal fasciculus and posterior corona radiata, above and beyond baseline white matter integrity. This finding suggests more definitively than previous cross-sectional studies that heavy drinking during adolescence may have harmful effects on white matter development.
These findings are consistent with other cross sectional studies from our lab implicating severity of substance use (alcohol use in particular) in structural tissue alterations in both association and projection fiber tracts (Bava et al. 2009; Jacobus et al., 2009;McQueeny et al., 2009; Medina et al., 2008); however, this is the first study to show that alcohol use may prospectively predict structural tissue changes independently of baseline DTI indices and marijuana use. Specifically, we found that, in youth who increased their drinking in late adolescence, mean diffusivity increased in the right and left superior longitudinal fasciculi. Research routinely shows this large fiber tract to undergo microstructural changes throughout adolescence and subserve improving neurocognitive functioning, spanning general intellectual functioning to working memory (Brauer et al., 2011). Alterations in microstructural and functional integrity in adolescent alcohol users in the superior longitudinal fasciculus and overlapping regions have been observed in both adolescents (Bava et al., 2009;Schweinsburg et al., 2010; Tapert et al., 2001), as well as adults (Pfefferbaum et al., 2009) and may represent alcohol-related neurotoxicity in cortical brain regions important for fronto-parietal-temporal networks.
We hypothesized both decreased FA and increased MD, together, would be seen in clusters showing between-group white matter differences. While we observed this to be the case in some regions (e.g., thalamic fiber tracts, posterior corona radiata), but did not see evidence for decreased anisotropic diffusion along with increased overall diffusion in all cases. Interestingly, more alcohol use over interscan interval was related to increased overall diffusivity (i.e., MD) in bilateral superior longitudinal fasciculus at follow-up, but not with changes in FA. It is possible that alcohol-related excitotoxic or pro-inflammatory stress (Crews and Nixon, 2009) leads to residual swelling or remodeling of myelin during this important developmental time period. Given that these predictive associations appear to be driven by mean diffusivity, it is possible that fluid accumulation in the extracellular space is related to alterations in myelin (vs. intracellular fluid or changes in interstitial complexity) (Pfefferbaum et al., 2009; Pfefferbaum and Sullivan, 2005). While this remains speculative, future projects utilizing advanced technology such as restriction spectrum imaging (RSI; White et al., in press) will allow us to make better inferences about the pathology driving changes in tissue microstructure (e.g., compromised myelination versus extracellular fluid versus reduced axon caliber).
We observed an increase in AD in the posterior corona radiata with greater alcohol use over interscan interval; although increased AD is typically thought to represent increased neuronal integrity and axonal development, unexpected positive relationships have been seen with substance using teens in other studies (Bava et al., 2009; De Bellis et al., 2008) and could reflect altered neurodevelopment, neural compensation, or a more complex interaction with neurodevelopment. We did not see prospective relationships with marijuana use severity and white matter integrity, similar to previous investigations (DeLisi et al., 2006).
Similarly, externalizing behaviors and non-substance use real-world risk taking behaviors at both time points predicted poorer white matter integrity in thalamic fiber tracts and splenium of the corpus callosum 18 months later. Notably, these predictors remained significant after controlling for white matter integrity at Time 1, months between scan session, and age. We did not find that severity or duration of marijuana use or other illicit substance use predicted white matter microstructural integrity in this sample. Positive relationships between externalizing and real-world risk taking behaviors and poorer white matter integrity were observed in several anterior fiber tracts, including prefrontal thalamic fiber tracts, and more posterior subcortical fibers (i.e., splenium). There have been investigations linking white matter integrity to more longstanding personality traits and psychopathology (Li et al., 2005). Yet, few studies have investigated the relationship between more “typical” risk taking (Jacobus et al., in press) and fiber architecture. It is possible that substance use affects ongoing myelination and neuroadaptive processes that leave these individuals vulnerable to risk taking behaviors (Casey et al., 2008); however these individuals may have different microstructural brain architecture, in addition to environmental influences, that predispose them to engage in high risk behaviors (substance-related and non-substance related risk taking behaviors). These behaviors may then make them increasingly vulnerable to brain changes, addiction, and mental health disorders.
Findings from this investigation may not generalize to those individuals excluded from study, particularly those individuals with more severe mental health problems and using psychotropic medications. Although findings represent a prospective investigation, we do not have baseline white matter indices on these teens prior to first initiation of substance use and therefore causation cannot be assumed. It is also likely that family history plays a role in the complex relationships between white matter integrity and substance use behaviors (Herting et al., 2011). It is important to note that understanding the complex white matter geometry in the developing brain can have limitations, as quantifying DTI indices in multiple fiber directions can bias diffusion estimates.
Understanding the behavioral correlates of white matter tissue integrity is important for clinical utilization of neuroimaging findings. If we can identify teens at high risk for substance and non-substance use related risk taking behaviors we may be able to develop strategies for preventing such problematic behaviors that can interfere with neurodevelopment and lead to addiction and more severe mental illness.
Acknowledgments
This research was supported by the National Institutes of Health R01 DA021182 and R01 AA013419to S.F. Tapert; T32 MH018399 and F32 DA032188 to J. Jacobus. We extend our appreciation to participants and their families, as well as to Diane Goldenberg, Amanda Gorlick, Tim McQueeny, and Anthony Scarlett whose support was vital to the completion of this research.
References
- Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- Andersson JL, Skare S. A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage. 2002;16:177–199. doi: 10.1006/nimg.2001.1039. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010;72:347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance use. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) Psychological Corporation; San Antonio, TX, USA: 1978. [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex. 2011;21:459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolscent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cox R. Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R, Glen D. Efficient, Robust, Nonlinear, and Guaranteed Positive Definite Diffusion Tensor Estimation, International Society of Magnetic Resonance in Medicine. Proc. ISMRM 14th Scientific Meeting; Seattle, WA. 2006. [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naïve youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009a;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim R, Bava S, Frank LR, Tapert SF. White matter integrity, substance use and risk taking in adolescence. Psychol Addict Behav. 2012 doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Li TQ, Mathew VP, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Ann NY Acad Sci. 2005;1064:184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolecent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC. Adolescent development. Annu Rev Psychol. 1988;39:583–607. doi: 10.1146/annurev.ps.39.020188.003055. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degredation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructre by excessive intracellular and extracellular fluid in alcohlism: Evidence from diffuion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Schwartz ES. Principles and implementation of diffusion-weighted and diffusion tensor imaging. Pediatr Radiol. 2007;37:739–748. doi: 10.1007/s00247-007-0516-z. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0 (DIS 4.0) Washington University; St. Louis, MO: 1996. [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1970. [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kinderman SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler abbreviated scale of intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- White NS, Leergaard TB, D’Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum Brain Mapp. doi: 10.1002/hbm.21454. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. The wide range achievement test-3 administration manual. Jastak Associates; Wilmington, DE, USA: 1993. [Google Scholar]