Abstract
Objectives
The objectives of this study are to determine the effect of aging, schizophrenia, and their interaction on cognitive function.
Design
Cross-sectional controlled study.
Setting
Community-living.
Participants
235 subjects with schizophrenia aged 19-79 and 333 comparison subjects aged 20-81.
Measurements
The Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB).
Results
Older age was associated with poorer performance on 9 of 10 MCCB tests in both subjects with schizophrenia and comparison subjects. Subjects with schizophrenia were impaired relative to comparison subjects on each of the 10 tests. However, there was no interaction between aging and schizophrenia on any test. Essentially the same results were observed when analyzing performance on the seven MCCB cognitive domains and MCCB global composite score.
Conclusions
Consistent with other reports, schizophrenia appears to be a disorder marked by generalized cognitive dysfunction. However, the rate of cognitive decline appears to be similar to that observed in healthy comparison subjects. They do not experience acceleration in cognitive aging which supports the hypothesis that schizophrenia is a syndrome of premature aging. Longitudinal studies including very old patients are needed to confirm and extend these findings.
Keywords: accelerated aging, cognition, life span, MATRICS, neuropsychology, old age, premature aging, schizophrenia
Objective
Cognitive deficits in schizophrenia are common, core features, and among the strongest predictors of functional disability (1). Functional disability contributes up to half of the costs in the treatment of schizophrenia (2) and these costs are particularly high as individuals with schizophrenia grow old (3). Considering that by 2025, 20% of individuals with schizophrenia will be age 65 or older (4), characterizing cognitive function and determining whether cognitive aging is accelerated in older individuals with schizophrenia, has a critical public health significance.
About 80% of older individuals with schizophrenia are community-dwellers (5). However, most of the longitudinal and controlled cross-sectional studies of cognition in older individuals with schizophrenia have assessed either chronically institutionalized individuals or a heterogeneous group of institutionalized and community-dwelling individuals. Hence, the generalizability of the findings from these studies to typical older community dwelling individuals with schizophrenia is limited. Further, due to the severe cognitive impairment observed in the institutionalized individuals, the cognitive assessment in such studies has been limited to brief cognitive screening measures that were developed originally to assess cognitive function in dementia (for a review, see (6). Hence, it is not possible to compare the findings from these studies with those from studies of younger individuals with schizophrenia who are typically assessed with traditional, comprehensive cognitive batteries.
With respect to the few studies that assessed cognition in exclusively community-dwelling older individuals with schizophrenia, they used global or screening measures of cognitive function (7-12); included samples in mid to late life rather than a fuller range of late life ages (13-18); or had both of these limitations (19-24). Further, 14 of these 18 publications came from one research group (for a review, see (6)). One cross-sectional study assessed three groups of community-dwelling individuals with schizophrenia aged 50-59, 60-69, and 70-85 and age-matched healthy comparison subjects. While this study showed an effect of both age and schizophrenia on cognition, it did not show an interaction between the two (25). Another cross-sectional study assessed four groups of community-dwelling individuals with schizophrenia aged 40-49, 50-59, 60-69, and 70 or above and two groups of healthy comparison subjects. This study found an interaction between aging and schizophrenia on speed of processing and verbal learning in the oldest group, aged 70 or above (26). However, both of these studies did not compare older with younger adults
To address these limitations in the literature and understand the effects of aging, schizophrenia, and their interaction on cognitive function, this study analyzed cognitive data obtained using the comprehensive Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) in 235 community-dwelling subjects with schizophrenia aged 19-79, and 333 comparison subjects aged 20-81. Based on the available literature (for review, see (6)), the a priori hypotheses were that there would be aging and schizophrenia effects on all cognitive domains and there would be an interaction effect on speed of processing and visual memory, such that relative to comparison subjects, impairment in these domains would significantly increase across the decades in older subjects with schizophrenia.
Considering the growing use of the MCCB, this study also contributes uniquely in extending the available data on the MCCB to old age in both subjects with schizophrenia and comparison subjects.
Methods
This study analyzed data obtained at the Centre for Addiction and Mental Health (CAMH) in Toronto, Canada, and during the MATRICS Psychometric and Standardization Study (PASS) (27, 28). Of 235 subjects with schizophrenia, 59 subjects aged 50 and above were recruited and assessed at CAMH, and 176 subjects were assessed during PASS. Of 333 comparison subjects, 33 aged 50 and above were recruited and assessed at CAMH and 300 were assessed during PASS. The methods used during MATRICS PASS are described in details in (27, 28). Thereafter, subjects assessed during the MATRICS PASS are referred to as MATRICS subjects, while those assessed at CAMH are referred to as CAMH subjects.
Setting and Subjects
CAMH is an academic hospital that provides psychiatric care to the Toronto urban population and serves as a tertiary referral center for a large suburban and rural population. CAMH subjects were recruited between May 2007 and November 2010 from advertisements and referral from providers. The study was approved by CAMH Research Ethics Board. All subjects signed a written informed consent.
Eligibility criteria for CAMH subjects with schizophrenia were: (1) age 50 years or above; (2) meeting DSM-IV TR criteria for a current diagnosis of schizophrenia or schizoaffective disorder; (3) clinically stable as operationalized by (a) not having been hospitalized within 3 months and (b) having had no change in antipsychotic medication dosage within 4 weeks prior to assessment; (4) ability and willingness to speak English; (5) corrected hearing and visual acuity; (6) ability and willingness to provide written informed consent; (7) not meeting criteria for a cognitive disorder secondary to a neurological disease or brain injury; (8) Mini-Mental State Examination (MMSE) (29) score of 18 or above because individuals with very low MMSE scores are unlikely to be able to complete a neuropsychological battery such as the MCCB; (9) not having a current major depressive or manic episode; (10) no alcohol or other substance use within the past 6 months; and (11) no electroconvulsive therapy within 6 months prior to assessment.
Eligibility criteria for CAMH comparison subjects were as above except that: (1) they did not meet any DSM IV TR psychiatric diagnosis except for simple phobias or an adjustment disorder; (2) they were not taking any psychotropic medication except for hypnotic at a stable dose for at least 4 weeks, and (3) they had no history of a primary psychotic disorder in a first-degree relative.
The setting and eligibility criteria for MATRICS subjects were not appreciably different from those for CAMH subjects except for the diagnosis criterion.
Measures
Clinical measures
Diagnosis was confirmed through the Structured Clinical Interview for DSM-IV Disorders. For CAMH subjects symptoms were assessed with the Positive And Negative Syndrome Scale (PANSS) for schizophrenia (30).
Cognitive measures
All subjects were administered the MCCB. The MCCB was developed using a structured consensus process and findings from the MATRICS PASS Phase 1 that was conducted in community-dwelling individuals with schizophrenia (27). Normative data were then generated in PASS Phase 2 using community samples of comparison subjects (28). The MCCB consists of ten tests that were administered in the same order as in the MATRICS PASS: Trail Making Test - Part A (TMA); Brief Assessment of Cognition in Schizophrenia Digit-Symbol-Coding (DSC); Hopkins Verbal Learning Test-Revised (HVLTR); Visual Memory Spatial Span (SS); Letter-Number Span (LNS); Neuropsychological Assessment Battery (NAB): Mazes (Mazes); Brief Visuospatial Memory Test-Revised (BVMTR); Category Fluency Test (CFT); Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions (MSCEIT); and Continuous Performance Test-Identical Pairs (CPT-IP). For CAMH subjects, the MCCB was administered by master’s degree level psychologists who received on-going supervision by a senior neuropsychologist (M.A.B.) in the administration of neuropsychological tests.
Data Analysis
First, demographic and clinical characteristics of CAMH subjects with schizophrenia were compared to those of CAMH comparison subjects using chi-square, t-tests, and Mann-Whitney-Wilcoxon as appropriate. Next, mean raw scores of each of the ten MCCB tests from CAMH subjects with schizophrenia and contols were compared using independent samples t-tests. Differences of means and their confidence intervals (CI’s) were calculated. Second, to assess the effects of schizophrenia, aging, and their interactions on cognitive function, data on data on 92 CAMH subjects and 476 MATRICS subjects were combined. MATRICS subjects with schizophrenia and comparison subjects ranged in age from 18-65, and 20-59, respectively with mean (SD) ages of 44.0 years (11.2) and 42.7 years (11.5), respectively. Thus, MATRICS subjects included few older subjects in contrast to CAMH subjects who were all age 50 and above. The total sample consisted of 235 subjects with schizophrenia ranging in age from 19 to 79 years and 333 comparison subjects ranging in age from 20 to 81 years. Following the approach of Kern et al. (28) this total sample was divided into four age groups: < 40, 40-49, 50-59, and >= 60. All subjects with schizophrenia and comparison subjects were compared across these four age groups on demographic and clinical characteristics using t- and chi-square statistic as indicated, and on individual cognitive tests using a series of general linear models while controlling for sex and education. Finally, T-scores were calculated for all subjects with schizophrenia based on the global normative values derived from the total sample of comparison subjects, i.e. the MATRICS and CAMH comparison subjects combined, for each cognitive test. Then, cognitive domain T-scores were calculated by summing the T-scores of the tests associated with each corresponding cognitive domain according to the MCCB manual, and standardizing the resulting sums to T-scores. In total, seven domain scores and one overall composite score were obtained through the summation and standardization of individual test T-scores as follows: (1) Speed of processing = CFT, DSC, TMA; (2) Attention / Vigilance = CPT-IP; (3) Working Memory = LNS, SSP; (4) Verbal Learning = HVLTR; (5) Visual Learning = BVMTR; (6) Reasoning and Problem Solving = Mazes; (7) Social Cognition = MSCEIT. A series of analyses of variances (ANOVAs) were performed to assess for age effects on all of the domains and the composite measure. All raw cognitive scores were normally distributed except for TMA for which natural logarithm transformation was applied in order to improve the distributional properties of this test. These transformed scores were then inversed in order to maintain a consistent direction with the other tests (i.e. higher scores indicate better performance). All data analyses were conducted using SAS 9.1.3.
Results
Demographic, clinical, and cognitive characteristics of CAMH subjects
CAMH subjects with schizophrenia and CAMH comparison subjects did not differ in age and sex distributions. However, subjects with schizophrenia had a significantly lower level of education, higher score on PANSS, and lower score on the MMSE. They were also impaired when contrasted with the CAMH comparison subjects of similar age and sex on all MCCB tests (see Table 1).
Table 1.
Demographic, Clinical, and Cognitive Characteristics of CAMH Subjects with Schizophrenia and CAMH Comparison Subjects
| CAMH Subjects with Schizophrenia | CAMH Comparison Subjects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | Difference of Means | Cohen’s d | 95% CI for Difference of Means | Statistic * | df | p | |
| Age | 59 | 63.5 | 6.8 | 33 | 63.4 | 7.7 | 0.09 | - | (-3.0, 3.2) | 0.06 | 90 | 0.9517 |
| Female | 28 | 47.5# | -- | 14 | 42.4 | -- | -- | - | -- | 0.22¥ | 1 | 0.64 |
| Education | 59 | 12.2 | 3.3 | 33 | 15.0 | 2.1 | -2.83 | - | (-4.1, -1.6) | -5.08 | 88.7 | <0.0001 |
| PANSS | 59 | 56.1 | 14.1 | 33 | 32.8 | 4.1 | 23.37 | - | - | 7.10 | - | <0.0001 |
| MMSE | 59 | 28.2 | 1.8 | 33 | 29.3 | 0.9 | -1.05 | 0.77 | - | -3.12 | - | 0.0018 |
| TMA | 58 | 64.5 | 29.2 | 33 | 34.4 | 16.2 | 30.1 | 1.19 | - | 5.77 | - | <0.0001 |
| DSC | 58 | 33.8 | 13.6 | 33 | 51.2 | 13.1 | -17.4 | 1.30 | (-23.2, -11.6) | -5.96 | 89 | <0.0001 |
| HVLTR | 58 | 18.1 | 5.0 | 33 | 23.2 | 5.8 | -5.1 | 0.96 | (-7.4, -2.8) | -4.42 | 89 | <0.0001 |
| SSP | 59 | 13.3 | 3.3 | 33 | 16.3 | 3.4 | -3.0 | 0.92 | (-4.5, -1.6) | -4.22 | 90 | <0.0001 |
| LNS | 58 | 11.0 | 3.9 | 33 | 15.3 | 3.4 | -4.3 | 1.15 | (-5.9, -2.7) | -5.27 | 89 | <0.0001 |
| Mazes | 58 | 5.3 | 4.8 | 33 | 10.1 | 5.6 | -4.8 | 0.93 | (-7.0, -2.5) | -4.26 | 89 | <0.0001 |
| BVMTR | 58 | 11.7 | 8.2 | 33 | 21.1 | 4.9 | -9.3 | 1.30 | (-12.5, -6.2) | -6.81 | 88.8 | <0.0001 |
| CFT | 59 | 14.6 | 4.7 | 33 | 21.1 | 5.0 | -6.5 | 1.35 | (-8.6, -4.4) | -6.20 | 90 | <0.0001 |
| MSCEIT | 59 | 87.6 | 10.2 | 33 | 98.1 | 10.7 | -10.4 | 1.00 | (-14.9, -5.9) | -4.61 | 90 | <0.0001 |
| CPT-IP | 58 | 1.90 | 0.74 | 33 | 2.61 | 0.74 | -0.7 | .095 | (-1.0, -0.4) | -4.36 | 89 | <0.0001 |
t-tests except for MMSE and TMA for which the Mann-Whitney-Wilcoxon test was used. The statistic reported for MMSE and TMA is a z-approximation to the Mann-Whitney-Wilcoxon U statistic;
percentage;
χ2
BVMTR: Brief Visuospatial Memory Test-Revised (Total recall score over three learning trials); CFT: Category Fluency (Total number of animals named in 60 seconds); CPT-IP: Continuous Performance Test-Identical Pairs (Mean d′ value across 2-, 3-, and 4-digit conditions); DSC: Digit Symbol Coding (Number of correct responses); HVLTR: Hopkins Verbal Learning Test-Revised (Total number of words recalled correctly over three learning trials); LNS: Letter Number Span (Number of correct trials); Mazes (Total raw score); MMSE: Mini-Mental State Examination; MSCEIT: Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions (Branch standard score using general consensus scoring); PANSS: Positive and Negative Syndrome Scale; SSP: Spatial Span (Sum of raw scores on forward and backward conditions); and TMA: Trails Making A (Time to completion – seconds).
Comparison of subjects with schizophrenia and healthy subjects on MCCB cognitive tests
The demographic characteristics of the total sample of subjects with schizophrenia and comparison subjects are summarized in Table 2.
Table 2.
Demographic Characteristics of All Subjects with Schizophrenia and All Comparison Subjects
| Characteristic | Schizophrenia | Comparison Subjects | t/chi-square, df | Statistical Significance |
|---|---|---|---|---|
|
| ||||
| Number of subjects | 235 | 333 | -- | -- |
|
| ||||
| Source of data | CAMH: 59 (25%) | MCAMH: 33 (10%) | chi-sq=23.44, df=1 | p<0.0001 |
| MATRICS: 176 (75%) | MATRICS: 300 (91%) | |||
|
| ||||
| Age (years) | 48.83 (13.35) | 44.68 (12.89) | t=3.73, df=566 | p=0.0002 |
|
| ||||
| Age group N (%), Mean Age (SD) | <40: 54 (23%), 29.6 (5.9) | <40: 100 (30%), 28.1 (5.8) | chi-sq=33.67, df=3 | p<0.0001 |
| 40-49: 56 (24%), 44.9 (2.7) | 40-49: 100 (30%), 45.3 (2.8) | |||
| 50-59: 70 (30%), 53.9 (2.7) | 50-59: 111 (33%), 54.5 (2.7) | |||
| 60+: 55 (23%), 65.3 (5.0)* | 60+: 22 (7%), 67.5 (5.7)* | |||
|
| ||||
| Sex | Male: 164 (70%) | Male: 57 (47%) | chi-sq=28.73, df=1 | p<0.0001 |
| Female: 71 (30%) | Female: 176 (53%) | |||
|
| ||||
| Years of education | 12.33 (2.68) | 14.39 (2.59) | t=9.20, df=565 | p<0.0001 |
|
| ||||
| Age of onset | 25.57 (9.68) | -- | -- | -- |
In these two groups, 21 subjects with schizophrenia and 13 comparison subjects were age 65 or older.
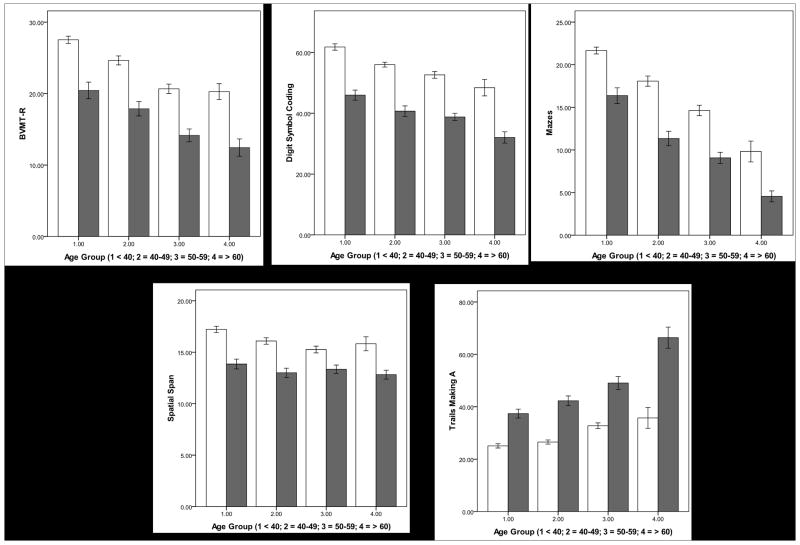
The general linear models did not reveal any interaction on any cognitive test. There was a schizophrenia effect on all ten cognitive tests, with schizophrenia associated with poor performance. There was an aging effect on all ten tests with aging associated with poor performance except for MSCEIT on which aging was associated with better performance. The aging effect was first observed in the 40-49 age group for BVMTR, DSC, Mazes, MSCEIT, SSP, and TMA, but in the 50-59 age group for CFT, CPT-IP, HVLTR, and LNS (see Table 3 and Figure 1). There was an education effect on all cognitive tests. There was a sex effect on DSC, HVLTR, Mazes, MSCEIT, and SSP with females performing better than males on DSC, HVLTR, and MSCEIT (see Table 3).
Table 3.
Schizophrenia, Aging, and their Interaction on Cognition across the Lifespan
| Schizophrenia × Aging* | Overall | Schizophrenia | Age Group | Sex | Education | |
|---|---|---|---|---|---|---|
|
| ||||||
| TMA | 1.35, df=3,552 | 73.19, df=6,555 | 143.16, df=1,555 | 37.82, df=3,555 | 0.72, df=1,555 | 24.09, df=1,555 |
| p=0.2584 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.3979 | p<0.0001 | |
|
| ||||||
| DSC | 0.09, df=3,556 | 87.07, df=6,559 | 133.51, df=1,559 | 38.21, df=3,559 | 6.91, df=1,559 | 64.19, df=1,559 |
| p=0.9678 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.0088 (F) | p<0.0001 | |
|
| ||||||
| HVLTR | 1.17, df=3,556 | 64.29, df=6,559 | 105.21, df=1,559 | 14.09, df=3,559 | 9.28, df=1,559 | 59.26, df=1,559 |
| p=0.3198 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.0024 (F) | p<0.0001 | |
|
| ||||||
| SSP | 0.99, df=3,556 | 32.70, df=6,559 | 58.67, df=1,559 | 6.45, df=3,559 | 15.07, df=1,559 | 46.00, df=1,559 |
| p=0.3948 | p<0.0001 | p<0.0001 | p=0.0003 | p=0.0001 (M) | p<0.0001 | |
|
| ||||||
| LNS | 1.74, df=3,554 | 48.98, df=6,557 | 92.63, df=1,557 | 3.52, df=3,557 | 2.82, df=1,557 | 67.13, df=1,557 |
| p=0.1577 | p<0.0001 | p<0.0001 | p=0.0150 | p=0.0934 | p<0.0001 | |
|
| ||||||
| Mazes | 0.66, df=3,556 | 84.86, df=6,559 | 96.58, df=1,559 | 79.83, df=3,559 | 9.87, df=1,559 | 21.20, df=1,559 |
| p=0.5797 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.0018 (M) | p<0.0001 | |
|
| ||||||
| BVMTR | 0.10, df=3,556 | 54.98, df=6,559 | 75.42, df=1,559 | 37.20, df=3,559 | 1.40, df=1,559 | 38.48, df=1,559 |
| p=0.9585 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.2378 | p<0.0001 | |
|
| ||||||
| CFT | 0.27, df=3,557 | 36.81, df=6,560 | 47.13, df=1,560 | 10.11, df=3,560 | <0.01, df=1,560 | 51.38, df=1,560 |
| p=0.8440 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.9981 | p<0.0001 | |
|
| ||||||
| MSCEIT | 0.55, df=3,551 | 37.88, df=6,554 | 109.07, df=1,554 | F=2.85, df=3,554 | 6.48, df=1,554 | 17.74, df=1,554 |
| p=0.6484 | p<0.0001 | p<0.0001 | p=0.0366 | p=0.0112 (F) | p<0.0001 | |
|
| ||||||
| CPT-IP | 0.93, df=3,553 | 37.43, df=6,556 | 60.02, df=1,556 | 7.93, df=3,556 | 2.55, df=1,556 | 49.85, df=1,556 |
| p=0.4236 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.1109 | p<0.0001 | |
The initial model included the interaction term. Then, due to the lack of significance of the interaction, it was removed and the model was refit excluding it. The remaining columns refer to the model parameters and significance associated with the model in which the interaction has been removed
All values are F-values except when indicated; F: females performed better than makes; M: males performed better than females; BVMTR: Brief Visuospatial Memory Test-Revised (Total recall score over three learning trials); CFT: Category Fluency (Total number of animals named in 60 seconds); CPT-IP: Continuous Performance Test-Identical Pairs (Mean d′ value across 2-, 3-, and 4-digit conditions); DSC: Digit Symbol Coding (Number of correct responses); HVLTR: Hopkins Verbal Learning Test-Revised (Total number of words recalled correctly over three learning trials); LNS: Letter Number Span (Number of correct trials); Mazes (Total raw score); MSCEIT: Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions (Branch standard score using general consensus scoring); SSP: Spatial Span (Sum of raw scores on forward and backward conditions); and TMA: Trails Making A (Time to completion – seconds).
Figure 1. Schizophrenia and Aging effects on the Ten Tests of MCCB.
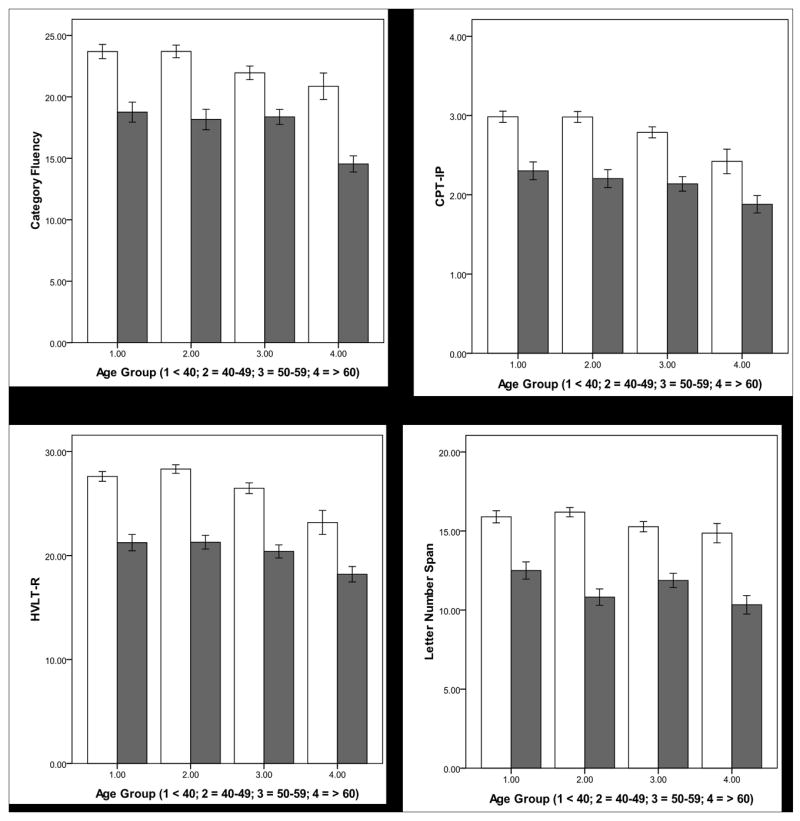
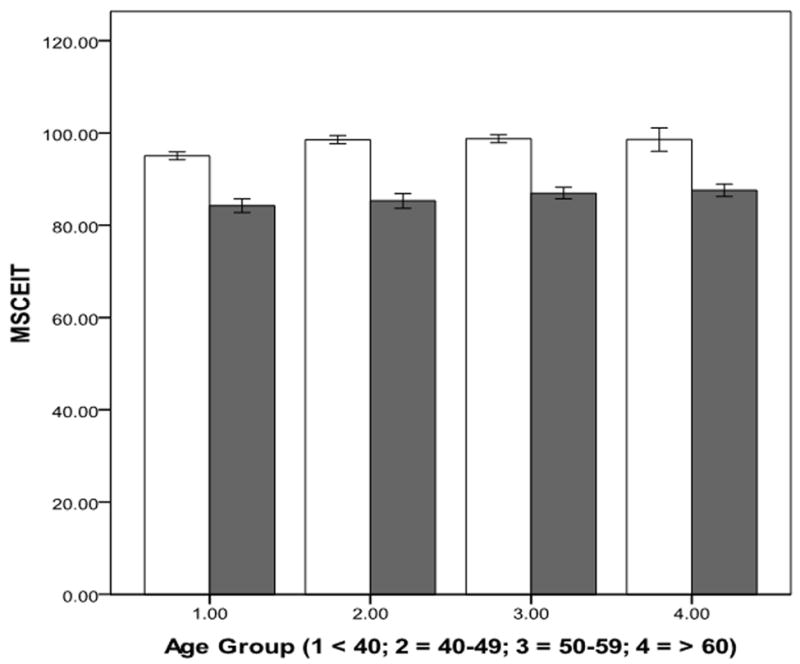
Schizophrenia is associated with poor performance effect on all cognitive tests. Aging is associated with poor performance on nine tests with an aging effect first observed in the 40-49 age group on five of them (upper panel), and in the 50-59 age group on four others (middle panel). Aging is associated with better performance on MSCEIT (lower panel) with aging effect first observed in the 40-49 age group. White bars: comparison subjects; Gray bars: participants with schizophrenia. BVMTR: Brief Visuospatial Memory Test-Revised (Total recall score over three learning trials); Category Fluency (Total number of animals named in 60 seconds); CPT-IP: Continuous Performance Test-Identical Pairs (Mean d′ value across 2-, 3-, and 4-digit conditions); Digit Symbol Coding (Number of correct responses); HVLTR: Hopkins Verbal Learning Test-Revised (Total number of words recalled correctly over three learning trials); Letter Number Span (Number of correct trials); Mazes (Total raw score); MSCEIT: Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions (Branch standard score using general consensus scoring); Spatial Span (Sum of raw scores on forward and backward conditions); and Trails Making A (Time to completion – seconds). Error bars = 1 standard error.
Performance of subjects with schizophrenia on MCCB cognitive domains and composite score
Using T-scores for subjects with schizophrenia based on the normative values derived from the comparison subjects, an additional series of ANOVAs were performed and found significant age effects on six domains: Speed of Processing (F=17.75, df=3,227, p<0.0001), Attention / Vigilance (F=2.73, df=3,228, p=0.0445), Working Memory (F=2.81, df=3,229, p=0.0401), Verbal Learning (F=3.97, df=3,230, p=0.0087), Visual Learning (F=11.16, df=3,230, p<0.0001), Reasoning and Problem Solving (F=38.21, df=3,230, p<0.0001); and Overall Composite Score (F=11.99, df=3,220, p<0.0001). No aging effect on Social Cognition was detected (F=1.11, df=3,229, p=0.3460) (see Figure 2).
Figure 2. Schizophrenia Group Mean Domain T-Scores by Age Group.
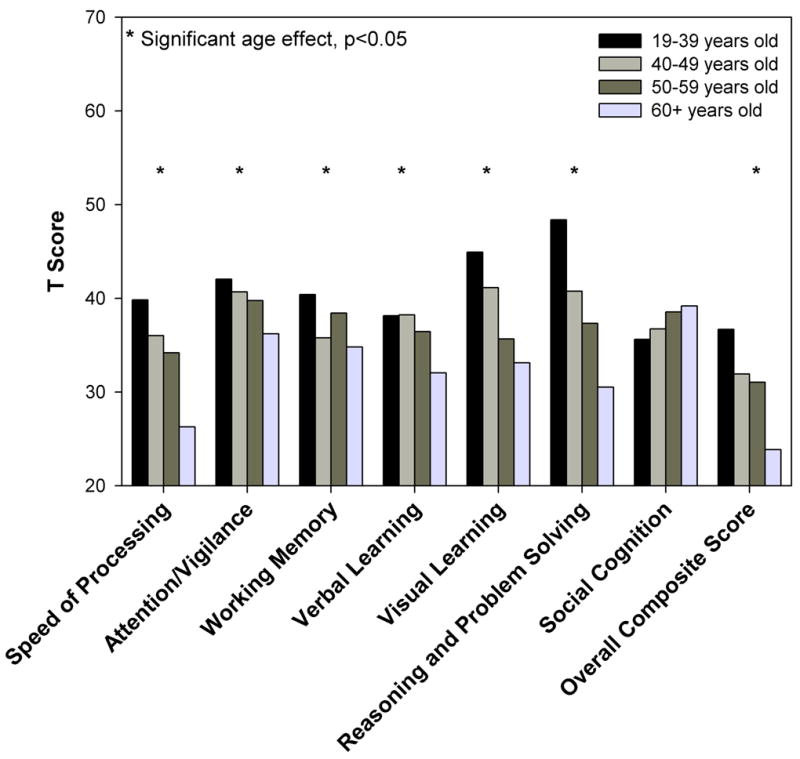
There is an aging on all cognitive domains and the overall composite score, except social cognition.
Conclusions
To our knowledge, this is the first published report on the use of the MCCB in a large group of older individuals with schizophrenia, extending the available data on this increasingly used cognitive battery. This is one of three published reports of community-dwelling individuals with schizophrenia in their 60’s and older assessed with a comprehensive cognitive battery (25, 26). This is also the first published report on the use of the MCCB to assess the effect of aging on cognitive function in individuals with schizophrenia across the adult lifespan. As predicted, CAMH older subjects were impaired compared to CAMH comparison subjects on all MCCB tests. As predicted, combining our data with those collected in the MATRICS PASS, we found an effect of schizophrenia and of aging on all tests. We did not find any interaction between age and schizophrenia on any cognitive test or domain.
To assess whether the study has enough power to detect an interaction between aging and schizophrenia, a series of Monte Carlo simulations were conducted examining the power to detect an interaction between aging and schizophrenia on each of our outcomes listed in Table 3, under a variety of effect size scenarios. 10,000 replications were carried out for each outcome/effect-size combination. Means and standard deviations in the comparison group were assumed to be consistent with those observed in the current sample. Means in the schizophrenia group were altered in a systematic manner while holding the observed standard deviations constant in order to generate a variety of potential effect sizes. Sample sizes for all interactions were also assumed to be consistent with the current study. Using these simulations, there was sufficient power (>80%) to detect an interaction effect on each of the outcomes listed in Table 3 at an alpha level of 0.05, provided that the effect sizes (eta-squared) associated with these interactions range between 0.010 and 0.025. Thus, these simulations support our finding that there is no meaningful interaction between aging and schizophrenia effect on any outcome measure.
While community-dwelling individuals with schizophrenia are impaired across the various cognitive tests and domains, their cognitive function followed the same trajectory and the same rate as those observed among healthy individuals, at least as late in life as the late 60’s. Such parallel progression is in contrast to what is observed among chronically institutionalized individuals with schizophrenia who start to experience more rapid decline in their 60’s (31). The differences in the trajectory of cognitive function between community-dwelling and chronically institutionalized individuals could be due to the fact that chronically institutionalized individuals have a more severe and aggressive disease that results in a rapid cognitive decline earlier than in community-dwelling individuals. On the other hand, the richer and more stimulating environmental exposure experienced by community-dwelling individuals could contribute to a delay in the manifestation of cognitive decline related to schizophrenia similar to what has been reported in Alzheimer disease (32). Such an environmental stimulation could protect cognitive reserve and promote compensatory mechanisms, thus postponing the manifestation of a progressive illness (33).
The stable magnitude of cognitive deficits of individuals with schizophrenia up to their seventh decade is also consistent with the hypothesis of schizophrenia as a syndrome of premature aging (34). Deficits occur around the time of onset and do not progress beyond what is observed due to effect of aging over the entire span of adult life. So, although individuals with schizophrenia decline at the same rate as those without the disorder, they cross the threshold of clinical impairment earlier, and thus exhibit “premature aging”. In addition to cognitive dysfunction, premature aging in schizophrenia is supported by a decreased lifespan (35), increased incidence of metabolic disease (36), increased pulse pressure (34), faster loss of gray matter (37), decline in white matter tracts integrity(38), decreased telomere length (39), and up- or down-regulation of genes implicated in both schizophrenia and aging (40). Still, premature aging in schizophrenia would be compatible with the possibility of rapid deterioration in certain cognitive functions very late in life (i.e., in one’s 70’s or 80’s): a rapid deterioration in memory and verbal ability has been observed in non-demented elders starting in their seventh decade (41) and could explain the aging by schizophrenia interaction observed in one study among individuals with schizophrenia aged 70 or above (26).
An advantageous aging effect on social cognition was observed starting in the 40-49 age group in the total sample of subjects. This is consistent with what was observed in the MATRICS PASS (28) and suggests that social cognition is highly stable or possibly even improves with age due to increased opportunity for social experiences. Alternatively, social cognition could be still declining with age but any decline is masked by a cohort or a survival effect that is impossible to disentangle in a cross-sectional study. The lack of any aging effect among the subjects with schizophrenia when analyzed separately could be due to the fact that the social impairment associated with schizophrenia masks any potential advantage associated with older age.
This study has some limitations: older comparison subjects had a higher level of education than older subjects with schizophrenia. Also, subjects were assessed at different sites: in particular, almost all of the older subjects were assessed at CAMH while almost all of the younger subjects were assessed during the MATRICS PASS. However, the MCCB is a standardized battery that was developed specifically to be able to compare findings across different studies. Another limitation is the use of the MCCB itself. While most community-dwelling studies showed no interactions between aging and schizophrenia using non-MCCB measures, there may still be non-MCCB cognitive measures that could be better suited to detect such interactions. For example, a recent study using the California Verbal Leaning Test demonstrated an interaction between aging and schizophrenia. However, this interaction is probably due to the fact that this study included a group of subjects age 70 or older rather than the use of his specific measure (26). One more limitation is that this is a cross-sectional study and therefore, the lack of an observed interaction between schizophrenia and aging could be due to a cohort effect. Older subjects with schizophrenia could have been functioning at a higher level when they were younger compared to the current younger subjects. Only a longitudinal study conducted over several decades could address this limitation. Finally, while our oldest subjects had a mean age in the late 60’s, an interaction in the 70’s or after cannot be ruled out as it has recently been suggested by another cross-sectional study (26).
Longitudinal and controlled studies would be necessary to disentangle the impact of aging and age-related factors such as psychiatric and medical comorbidities, from the impact of schizophrenia and schizophrenia-related factors. Further, assessing individuals in their 70’s or later in life is necessary to assess for acceleration in cognitive decline later in life as the general population including individuals with schizophrenia continues to age. Finally, comparing only individuals with schizophrenia to comparison subjects precludes the determination of whether identified deficits are due to the non-specific effect of a severe mental illness, or to the specific effect of schizophrenia. A comparison group that consists of individuals with another severe mental illness such as a bipolar disorder will be required to address this issue.
In conclusion, older individuals with schizophrenia experience deficits in all cognitive domains. These deficits are comparable in magnitudes to those observed earlier in life as cognitive functions seem to decline similarly with both normal aging and schizophrenia across all cognitive domains except social cognition up to the seventh decade. Future longitudinal studies are needed to confirm these findings and to explore whether aging accelerates in schizophrenia very late in life.
Acknowledgments
Sources of Funding: Dr. Butters has received remuneration for providing neuropsychological assessment services for a clinical trial funded by Medtronic and as a consultant for Fox Learning Systems, and received salary support from NIH MH R01 072947. Dr. Kern is an officer with MATRICS Assessment, Inc. and he receives financial compensation for his role in the non-profit organization. Funding for the MATRICS initiative was provided through NIMH contract N01MH22006 to the University of California, Los Angeles. Dr. Rajji was funded through a Canadian Institutes of Health Research (CIHR180087).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest. The other authors report no financial relationships with commercial interests.
References
- 1.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Wu EQ, Birnbaum HG, Shi LZ, Ball DE, Kessler RC, Moulis M, Aggarwal J. The economic burden of schizophrenia in the United States in 2002. Journal of Clinical Psychiatry. 2005;66(9):1122–1129. doi: 10.4088/jcp.v66n0906. [DOI] [PubMed] [Google Scholar]
- 3.Cuffel BJ, Jeste DV, Halpain M, Pratt C, Tarke H, Patterson TL. Treatment costs and use of community mental health services for schizophrenia by age cohorts. American Journal of Psychiatry. 1996;153(7):870–876. doi: 10.1176/ajp.153.7.870. [DOI] [PubMed] [Google Scholar]
- 4.Goeree R, Farahati F, Burke N, Blackhouse G, O’Reilly D, Pyne J, Tarride JE. The economic burden of schizophrenia in Canada in 2004. Current Medical Research and Opinion. 2005;21(12):2017–2028. doi: 10.1185/030079905X75087. [DOI] [PubMed] [Google Scholar]
- 5.Gurland BJ, Cross PS. Epidemiology of Psycho-Pathology in Old-Age - Some Implications for Clinical Services. Psychiatric Clinics of North America. 1982;5(1):11–82. [PubMed] [Google Scholar]
- 6.Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: A systematic review. Schizophrenia Research. 2008;102(1-3):122–140. doi: 10.1016/j.schres.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Bankole AO, Cohen CI, Vahia I, Diwan S, Kehn M, Ramirez PM. Factors affecting quality of life in a multiracial sample of older persons with schizophrenia. American Journal of Geriatric Psychiatry. 2007;15(12):1015–1023. doi: 10.1097/JGP.0b013e31805d8572. [DOI] [PubMed] [Google Scholar]
- 8.Brodaty H, Sachdev P, Koschera A, Monk D, Cullen B. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. British Journal of Psychiatry. 2003;183:213–219. doi: 10.1192/bjp.183.3.213. [DOI] [PubMed] [Google Scholar]
- 9.Cohen CI, Stastny P, Perlick D, Samuelly I, Horn L. Cognitive Deficits Among Aging Schizophrenic-Patients Residing in the Community. Hospital and Community Psychiatry. 1988;39(5):557–559. doi: 10.1176/ps.39.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Evans JD, Negron AE, Palmer BW, Paulsen JS, Heaton RK, Jeste DV. Cognitive deficits and psychopathology in institutionalized versus community-dwelling elderly schizophrenia patients. Journal of Geriatric Psychiatry and Neurology. 1999;12(1):11–15. doi: 10.1177/089198879901200104. [DOI] [PubMed] [Google Scholar]
- 11.Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Archives of General Psychiatry. 2001;58(1):24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Laks J, Fontenelle LF, Chalita A, Mendlowicz MV. Absence of dementia in late-onset schizophrenia - A one year follow-up of a Brazilian case series. Arquivos de Neuro-Psiquiatria. 2006;64(4):946–949. doi: 10.1590/s0004-282x2006000600011. [DOI] [PubMed] [Google Scholar]
- 13.Depp CA, Moore DJ, Sitzer D, Palmer BW, Eyler LT, Roesch S, Lebowitz BD, Jeste DV. Neurocognitive impairment in middle-aged and older adults with bipolar disorder: Comparison to schizophrenia and normal comparison subjects. Journal of Affective Disorders. 2007;101(1-3):201–209. doi: 10.1016/j.jad.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans JD, Heaton RK, Paulsen JS, Palmer BW, Patterson T, Jeste DV. The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenia patients. Biological Psychiatry. 2003;53(5):422–430. doi: 10.1016/s0006-3223(02)01476-2. [DOI] [PubMed] [Google Scholar]
- 15.Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris MJ, Jeste DV. Neuropsychological Deficits in Schizophrenics - Relationship to Age, Chronicity, and Dementia. Archives of General Psychiatry. 1994;51(6):469–476. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- 16.Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R. Clinical and Neuropsychological Characteristics of Patients with Late-Onset Schizophrenia. American Journal of Psychiatry. 1995;152(5):722–730. doi: 10.1176/ajp.152.5.722. [DOI] [PubMed] [Google Scholar]
- 17.Moore R, Blackwood N, Corcoran R, Rowse G, Kinderman P, Bentall R, Howard R. Misunderstanding the intentions of others: An exploratory study of the cognitive etiology of persecutory delusions in very late-onset schizophrenia-like psychosis. American Journal of Geriatric Psychiatry. 2006;14(5):410–418. doi: 10.1097/01.JGP.0000200604.47367.38. [DOI] [PubMed] [Google Scholar]
- 18.Palmer BW, Bondi MW, Twamley EW, Thal L, Golshan S, Jeste DV. Are Late-Onset Schizophrenia Spectrum Disorders Neurodegenerative Conditions? Annual Rates of Change on Two Dementia Measures. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(1):45–52. doi: 10.1176/jnp.15.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, Kim K, Meeks T, Kraemer HC. A new brief instrument for assessing decisional capacity for clinical research. Archives of General Psychiatry. 2007;64(8):966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- 20.Moore DJ, Palmer BW, Jeste DV. Use of the Mini-Mental State Exam in Middle-Aged and Older Outpatients With Schizophrenia: Cognitive Impairment and Its Associations. American Journal of Geriatric Psychiatry. 2004;12(4):412–419. doi: 10.1176/appi.ajgp.12.4.412. [DOI] [PubMed] [Google Scholar]
- 21.Palmer BW, Dunn LB, Appelbaum PS, Jeste DV. Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Archives of General Psychiatry. 2004;61(3):230–236. doi: 10.1001/archpsyc.61.3.230. [DOI] [PubMed] [Google Scholar]
- 22.Patterson TL, Klapow JC, Eastham JH, Heaton RK, Evans JD, Koch WL, Jeste DV. Correlates of functional status in older patients with schizophrenia. Psychiatry Research. 1998;80(1):41–52. doi: 10.1016/s0165-1781(98)00060-2. [DOI] [PubMed] [Google Scholar]
- 23.Savla GN, Moore DJ, Roesch SC, Heaton RK, Jeste DV, Palmer BW. An evaluation of longitudinal neurocognitive performance among middle-aged and older schizophrenia patients: Use of mixed-model analyses. Schizophrenia Research. 2006;83(2-3):215–223. doi: 10.1016/j.schres.2005.12.851. [DOI] [PubMed] [Google Scholar]
- 24.Zorrilla LTE, Heaton RK, McAdams LA, Zisook S, Harris MJ, Jeste DV. Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: No differences in age-related cognitive decline. American Journal of Psychiatry. 2000;157(8):1324–1326. doi: 10.1176/appi.ajp.157.8.1324. [DOI] [PubMed] [Google Scholar]
- 25.Bowie CR, Reichenberg A, McClure MM, Leung WL, Harvey PD. Age-associated differences in cognitive performance in older community dwelling schizophrenia patients: Differential sensitivity of clinical neuropsychological and experimental information processing tests. Schizophrenia Research. 2008;106(1):50–58. doi: 10.1016/j.schres.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loewenstein DA, Czaja SJ, Bowie CR, Harvey PD. Age-Associated Differences in Cognitive Performance in Older Patients With Schizophrenia: A Comparison With Healthy Older Adults. American Journal of Geriatric Psychiatry. 2012;20(1):29–40. doi: 10.1097/JGP.0b013e31823bc08c. [DOI] [PubMed] [Google Scholar]
- 27.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 28.Kern RS, Nuechterlein KH, Green MF, Laade LE, Fenton WS, Gold JM, Keefe RSE, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS consensus cognitive battery, part 2: Co-norming and standardization. American Journal of Psychiatry. 2008;165(2):214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State - Practical Method for Grading Cognitive State of Patients for Clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenics. Psychiatry Research. 1988;23(1):99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 31.Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, White L, Adler D, Davis KL. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: A comparison with Alzheimer’s disease and normal aging. American Journal of Psychiatry. 2001;158(9):1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, de Leon CFM, Evans DA. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology. 2010;75(11):990–996. doi: 10.1212/WNL.0b013e3181f25b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease & Associated Disorders. 2006;20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 34.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is Schizophrenia a Syndrome of Accelerated Aging? Schizophrenia Bulletin. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. American Heart Journal. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Ryan MCM, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. American Journal of Psychiatry. 2003;160(2):284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- 37.Ho B, Nopoulos P, Arndt S, Pierson R, Ziebell S, Andreasen NC. Longitudinal study of MRI brain morphology in schizophrenia involving multiple within-subject assessments. Schizophrenia Bulletin. 2007;33(2):335–335. [Google Scholar]
- 38.Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Mulsant BH, Pollock BG, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133:1494–1504. doi: 10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao HT, Cawthon RM, DeLisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Porton B. Rapid telomere erosion in schizophrenia. Molecular Psychiatry. 2008;13(2):118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- 40.Glorioso C, Sibille E. Between destiny and disease: Genetics and molecular pathways of human central nervous system aging. Progress in Neurobiology. 2011;93(2):165–181. doi: 10.1016/j.pneurobio.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5(2):87–U12. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]