Abstract
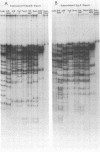
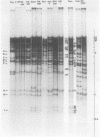
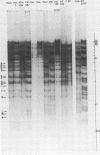
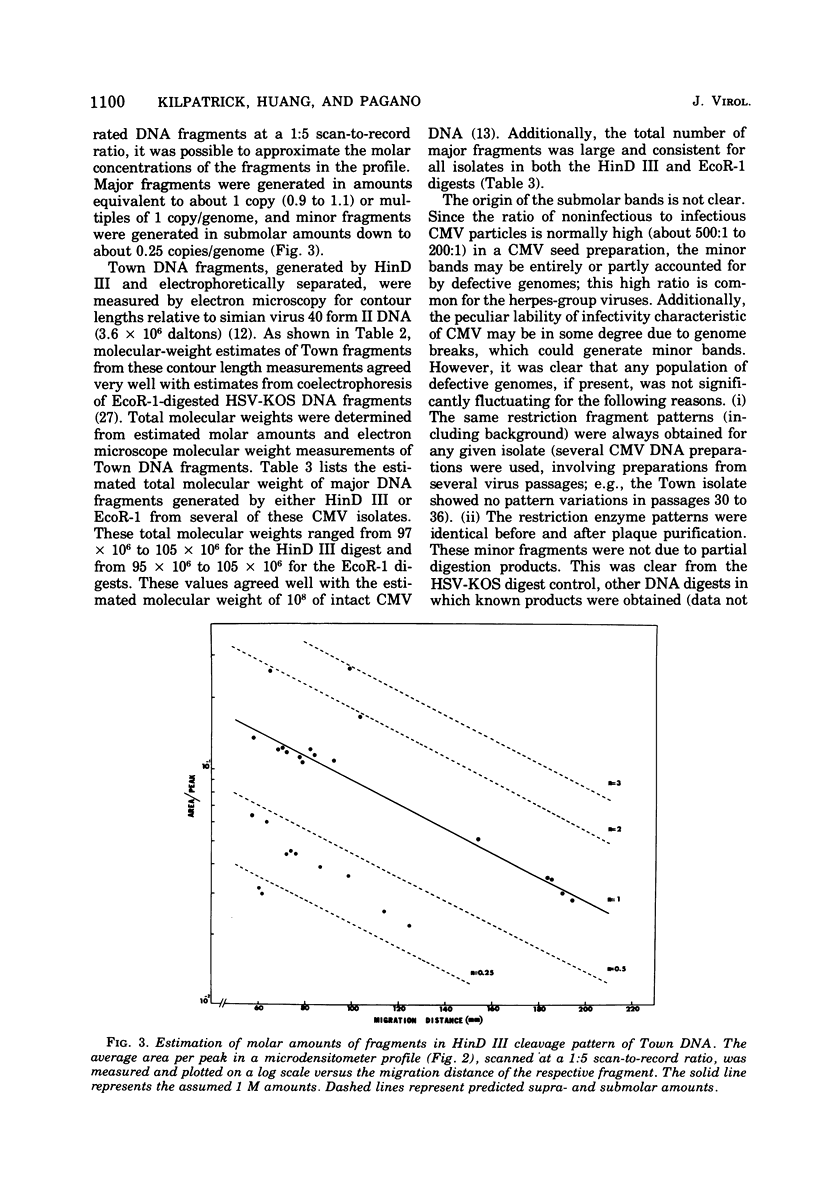
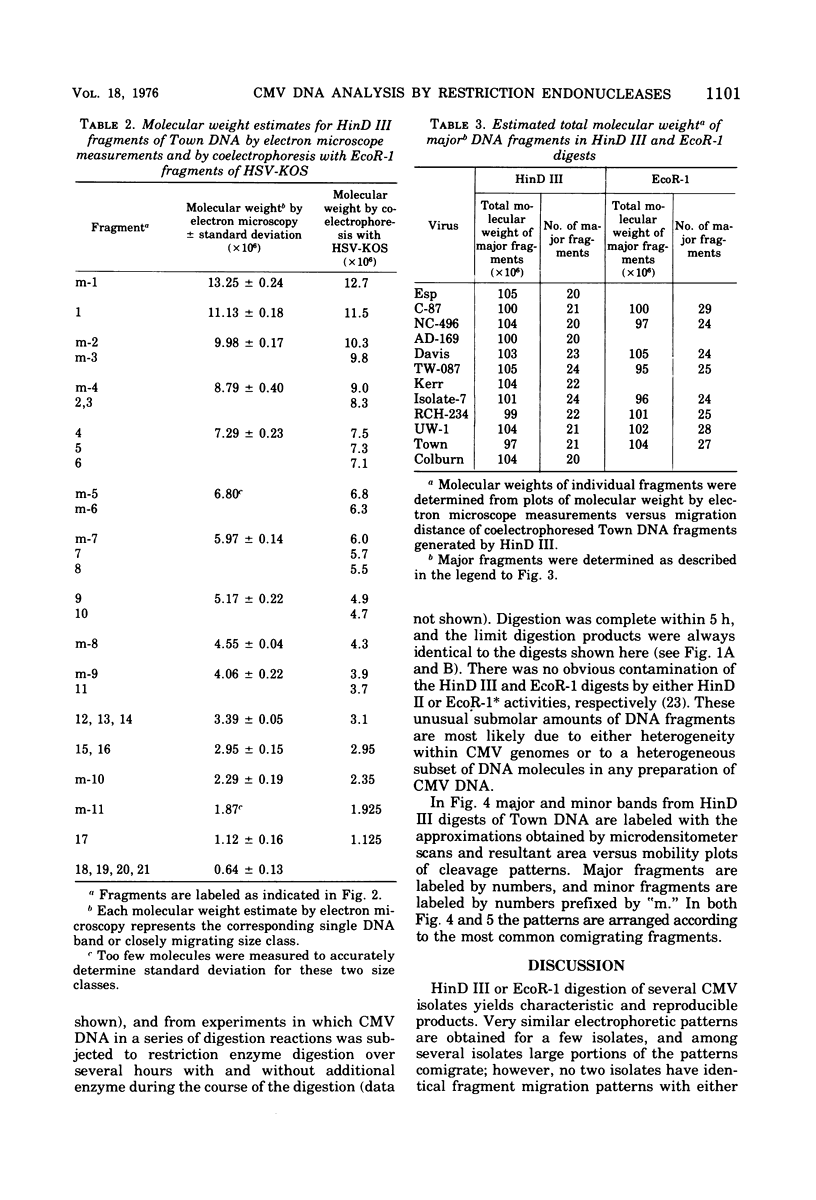
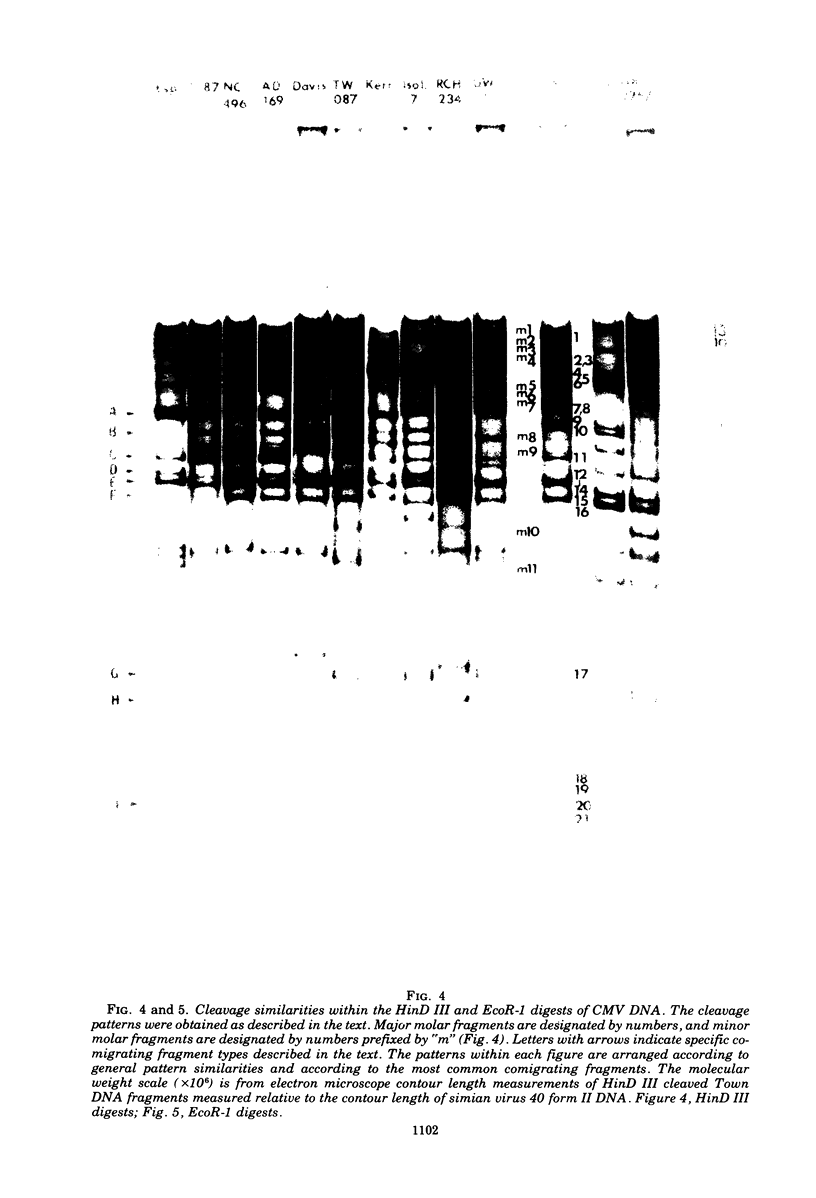
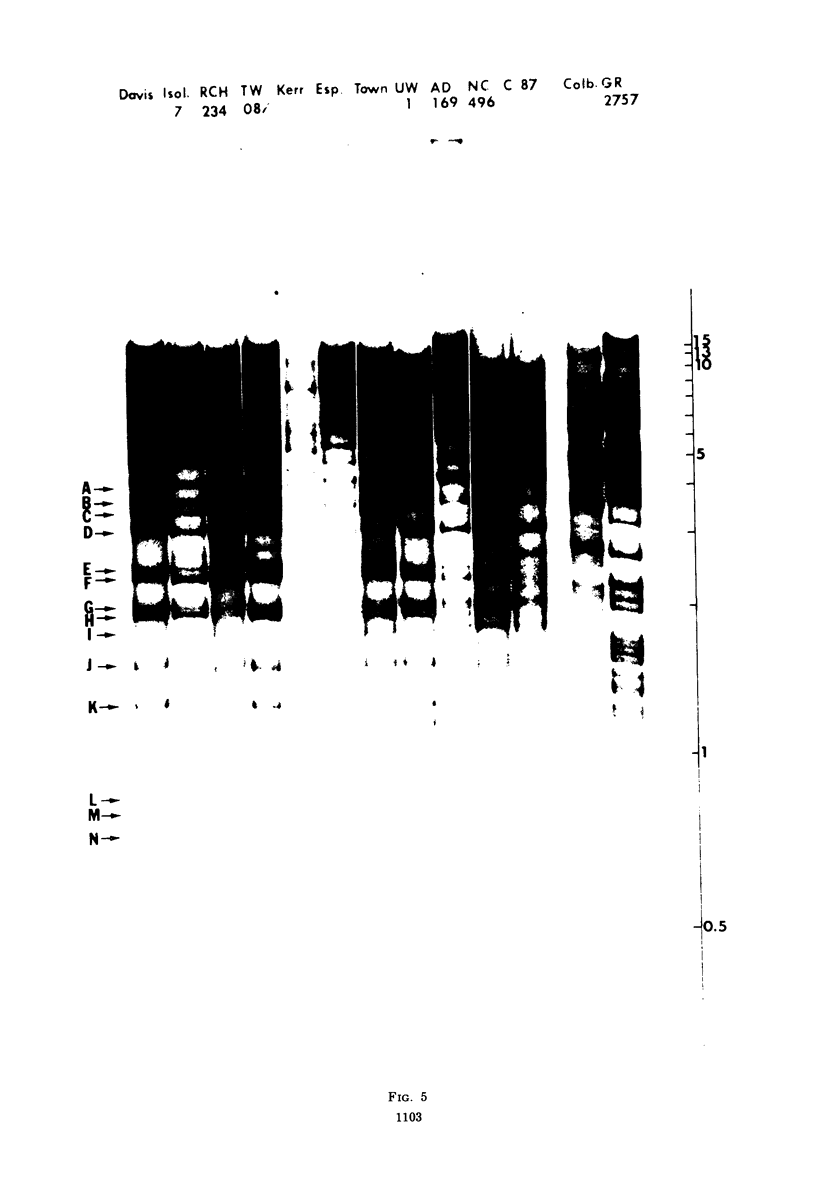
Cleavage of genomes of eleven human, one simian, and one simian-related cytomegalovirus (CMV) isolate by the restriction endonucleases HinD III and EcoR-1 generated reproducible DNA fragments. The size range of CMV DNA fragments as estimated by contour length measurements in comparison with simian virus 40 form II DNA and by coelectrophoresis with EcoR-1 fragments of herpes simplex virus DNA varied between 15 X 10(6) and 0.5 X 10(6) daltons. Comparison of the cleavage products of each isolate in 1% agarose slab gels showed extensive comigration of fragments among the human CMV isolates. In the HinD III digests, three fragment bands comigrated among all human CMV isolates, and six fragments comigrated among most, but not all, human CMV isolates. In the EcoR-1 digests, nine fragment bands comigrated among all human CMV isolates, and five bands comigrated among most, but not all human isolates. Each isolate had a distinctive electrophoretic profile with either HinD III or EcoR-1 digests. No two isolates had identical HinD III or EcoR-1 patterns although some isolates did share more general pattern similarities than others. No clear-cut subgrouping of isolates based on cleavage pattern characteristics could be discerned. Comparison of HinD III and EcoR-1 patterns showed that human isolates differ greatly from nonhuman CMV isolates. HinD III and EcoR-1 digests of each isolate contained both major and minor molar classes of DNA fragments that ranged from about 1 and multiples of 1 M down to about 0.25 M; however, the summed molecular weights for major molar fragments resulting from HinD III or EcoR-1 digests of several isolates closely approximated the molecular weight of 10(8) of the intact genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- BENYESH-MELNICK M., ROSENBERG H. S., WATSON B. VIRUSES IN CELL CULTURES OF KIDNEYS OF CHILDREN WITH CONGENITAL HEART MALFORMATIONS AND OTHER DISEASES. Proc Soc Exp Biol Med. 1964 Nov;117:452–459. doi: 10.3181/00379727-117-29607. [DOI] [PubMed] [Google Scholar]
- Chiang W. T., Wentworth B. B., Alexander E. R. The use of an immunofluorescence technique for the determination of antibodies to cytomegalovirus strains in human sera. J Immunol. 1970 Apr;104(4):992–999. [PubMed] [Google Scholar]
- Diosi P., Moldovan E., Tomescu N. Latent cytomegalovirus infection in blood donors. Br Med J. 1969 Dec 13;4(5684):660–662. doi: 10.1136/bmj.4.5684.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Frenkel N., Roizman B. Anatomy of herpes simplex virus DNA: strain differences and heterogeneity in the locations of restriction endonuclease cleavage sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1768–1772. doi: 10.1073/pnas.72.5.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Byrne J. C., Takemoto K. K., Martin M. A. Physical map of the BK virus genome. J Virol. 1975 Oct;16(4):959–973. doi: 10.1128/jvi.16.4.959-973.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Newbold J. E., Pagano J. S. Analysis of simian virus 40 DNA with the restriction enzyme of Haemophilus aegyptius, endonuclease Z. J Virol. 1973 Apr;11(4):508–514. doi: 10.1128/jvi.11.4.508-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Pagano J. S. Human cytomegalovirus. II. Lack of relatedness to DNA of herpes simples I and II, Epstein-Barr virus, and nonhuman strains of cytomegalovirus. J Virol. 1974 Mar;13(3):642–645. doi: 10.1128/jvi.13.3.642-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krech U., Jung M. The development of neutralizing antibodies in guinea pigs following immunization with human cytomegalovirus. Arch Gesamte Virusforsch. 1969;28(2):248–250. doi: 10.1007/BF01249391. [DOI] [PubMed] [Google Scholar]
- Lang D. J. Cytomegalovirus infections in organ transplantation and post transfusion. An hypothesis. Arch Gesamte Virusforsch. 1972;37(4):365–377. doi: 10.1007/BF01241460. [DOI] [PubMed] [Google Scholar]
- Montgomery R., Youngblood L., Medearis D. N., Jr Recovery of cytomegalovirus from the cervix in pregnancy. Pediatrics. 1972 Apr;49(4):524–531. [PubMed] [Google Scholar]
- Nigida S. M., Jr, Falk L. A., Wolfe L. G., Deinhardt F., Lakeman A., Alford C. A. Experimental infection of marmosets with a cytomegalovirus of human origin. J Infect Dis. 1975 Nov;132(5):582–586. doi: 10.1093/infdis/132.5.582. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. P., Summers W. C. Structure and function of herpesvirus genomes. I. comparison of five HSV-1 and two HSV-2 strains by cleavage their DNA with eco R I restriction endonuclease. J Virol. 1975 Apr;15(4):726–732. doi: 10.1128/jvi.15.4.726-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyeshmelnick M. Thermoinactivation of human cytomegalovirus. J Bacteriol. 1966 Jan;91(1):221–226. doi: 10.1128/jb.91.1.221-226.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]
- Weller T. H. Review. Cytomegaloviruses: the difficult years. J Infect Dis. 1970 Dec;122(6):532–539. doi: 10.1093/infdis/122.6.532. [DOI] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]