Abstract
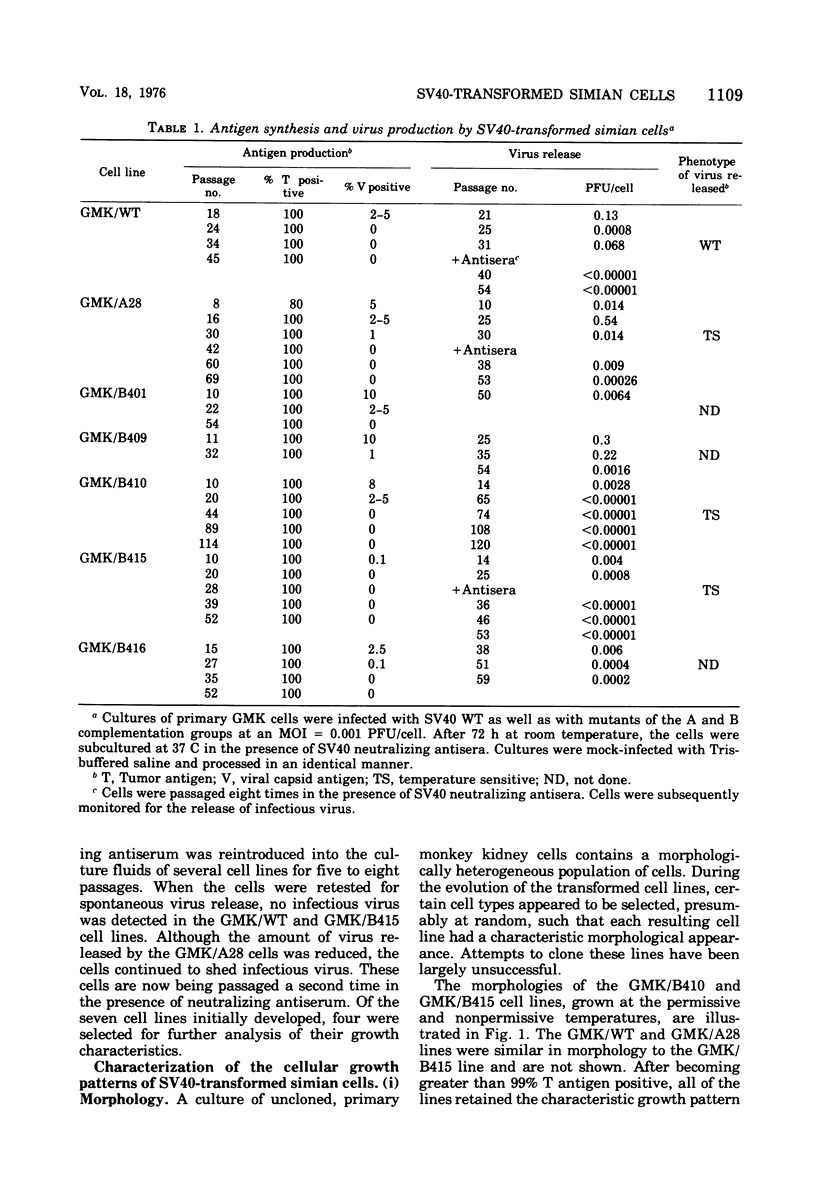
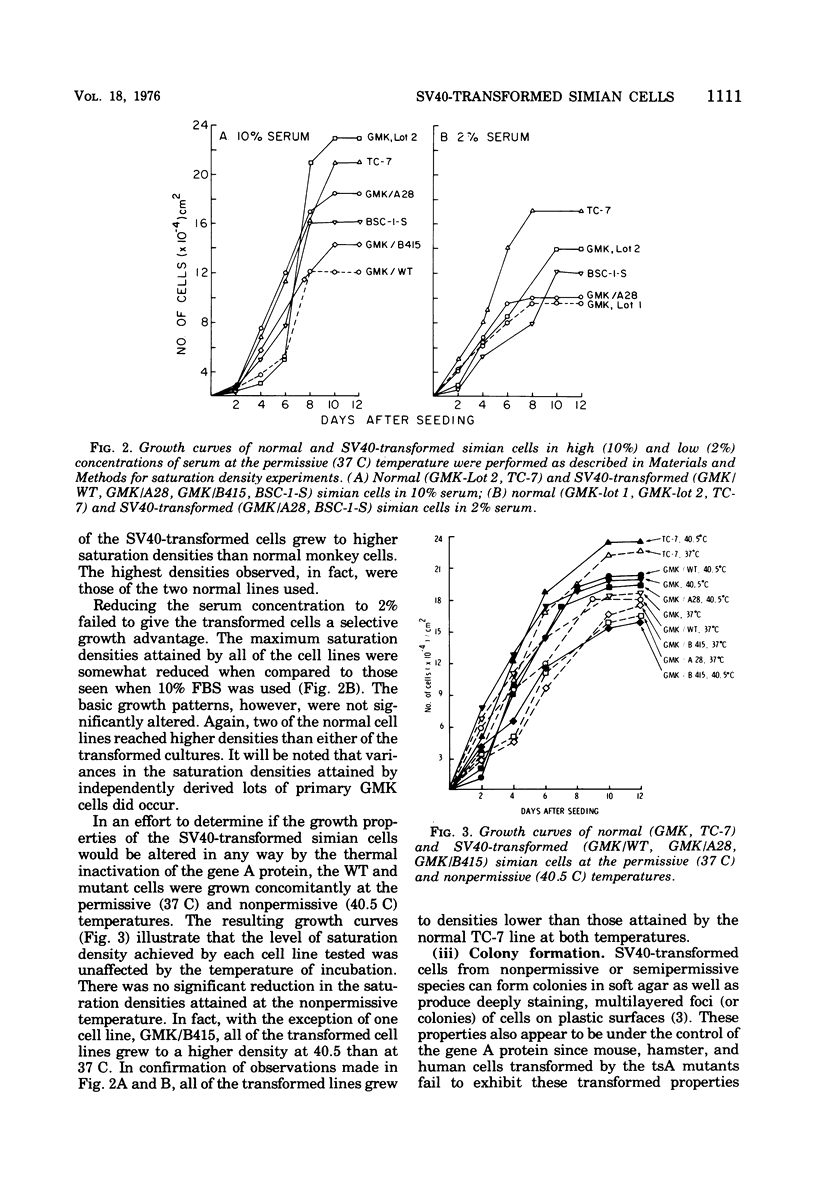
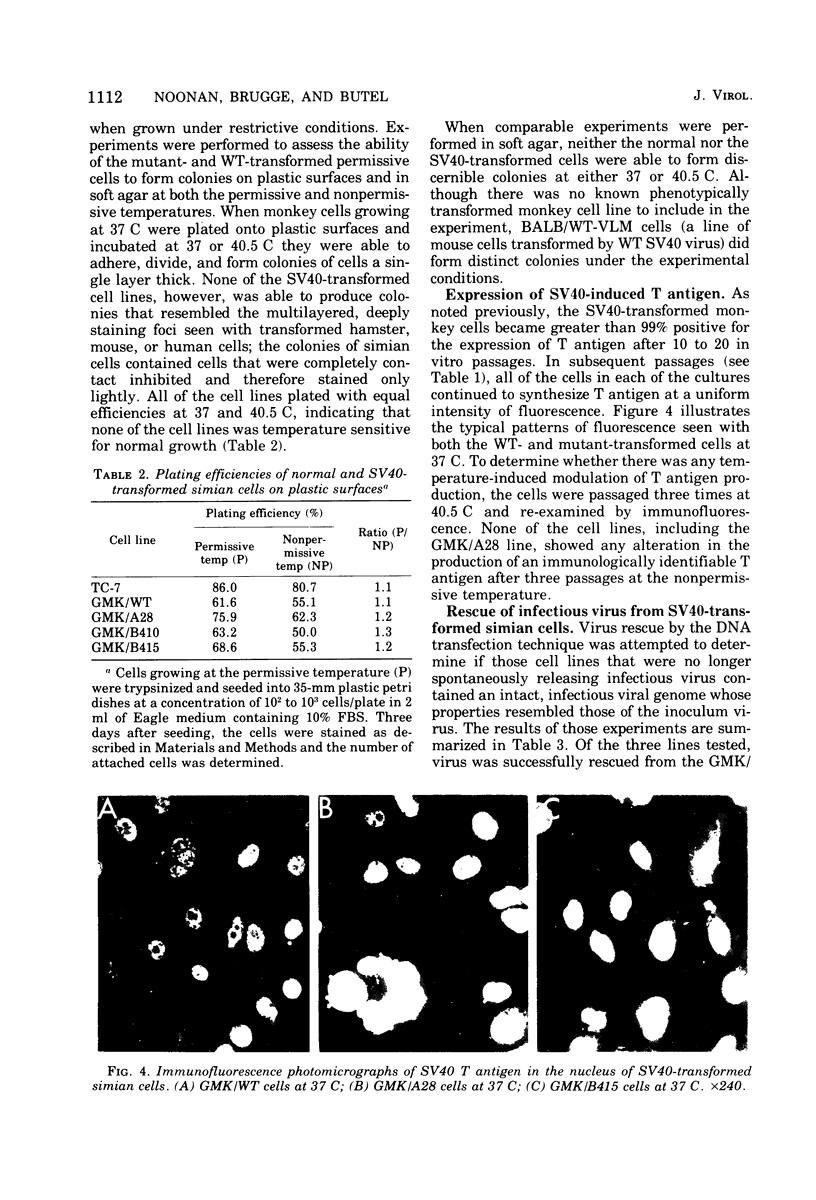
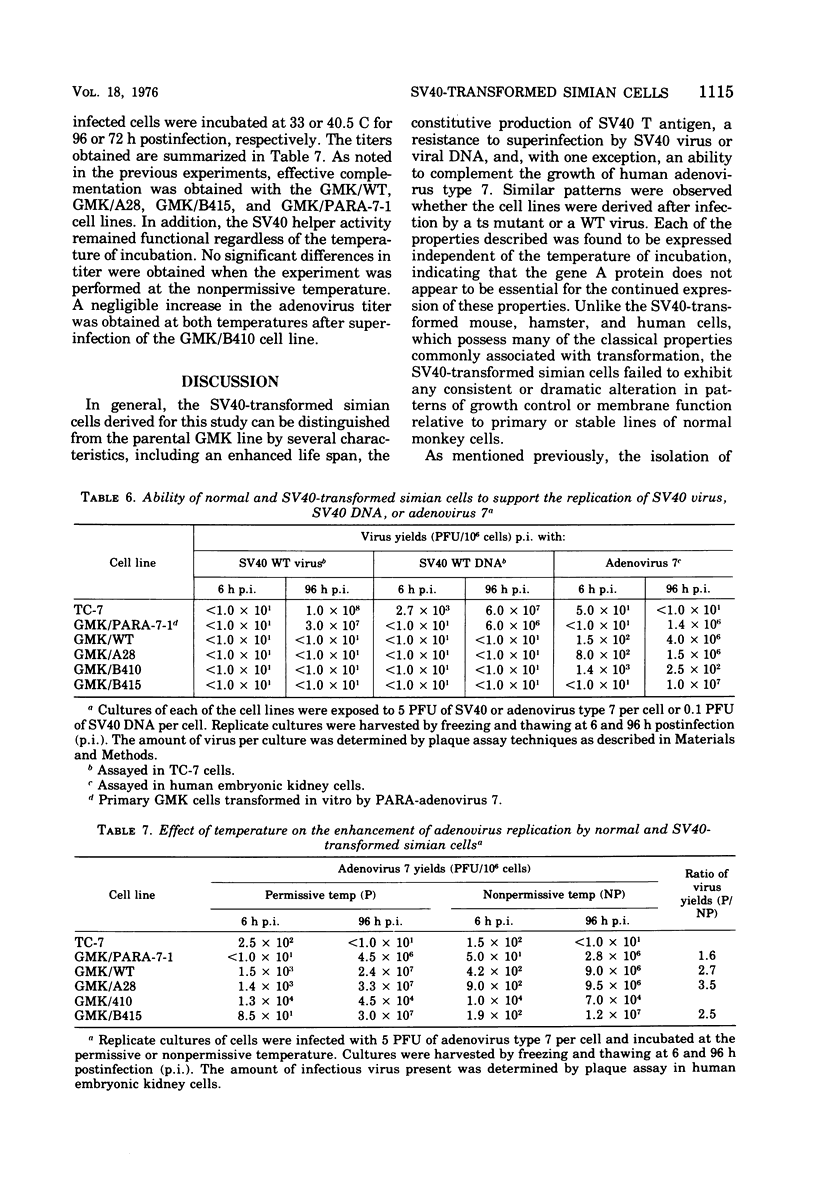
Seven lines derived from primary African green monkey kidney cells, which had survived lytic infection by wild-type simian virus 40 (SV40) or temperature-sensitive mutants belonging to the A and B complementation groups, were established. These cultures synthesize SV40 tumor (T) antigen constitutively and have been passaged more than 60 times in vitro. The cells released small amounts of virus even at high passage levels but eventually became negative for the spontaneous release of virus. Virus rescued from such "nonproducer" cells by the transfection technique exhibited the growth properties of the original inoculum virus. Four of the cell lines were tested for the presence of altered growth patterns commonly associated with SV40-induced transformation. Although each of the cell lines was greater than 99% positive for T antigen, none of the cultures could be distinguished from primary or stable lines of normal simian cells on the basis of morphology, saturation density in high or low serum concentrations, colony formation on plastic or in soft agar, hexose transport, or concanavalin A agglutinability. However, the cells could be distinguished from the parental green monkey kidney cells by a prolonged life span, the presence of T antigen, a resistance to the replication of superinfecting SV40 virus or SV40 viral DNA, and, with three of the four lines, an ability to complement the growth of human adenovirus type 7. These properties were expressed independent of the temperature of incubation. These results indicate that the presence of an immunologically reactive SV40 T antigen is not sufficient to ensure induction of phenotypic transformation and suggest that a specific interaction between viral and cellular genes and/or gene products may be a necessary requirement.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd V. A., Butel J. S. Demonstration of infectious deoxyribonucleic acid in transformed cells. I. Recovery of simian virus 40 from yielder and nonyielder transformed cells. J Virol. 1972 Sep;10(3):399–409. doi: 10.1128/jvi.10.3.399-409.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Rapp F. Replication in simian cells of defective viruses in an SV40-adenovirus "hybrid" population. J Bacteriol. 1966 Jan;91(1):278–284. doi: 10.1128/jb.91.1.278-284.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Richardson L. S., Melnick J. L. Variation in properties of SV40-transformed simian cell lines detected by superinfection with SV40 and human adenoviruses. Virology. 1971 Dec;46(3):844–855. doi: 10.1016/0042-6822(71)90085-7. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H., Foley G. E., Koprowski H., Lazarus H., Levine E. M., Adams R. A. Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med. 1970 Apr 1;131(4):863–879. doi: 10.1084/jem.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders J. F. Cell transformation by viruses as illustrated by the response of human and hamster renal cells to Simian virus 40. Harvey Lect. 1965;59:113–153. [PubMed] [Google Scholar]
- Feldman L. A., Butel J. S., Rapp F. Interaction of a simian papovavirus and adenoviruses. I. Induction of adenovirus tumor antigen during abortive infection of simian cells. J Bacteriol. 1966 Feb;91(2):813–818. doi: 10.1128/jb.91.2.813-818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M. V., Moorhead P. S. Transformation of African green monkey kidney cultures infected with simian vacuolating virus (SV40). Tex Rep Biol Med. 1965 Jun;23(Suppl):242–258. [PubMed] [Google Scholar]
- Fox R. I., Baum S. G. Posttranscriptional block to adenovirus replication in nonpermissive monkey cells. Virology. 1974 Jul;60(1):45–53. doi: 10.1016/0042-6822(74)90364-x. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Baum S. G. Synthesis of viral ribonucleic acid during restricted adenovirus infection. J Virol. 1972 Aug;10(2):220–227. doi: 10.1128/jvi.10.2.220-227.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huebner K., Santoli D., Croce C. M., Koprowski H. Characterization of defective SV40 isolated from SV40-transformed cells. Virology. 1975 Feb;63(2):512–522. doi: 10.1016/0042-6822(75)90324-4. [DOI] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Brown M., Dubbs D. R. Identification of the simian virus 40 which replicates when simian virus 40-transformed human cells are fused with simian virus 40-transformed mouse cells or superinfected with simian virus 40 deoxyribonucleic acid. J Virol. 1970 Jul;6(1):69–77. doi: 10.1128/jvi.6.1.69-77.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Ishitsuka H., Oda K. An SV40-induced initiation factor for protein synthesis concerned with the regulation of permissiveness. Nature. 1974 Dec 20;252(5485):649–653. doi: 10.1038/252649a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Rapp F., Feldman L. A., Mandel M. Synthesis of virus deoxyribonucleic acid during abortive infection of simian cells by human adenoviruses. J Bacteriol. 1966 Oct;92(4):931–936. doi: 10.1128/jb.92.4.931-936.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Pauluzzi S., Butel J. S. Variation in properties of plaque progeny of PARA (defective simian papovavirus 40)-adenovirus 7. J Virol. 1969 Nov;4(5):626–631. doi: 10.1128/jvi.4.5.626-631.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Trulock S. C. Susceptibility to superinfection of simian cells transformed by SV 40. Virology. 1970 Apr;40(4):961–970. doi: 10.1016/0042-6822(70)90142-x. [DOI] [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Swetly P., Brodano G. B., Knowles B., Koprowski H. Response of simian virus 40-transformed cell lines and cell hybrids to superinfection with simian virus 40 and its deoxyribonucleic acid. J Virol. 1969 Oct;4(4):348–355. doi: 10.1128/jvi.4.4.348-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. Viral transformation of monkey kidney cell cultures. Nature. 1967 Feb 25;213(5078):768–770. doi: 10.1038/213768a0. [DOI] [PubMed] [Google Scholar]





