Abstract
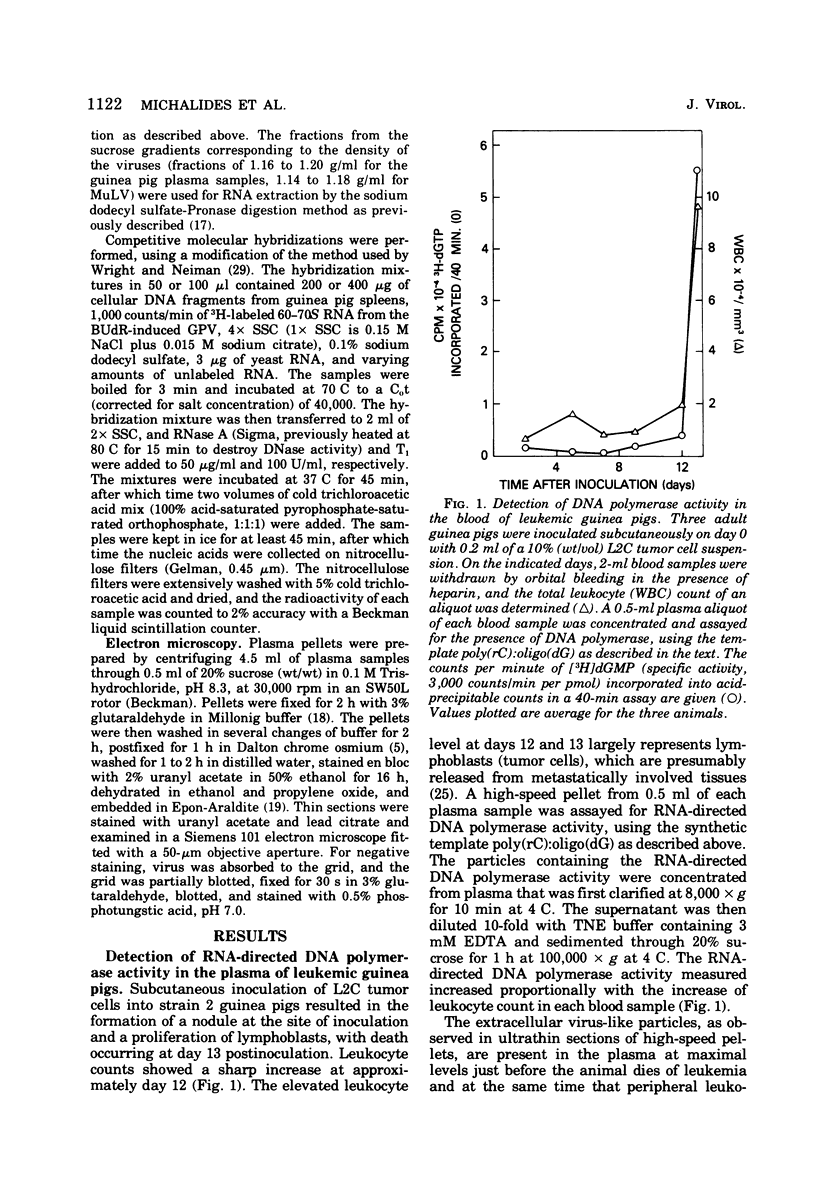
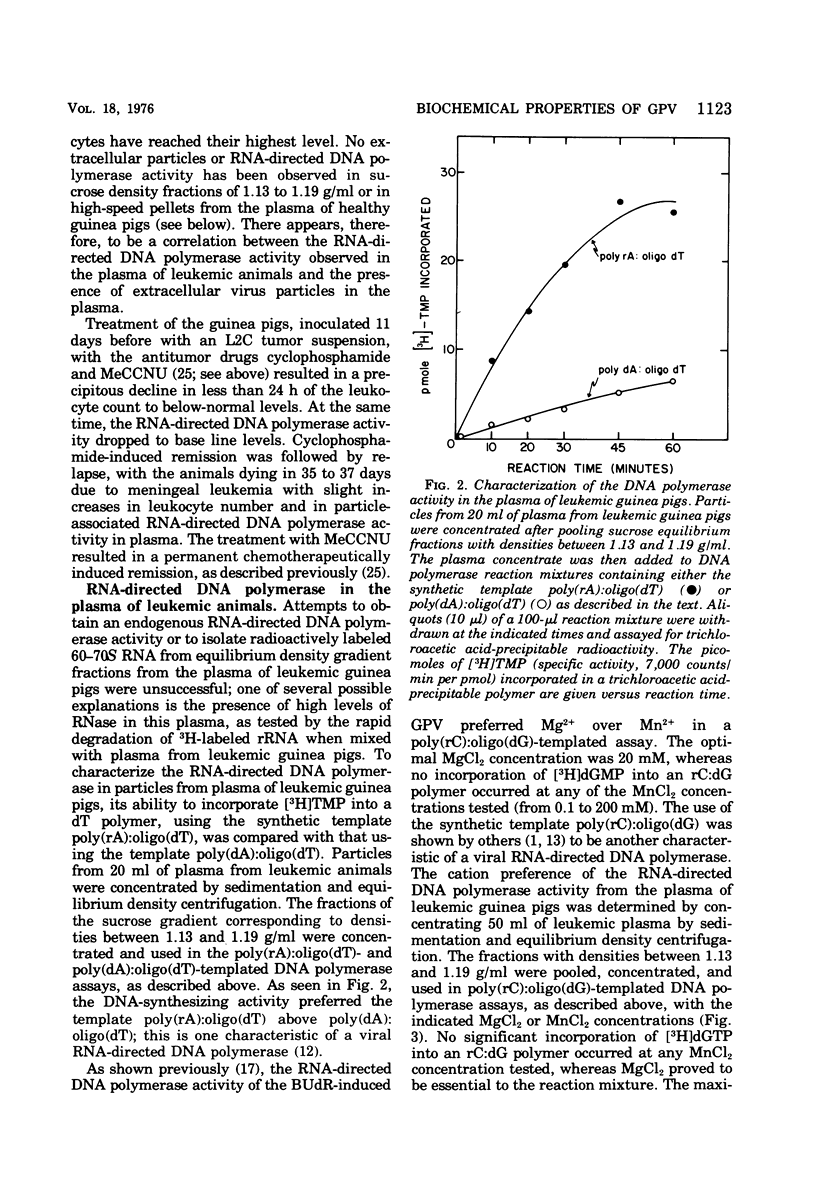
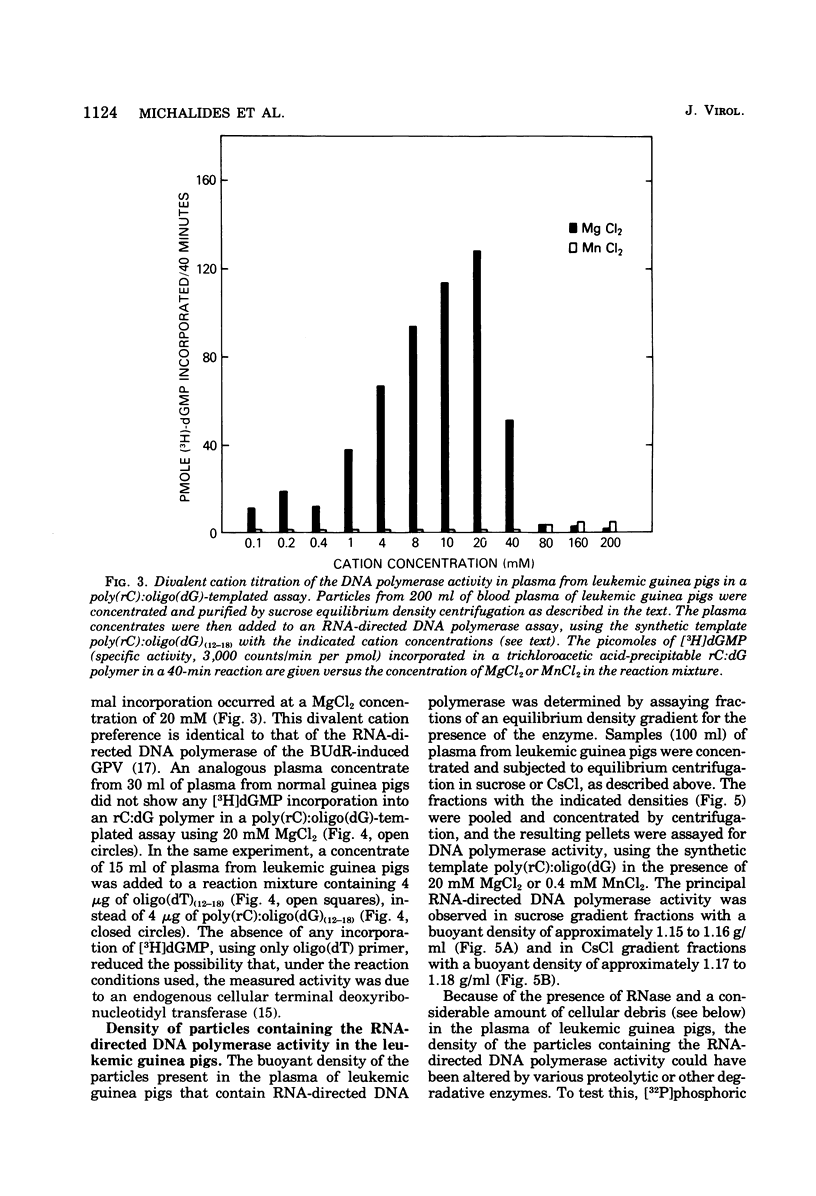
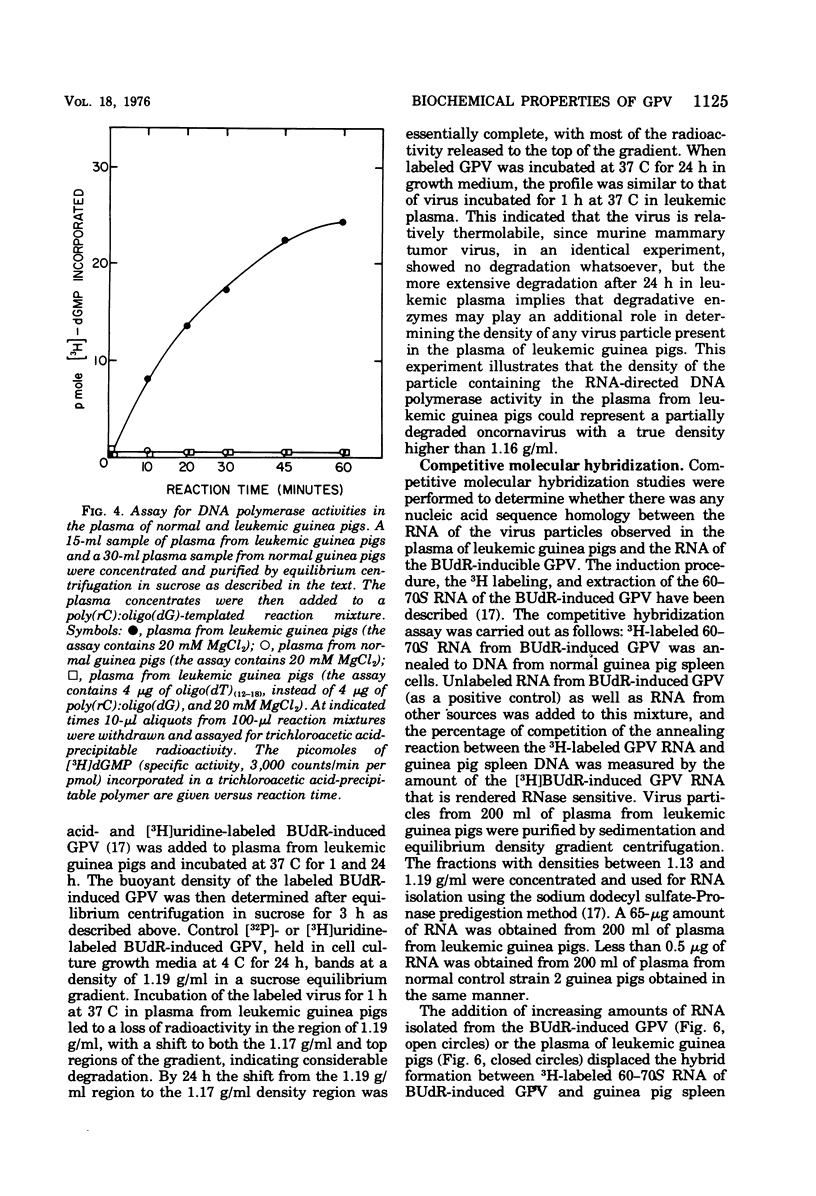
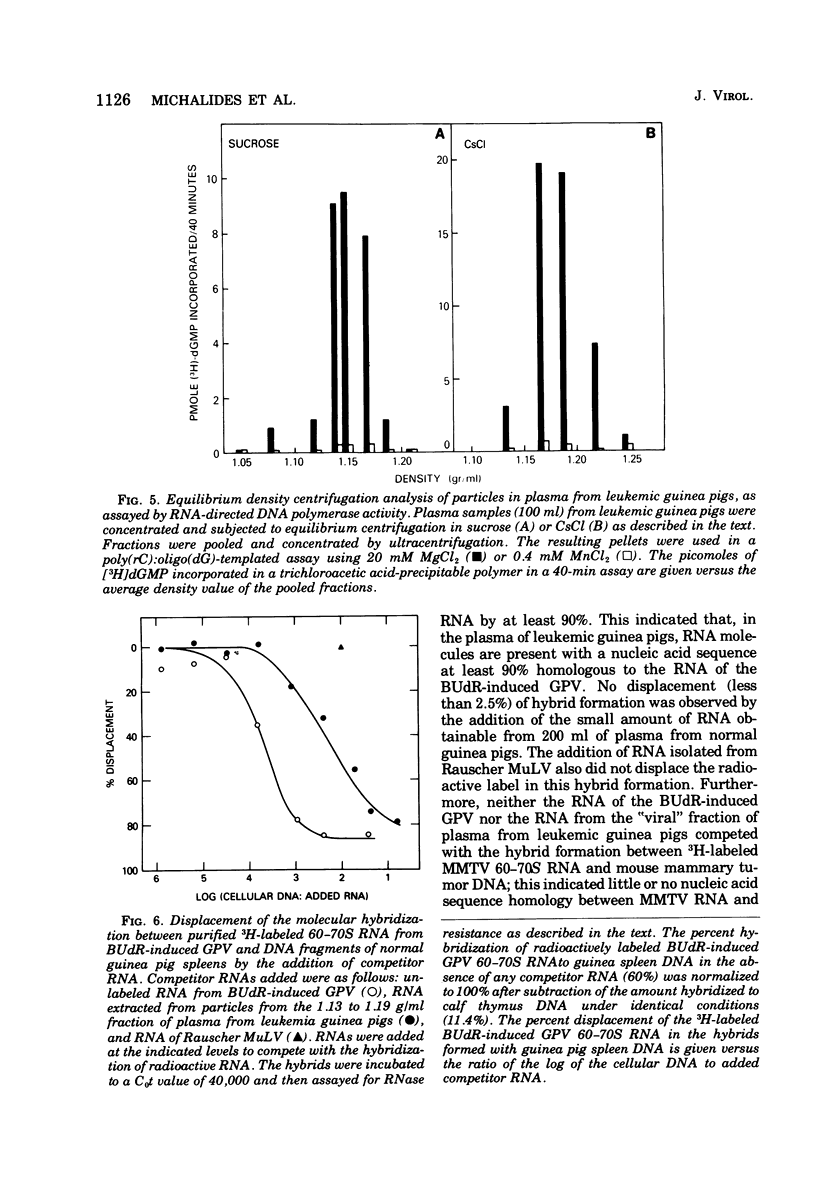
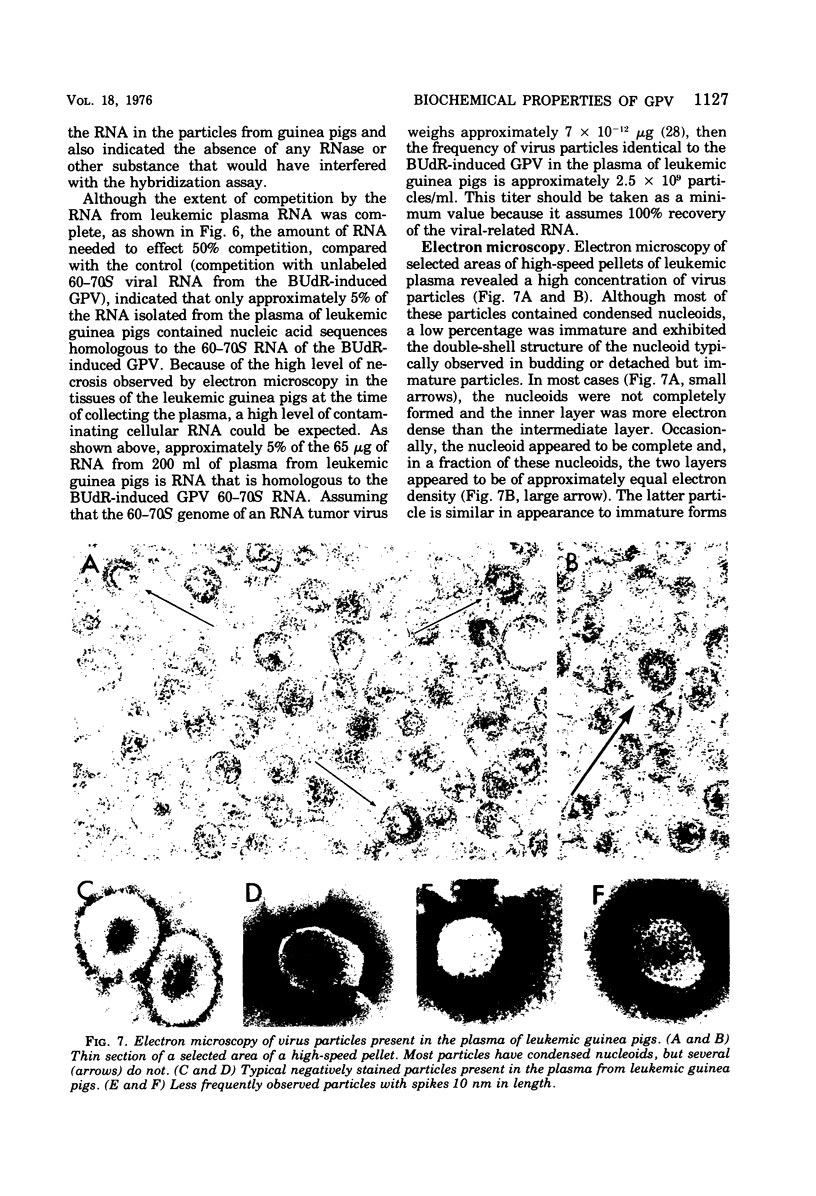
The inoculation of L2C guinea pig leukemia cells into strain 2 guinea pigs results in the death of the animals within 12 to 15 days. Death is preceded by the simultaneous appearance in the plasma of (i) elevated leukocyte levels, (ii) extracellular virus particles, and (iii) a particle-associated RNA-directed DNA polymerase. This enzyme activity has a cation preference identical to that of the type B bromodeoxyuridine-induced guinea pig virus, i.e., an Mg2+ optimum at 20 mM and no activity using Mn2+. Competitive molecular hybridization studies also revealed that the plasma of leukemic guinea pigs contained approximately 2 X 10(9) genome equivalents per ml of an RNA that is homologous to the RNA of the bromodeoxyuridine-induced guinea pig virus. Morphological observations indicate that most, but not all, of the extracellular particles observed in leukemia plasma are derived from the intracisternal particles seen in the L2C tumor cells. The possibilities that either two viral populations are present or that the in vivo morphogenesis of the type B bromodexoyuridine-inducible guinea pig virus is markedly different from its in vitro morphogenesis are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black V. H. Virus particles in primordial germ cells of fetal guinea pigs. J Natl Cancer Inst. 1974 Feb;52(2):545–551. doi: 10.1093/jnci/52.2.545. [DOI] [PubMed] [Google Scholar]
- CONGDON C. C., LORENZ E. Leukemia in guinea-pigs. Am J Pathol. 1954 Mar-Apr;30(2):337–359. [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Perk K., Dalton A. J. Virus-like particles induced in guinea pig cells by 5-bromo-2'-deoxyuridine are morphologically similar to murine B-type virus. Nature. 1974 Jun 28;249(460):828–830. doi: 10.1038/249828a0. [DOI] [PubMed] [Google Scholar]
- Dunkel V. C., Bast R. C., Jr, Gerwin B. I., Heine U., Cottler-Fox M., Borsos T. Presence of A-type and absence of C-type virus particles in a chemically induced guinea pig hepatoma. J Natl Cancer Inst. 1974 Aug;53(2):591–593. doi: 10.1093/jnci/53.2.591. [DOI] [PubMed] [Google Scholar]
- Feldman D. G., Gross L. Electron microscopic study of the guinea pig leukemia virus. Cancer Res. 1970 Nov;30(11):2702–2711. [PubMed] [Google Scholar]
- Fridlender B., Fry M., Bolden A., Weissbach A. A new synthetic RNA-dependent DNA polymerase from human tissue culture cells (HeLa-fibroblast-synthetic oligonucleotides-template-purified enzymes). Proc Natl Acad Sci U S A. 1972 Feb;69(2):452–455. doi: 10.1073/pnas.69.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Gallagher R. E., Miller N. R., Mondal H., Saxinger W. C., Mayer R. J., Smith R. G., Gillespie D. H. Relationships between components in primate RNA tumor viruses and in the cytoplasm of human leukemic cells: implications to leukemogenesis. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):933–961. doi: 10.1101/sqb.1974.039.01.109. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D. Activation of guinea pig C-type virus in cultured spleen cells by 5-bromo-2'-deoxyuridine. J Natl Cancer Inst. 1972 Aug;49(2):567–570. [PubMed] [Google Scholar]
- Kacian D. L., Spiegelman S. Purification and detection of reverse transcriptase in viruses and cells. Methods Enzymol. 1974;29:150–173. doi: 10.1016/0076-6879(74)29018-9. [DOI] [PubMed] [Google Scholar]
- Lewis B. J., Abrell J. W., Smith R. G., Gallo R. C. Human DNA polymerase 3 (R-DNA polymerase): distinction from DNA polymerase I and reverse transcriptase. Science. 1974 Mar 1;183(4127):867–869. doi: 10.1126/science.183.4127.867. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Ma B. I., Swartzendruber D. C., Murphy W. H. Detection of virus-like particles in germinal centers of normal guinea pigs. Proc Soc Exp Biol Med. 1969 Feb;130(2):586–590. doi: 10.3181/00379727-130-33613. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Bohn E. W., Wilson S. H. On the DNA polymerase III of mouse myeloma: partial purification and characterization. Biochemistry. 1975 Mar 11;14(5):1006–1020. doi: 10.1021/bi00676a020. [DOI] [PubMed] [Google Scholar]
- McCaffrey R., Smoler D. F., Baltimore D. Terminal deoxynucleotidyl transferase in a case of childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1973 Feb;70(2):521–525. doi: 10.1073/pnas.70.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Schlom J., Dahlberg J., Perk K. Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol. 1975 Oct;16(4):1039–1050. doi: 10.1128/jvi.16.4.1039-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Nayak D. P. Characterization of bromodeoxyuridine-induced endogenous guinea pig virus. J Virol. 1974 Sep;14(3):679–688. doi: 10.1128/jvi.14.3.679-688.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel E., Banfield W., Burstein S., Tousimis A. J. Virus particles associated with strain 2 guinea pig leukemia (L2C/N-B). J Natl Cancer Inst. 1967 Jun;38(6):979–981. [PubMed] [Google Scholar]
- Nayak D. P., Murray P. R. Induction of type C viruses in cultured guinea pig cells. J Virol. 1973 Jul;12(1):177–187. doi: 10.1128/jvi.12.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. W., Perk K., Chirigos M. A., Torgersen J. A. Drug therapy against a transplantable guinea pig leukemia. Cancer Res. 1975 Apr;35(4):1093–1098. [PubMed] [Google Scholar]
- Perk K., Dahlberg J. E. Murine intracisternal A type particles fail to separate from the membrane of the endoplasmic reticulum. J Virol. 1974 Nov;14(5):1304–1306. doi: 10.1128/jvi.14.5.1304-1306.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Duh F. G., Cho H. Y., Wuu K. D., Vernon M. L. Brief communication: Activation by 5-bromo-2'-deoxyuridine of particles resembling guinea-pig leukemia virus from guinea-pig nonproducer cells. J Natl Cancer Inst. 1973 Oct;51(4):1327–1331. doi: 10.1093/jnci/51.4.1327. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Avian tumor viruses. Adv Virus Res. 1965;11:293–385. doi: 10.1016/s0065-3527(08)60549-7. [DOI] [PubMed] [Google Scholar]
- Wright S. E., Neiman P. E. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. doi: 10.1021/bi00704a035. [DOI] [PubMed] [Google Scholar]



