Abstract
Gastroparesis is a clinical disorder characterized by upper gastrointestinal symptoms related with delayed gastric emptying of solids and liquids in the absence of mechanical obstruction. Diabetes mellitus has been the most common cause of gastroparesis and idiopathic gastroparesis also accounts for a third of all chronic cases. The most important mechanisms of gastroparesis, as understood to date, are loss of expression of neuronal nitric oxide synthase and loss of the interstitial cells of Cajal. However, the pathogenesis of gastroparesis is poorly understood. There have been several studies on specific molecules related to the pathogenesis of gastroparesis. Additionally, the Gastroparesis Clinical Research Consortium of the National Institutes of Health has achieved several promising results regarding the pathophysiology of gastroparesis. As the progress in the pathophysiology of gastroparesis has been made, a promising new drug therapy has been found. The pathophysiology and drug therapy of gastroparesis are focused in this review. Until now, the real-world medication options for treatment of gastroparesis are limited. However, it is expected to be substantially improved as the pathophysiology of gastroparesis is elucidated.
Keywords: Diabetes mellitus, Etiology, Gastroparesis, Physiopathology, Therapy
Introduction
Gastroparesis is a condition that delays gastric emptying of solids and liquids in cases where there is no mechanical obstruction.1 A variety of mechanisms, such as vagal nerve dysfunction, sympathetic nerve dysfunction, damage to the enteric nerve system, as well as hyperglycemia itself, impede gastrointestinal (GI) function. The most common disease associated with gastroparesis is diabetes although idiopathic cases are just as frequent if not more so. Rarer associations include postsurgical conditions, collagen vascular diseases, and neurological disorders.2 Diabetic gastroparesis (DG), first reported in patients with type I diabetes in 1958, has a significantly negative effect on quality of life and is a chronic and often debilitating disorder.3 Because the symptoms of DG and gastric emptying are as yet poorly correlated, its epidemiology is difficult to assess. Fifty percent of patients with type I diabetes of 10 years' duration had abnormal gastric emptying;4 13% of Korean diabetic patients had dyspepsia.5 A population-based survey of 15,000 adults showed that 11 to 18% of gastroparesis patients had upper GI symptoms.6 A population-based epidemiologic study from Olmsted County, using a combined definition of delayed gastric emptying and symptoms, found a prevalence of 24.2 per 100,000 inhabitants and an incidence of 6.3 per 100,000 persons per year, which indicates that gastroparesis is an uncommon condition in the community compared with tertiary hospital settings.7 Another population-based cohort study confirmed the relative uncommonness of gastroparesis, showing cumulative proportions, over a 10-year time period, of 5.2% for type 1 DM, 1.0% for type 2 DM and 0.2% among controls.8 However, a different study estimated that 1.8% of community residents had delayed gastric emptying whereas the prevalence of diagnosed gastroparesis was low (0.02%).9 On the basis of these reults, the authors suggested that known gastroparesis is likely just the tip of a large hidden iceberg. In other words, the prevalence of gastroparesis undoubtedly is higher than reported, due to the fact that many gastroparesis sufferers remain undiagnosed. In addition, as the incidence rate of diabetes rises, so too will that of gastroparesis. Recently, progress in the pathophysiology of gastroparesis has been made, and a promising new drug therapy has been found. These are the subjects of the present review.
Pathogenesis
The pathogenesis of gastroparesis is poorly understood. Gastric emptying entails interaction among smooth muscle, enteric and extrinsic autonomic nerves, and the interstitial cells of Cajal (ICC).10 Traditionally, autonomic neuropathy was considered to be the main mechanism of DG, because it was assumed to be a symptom related to diabetic neuropathy. Vagus nerve dysfunction reduces pyloric relaxation and thereby prohibits passage of foods, which are effects similar to the consequences of subdiaphragmatic vagotomy.11 However, some patients have gastroparesis without evidence of generalized autonomic neuropathy,12 although these patients may have more subtle and specific disturbances in gastric autonomic innervation. Much recent attention has been focused on intrinsic nerves in the stomach. The most important mechanisms of gastroparesis, as understood to date, are loss of expression of neuronal nitric oxide synthase (nNOS) and loss of ICC.13
Inhibitory nitrergic neurons in the gastric wall secret nitric oxide (NO). NO is an important cellular signaling molecule; its various functions include relaxation of smooth muscle and, consequently, accommodation of the fundus and relaxation of pylorus.14 NO is synthesized by nNOS, which is expressed in the enteric nerve. The major functionality of nNOS is control of the muscle tone of the lower esophageal sphincter, the pylorus, the sphincter of Oddi, and the anus.15 Additionally, it modulates the accommodative reflex of the fundus and the peristaltic reflex of the small intestine.16 The molecular change of enteric nervous system effects gastric emptying delay through depletion of nNOS. Animal models showed that there is a loss of function of NOS neurons both in spontaneously diabetic rats and streptozotocin diabetic rats.17,18 DG was correlated with the loss of nNOS mRNA and protein.19 Recent studies suggest that hyperlipidemia, shown to impair gastric motility functions in low-density lipoprotein receptor knockout mice and apolipoprotein E knockout mice, is a potential cause of developing gastroparesis.20,21 Although the expression of nNOS is decreased in DG, the loss of nNOS itself does not predict delayed gastric emptying.22 In a study on female diabetic rats, gastric relaxation was better correlated with the level of the dimerised form of nNOS than with the absolute nNOS level.23 This means that the absolute nNOS level is less important than post-translational modification of nNOS. Furthermore, it can explain why the incidence of gastroparesis is significantly higher among young women.24
Loss of nNOS, significantly, is related to the loss of ICC in the stomach. ICC generate a slow wave in the stomach and transmit it to smooth muscle, thereby enabling phasic contraction. Loss of ICC in fact is one of the main histological findings in DG. Specifically, ICC were greatly reduced in the distal stomach in diabetic mice manifesting delayed gastric emptying, impaired electrical pacemaking, and reduced motor neurotransmission.25 Another study found that myenteric-ganglia-related ICC were decreased in 50-70% of all nNOS-/- mice and further, the ICC derived from knockout mice were increased by NO donors.26 However, the increase of NO by sildenafil did not help humans.27
There have been several studies on specific molecules related to the pathogenesis of gastroparesis. One study investigated ultrastructural fibroblast-like cells (FLCs), which are interstitial cells existing near the human small intestine and in close proximity to ICC, but different from them.28 FLC growth is stimulated by platelet-derived growth factor receptor alpha (PDGFRα) in human gastric smooth muscle. However, a very recent investigation observed no differences in the distribution, morphology, or overall numbers of PDGFRα-immunoreactive FLC relative to ICC in gastroparesis patients.29 Further studies elucidating the role of FLC in human GI function are needed. Recent work has emphasized the potential role of immune cells in the pathophysiology of gastroparesis. One such study reported increases in the immunoreactivity of CD45 (a general hematopoietic cell marker) and CD68 (a selective marker for macrophages) for both patients with DG and idiopathic gastroparesis (IG).30 Another study found that in DG patients, heme oxygenase-2 (HO-2), which, like nNOS, is an endogenous gaseous neuromodulator inhibiting GI motility, was decreased.31 Choi et al22 suggested that the decrease of heme oxygenase-1, which is cytoprotective against oxidative injury, increases oxidative stress and induces DG in non-obese diabetic mice. Another study confirmed that increased heme oxygenase-1 expression prevents delayed gastric emptying in diabetic mice.32 Additionally, it has been reported that the decrease of tetrahydrobiopterin, a major cofactor in nNOS activity and NO synthesis, causes delayed gastric emptying in female rats.33
Human studies remain insufficient, though one showed that among 14 patients with refractory gastroparesis, 5 showed an absence of ICC and 9 had an ICC/normal-cell ratio of 20%.34 Another study found that the ICC density, along with the expression of nNOS and substance P, was decreased in the gastric antrum of diabetic patients, which might explain dysmotility symptoms observed in diabetic patients.35 Loss of ICC in both human and murine DG, in any case, has been verified.25,35,36 The Ca2+-activated Cl- channels are vital for smooth muscle contraction and secretion. ICC selectively express Ano1, which is related to classical Ca2+-activated Cl- currents.37 Ano1, for determinations of the ICC distribution in the human and mouse GI tracts, is a better selective marker of ICC than mast cells.38 A recent investigation involving DG patients and Ano1 splicing showed the changes Ano1 expression brought on DG; Ano1, therefore, could be a new molecular target in terms of both the etiologic and therapeutic aspects.39
In diabetic patients, attention also has to be paid to the association between hyperglycemia and gastric emptying. Hyperglycemia stimulates pyloric contraction and inhibits antral contraction, thereby delaying the gastric emptying.40 Advanced glycation end-products (AGEs), produced during hyperglycemia, can inhibit the expression of intestinal nNOS in vitro.41 Generation of AGEs in diabetic rat results in loss of intestinal nNOS expression, thereby inhibitors of AGEs might be useful in the treatment of GI complications of diabetes.42
The Gastroparesis Clinical Research Consortium (GPCRC) of the National Institutes of Health (NIH) has achieved several promising results regarding the pathophysiology of gastroparesis. An investigation of the cellular changes in patients with DG (n = 20) and IG (n = 20), referencing full-thickness biopsy specimens, discovered that 83% of patients with gastroparesis had histological abnormalities such as loss of ICC and increase in CD45 and CD68 immunoreactivity. There were no differences between the 2 disorders, except that most of the cases of nNOS-expression reduction were found in patients with IG (40%; DG patients: 20%). Connective-tissue stroma was significantly increased in both disorders, according to the results of electron microscopy.30 However, Faussone-Pellegrini et al43 suggested that the difference between the 2 disorders lies in the ultrastructural changes in ICC and nerves. They determined that a thickened basal lamina around smooth muscle cells and nerves was the distinguishing feature of DG, whereas in the case of IG, fibrosis around the nerves was dispositive. They also found that damage to ICC and nerves was more severe in IG. They documented the contrasting ultrastructural changes between the disorders, from which results potential target therapies might be developed.43 Grover et al. reported that ICC and enteric nerves were decreased in both disorders compared with healthy controls; however they did not correlate these findings with symptoms severity. Symptoms severity and nausea are related to myenteric immune infiltration in IG. Loss of ICC delays gastric emptying in DG, whereas in IG, these factors are unrelated.44
Treatment
Prokinetic agents are the mainstay of treatment, because they accelerate gastric emptying by increasing antral contractility and improving the effectiveness of gastropyloroduodenal motility. Their other actions include centrally mediated antiemetic effects, proximal gastric relaxation, suppression of visceral sensation, and improvement in gastric dysrhythmias.45 Metoclopramide, a prokinetic and antinauseant agent approved by the Food and Drug Administration (FDA) for treatment of DG, is a potent central and peripheral dopamine receptor antagonist. The FDA recommends only short-term treatment (4-12 weeks), as metoclopramide crosses the blood/brain barrier, producing CNS side effects such as anxiety, agitation, somnolence, insomnia, and intractable tardive dyskinesia.46,47 Older people and women should be especially cautious in its use. Domperidone, another dopamine (D2) antagonist, enhances stomach contraction by antagonizing the peripheral receptors in the stomach.48 Its utility is similar to that of metoclopramide, but without the CNS side effects, and is widely available in most countries. Although the drug is not approved in the USA, the FDA makes it available for use via an investigational new drug application.49 A recent study demonstrated that the effect of domperidone is associated with age and genetic polymorphisms in the potassium channel KCNH2 gene and the alpha1D adrenoceptor ADRA1D gene.50 Erythromycin is a motilin receptor agonist that functions as a potent prokinetic agent. Unfortunately, tachyphlaxis appears to limit its benefits to short-term. The focus therefore has shifted to alternative motilides. Mitemcinal, a motilin agonist, enhanced gastric emptying in a randomized double-blind study on 106 patients with gastroparesis.51 However, symptomatic improvement was no better than placebo. These and other studies on prokinetics suggests that simply enhancing gastric emptying may not provide the hoped therapeutic outcome and bring into question about the relationship between emptying and symptoms. Nevertheless, the quest for other prokinetics continues. GSK962040 is a small-molecule, selective motilin receptor agonist that stimulates GI motility in humans and rabbits.52 The results of a phase II study on its single-dose safety, tolerability, and pharmacokinetics are anticipated. The serotonin type 4 (5-HT4) receptor, with its location on the cholinergic nerve endings of interneurons and motor neurons, is a major target for enhancement of GI motility. However, there is no available data on the use of the serotonin 5-HT4 receptor agonist prucalopride/TD-5108 and gastroparesis.
Ghrelin, synthesized in the endocrine cells of the gastric mucosa, stimulates growth hormone release, gastric motility and food intake. Ghrelin has antioxidant and anti-inflammatory effects,53 and enhances gastric emptying in DG patients.54 Specifically, TZP-101, a selective intravenously administered agonist has been the subject of keen interest. A report on a trial with healthy volunteers documented good safety profile55 in enhancing gastric emptying and improving symptoms in DG. In 10 symptomatic diabetic patients (type 1, n = 7; type 2, n = 3), solid-meal half emptying was reduced by 20%, and postprandial fullness by 37%, after intravenous TZP-101 administration.56 A double-blind, randomized and placebo-controlled study on TZP-101 showed that 80 µg/kg is the most effective as well as safe and well-tolerated dose.57 In patients with severe nausea and vomiting, TZP-101 reduced the severity and frequency of both.58 Additionally, an oral preparation, TZP-102, has been formulated, and a phase II study is in progress. A 4-week double-blind, placebo-controlled study demonstrated significant improvements in nausea, early satiety, postprandial fullness, and the total Gastroparesis Cardinal Symptom Index.59 Table summarized the information from new prokinetics under evaluation.
Table.
Recent Studies on New Prokinetics for Gastroparesis
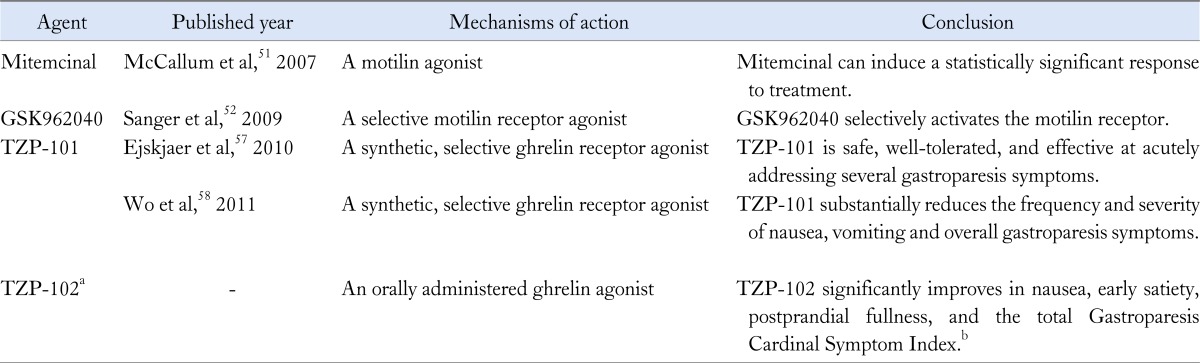
aTZP-102 is under evaluation in a 12-week, phase 2b trial; bThis result has come from a 4-week double-blind, placebo-controlled study.
Antiemetic drugs have been used successfully in clinical practice to treat the symptoms of gastroparesis in spite of insufficient scientific evidences. The most commonly used antiemetic drugs are phenothiazines such as prochlorperazine and thiethylperazine. They can be used in combination with prokinetic agents. Most standard antiemetic agents have no effect on gastric motor function.60 Ondansetron, a 5-HT3 receptor antagonist, is effective in controlling nausea and vomiting, but has not been shown to improve gastric emptying.61 Mirtazapine is an antidepressant that is active on the 5-HT3 receptor; it has been used for the treatment of nausea in patients with gastroparesis refractory to conventional prokinetic therapies.62,63 Tricyclic antidepressants (TCAs) are efficacious in functional nausea and vomiting and irritable bowel syndrome.64,65 In a retrospective review of 24 diabetic patients presenting with nausea and vomiting and unresponsive to prokinetic therapy, 88% reported moderate symptom improvement with TCAs.66 Tricyclic medications in low doses can reduce pain associated neuropathic pain.67 This may also account for their beneficial effects in gastroparesis based on the hypothesis that the nausea in this condition results from a vagal sensory neuropathy. However, the actual mechanism is poorly understood. The available studies on TCAs considered only small numbers of patients, and were not randomized.68 The NIH funded gastroparesis research consortium, GPCRC, has recently conduct a placebo controlled randomized trial of low dose nortriptyline in patients with idiopathic gastroparesis, and the results are eagerly awaited.
Gastric electrical stimulation (GES) is an alternative option for the treatment of medically refractory gastroparesis. The gastric stimulation device is implanted subcutaneously into the abdominal wall, and the electrodes are placed in the serosa. In fact, GES by short pulses and low energy (Enterra Therapy System, Medtronic, Minneapolis, MN, USA) has been approved as a therapeutic option by the US FDA for the treatment of diabetic and idiopathic etiologies of gastroparesis that are refractory to all medical management. Most published data has come from openlabel studies, though a double-blind crossover design showed significantly decreased vomiting frequency and GI symptoms as well as improved quality of life in patients with severe gastroparesis.69 Another crossover prospective study found that six weeks of GES therapy with Enterra significantly reduced vomiting and gastroparetic symptoms, and, after 12 months of GES, the subjective and objective parameters had improved compared with the baseline.70 Meta-analysis also has suggested that high-frequency GES, on the basis of demonstrated substantial and significant improvement of symptoms and gastric emptying, is an effective and safe method for treating refractory gastroparesis.71 There is some new research in this field as well. As an alternative to single-channel gastric pacing, which can normalize gastric dysryhthmia and improve gastric emptying in patients with gastroparesis, 2-channel gastric pacing can be used to normalize and enhance gastric slow-wave activity as well as accelerate gastric emptying safely in DG patients.72 In 2005, a temporary GES technique showed rapid, significant, and sustained symptom improvement.73 A more recent study showed that endoscopically implanted temporary GES can relieve gastroparesis symptoms and clinically predict a need for permanent GES.74 Clinical-field use of GES devices should proceed cautiously and in accordance with important considerations, among which is the fact that some patients might develop implantation related infections.75 Also, it is not a treatment option available for all medically refractory cases of gastroparesis; rather, there are several factors favorable to positive clinical response including (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom and (3) independence from narcotic analgesics prior to stimulator implantation.76 Moreover, GES-implantation treatment has not been shown to be clearly superior to placebo for lack of control. Another problem is its high cost.
Conclusion
The real-world treatment options for gastroparesis are limited; however, it is expected that this situation will be substantially improved as the pathophysiology of gastroparesis comes to be better understood.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Jung Hwan Oh and Pankaj J Pasricha were involved in planning the study and drafting the manuscript.
References
- 1.Parkman HP, Hasler WL, Fisher RS American Gastroenterological Association. Medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589–1591. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 2.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716–1726. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horowitz M, Edelbroek M, Fraser R, Maddox A, Wishart J. Disordered gastric motor function in diabetes mellitus. Recent insights into prevalence, pathophysiology, clinical relevance, and treatment. Scand J Gastroenterol. 1991;26:673–684. doi: 10.3109/00365529108998583. [DOI] [PubMed] [Google Scholar]
- 5.Oh JH, Choi MG, Kang MI, et al. The prevalence of gastrointestinal symptoms in patients with non-insulin dependent diabetes mellitus. Korean J Intern Med. 2009;24:309–317. doi: 10.3904/kjim.2009.24.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 7.Jung HK, Choung RS, Locke GR, 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rey E, Choung RS, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR., 3rd Prevalence of hidden gastroparesis in the community: the gastroparesis "iceberg". J Neurogastroenterol Motil. 2012;18:34–42. doi: 10.5056/jnm.2012.18.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguchi T, Nakajima M, Sone H, Tada H, Kumagai AK, Takahashi T. Gastric distension-induced pyloric relaxation: central nervous system regulation and effects of acute hyperglycaemia in the rat. J Physiol. 2001;533(Pt 3):801–813. doi: 10.1111/j.1469-7793.2001.t01-1-00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clouse RE, Lustman PJ. Gastrointestinal symptoms in diabetic patients: lack of association with neuropathy. Am J Gastroenterol. 1989;84:868–872. [PubMed] [Google Scholar]
- 13.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah V, Lyford G, Gores G, Farrugia G. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126:903–913. doi: 10.1053/j.gastro.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. Gastroenterology. 2008;135:1258–1266. doi: 10.1053/j.gastro.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421–430. doi: 10.1007/s00535-003-1094-y. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–1544. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 18.Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42:2106–2110. doi: 10.1023/a:1018830820537. [DOI] [PubMed] [Google Scholar]
- 19.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:803. doi: 10.1172/jci8273c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil. 2011;23:773–e335. doi: 10.1111/j.1365-2982.2011.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravella K, Yang H, Gangula PR. Impairment of gastric nitrergic and NRF2 system in apolipoprotein E knockout mice. Dig Dis Sci. 2012;57:1504–1509. doi: 10.1007/s10620-012-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. 2064.e1–2064.e2. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–G733. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 26.Choi KM, Gibbons SJ, Roeder JL, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–595. doi: 10.1111/j.1365-2982.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 27.Dishy V, Cohen Pour M, Feldman L, et al. The effect of sildenafil on gastric emptying in patients with end-stage renal failure and symptoms of gastroparesis. Clin Pharmacol Ther. 2004;76:281–286. doi: 10.1016/j.clpt.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Vanderwinden JM, Rumessen JJ, De Laet MH, Vanderhaeghen JJ, Schiffmann SN. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest. 1999;79:59–65. [PubMed] [Google Scholar]
- 29.Grover M, Bernard CE, Pasricha PJ, et al. Platelet-derived growth factor receptor alpha (PDGFRalpha)-expressing "fibroblast-like cells" in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil. 2012;24:844–852. doi: 10.1111/j.1365-2982.2012.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1575.e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409.e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gangula PR, Mukhopadhyay S, Ravella K, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G692–G699. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–108. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–1087. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 36.Horváth V, Vittal H, Lörincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Large WA, Wang Q. Characteristics and physiological role of the Ca(2+)-activated Cl- conductance in smooth muscle. Am J Physiol. 1996;271(2 Pt 1):C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzone A, Bernard CE, Strege PR, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem. 2011;286:13393–13403. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraser R, Horowitz M, Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut. 1991;32:475–478. doi: 10.1136/gut.32.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392–400. doi: 10.1111/j.1365-2982.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 42.Jeyabal PV, Kumar R, Gangula PR, Micci MA, Pasricha PJ. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol Motil. 2008;20:253–261. doi: 10.1111/j.1365-2982.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 43.Faussone-Pellegrini MS, Grover M, Pasricha PJ, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24:531–539.e249. doi: 10.1111/j.1365-2982.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoo J, Rayner CK, Jones KL, Horowitz M. Pathophysiology and management of gastroparesis. Expert Rev Gastroenterol Hepatol. 2009;3:167–181. doi: 10.1586/egh.09.10. [DOI] [PubMed] [Google Scholar]
- 46.Tonini M, Cipollina L, Poluzzi E, Crema F, Corazza GR, De Ponti F. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004;19:379–390. doi: 10.1111/j.1365-2036.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 47.Parkman HP, Hasler WL, Fisher RS American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 48.Dumitrascu DL, Weinbeck M. Domperidone versus metoclopramide in the treatment of diabetic gastroparesis. Am J Gastroenterol. 2000;95:316–317. doi: 10.1111/j.1572-0241.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- 49.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 50.Parkman HP, Jacobs MR, Mishra A, et al. Domperidone treatment for gastroparesis: demographic and pharmacogenetic characterization of clinical efficacy and side-effects. Dig Dis Sci. 2011;56:115–124. doi: 10.1007/s10620-010-1472-2. [DOI] [PubMed] [Google Scholar]
- 51.McCallum RW, Cynshi O Investigative Team. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26:1121–1130. doi: 10.1111/j.1365-2036.2007.03461.x. [DOI] [PubMed] [Google Scholar]
- 52.Sanger GJ, Westaway SM, Barnes AA, et al. GSK962040: a small molecule, selective motilin receptor agonist, effective as a stimulant of human and rabbit gastrointestinal motility. Neurogastroenterol Motil. 2009;21:657–664.e30-e31. doi: 10.1111/j.1365-2982.2008.01270.x. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122–125. doi: 10.3164/jcbn.10-16GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lasseter KC, Shaughnessy L, Cummings D, et al. Ghrelin agonist (TZP-101): safety, pharmacokinetics and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol. 2008;48:193–202. doi: 10.1177/0091270007310380. [DOI] [PubMed] [Google Scholar]
- 56.Ejskjaer N, Vestergaard ET, Hellström P, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 57.Ejskjaer N, Dimcevski G, Wo J, et al. Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2010;22:1069–e281. doi: 10.1111/j.1365-2982.2010.01519.x. [DOI] [PubMed] [Google Scholar]
- 58.Wo JM, Ejskjaer N, Hellström P, et al. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting - randomised clinical study subset data. Aliment Pharmacol Ther. 2011;33:679–688. doi: 10.1111/j.1365-2036.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- 59.Tack J, Janssen P. Emerging drugs for functional dyspepsia. Expert Opin Emerg Drugs. 2011;16:283–292. doi: 10.1517/14728214.2011.558502. [DOI] [PubMed] [Google Scholar]
- 60.Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen OH, Hvid-Jacobsen K, Lund P, Langoholz E. Gastric emptying and subjective symptoms of nausea: lack of effects of a 5-hydroxytryptamine-3 antagonist ondansetron on gastric emptying in patients with gastric stasis syndrome. Digestion. 1990;46:89–96. doi: 10.1159/000200337. [DOI] [PubMed] [Google Scholar]
- 62.Kim SW, Shin IS, Kim JM, et al. Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment. Psychosomatics. 2006;47:440–442. doi: 10.1176/appi.psy.47.5.440. [DOI] [PubMed] [Google Scholar]
- 63.Johnstone M, Buddhdev P, Peter M, Diggory R. Mirtazapine: a solution for postoperative gastroparesis? [accessed December 2012];BMJ Case Rep. 2009 doi: 10.1136/bcr.02.2009.1579. Available from URL: http://casereports.bmj.com/content/2009/bcr.02.2009.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prakash C, Lustman PJ, Freedland KE, Clouse RE. Tricyclic antidepressants for functional nausea and vomiting: clinical outcome in 37 patients. Dig Dis Sci. 1998;43:1951–1956. doi: 10.1023/a:1018878324327. [DOI] [PubMed] [Google Scholar]
- 65.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 66.Sawhney MS, Prakash C, Lustman PJ, Clouse RE. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–424. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 67.Gorelick AB, Koshy SS, Hooper FG, Bennett TC, Chey WD, Hasler WL. Differential effects of amitriptyline on perception of somatic and visceral stimulation in healthy humans. Am J Physiol. 1998;275(3 Pt 1):G460–G466. doi: 10.1152/ajpgi.1998.275.3.G460. [DOI] [PubMed] [Google Scholar]
- 68.Stapleton J, Wo JM. Current treatment of nausea and vomiting associated with gastroparesis: antiemetics, prokinetics, tricyclics. Gastrointest Endosc Clin N Am. 2009;19:57–72. vi. doi: 10.1016/j.giec.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 70.McCallum RW, Snape W, Brody F, Wo J, Parkman HP, Nowak T. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–954. doi: 10.1016/j.cgh.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 71.Chu H, Lin Z, Zhong L, McCallum RW, Hou X. Treatment of high-frequency gastric electrical stimulation for gastroparesis. J Gastroenterol Hepatol. 2012;27:1017–1026. doi: 10.1111/j.1440-1746.2011.06999.x. [DOI] [PubMed] [Google Scholar]
- 72.Lin Z, Sarosiek I, Forster J, Ross RA, Chen JD, McCallum RW. Two-channel gastric pacing in patients with diabetic gastroparesis. Neurogastroenterol Motil. 2011;23:912–e396. doi: 10.1111/j.1365-2982.2011.01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ayinala S, Batista O, Goyal A, et al. Temporary gastric electrical stimulation with orally or PEG-placed electrodes in patients with drug refractory gastroparesis. Gastrointest Endosc. 2005;61:455–461. doi: 10.1016/s0016-5107(05)00076-3. [DOI] [PubMed] [Google Scholar]
- 74.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496–503.e3. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cutts TF, Luo J, Starkebaum W, Rashed H, Abell TL. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 76.Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078. doi: 10.1007/s10620-007-0124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


