Abstract
Background/Aims
A selective 5-hydroxytryptamine (5-HT) type 3 receptor antagonist, ramosetron, inhibits stress-induced abnormal defecation in animals and is currently used as a therapeutic drug for irritable bowel syndrome with diarrhea. The aim of this study is to investigate the effect of ramosetron on altered gastrointestinal (GI) transit.
Methods
Male guinea pigs weighing approximately 300 g were used. The effect of ramosetron was investigated on altered GI transit induced by thyrotropin-releasing hormone (TRH), 5-HT, or mustard oil (MO). GI transit was evaluated by the migration of charcoal mixture from the pylorus to the most distal point, and expressed as a percentage (%) of charcoal migration (cm) of the total length of total small intestine (cm).
Results
The average charcoal transit was 51.3 ± 20.1% in the control (vehicle) group, whereas in the ramosetron group charcoal moved 56.6 ± 21.9%, 46.9 ± 9.14% and 8.4 ± 5.6% of the total small intestine at the concentrations of 10, 30 and 100 µg/kg, respectively. GI transit after administration of TRH (100 µg/kg), 5-HT (10 mg/kg) or MO (10 mg/kg) was accelerated compared to vehicle (5-HT, 94.9 ± 9.22%; TRH, 73.4 ± 14.7%; MO, 81.0 ± 13.7%). Ramosetron inhibited GI transit altered by 5-HT, TRH or MO.
Conclusions
Ramosetron modulated GI transit. We suggest that ramosetron may be therapeutically useful for those with accelerated upper GI transit.
Keywords: Gastrointestinal transit, Mustard oil, Ramosetron, Serotonin 5-HT3 receptor antagonist, Thyrotropin-releasing hormone
Introduction
As a potent and selective 5-hydroxytryptamine type 3 receptor antagonist (5-HT3RA), ramosetron hydrochloride (ramosetron), has been introduced in Japan to treat gastrointestinal (GI) symptoms such as nausea and vomiting caused by antineoplastic agents.1 5-HT3RAs (e.g., ondansetron and granisetron) have been reported to inhibit GI motility and reduce visceral sensitivity.2,3 Ramosetron also has a long-acting inhibitory effect on stress-induced abnormal defecation in rats, though the drug does not influence normal defecation.4 Unlike existing antidiarrheal and spasmolytic agents, 5-HT3RAs work through inhibition of colonic hyperalgesia.5 Therefore, ramosetron is currently used as a therapeutic drug for irritable bowel syndrome with diarrhea (IBS-D).6,7 However, few studies have evaluated the effect of ramosetron on upper GI transit.
This study investigated the effects of ramosetron on GI transit with 5-HT, thyrotropin-releasing hormone (TRH) and mustard oil (MO). The latter two were selected as TRH enhances serotonin-induced GI motility,8,9 and as MO activates transient receptor potential ankyrin-1 (TRPA-1) to induce 5-HT release from enterochromaffin (EC) cells in vitro.10 Furthermore, our study verified the inhibitory effects of ramosetron on altered GI transit induced by 5-HT, TRH and MO in guinea pigs.
Materials and Methods
Animals
Adult male Hartley guinea pigs (250-350 g, Orient Bio, Inc., Seoul, Korea) were acclimated to their holding room (temperature controlled at 21 ± 1℃, 50 ± 10% humidity and 12-hour light/dark cycle). A standard guinea pig diet (7006; Teklad Guinea Pig Diet, Harlan Laboratories, Madison, WI, USA) and drinking water were provided ad libitum. Guinea pigs were deprived of food overnight before the experiment but were allowed free access to water. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals provided by the Animal Laboratory Ethics Committees of the Department of Laboratory Animal Medicine, Medical Research Center, Yonsei University College of Medicine.
Drugs and Chemicals
The following drugs and chemicals were used: pentobarbital sodium (Hanlim Pharmaceuticals, Seoul, Korea), charcoal (Sigma, St. Louis, MO, USA), ramosetron hydrochloride (Astellas Pharma, Inc., Tokyo, Japan), 5-HT (Sigma), TRH (Sigma) and MO (Sigma). Ramosetron, 5-HT and MO were administrated orally with a charcoal mixture. For experiments involving TRH, this drug was mixed in saline and injected subcutaneously (SC) at total dose of 2 mg/kg prior to charcoal mixture administration.
Experimental Design
Assessment of gastrointestinal transit
After being fasted for 24 hours with free access to water, guinea pigs were anesthetized by intraperitoneal (i.p.) injection of pentobarbital sodium (40 mg/kg). GI transit was measured by using the charcoal transit assay. The charcoal mixture consisted of charcoal, barium and normal saline mixed in a 1:2:6 ratio. The guinea pigs received an intragastric administration of charcoal mixture combined with ramosetron, 5-HT and MO through orogastric cannula. TRH was injected SC. After 2 hours, the guinea pigs were sacrificed. The abdomens were opened and the intestines were removed from the pyloric junction to the ileocecal valve. GI transit was evaluated as the migration of charcoal mixture from the pylorus to the most distal point of migration and expressed as a percentage (%) of charcoal migration (cm) through the total length of small intestine (cm). The distance moved through the small intestine represents both gastric transit and small bowel transit. Additionally, small intestine transit is used interchangeably with upper GI transit.11
Effect of ramosetron, 5-hydroxytryptamine, thyrotropin-releasing hormone or mustard oil on gastrointestinal transit
Ramosetron (10, 30 and 100 µg/kg) was administered via charcoal mixture through an orogastric cannula. Likewise, 5-HT (1, 5 and 10 mg/kg) and MO (0.1, 1 and 10 mg/kg) were also administered by the same method. TRH (1, 10 and 100 µg/kg) was administered SC. Unlike the 5-HT and MO group, which were given the drug per os, TRH group had its agent subcutaneously injected. To offset the effect due to the difference of administration, 2 control group was formed. Doses were selected based upon the results of preliminary experiments as well as on previously published data.12-15
Effect of ramosetron on altered gastrointestinal transit induced by 5-hydroxytrytamine, thyrotropin-releasing hormone or mustard oil
Ramosetron (10, 30 and 100 µg/kg) was administered via charcoal mixture combined with 5-HT (10 mg/kg) or MO (10 mg/kg) through an orogastric cannula. TRH (100 µg/kg) was concurrently administered via charcoal mixture combined with ramosetron (10, 30 and 100 µg/kg) through an orogastric cannula.
Statistical Methods
Different doses of the same treatment protocol were given at the same time-point to determine that the effects of drug treatment on charcoal transit were dose-dependent. The 'within group' multiple comparisons were assessed by one-way analysis of variance. The mean and standard deviation of the mean of charcoal transit rate for each treatment group were calculated. The student's t test was used to compare individual treatment groups. A P-value of less than 0.05 (P < 0.05) was considered to be statistically significant. All data were analyzed using the SPSS version 17.0 for Windows software (SPSS Inc., Chicago, IL, USA).
Results
Effect of Ramosetron on Gastrointestinal Transit
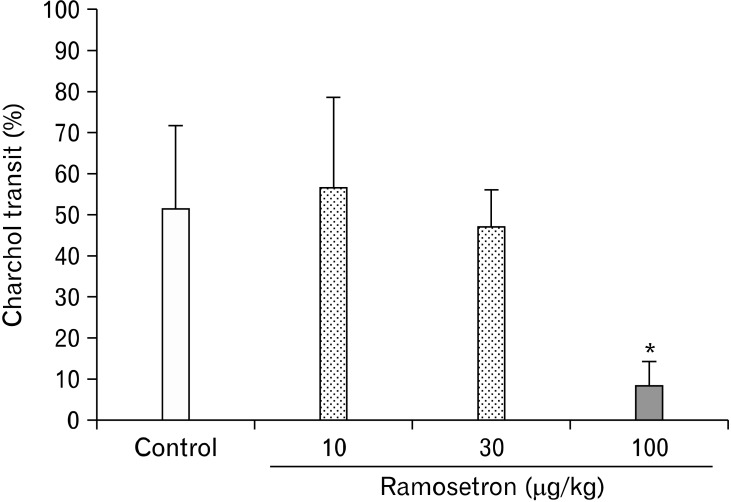
The average charcoal transit was 51.3 ± 20.1% in the control (vehicle) group (n = 7) (Fig. 1). After oral administration of ramosetron, the transit was 56.6 ± 21.9% (n = 6), 46.9 ± 9.1% (n = 6) and 8.4 ± 5.6% (n = 6), at ramosetron doses of 10, 30 and 100 µg/kg, respectively (P < 0.01).
Figure 1.
The effect of ramosetron on gastrointestinal (GI) transit. Ramosetron inhibits GI transit. *P < 0.01 compared with the control group (n = 7).
Effect of 5-hydroxytryptamine, Thyrotropin-releasing Hormone or Mustard Oil on Gastrointestinal Transit
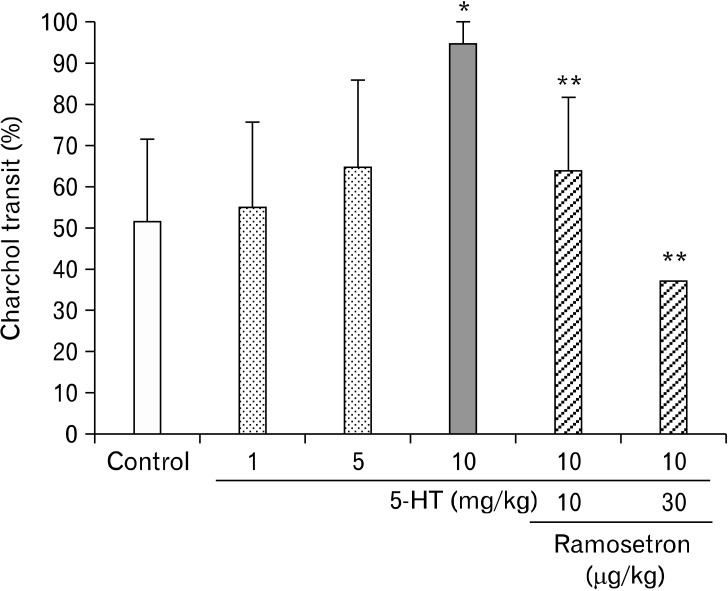
Oral administration of 5-HT at doses of 1, 5 and 10 mg/kg accelerated GI transit of charcoal in a dose-dependent fashion (Fig. 2). In the control group (n = 7) the charcoal moved 51.3 ± 20.1%, whereas the transit for the 5-HT group was 55.1 ± 20.5% (n = 6), 64.7 ± 20.9% (n = 6) and 94.9 ± 9.2% (n = 6) at doses of 1, 5 and 10 mg/kg, respectively. A significant change was observed at a 5-HT dose of 10 mg/kg (P < 0.01).
Figure 2.
The effect of ramosetron on altered gastrointestinal (GI) transit induced by 5-hydroxytryptamine (5-HT). The 5-HT accelerated GI charcoal transit dose-dependently. The maximum effect was observed at a dose of 10 mg/kg (*P < 0.01 compared with the control group [n = 7]). Ramosetron significantly inhibited the accelerated GI transit caused by 5-HT (**P < 0.01 compared with the 5-HT group [n = 6]).
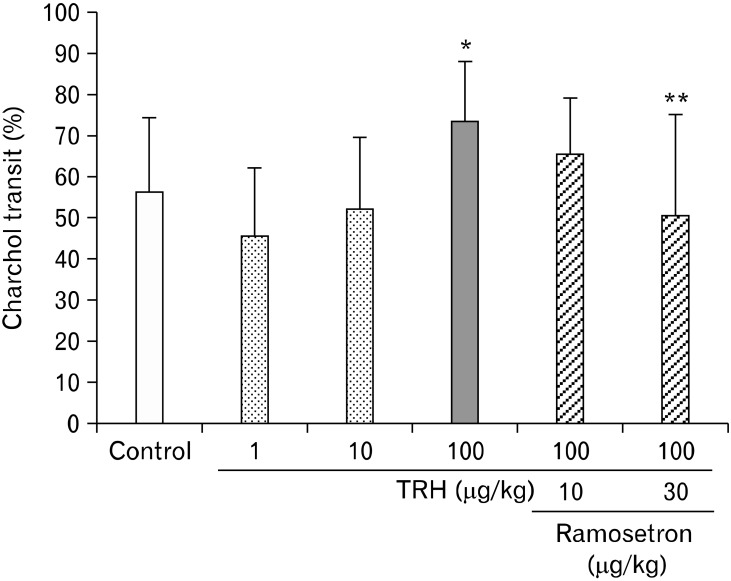
Similarly, subcutaneous administration of TRH accelerated charcoal transit (Fig. 3). The charcoal moved 56.1 ± 18.4% in the control group (n = 6), whereas for the TRH group the charcoal transit was 45.5 ± 16.9% (n = 6), 52.1 ± 17.4% (n = 6) and 73.5 ± 14.7% (n = 6) at doses of 1, 10 and 100 µg/kg, respectively. The maximum effect was achieved for TRH at a dose of 100 µg/kg (P = 0.102).
Figure 3.
The effect of ramosetron on altered gastrointestinal (GI) transit induced by thyrotropin-releasing hormone (TRH). TRH accelerated GI charcoal transit. The maximum effect was observed in TRH at a dose of 100 µg/kg (*P = 0.102 compared with the control group [n = 6]). Ramosetron significantly inhibited the accelerated GI transit caused by TRH (**P < 0.05 compared with the TRH group [n = 6]).
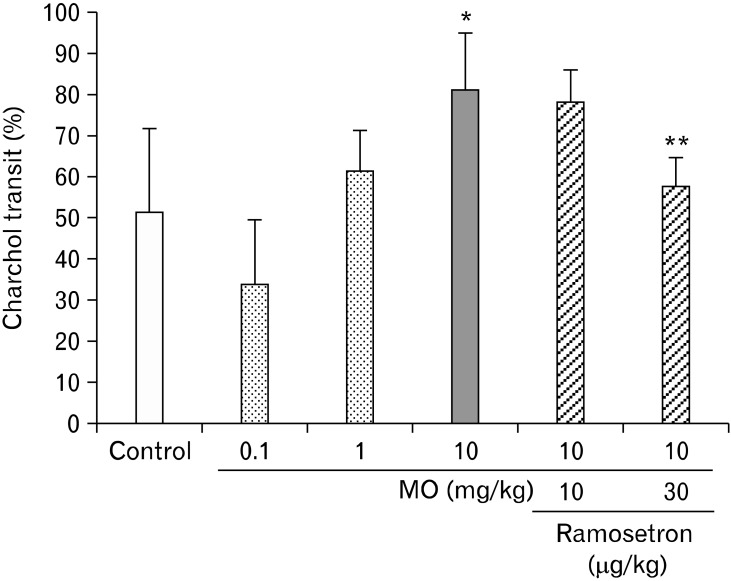
Oral administration of MO also accelerated GI transit (Fig. 4). In the control group (n = 7) the charcoal moved 51.3 ± 20.1%, and in the MO group the charcoal transit was 33.9 ± 15.3% (n = 6), 61.3 ± 9.8% (n = 6) and 81.0 ± 13.7% (n = 6) at doses of 0.1 (n = 6), 1 and 10 mg/kg, respectively. A significant difference was observed for MO at a dose of 10 mg/kg (P < 0.01).
Figure 4.
The effect of ramosetron on altered gastrointestinal (GI) transit induced by mustard oil (MO). MO accelerated GI charcoal transit. The maximum effect was observed in MO at a dose of 10 mg/kg (*P < 0.01 compared with the control group [n = 7]). Ramosetron significantly inhibited the accelerated GI transit caused by MO (**P < 0.01 compared with the MO group [n = 7]).
Effect of Ramosetron on Altered Gastrointestinal Transit Induced by 5-hydroxytryptamine, Thyrotropin-releasing Hormone or Mustard Oil
Oral administration of ramosetron at doses of 10 and 30 µg/kg inhibited 5-HT, TRH and MO-induced accelerated GI transit (Fig. 2-4). In particular, ramosetron at a dose of 30 µg/kg significantly inhibited the accelerated GI transit caused by 5-HT at a dose of 10 mg/kg (P < 0.01, n = 6), TRH at a dose of 100 µg/kg (P < 0.01, n = 6) and MO at a dose of 10 mg/kg (P < 0.01, n = 7).
Discussion
Ramosetron, a selective 5-HT3RA, inhibits GI transit. This effect was well demonstrated by its inhibitory effects on accelerated GI transit caused by 5-HT, TRH and MO in this study.
The 5-HT is an important signaling molecule that involves peristaltic, secretory, vagal and nociceptive reflexes.16 This signaling molecule is found in the GI tract in the interneurons that terminate in the myenteric and submucosal plexuses,17 is released from EC cells by vagus nerve stimulation, and induces a release of acetylcholine from excitatory (cholinergic) neurons.18 TRH stimulates the centers of the brain which control the vagal-enteric nervous systems, and involves cholinergic and serotonergic mechanisms.19 The smooth muscle contraction that is elicited is influenced by changes of neurotransmitter release, either positively or negatively.17 TRH also induces release of serotonin, which results in accelerated GI transit and causes diarrhea.13,20 Similarly, MO induces 5-HT release from EC cells in vitro by way of TRPA-1, which is highly expressed in both the intestine and the stomach.10 TRPA-1 agonists are reported to have a contractile effect in isolated mouse intestine in vivo, but the effect of TRPA-1 agonists on GI motility in guinea pigs has not been verified.21 In a previous experiment in rabbits, the contractile responses of allyl isothiocyanate, which is a TRPA-1 agonist, were reduced by ramosetron.22
The 5-HT3 receptors (the original 5-HT M receptor) are distributed widely in both the GI tract and the central nerve system, and result in increased intestinal secretion and alteration of peristaltic activity.16,23 In animals, 5-HT3RAs have been found to inhibit stress-induced abnormal defecation in animals.9,12 Furthermore, 5-HT3RAs already have a therapeutic use for IBS-D, and their effectiveness is well-known. 5-HT3RAs have also been confirmed to have an inhibitory effect on lower GI transit,5,12 but their effects and mechanism on the upper GI tract (stomach and small intestine) have not been verified.
Previously, Doihara et al24 showed that intragastric administration of 1 mg/kg allyl isothiocyanate facilitated phasic contractions in the gastric antrum and jejunum, and that these effects were inhibited by pretreatment with ruthenium red, a TRPA-1 antagonist. However, in another study involving guinea pigs, the selective 5-HT3RA ondansetron increased the rate of gastric emptying in vivo,25 although the precise mechanisms have not been elucidated.
Ramosetron is a potent and selective 5-HT3RA. Ohta et al26 have suggested that ramosetron may achieve long-lasting binding to 5-HT3 receptors because ramosetron possesses the distinctive ability to maintain an active 3-dimensional chemical conformation. This drug has already proven to be effective for IBS-D in both animal and clinical studies. Recently, although Hirata et al27 showed that ramosetron significantly inhibited the delayed gastric emptying in a corticotrophin releasing factor and soybean oil-induced rat model, few studies have investigated the effects of ramosetron on altered upper GI transit. Therefore, we hypothesized that ramosetron may affect the motor function of the small bowel as well as the stomach, which was investigated by using charcoal GI transit. Aquiring the data on net effect of the gastric and small intestinal charcoal transit makes our study different from previous studies.
Our study demonstrated that ramosetron inhibits normal GI transit and that it also inhibits the accelerated GI transit induced by 5-HT, TRH and MO in guinea pigs. Along these lines, previous animal and preliminary human studies have indicated that 5-HT3RAs may facilitate activity dependent on the degree of basal tone.17,23 A few studies have also shown that 5-HT3RAs accelerated delayed gastric emptying in animal models.28 On the other hand, Talley et al29 reported that ondansetron, a selective 5-HT3RA, did not significantly alter small intestine transit and oral to cecal transit time in healthy volunteers. Troisetron, a selective 5-HT3RA, has been found to either modestly increase gastric emptying or reduce it in healthy volunteers.30 The action of 5-HT3RAs to modify contraction-relaxation responses would be dependent on the relative balance of excitatory-inhibitory tone.17,23 Therefore, 5-HT3RAs might reduce motor activity in the presence of an abnormally increased basal activity. The transit time at lower doses of TRH and MO is actually slower compared to the control group in this study. Different doses of TRH and MO do affect GI transit. It was shown in higher doses that they accelerated GI transit. Perhaps this is due to a balance of inhibitory and excitatory tone at the neuromuscular junction. It will necessitate further investigation and more studies to explain the odd phenomena that lower doses of TRH and MO causes apparent inhibitory effect on GI transit.
In conclusion, this stuty showed that 5-HT, TRH and MO accelerated GI transit. Ramosetron, which is a 5-HT3RA, inhibited 5-HT, TRH and MO-induced accelerated GI transit. Therefore, we suggest that ramosetron may be therapeutically useful for accelerated upper and lower GI transit.
Footnotes
This study was supported in part by a research fund from Astellas Pharma, Inc., Korea.
Yoo Mi Park and Hyojin Park planned this study. Yoo Mi Park and Young Ju Lee was conducted the study. Young Ho Lee and Tae Il Kim contributed to revise and draft the manuscript. Yoo Mi Park wrote the paper.
References
- 1.Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc) 2002;38:75–89. doi: 10.1358/dot.2002.38.2.820104. [DOI] [PubMed] [Google Scholar]
- 2.Steadman CJ, Talley NJ, Phillips SF, Zinsmeister AR. Selective 5-hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea-predominant irritable bowel syndrome: a pilot study. Mayo Clin Proc. 1992;67:732–738. doi: 10.1016/s0025-6196(12)60797-6. [DOI] [PubMed] [Google Scholar]
- 3.Prior A, Read NW. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1993;7:175–180. doi: 10.1111/j.1365-2036.1993.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 4.Shafik A, El-Sibai O, Ahmed I. Parasympathetic extrinsic reflex: role in defecation mechanism. World J Surg. 2002;26:737–740. doi: 10.1007/s00268-002-6285-9. discussion 741. [DOI] [PubMed] [Google Scholar]
- 5.Hirata T, Keto Y, Nakata M, et al. Effects of serotonin 5-HT3 receptor antagonists on stress-induced colonic hyperalgesia and diarrhoea in rats: a comparative study with opioid receptor agonists, a muscarinic receptor antagonist and a synthetic polymer. Neurogastroenterol Motil. 2008;20:557–565. doi: 10.1111/j.1365-2982.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 6.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77:225–235. doi: 10.1159/000150632. [DOI] [PubMed] [Google Scholar]
- 7.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1202–1211. doi: 10.1080/00365520802240255. [DOI] [PubMed] [Google Scholar]
- 8.Taché Y, Yoneda M, Kato K, Király A, Sütö G, Kaneko H. Intracisternal thyrotropin-releasing hormone-induced vagally mediated gastric protection against ethanol lesions: central and peripheral mechanisms. J Gastroenterol Hepatol. 1994;9(suppl 1):S29–S35. doi: 10.1111/j.1440-1746.1994.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyata K, Kamato T, Nishida A, et al. Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther. 1992;261:297–303. [PubMed] [Google Scholar]
- 10.Nozawa K, Kawabata-Shoda E, Doihara H, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci USA. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimball ES, Wallace NH, Schneider CR, D'Andrea MR, Hornby PJ. Small intestinal cannabinoid receptor changes following a single colonic insult with oil of mustard in mice. Front Pharmacol. 2010;1:132. doi: 10.3389/fphar.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata T, Funatsu T, Keto Y, Akuzawa S, Sasamata M, Miyata K. Inhibitory effects of ramosetron, a potent and selective 5-HT3-receptor antagonist, on conditioned fear stress-induced abnormal defecation and normal defecation in rats: comparative studies with antidiarrheal and spasmolytic agents. J Pharmacol Sci. 2008;106:264–270. doi: 10.1254/jphs.fp0071943. [DOI] [PubMed] [Google Scholar]
- 13.Pillai NP, Bhargava HN. The effect of thyrotropin releasing hormone and morphine on gastrointestinal transit. Peptides. 1984;5:1055–1059. doi: 10.1016/0196-9781(84)90170-0. [DOI] [PubMed] [Google Scholar]
- 14.Nagakura Y, Kontoh A, Tokita K, Tomoi M, Shimomura K, Kadowaki M. Combined blockade of 5-HT3- and 5-HT4-serotonin receptors inhibits colonic functions in conscious rats and mice. J Pharmacol Exp Ther. 1997;281:284–290. [PubMed] [Google Scholar]
- 15.Kojima R, Doihara H, Nozawa K, Kawabata-Shoda E, Yokoyama T, Ito H. Characterization of two models of drug-induced constipation in mice and evaluation of mustard oil in these models. Pharmacology. 2009;84:227–233. doi: 10.1159/000236524. [DOI] [PubMed] [Google Scholar]
- 16.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costall B, Naylor RJ. 5-Hydroxytryptamine: new receptors and novel drugs for gastrointestinal motor disorders. Scand J Gastroenterol. 1990;25:769–787. doi: 10.3109/00365529008999215. [DOI] [PubMed] [Google Scholar]
- 18.Talley NJ. Review article: 5-hydroxytryptamine agonists and antagonists in the modulation of gastrointestinal motility and sensation: clinical implications. Aliment Pharmacol Ther. 1992;6:273–289. doi: 10.1111/j.1365-2036.1992.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa T, Yang H, Taché Y. Medullary sites of action of the TRH analogue, RX 77368, for stimulation of gastric acid secretion in the rat. Gastroenterology. 1988;95:1470–1476. doi: 10.1016/s0016-5085(88)80065-9. [DOI] [PubMed] [Google Scholar]
- 20.Horita A, Carino MA. Centrally administered thyrotropin-releasing hormone (TRH) stimulates colonic transit and diarrhea production by a vagally mediated serotonergic mechanism in the rabbit. J Pharmacol Exp Ther. 1982;222:367–371. [PubMed] [Google Scholar]
- 21.Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:353–357. doi: 10.1007/s00210-009-0435-7. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Hidaka K, Miyata K, Kamato T, Nishida A, Honda K. Characterization of YM060, a potent and selective 5-hydroxytryptamine3 receptor antagonist, in rabbit nodose ganglion and N1E-115 neuroblastoma cells. J Pharmacol Exp Ther. 1992;263:1127–1132. [PubMed] [Google Scholar]
- 23.Farthing MJ. 5-Hydroxytryptamine and 5-hydroxytryptamine-3 receptor antagonists. Scand J Gastroenterol Suppl. 1991;188:92–100. doi: 10.3109/00365529109111236. [DOI] [PubMed] [Google Scholar]
- 24.Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur J Pharmacol. 2009;617:124–129. doi: 10.1016/j.ejphar.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Gore S, Gilmore IT, Haigh CG, Brownless SM, Stockdale H, Morris AI. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther. 1990;4:139–144. doi: 10.1111/j.1365-2036.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohta M, Suzuki T, Ohmori J, et al. Novel 5-hydroxytryptamine (5-HT3) receptor antagonists. II. Synthesis and structure-activity relationships of 4,5,6,7-tetrahydro-1H-benzimidazole derivatives. Chem Pharm Bull (Tokyo) 1996;44:1000–1008. doi: 10.1248/cpb.44.1000. [DOI] [PubMed] [Google Scholar]
- 27.Hirata T, Keto Y, Yamano M, Yokoyama T, Sengoku T, Seki N. Inhibitory effect of ramosetron on CRF- and soybean oil-induced delays in gastric emptying in rats. J Gastroenterol Hepatol. 2012;27:1505–1511. doi: 10.1111/j.1440-1746.2012.07172.x. [DOI] [PubMed] [Google Scholar]
- 28.Costall B, Gunning SJ, Naylor RJ, Tyers MB. The effect of GR38032F, novel 5-HT3-receptor antagonist on gastric emptying in the guinea-pig. Br J Pharmacol. 1987;91:263–264. doi: 10.1111/j.1476-5381.1987.tb10280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talley NJ, Phillips SF, Haddad A, et al. Effect of selective 5HT3 antagonist (GR 38032F) on small intestinal transit and release of gastrointestinal peptides. Dig Dis Sci. 1989;34:1511–1515. doi: 10.1007/BF01537102. [DOI] [PubMed] [Google Scholar]
- 30.Stacher G, Bergmann H, Schneider C, et al. Effects of the 5-HT3 receptor antagonist ICS 205-930 on fat-delayed gastric emptying and antral motor activity. Br J Clin Pharmacol. 1990;30:41–48. doi: 10.1111/j.1365-2125.1990.tb03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]