Abstract
Background/Aims
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders and when compared to the vast knowledge pertaining to adults with IBS, very little is known about IBS in children and adolescents. We aimed to explore the prevalence of IBS, identify symptoms and contributing factors and also to examine the efficacy of trimebutine maleate in children and adolescents.
Methods
The study involved 345 children and adolescents (4-18 years) and parents were requested to fill in a questionnaire, Rome III criteria was used to diagnose IBS. To exclude organic disease, all patients underwent medical investigations. Half of the randomly selected IBS patients were treated with trimebutine maleate while the rest of IBS patients were not. The IBS patients were reevaluated at the end of 3 weeks.
Results
The prevalence of IBS according to Rome III criteria in children and adolescents was 22.6% and IBS with constipation was the predominant subtype. Back pain (OR, 6.68), headache (OR, 4.72) and chronic fatigue (OR, 3.74) were significantly higher in IBS group. The prevalence of IBS in both parents and depression in mothers was greater for the patient group than the healthy controls (P < 0.0001). The prevalence of functional dyspepsia in IBS group was 80.8% and was significantly higher than control group. Clinical recovery was seen in 94.9% of the trimebutine maleate group versus spontaneous recovery in 20.5% of the non-medicated group. The difference was significant (P < 0.0001).
Conclusions
IBS is a common disorder in children and adolescents. IBS is closely associated with somatic and familial factors. Trimebutine maleate is effective for pediatric IBS patients.
Keywords: Child, Irritable bowel syndrome, Trimebutine
Introduction
Irritable bowel syndrome (IBS) is one of the most common causes of recurrent abdominal pain in the pediatric population.1 Studies have estimated the prevalence of IBS to range between 6% and 14% in children and between 22.0% and 35.5% in adolescents.2-4 Furthermore, IBS has a significant impact on quality of life of affected children,5 which highlights the clinical importance of the disease.
IBS as described by the Rome III criteria includes weekly symptoms of abdominal pain or discomfort accompanied by changes in bowel patterns. These include changes in bowel movement form or frequency at the onset of pain and/or relief of pain with defecation.6
Trimebutine maleate, an opioid agonist that acts on the peripheral delta, mu and kappa receptors, is used in the treatment of IBS.7
We examined the incidence, distribution, symptoms, and factors triggering IBS according to the Rome III criteria in patients visiting a pediatric clinic for any reason. We also examined the efficacy of trimebutine maleate treatment in randomly selected children with IBS in a randomized controlled study.
Materials and Methods
Our study was conducted at Istanbul University Cerrahpaşa Medical Faculty Pediatric Gastroenterology and Hepatology and Nutrition Department from August 2007 to May 2008. The protocol for use of the human subjects in this study was approved by the local ethics committee of Cerrahpaşa Medical Faculty (Ref. No. 35787).
The study enrolled 345 children who were admitted to the general Pediatrics Clinics of the Cerrahpaşa Medical Faculty Pediatric Department for various reasons from August, 2007 to May, 2008. Their ages ranged from 4-18 years. The children were selected randomly. To investigate the epidemiology of IBS, their parents were asked to complete a modified version of the questionnaire on Pediatric Gastrointestinal Symptoms, Rome III version, consisting of 35 questions. Based on the questionnaire results, those patients who met the Rome III criteria, with a normal physical examination and growth curve, were pre-diagnosed with IBS. All patients underwent a complete evaluation including blood count, erythrocyte sedimentation rate, and stool examination for occult blood, leukocytes, ova and parasites (independently collected 3 stool specimens were examined).
The 78 patients with normal laboratory results were diagnosed with IBS and designated as the patient group. Of these, 39 selected at random were administered with trimebutine maleate (3 mg/kg/day, 3 doses in a day) for 3 weeks. The remaining 39 patients did not take any medication. After 3 weeks, the patients and their parents were reevaluated regarding symptoms. We used 'adequate relief' as the primary outcome measure at the end of the 3 weeks of trimebutine maleate treatment. Parents were asked to answer 'Did your child have adequate relief of his/her IBS pain and discomfort in the past 7 days?' and subjects could answer 'yes' or 'no.' Responder was defined as a subject whose parent answered 'yes'.
Based on the questionnaire, 214 cases with no abdominal pain for at least for 2 months were considered as the healthy control group.
Statistical Methods
Numerical data (quantitative) are reported as means ± SD. Categorical data (nominal) are reported as the number of people in a group (n) or percentage (%). Categorical variables in the patient and healthy control groups were compared using the chi-square test. The Mann-Whitney U test was used for nominal variables. Results were assessed by the 95% CI and significance at P < 0.05. Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS version 15.0 for windows; SPSS Inc., Chicago, IL, USA).
Results
The study group comprised of 184 (53.3%) girls and 161 (46.7%) boys with a mean age of 9.50 ± 3.44 years (range, 4-18 years). The healthy control group comprised of 106 (49.5%) girls and 108 (50.5%) boys with a mean age of 9.49 ± 3.49 years. The 78 patients diagnosed with IBS comprised of 47 (60.3%) females and 31 (39.7%) males with a mean age of 9.79 ± 3.45 years.
In our study, the prevalence of IBS in patients admitted to general pediatrics clinics in our tertiary center was 22.6%, as diagnosed based on the Rome III criteria.
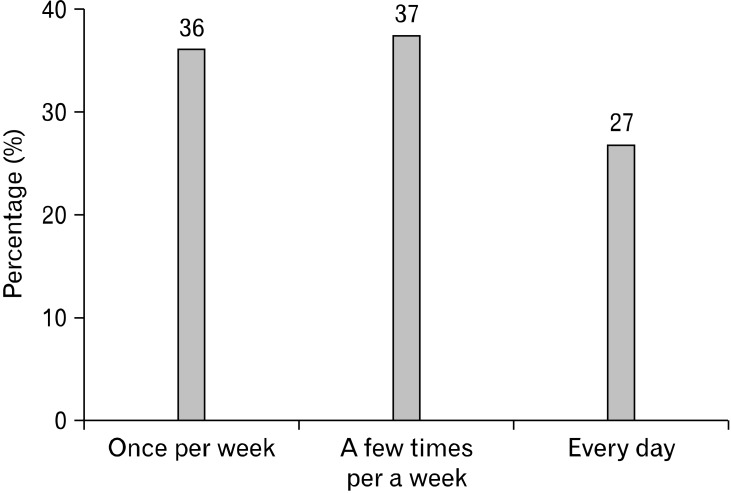
Figure 1 shows the distribution of abdominal pain frequency in IBS patients. In addition, gatrointestinal system symptoms in the IBS patients and healthy controls are compared in Table 1. Symptoms not related to the gastrointestinal system in the IBS patients and healthy controls are compared in Table 2.
Figure 1.
Distribution of abdominal pain frequency in irritable bowel syndrome patients.
Table 1.
Comparison of Irritable Bowel Syndrome Patients With the Control Group According to Their Gastrointestinal Symptoms
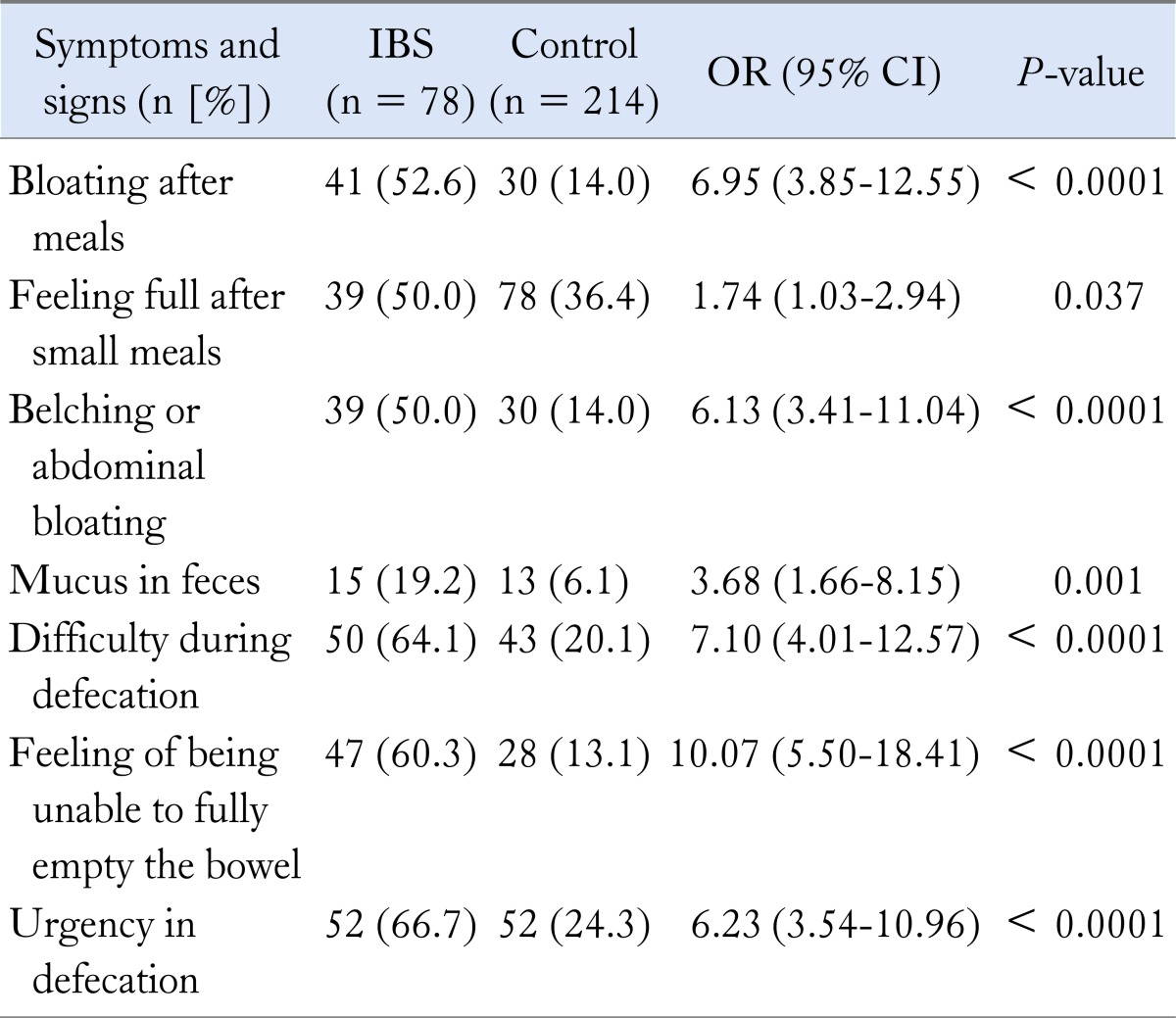
IBS, irritable bowel syndrome.
Table 2.
Comparison of Irritable Bowel Syndrome Patients With the Control Group According to Symptoms Not Related to the Gastrointestinal System
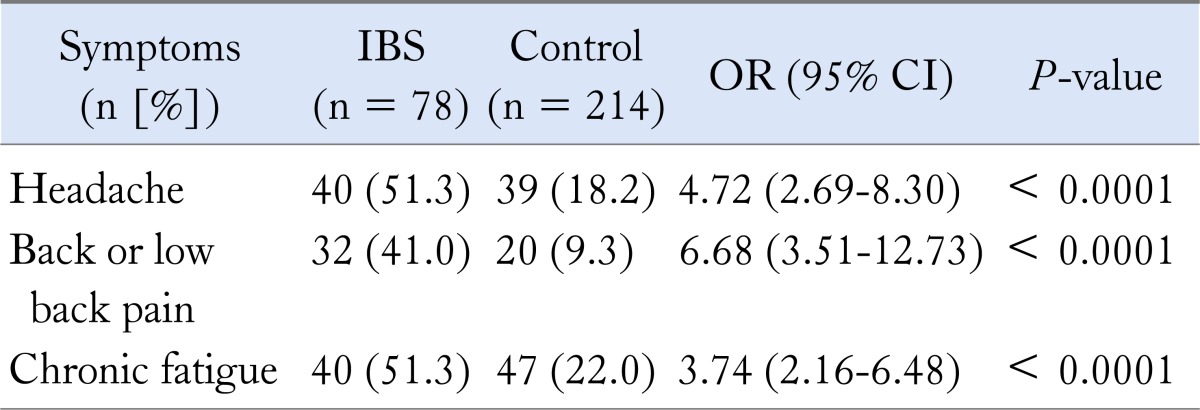
IBS, irritable bowel syndrome.
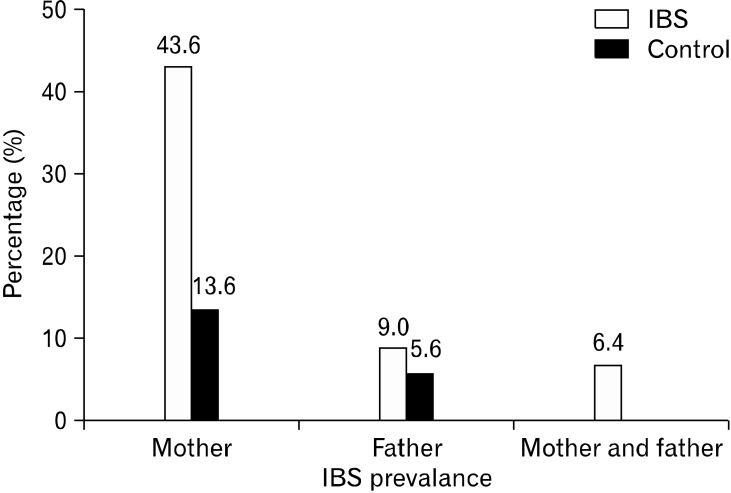
The prevalence of IBS in parents was greater for the patient group than the healthy controls (P < 0.0001). The Rome III criteria for adults were used to diagnose IBS in the parents of the children studied (Fig. 2).
Figure 2.
Comparison of the prevalence of irritable bowel syndrome in the parents of the patient and control groups (P < 0.0001). IBS, irritable bowel syndrome.
Of the 78 patients, 33 (42.3%) had constipation primarily, 33.3% had diarrhea and 12.8% had episodes of both constipation and diarrhea. In 11.5% of IBS patients, the symptoms were not classifiable.
Mothers of IBS children and control group were referred to psychiatrist and Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) test was used for diagnosis of depression. Of the mothers of children with IBS, 41% had a diagnosis of depression. This was significantly higher than in the control group (8.4%, P < 0.0001).
We found that the prevalence of functional dyspepsia (FD) in IBS patients was 80.8%, which was significantly higher than in the control group (20.6%, P < 0.0001).
Clinical recovery was seen in 94.9% of the trimebutine maleate group versus spontaneous recovery in 20.5% of the non-medicated group. The difference was significant (P < 0.0001).
Discussion
According to the studies, the prevalence of IBS ranges from 6-14% in children and 22.0-35.5% in adolescents in Western countries.2-4 A recent Chinese survey of students determined that the prevalence of IBS in children and adolescents was 20.7% according to the Rome III criteria.8 In our study, the prevalence of IBS in children 4 to 18 years old was 22.6%, which is higher than that detected using the Rome I and II criteria. The main difference between the pediatric Rome III and II criteria is that the duration of abdominal pain was reduced from 3 to 2 months. In a study conducted in Sri Lanka, the Rome II and III criteria were used to diagnose functional gastrointestinal system disorders; 60% and 71% of children were diagnosed with functional gastrointestinal system disorders using the respective criteria.9 Therefore, the Rome III criteria are considered more effective for diagnosis of functional gastrointestinal system disorders.
Our study was patient based and done in a tertiary hospital. Also children and adolescents in Turkey are devoted to their studies, and many attend private cramming schools on weekends to increase their probability of passing the entrance examinations for prestigious senior high schools and colleges. This, and the changing lifestyle due to urbanization, put them under stress. These factors might explain the high prevalence we report here.
In a Sri Lanken study using Rome III criteria, 27.1% of IBS patients were constipation predominant, 28% diarrhea predominant and 27.1% were mixed type.10 A recent study using the Rome III criteria classified IBS patients as 20.1% constipation predominant, 18.5% diarrhea predominant, 10.3% alternating diarrhea and constipation, and 51.1% undetermined.8 In our study, constipation was also the most common subtype (42.3%), with diarrhea predominant in 33.3%, alternating attacks of both in 12.8%, and 11.5% unclassified.
In one study, the prevalence of IBS in the parents of children with IBS was significantly higher than in those of children without IBS. There was an association between the types of gastrointestinal disorder found in children and their parents. Of the mothers of children with IBS, 41% were diagnosed with depression. This was significantly higher than in the control group.
Clinical overlap of FD and IBS according to the Rome III criteria is very common. One risk factor for FD-IBS overlap is the presence of postprandial fullness symptom.11 In a study of adults with IBS, 87% met the criteria for dyspepsia.12 Overlap between FD and IBS has been reported in pediatric studies.13 We found that the prevalence of FD in IBS patients was 80.8%, which was significantly higher than in the control group.
One meta-analysis of 4 controlled studies compared smooth muscle relaxants such as trimebutine maleate and placebo. The data suggested that trimebutine was significantly more effective than placebo for controlling pain and flatus. There was no difference in side effects between the trimebutine and placebo groups.14 In our study, the clinical recovery was significantly higher in the trimebutine maleate group. Although our study is not placebo controlled, and it seems like a limitation of our study, the significant difference between the treated and non-treated group may implicate the efficacy of trimebutine maleate in IBS children.
IBS is common in the pediatric population visiting general pediatrics clinics. IBS is closely associated with somatic and familial factors. Trimebutine maleate is effective for treating pediatric IBS patients.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Gülcan S Karabulut: data collecting and writing. Ömer F Beşer: writing. Ethem Erginöz: statistical analysis. Tufan Kutlu and Fügen Ç Çokuğraş: literature review. Tülay Erkan: literature review and supervisor.
References
- 1.Chiou E, Nurko S. Functional abdominal pain and irritable bowel syndrome in children and adolescents. Therapy. 2011;8:315–331. doi: 10.2217/thy.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129:220–226. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 3.Caplan A, Walker L, Rasquin A. Validation of the pediatric Rome II criteria for functional gastrointestinal disorders using the questionnaire on pediatric gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;41:305–316. doi: 10.1097/01.mpg.0000172749.71726.13. [DOI] [PubMed] [Google Scholar]
- 4.Miele E, Simeone D, Marino A, et al. Functional gastrointestinal disorders in children: an Italian prospective survey. Pediatrics. 2004;114:73–78. doi: 10.1542/peds.114.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Varni JW, Lane MM, Burwinkle TM, et al. Health-related quality of life in pediatric patients with irritable bowel syndrome: a comparative analysis. J Dev Behav Pediatr. 2006;27:451–458. doi: 10.1097/00004703-200612000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delvaux M, Wingate D. Trimebutine: mechanism of action, effects on gastrointestinal function and clinical results. J Int Med Res. 1997;25:225–246. doi: 10.1177/030006059702500501. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Li D, Cheng G, Fan J, Lu H. An epidemiologic study of irritable bowel syndrome in adolescents and children in South China: a school-based study. Child Care Health Dev. 2010;36:781–786. doi: 10.1111/j.1365-2214.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 9.Devanarayana NM, de Silva DG, de Silva HJ. Aetiology of recurrent abdominal pain in a cohort of Sri-Lankan children. J Paediatr Child Health. 2008;44:195–200. doi: 10.1111/j.1440-1754.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- 10.Rajindrajith S, Devanarayana NM. Subtypes and symptomatology of irritable bowel syndrome in children and adolescents: a school-based survey using Rome III criteria. J Neurogastroenterol Motil. 2012;18:298–304. doi: 10.5056/jnm.2012.18.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang A, Liao X, Xiong L, et al. The clinical overlap between functional dyspepsia and irritable bowel syndrome based on Rome III criteria. BMC Gastroenterol. 2008;8:43. doi: 10.1186/1471-230X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 13.Schurman JV, Friesen CA, Danda CE, et al. Diagnosing functional abdominal pain with the Rome II criteria: parent, child, and clinician agreement. J Pediatr Gastroenterol Nutr. 2005;41:291–295. doi: 10.1097/01.mpg.0000178438.64675.c4. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:355–361. doi: 10.1046/j.1365-2036.2001.00937.x. [DOI] [PubMed] [Google Scholar]