Abstract
Background:
Surfactant dysfunction may contribute to the development of bronchopulmonary dysplasia (BPD) in persistently ventilated preterm infants. We conducted a multicenter randomized, blinded, pilot study to assess the safety and efficacy of late administration of doses of a surfactant protein-B (SP-B)-containing surfactant (calfactant) in combination with prolonged inhaled nitric oxide (iNO) in infants ≤1,000 g birth weight (BW).
Methods:
We randomized 85 preterm infants ventilated at 7–14 d after birth to receive either late administration of surfactant (up to 5 doses) plus prolonged iNO or iNO alone. Large aggregate surfactant was isolated from daily tracheal aspirates (TAs) for measurement of SP-B content, total protein, and phospholipid (PL).
Results:
Late administration of surfactant had minimal acute adverse effects. Clinical status as well as surfactant recovery and SP-B content in tracheal aspirate were transiently improved as compared to the controls; these effects waned after 1 d. The change in SP-B content with surfactant dosing was positively correlated with SP-B levels during treatment (r = 0.50, P = 0.02).
Conclusion:
Low SP-B values increased with calfactant administration, but the relationship of this response to SP-B levels suggests that degradation is a contributing mechanism for SP-B deficiency and surfactant dysfunction. We conclude that late therapy with surfactant in combination with iNO is safe and transiently increases surfactant SP-B content, possibly leading to improved short- and long-term respiratory outcomes.
Prolonged mechanical ventilation and bronchopulmonary dysplasia (BPD), a common morbidity of extreme prematurity, are important contributors to adverse outcomes in extremely premature newborns (1,2,3). Several pharmacologic therapies, including those involving the administration of vitamin A and caffeine, have demonstrated efficacy in the prevention of BPD (4,5). We have previously demonstrated an improvement in survival without BPD in newborns after prolonged administration of inhaled nitric oxide (iNO), the most pronounced beneficial effects being in infants enrolled at 7–14 d after birth (6,7). There was a concomitant decrease in the duration of assisted ventilation and pulmonary morbidity at 1 y in these infants (8,9). BPD is of multifactorial origin, however, and substantial morbidity remains even after iNO treatment (8,10). There is therefore a need for new therapeutic approaches and combination therapies to prevent the disorder.
We have previously described surfactant dysfunction (defined as elevation in the values of minimum surface tension in vitro) in high proportions (43–76%) of preterm infants who remain intubated and ventilated at 1–2 wk of age (11,12,13). Infants are twice as likely to develop surfactant dysfunction during episodes of respiratory deterioration or infection, and higher minimum surface tension is directly correlated with an index of lung disease severity (the respiratory severity score expressed as: RSS = mean airway pressure × concentration of inspired oxygen) (11,12). In these ventilated preterm infants, elevated minimum surface tension as measured in tracheal aspirates (TAs) was associated with altered lipid composition, lower total protein in the surfactant fraction, and markedly lower content of surfactant proteins (SPs) B and C. SP-B content had the strongest correlation with surface tension and was inversely related (11). Similar findings relating SP-B content to surfactant dysfunction have been described in acute lung injury, thereby supporting the validity of using SP-B content as an indicator of surfactant function (14).
We as well as others have described transient improvements in respiratory status, including a decrease in RSS, with later administration (after initial treatment of respiratory distress syndrome) of an SP-B-containing surfactant (13,15,16,17). In our previous open-label pilot study of late surfactant (calfactant) administration, we treated high-risk preterm infants with 2 or 3 doses of surfactant. There was a nonsignificant increase in the proportion of without BPD survivors when we increased the number of late doses (36.6 vs. 19.5%) (13).
In a previous study, we had found only transient improvement in surfactant function with iNO therapy; we therefore hypothesized that iNO improves respiratory outcomes in high-risk preterm infants through other mechanisms (12). Consequently, in our current study, we sought to examine the potential benefit of combined therapy involving iNO along with up to 5 doses of surfactant. We conducted a randomized, masked, pilot study in extremely low birth weight (BW) infants to quantify the effects of later administration of surfactant on SP-B content in these high-risk ventilated infants receiving iNO for prevention of BPD (6,7). We hypothesized that late doses of an SP-B-containing surfactant (calfactant, Infasurf, ONY, Amherst, NY) would increase the SP-B content (as measured in TAs) in the treated infants, and would be safe and well tolerated. We also hypothesized that surfactant treatment would increase the delivery of iNO (as measured by change in excretion levels of urinary NO metabolites), given its beneficial effects on alveolar recruitment.
Results
Study Population, and Safety and Tolerability of Surfactant Dosing
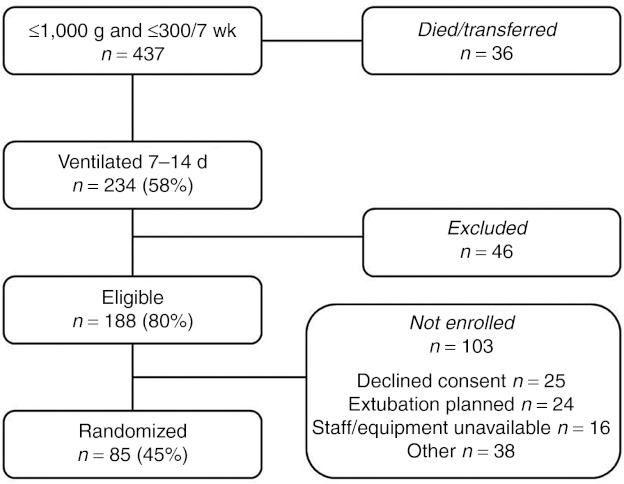
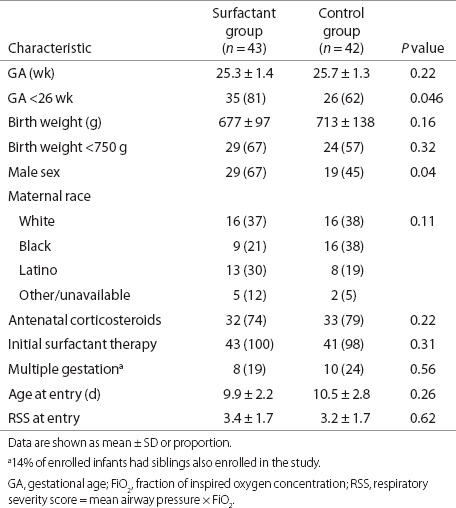
A total of 437 infants who met the study's gestational age (GA) and BW criteria were admitted to study hospitals and screened for enrollment. Of these, 59% were alive and ventilated in study hospitals between day of life 7 and 14. Of 188 eligible infants without exclusion criteria, 85 were enrolled and randomized (Figure 1) with 43 in the Surfactant arm of the study and 42 in the Control arm (iNO-alone). The characteristics of the enrolled infants are shown by treatment group (Table 1). The two groups were well matched except that the Surfactant treatment group had a significantly greater proportion of infants with GA <26 wk and also a significantly greater proportion of male infants.
Figure 1.
Enrollment flow diagram for infants admitted to the nine study hospitals during the recruitment period, January 2008–October 2009.
Table 1. Characteristics of study population.
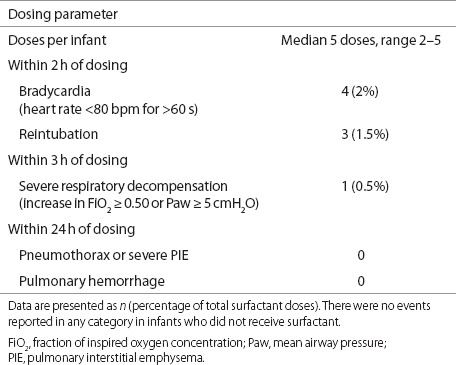
Overall, late dosing with surfactant was well tolerated. A total of 201 doses of surfactant were administered to the 43 infants enrolled in the Surfactant group during the entire study (median, 5 doses per infant). Adverse events (bradycardia, need for reintubation, and respiratory decompensation) were associated with ≤ 2% of the administered doses (Table 2), and there were no adverse events in the Control infants. We also examined co-morbidities of prematurity, to determine whether there were any potential additional effects of late dosing of surfactant. There were no significant differences in the Surfactant arm vs. the Control arm as regards rates of culture-positive sepsis (37 vs. 43%), necrotizing enterocolitis (14 vs. 10%), severe intraventricular hemorrhage or periventricular leukomalacia (14 vs. 10%), and retinopathy of prematurity requiring surgery (21 vs. 33%). After enrollment, there was a trend toward more frequent treatment for patent ductus arteriosus in the Surfactant group as compared to the Control group (49 vs. 32%, P = 0.14). After adjustment for GA and sex, the treatment effect remained nonsignificant. There was also nonsignificant difference in death rates in the two treatment groups (12 vs. 17%, for Surfactant vs. Control).
Table 2. Tolerance of 201 surfactant doses in 43 infants treated with surfactant.
Clinical Response to Surfactant Treatment
We evaluated the clinical response to surfactant treatment at 1, 2, 24, and 48 h after study drug dosing by comparing Surfactant and Control groups with respect to absolute changes in RSS relative to the pre-dose values. Only infants who remained intubated and ventilated received study drug doses. Mixed linear effects models were adjusted for study drug dose number (dose 1–5). There was no significant interaction between treatment and dose number. Surfactant treatment resulted in modest significant decreases in RSS at 1 h and 2 h after the study drug dose; differences at 24 and 48 h were no longer statistically significant (mean RSS differences: −0.25, P = 0.03 at 1 h; −0.33, P = 0.01 at 2 h; −0.24, P = 0.16 at 24 h; −0.33, P = 0.12 at 48 h; n = 369 study dose procedures).
At a postmenstrual age (PMA) of 36 wk, 37% (16/43) of the infants in the Surfactant group and 33% (14/42) of those in the Control group were alive without BPD (relative risk 1.14, 95% confidence interval 0.62, 2.10). Adjustment for GA and sex yielded no changes in the estimates of treatment effect (data not shown).
Effects of Late Administration of Surfactant on Surfactant Parameters
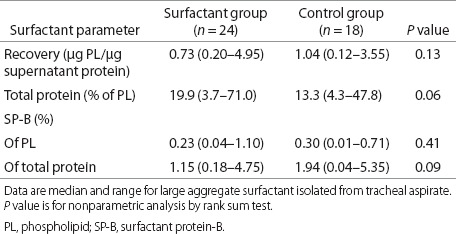
We focused our analysis on data relating to surfactant parameters from daily TA sample collection. Surfactant recovery and composition values before study drug dosing are presented in Table 3. Although none of the differences between the Surfactant and Control groups was statistically significant, there were trends toward a more abnormal surfactant profile at baseline in surfactant-treated infants, characterized by lower surfactant recovery, higher levels of total protein in surfactant, and lower SP-B content. Of note, at baseline, total protein in the surfactant fraction had a moderate inverse correlation with BW (r = −0.35, P = 0.02) and a positive correlation with RSS (r = 0.34, P = 0.02), suggesting that increased alveolar protein impacts severity of lung disease.
Table 3. Characteristics of surfactant at baseline for infants with daily tracheal aspirate sampling.
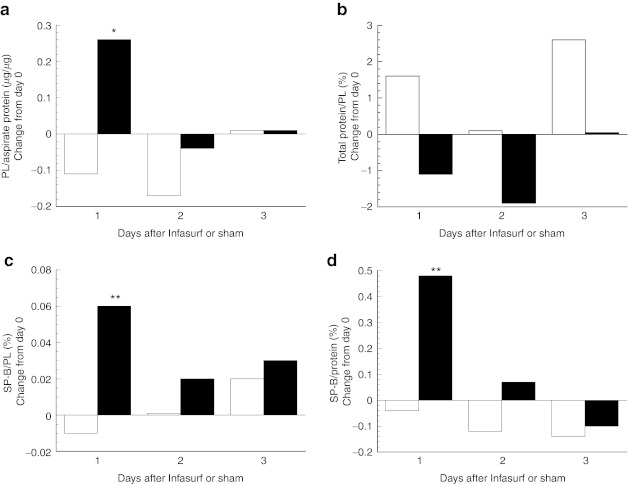
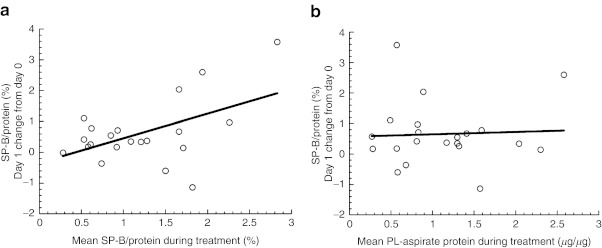
We evaluated the changes in surfactant characteristics in response to study drug dosing in terms of the absolute change from baseline, and compared the responses in surfactant-treated infants with those in the Controls (Figure 2). We found a significant improvement in surfactant recovery (Figure 2a) and an increase in SP-B content (Figure 2c,d) with surfactant treatment at day 1 after study drug dosing, with waning, nonsignificant differences on day 2, and minimal differences on day 3. There was also a favorable decrease in total protein in the surfactant fraction with treatment (Figure 2b), which was not statistically significant (P = 0.13). Furthermore, we investigated the variability observed in SP-B content levels after surfactant administration (range:−1.2 to +3.5% in treated infants). There was a moderately strong linear correlation between the mean levels of SP-B content (normalized to total protein in surfactant) at day 1 after surfactant dosing and the mean levels on other days during treatment (r = 0.50, P = 0.02, Figure 3a), suggesting that changes in SP-B content with surfactant dosing are dependent on SP-B levels. In contrast, there was no relationship between the change in SP-B content at day 1 after surfactant dosing and surfactant recovery on other days during treatment. That is, the increment in SP-B content after surfactant dosing was independent of surfactant recovery (r = 0.05, P = 0.83, Figure 3b).
Figure 2.
Absolute change in surfactant parameters following study drug dosing (days 1–3). Data are median values for the Control (white bars, n = 43–47 from 20 infants) and Surfactant (black bars, n = 55–64 from 27 infants) groups. (a) Surfactant recovery, phospholipid (PL) normalized to total protein content of tracheal aspirate; (b) total protein in surfactant pellet as a percentage of PL; (c) surfactant protein-B (SP-B) content of surfactant pellet, normalized to PL; and (d) SP-B content normalized to total protein in surfactant pellet. P values are for comparison of the change between groups, *P = 0.04, **P ≤ 0.002.
Figure 3.
Absolute change in surfactant parameters in infants treated with surfactant (n = 21) on day 1 vs. mean level of surfactant parameters on subsequent days prior to dosing (days 2–4). Surfactant protein-B (SP-B) content (%) normalized to total protein in surfactant: (a) vs. SP-B content r = 0.50, P = 0.02, and (b) surfactant recovery (μg phospholipid (PL)/μg tracheal aspirate protein): r = 0.05, P = 0.83.
We hypothesized that late treatment with surfactant might increase iNO delivery secondary to improved alveolar recruitment. However, we saw no effect of surfactant treatment on urinary excretion of NO metabolites (NOx) or on cyclic guanosine monophosphate at iNO doses of 10–20 ppm. The following are the data, expressed as median and range, n = 16–17 per group, for the Surfactant and Control arms, respectively: the multiple of increase in the ratio of NOx to creatinine relative to baseline value was 3.1 (1.2–9.4) vs. 3.3 (1.0–9.4), P = 0.81, and the multiple of increase in the ratio of cyclic guanosine monophosphate to creatinine relative to baseline value was 2.8 (0.3–8.3) vs. 1.9 (0.6–11.1), P = 0.34.
Discussion
In this randomized, blinded, controlled, pilot study, we have demonstrated that late administration of an SP-B-containing surfactant transiently increases SP-B content in the lung aspirates in treated infants as compared to controls. Respiratory status, as assessed in terms of RSS, was also improved; however, both biochemical and clinical effects waned by day 2 after surfactant dosing. Late dosing of surfactant was well tolerated, and there was no evidence of adverse effects as regards the incidence of neonatal comorbidities of prematurity or of mortality.
This study was designed to take advantage of the transient effects observed in our previous open-label study of late administration of surfactant, wherein infants received 2 or 3 doses of calfactant (13). We hoped to augment the clinical response to surfactant treatment by administering up to 5 doses of surfactant. As we had previously increased the number of doses from 2 to 3, we subsequently decreased the dosing interval to 3 d in this study. However, we found that this dosing interval was in fact insufficient to ensure persistent restoration of adequate SP-B levels (and therefore surfactant function) among preterm infants with acquired surfactant dysfunction. Although the acute effect we demonstrated on RSS was modest, it was similar to that observed in our earlier pilot study and the experience reported by Katz and Klein (13,15). Bissinger described a more pronounced improvement in RSS with late administration of surfactant when it was carried out during an acute respiratory decompensation (16). It is not surprising that the response to replacement surfactant during an acute deterioration would be greater than the effect in chronically ventilated infants who are free of acute decompensation. These findings are consistent with those from experimental studies in chronically ventilated preterm lambs, which had a greater amount of water in the lungs as compared with controls, and showed further increases in fluid and protein during episodes of acute infection (consistent with worsening pulmonary edema) (18).
We found that late administration of surfactant doses increased surfactant recovery and SP-B content, regardless of whether SP-B was normalized either to phospholipid (PL) or to total protein in surfactant. However, as noted, this effect was transient. An additional interesting finding lends insight into the mechanism of acquired surfactant deficiency in ventilated extremely low BW infants. We expected that the incremental increases in SP-B at day 1 after treatment would be equal, regardless of SP-B levels. However, we found instead that the change in SP-B content with dosing was dependent on mean SP-B content levels, whereas SP-B content was not related to surfactant recovery during treatment. These data are consistent with a faster rate of SP-B turnover as a contributor to low SP-B in these infants, suggesting that additional proteins in the surfactant fraction could be impairing surfactant function through SP-B degradation. In contrast, on the basis of surfactant recovery, there was no indication of faster PL turnover associated with low SP-B increase after treatment. Although specific degradation pathways for SP-B have not been identified, imbalances between proteases and their inhibitors are noted in the ventilated immature lung in experimental models (19). This situation may be analogous to specific changes in the surfactant lipid profile mediated by elevated phospholipase A2 activity after oleic acid–induced lung injury in rodents (20). Another consideration is that the biophysical interaction between SP-B and surfactant lipids, which determines lipid organization and surface activity, is influenced by both the concentration of peptide and composition of the lipids (21). Alterations in surfactant lipid composition occur in newborn premature infants who have surfactant deficiency (22) as well as in those with later-acquired dysfunction, as we describe in this study (11). It is therefore likely that variability in the response to late therapy with surfactant depends on both the level of SP-B (which is determined by synthetic and clearance rates) and the lipid composition of endogenous surfactant.
It was also interesting to note that total protein in the surfactant fraction fell with surfactant treatment (albeit not to a statistically significant extent), suggesting that there was an amelioration of pulmonary edema or epithelial injury and sloughing with surfactant therapy. Pulmonary edema has been described in chronically ventilated preterm lambs (18); it occurs in experimental SP-B deficiency and is associated with increase alveolar protein levels (23). However, the relationship between alveolar protein content and surfactant is variable per data from animal studies. In the setting of high tidal volume ventilation in rodents, the increase in total lavage protein was associated with decreased surfactant function, despite increase in surfactant levels (24). Other studies of various forms of augmented surfactant administration have shown anti-inflammatory effects but no differences in total aspirate protein levels (25,26). In our previous open-label study of the effects of calfactant administration, late administration surfactant had no sustained effect on inflammatory mediators in lung aspirate fluid (13). However, in that study, we did not collect daily tracheal aspirate samples during dosing and we had no control group to assess the effects of surfactant administration on the inflammatory profile in chronically ventilated infants.
Pathophysiologic factors that probably contribute to the development of BPD include lung injury secondary to hyperoxia, barotrauma from mechanical ventilation, and atelectasis (18,27,28). Given the multifactorial nature of BPD, a dosing regimen that failed to achieve persistent maintenance of SP-B content, and the smallness of our sample for this feasibility study, we were not surprised to observe only a modest, nonsignificant effect on clinical outcome in the infants at PMA of 36 wk. to compare these outcomes with those of infants enrolled in our previous iNO-only study (nitric oxide for the prevention of chronic lung disease), we extracted unpublished outcome data relating to infants who were similar to those enrolled in this pilot study (BW ≤1,000 g, intubated at the time of study enrollment, and having study treatment initiated at day of life 7–14) (6,7). There were 92 infants meeting these criteria in the iNO group and 97 infants in the placebo group; these represented only one-third of the total study enrollment. At a PMA of 36 wk, 46% of the iNO-treated infants and 25% of the placebo-treated infants had survived and without a diagnosis of BPD. Although our outcomes in our study approached those of iNO-treated infants in nitric oxide for the prevention of chronic lung disease, due to the multifactorial nature of BPD, we had hoped to improve upon these outcomes with combination therapy. Data from our various studies have suggested that there has been an evolution in this patient population. In our earlier (1997–2001) descriptive study, 47% of the infants had surfactant dysfunction (11). The prevalence of surfactant dysfunction in a subset of infants enrolled in the nitric oxide for the prevention of chronic lung disease study (2000–2005) was only 43%, whereas it was 76% in our later surfactant study (2004–2007) (12,13). In the earlier descriptive study, SP-B content averaged 0.98% PL with normal surface tension and 0.20% PL with abnormal surface tension (11), whereas in this later study, SP-B content at baseline trended even lower than in our previous open-label study: median values were 0.23% PL and 0.30% PL in the Surfactant and Control arms, respectively (Table 3), as compared to 0.34% PL in our recent open-label study (13). These differences may reflect trends toward increasing use of non-invasive ventilation in neonatal intensive care, which could have an impact on the characteristics of infants who remain intubated at day of life 7–14 (29). In two additional cohorts of extremely preterm infants born between the years 2000 and 2004, mechanical ventilation at days 7–14 was an important and independent risk factor for the development of BPD (30,31). Although the level of SP-B required to prevent BPD is not known, experimental models of SP-B deficiency demonstrate that, although lung dysfunction is attenuated when SP-B is restored to ~30% of control levels, normal surface tension is not restored until SP-B content returns to normal (23).
In this pilot study, we did not assess the later effects of late treatment with surfactant on pulmonary morbidity, an important finding of the impact of iNO in the nitric oxide for the prevention of chronic lung disease study, even in the subsets of infants who showed less evidence of treatment benefit at a PMA of 36 wk (6,7,8). Other studies have demonstrated decreased morbidity at later follow-up, despite there having been no significant benefit at a PMA of 36 wk (32). Our study found that the safety profile of late administration of calfactant was reassuring. When compared with data from a similar trial of lucinactant administration, bradycardia episodes were less frequent in our study; however, the threshold for reporting such instances was somewhat lower in our study (<80 bpm with a duration of at least 60 s) (17).
The change in urinary excretion of NOx from baseline of 10–20 ppm in the iNO group was similar in magnitude to changes in plasma NOx that we have previously reported at these iNO doses (33). We had not previously reported changes in cyclic guanosine monophosphate. These demonstrated a similar effect size to that of NOx, indicating the biological activity of iNO in this patient population. We hypothesized that we might see improved iNO delivery and biological activity with late administration of surfactant, through recruitment of additional lung surface area. However, any differences that might have occurred were not detectable with our sampling scheme, which coincided with the timing of our late dosing of surfactant.
In conclusion, in this pilot study we found that a dosing interval of 3 d was insufficient to restore and maintain adequate SP-B levels. These data suggest that a more appropriate dosing interval for late administration of surfactant would be 1–2 d. Although the continuous effects of late administration of surfactant may not be a prerequisite for long-term benefit, the additive effects of recurrent dosing at these shortened dosing intervals, with the resultant improvements in lung function, could allow for more rapid weaning from mechanical ventilation, leading to improved pulmonary outcomes, both short-term and long-term. We are studying this modified regimen in an ongoing trial powered to identify an improvement in the rate of BPD at a PMA of 36 wk in extremely low GA newborns. We intend to follow up the pulmonary and developmental outcomes in these infants up to 2 y of corrected age (NCT01022580).
Methods
Enrollment and Study Population
The study was undertaken in nine hospitals having tertiary care intensive care nurseries in seven cities in the United States between January 2008 and October 2009. Written, informed consent was obtained from the families of all the participating infants, and all research activities were overseen by local institutional review boards (University of California, San Francisco (lead center), Alta Bates Medical Center, Children's Hospital of Oakland Research Institute, Children's Memorial Hospital, Children's Mercy Hospital, Northwestern Hospital, Stony Brook University Medical Center, Texas Children's Hospital, and Women's and Children's Hospital of Buffalo). The study was registered at ClincalTrials.gov (NCT00569530). Eligible infants were those with BW ≤1,000 g and GA ≤ 30 0/7 wk who remained intubated and ventilated at day of life 7–14. The exclusion criteria were active pulmonary hemorrhage or air leak (pneumothorax requiring thoracostomy tube drainage or severe pulmonary interstitial emphysema), bilateral grade IV intraventricular hemorrhage, life expectancy <7 d, serious congenital malformations, prior treatment with iNO, or surfactant treatment within 48 h of enrollment.
Study Protocol
The infants were randomized in the investigational pharmacy to either combination treatment with iNO and calfactant (Surfactant arm), or iNO alone (Control arm). Infants who were products of multiple gestation were randomized together (effectively, the mother was randomized), given the usual parental preference for the siblings to be in the same treatment group. Randomization was stratified by study hospital only, without considering BW strata. All infants were treated with iNO per the regimen previously described (6,7): 20 ppm × 3–4 d, weaned to 10 ppm × 7 d, 5 ppm × 7 d, 2 ppm × 7 d, and then weaned off. Infants who were extubated also completed the same iNO schedule with nasal cannula flow maintained at ≥1 l/min. To maintain blinding, study drug dosing (calfactant or sham) was undertaken behind a bedside screen, with clinical staff away from the bedside and all monitors silenced. Two research dosing staff attended the infant, and the screen remained in place for at least 20 min regardless of whether surfactant was administered or not. Infants assigned to surfactant treatment (iNO and calfactant) were treated with the standard calfactant dose of 3 ml/kg through an endotracheal tube. Infants randomized to iNO alone (Controls) had minimal intervention, consisting of placement of the in-line surfactant dosing catheter or no manipulation at all, depending on local institutional review boards guidance.
Study drug doses were initially timed to coincide with the iNO weaning schedule: study day 0, study days 3–4, and then weekly, up to 5 doses if the infant remained intubated (“Weekly dosing” n = 30 enrolled, 14 Surfactant, 16 Control). After further analysis of lung aspirates from our previous open-label study of calfactant (13), the dosing interval was shortened to 3 ± 1 d, with a maximum of 5 doses (“3-d dosing” n = 55 enrolled, 29 Surfactant, 26 Control). TA samples were initially collected only immediately before study drug dosing. The sample collection schedule was modified during the “3-d dosing” protocol to allow for a better understanding of SP-B kinetics, with daily TA collection for the first 10 d of the study.
Urine samples were collected by extraction from cotton balls placed in the diaper prior to iNO initiation (if possible), and on days of study procedures. Any sample potentially contaminated by stool was discarded. NOx (nitrates + nitrites, pmol/ml) were measured using chemiluminescence (NOA 280, Sievers Instruments, Boulder CO), and cyclic guanosine monophosphate was determined by means of enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor MI).
Clinical data were collected, including perinatal demographic characteristics, daily respiratory support (to calculate RSS), comorbidities of prematurity, and status at PMA of 36 wk and at the time of discharge from the hospital. The diagnosis of BPD was determined at a PMA of 36 ± 1 wk by evaluation of clinical status and a physiologic challenge of oxygen and flow reduction for infants receiving effective inspired oxygen concentration (fraction of inspired oxygen concentration) ≤ 0.30 without assisted ventilation (34).
Processing of Tracheal Aspirate and Measurement of Surfactant Parameters
Lung aspirate fluid was collected by means of routine suctioning of the endotracheal tube. Specimens were collected after instillation of 0.5 ml saline via the endotracheal tube, followed by a brief period of ventilation before suctioning to recover the saline along with a sample of lung epithelial lining fluid. This procedure was repeated and the collection apparatus was cleared with saline to standardize the volume of the collected TA sample. Infants who did not tolerate saline instillation underwent the procedure without this step, if secretions could be effectively cleared. The TA sample was stored on ice and immediately processed, or refrigerated (4̊ C) if processing could not be undertaken immediately. TA processing consisted of a high-speed (3,000 rpm × 5 min) centrifugation to isolate the large aggregate surfactant pellet, followed by freezing of supernatant and pellet after removal of any frank mucous.
Surfactant and supernatant fractions were assayed. The total PL content of surfactant was measured by lipid extraction and phosphorus assay (35,36). Total protein in supernatant and surfactant were quantified using the Bradford method. SP-B was assayed by immuno-dot, with Infasurf (0.74% of PL) as standard (37). The results are presented as follows: surfactant recovery is expressed as PL per total protein in supernatant (µg/µg), the total protein in surfactant fraction (%) is normalized to PL content of surfactant, and the SP-B content is expressed as a percentage using two denominators: PL and total protein in the surfactant fraction.
Statistical Analyses
This study was designed to assess the biochemical end point of increased lung aspirate SP-B content in infants treated with late administration of surfactant, as compared with the control group. Given the non-normal distribution, these changes were assessed using non-parametric methods. Other univariate analyses utilized standard χ-square tests, t-tests, and correlations, as appropriate. Clinical outcomes of effect size were analyzed using generalized estimating equations to account for treatment clustered by siblings, and mixed linear effects models were used to assess RSS response to study dose procedures.
Statement of Financial Support
Support for this study was received from K23HL079922 (to R.L.K.), the Pulmonary Hypertension Association (to R.L.K.), the Clinical Translational Research Awards UL RR024131 (University of California, San Francisco), and M01 RR10710 (Stony Brook University Medical Center). Unrestricted research grants were also received from Forrest Laboratories and IKARIA (to R.A.B.).
Disclosure
R.A.B. holds the Investigational New Drug application for combined therapy with inhaled nitric oxide and late calfactant for prevention of BPD.
Acknowledgments
We thank the physicians, nurses, respiratory therapists, and participating patients and their families at the following participating neonatal intensive care units for their cooperation and assistance in conducting the trial: University of California, San Francisco Benioff Children's Hospital, San Francisco, CA; Alta Bates Summit Medical Center, Berkeley, CA; Children's Hospital and Research Center, Oakland, CA; Children's Memorial and Northwestern Memorial Hospitals, Chicago, IL; Texas Children's Hospital, Houston, TX; Women and Children's Hospital of Buffalo, Buffalo, NY; Children's Mercy Hospital and Clinics, Kansas City, MO; and Stony Brook University Hospital, Stony Brook, NY. We also thank Jeanette Asselin and the site coordinators for trial coordination, Lisa Palermo for expert statistical analysis, and Cheryl Chapin, Hart Horneman, and Marquesa Finch for their technical assistance. This trial was registered on ClinicalTrials.gov (NCT00569530).
References
- Ehrenkranz RA, Walsh MC, Vohr BR.et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia Pediatrics 20051161353–60. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P.et al. Long-term effects of caffeine therapy for apnea of prematurity N Engl J Med 20073571893–902. [DOI] [PubMed] [Google Scholar]
- Laughon M, Allred EN, Bose C.et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants Pediatrics 20091231124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JE, Wright LL, Oh W.et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network N Engl J Med 19993401962–8. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P.et al. Caffeine therapy for apnea of prematurity N Engl J Med 20063542112–21. [DOI] [PubMed] [Google Scholar]
- Ballard RA, Truog WE, Cnaan A.et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation N Engl J Med 2006355343–53. [DOI] [PubMed] [Google Scholar]
- Ballard RA. Inhaled nitric oxide in preterm infants–correction. N Engl J Med. 2007;357:1444–5. doi: 10.1056/NEJMc076350. [DOI] [PubMed] [Google Scholar]
- Hibbs AM, Walsh MC, Martin RJ.et al. One-year respiratory outcomes of preterm infants enrolled in the nitric oxide (to prevent) chronic lung disease trial J Pediatr 2008153525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic JA, Hibbs AM, Palermo L.et al. Economic evaluation of inhaled nitric oxide in preterm infants undergoing mechanical ventilation Pediatrics 20091241325–32. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Hibbs AM, Martin CR.et al. Two-year neurodevelopmental outcomes of ventilated preterm infants treated with inhaled nitric oxide J Pediatr 2010156556–61.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JD, Ballard RA, Cnaan A.et al. Dysfunction of pulmonary surfactant in chronically ventilated premature infants Pediatr Res 200456918–26. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Merrill JD, Truog WE.et al. Surfactant function and composition in premature infants treated with inhaled nitric oxide Pediatrics 2007120346–53. [DOI] [PubMed] [Google Scholar]
- Merrill JD, Ballard PL, Courtney SE.et al. Pilot trial of late booster doses of surfactant for ventilated premature infants J Perinatol 201131599–606. [DOI] [PubMed] [Google Scholar]
- Günther A, Schmidt R, Harodt J.et al. Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: impact on biophysical and biochemical surfactant properties Eur Respir J 200219797–804. [DOI] [PubMed] [Google Scholar]
- Katz LA, Klein JM. Repeat surfactant therapy for postsurfactant slump. J Perinatol. 2006;26:414–22. doi: 10.1038/sj.jp.7211533. [DOI] [PubMed] [Google Scholar]
- Bissinger R, Carlson C, Michel Y, Dooley C, Hulsey T, Jenkins D. Secondary surfactant administration in neonates with respiratory decompensation. J Perinatol. 2008;28:192–8. doi: 10.1038/sj.jp.7211909. [DOI] [PubMed] [Google Scholar]
- Laughon M, Bose C, Moya F.et al. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia Pediatrics 200912389–96. [DOI] [PubMed] [Google Scholar]
- Bland RD, Albertine KH, Carlton DP.et al. Chronic lung injury in preterm lambs: abnormalities of the pulmonary circulation and lung fluid balance Pediatr Res 20004864–74. [DOI] [PubMed] [Google Scholar]
- Altiok O, Yasumatsu R, Bingol-Karakoc G.et al. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia Am J Respir Crit Care Med 2006173318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue S, Kuwabara K, Mikawa K.et al. Crucial role of group IIA phospholipase A(2) in oleic acid-induced acute lung injury in rabbits Am J Respir Crit Care Med 19991601292–302. [DOI] [PubMed] [Google Scholar]
- Farver RS, Mills FD, Antharam VC, Chebukati JN, Fanucci GE, Long JR. Lipid polymorphism induced by surfactant peptide SP-B(1-25) Biophys J. 2010;99:1773–82. doi: 10.1016/j.bpj.2010.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley SA, Kovacevic M, Paciga JE, Balis JU. Sequential changes of surfactant phosphatidylcholine in hyaline-membrane disease of the newborn. N Engl J Med. 1979;300:112–6. doi: 10.1056/NEJM197901183000303. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Whitsett JA, Martis PC, Weaver TE. Reversibility of lung inflammation caused by SP-B deficiency. Am J Physiol Lung Cell Mol Physiol. 2005;289:L962–70. doi: 10.1152/ajplung.00214.2005. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Malloy J, McCaig L.et al. Mechanical ventilation of isolated septic rat lungs: effects on surfactant and inflammatory cytokines J Appl Physiol 200191811–20. [DOI] [PubMed] [Google Scholar]
- Walker MG, Tessolini JM, McCaig L, Yao LJ, Lewis JF, Veldhuizen RA. Elevated endogenous surfactant reduces inflammation in an acute lung injury model. Exp Lung Res. 2009;35:591–604. doi: 10.1080/01902140902780460. [DOI] [PubMed] [Google Scholar]
- Sato A, Whitsett JA, Scheule RK, Ikegami M. Surfactant protein-d inhibits lung inflammation caused by ventilation in premature newborn lambs. Am J Respir Crit Care Med. 2010;181:1098–105. doi: 10.1164/rccm.200912-1818OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coalson JJ, Winter VT, Gerstmann DR, Idell S, King RJ, Delemos RA. Pathophysiologic, morphometric, and biochemical studies of the premature baboon with bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;145 4 Pt 1:872–81. doi: 10.1164/ajrccm/145.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–46. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- Bancalari E, Claure N. Non-invasive ventilation of the preterm infant. Early Hum Dev. 2008;84:815–9. doi: 10.1016/j.earlhumdev.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Laughon M, Bose C, Allred EN.et al. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants Arch Dis Child Fetal Neonatal Ed 201196F114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon MM, Langer JC, Bose CL.et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants Am J Respir Crit Care Med 20111831715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–76. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- Posencheg MA, Gow AJ, Truog WE.et al. Inhaled nitric oxide in premature infants: effect on tracheal aspirate and plasma nitric oxide metabolites J Perinatol 201030275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Yao Q, Gettner P.et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates Pediatrics 20041141305–11. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bernhard W, Linck M, Creutzburg H.et al. High-performance liquid chromatographic analysis of phospholipids from different sources with combined fluorescence and ultraviolet detection Anal Biochem 1994220172–80. [DOI] [PubMed] [Google Scholar]
- Notter RH, Wang Z, Egan EA, Holm BA. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids. 2002;114:21–34. doi: 10.1016/s0009-3084(01)00197-9. [DOI] [PubMed] [Google Scholar]