Abstract
Purpose
Placenta growth factor (PlGF) is a member of the vascular endothelial growth factor (VEGF) family. PlGF is implicated in several pathologic processes, including the growth and spread of cancer and tumor angiogenesis. The aim of this study was to evaluate the expression and the clinical implications of PlGF in colorectal cancer.
Methods
In order to ascertain the clinical significance of PlGF expression in colorectal cancer, the researcher analyzed the expression pattern of PlGF by using an immunohistochemical method and attempted to establish if a relationship existed between PlGF expression and microvessel density (MVD), and subsequently between PlGF expression and the predicted prognosis. A total of 83 patients with colorectal cancer were included for immunohistochemical staining. Clinicopathological characteristics were defined according to the tumor-node-metastasis (TNM) criteria of the Union for International Cancer Control. Clinicopathologic factors, such as age, sex, histological types of tumors, tumor cell grade, TNM stage, lymphovascular invasion, and lymph-node metastasis, were reviewed.
Results
In this study, the PlGF protein expression level was significantly correlated with MVD, patient survival, and clinicopathological factors such as lymph-node metastasis, TNM staging, lymphatic invasion and vascular invasion.
Conclusion
PlGF may be an important angiogenic factor in human colorectal cancer, and in this study, PlGF expression level was significantly correlated with positive lymph-node metastases, tumor stage, and patient survival. These findings suggest that PlGF expression correlates with disease progression and may be used as a prognostic marker for colorectal cancer.
Keywords: Placenta growth factor, Colorectal neoplasms
INTRODUCTION
Colorectal cancer is the second most common form of malignant tumor for men and the third among women in Korea. Its incidence has recently been showing an increasing trend due to changes in dietary habit and lifestyle [1]. Various factors have been identified as being among the most commonly known prognostic factors of colorectal cancer, including from Duke's stages and the tumornode-metastasis (TNM) staging of the American Joint of Committee on Cancer (AJCC). Moreover, in recent studies on molecular biology, new factors have been raised as other prognostic factors for colorectal cancer.
Adjacent blood vessels are essential for all cells composing the human body, providing oxygen and nutrients and eliminating metabolic wastes. Therefore, a network of micro-blood vessels is spread all over the body, reaching about a total of 100,000 km in length and maintaining the normal functions of cells and metabolic activities. Such blood vessels are formed by a de novo mechanism through the division and differentiation of angioblasts, or they are formed through angiogenesis, with theformation of new peripheral blood vessels from existing blood vessels [2].
Oxygen and nutrition supply through angiogenesis is critical to tumor growth, and it is known to be a prerequisite for tumor metastasis. Vascular endothelial growth factor (VEGF) is recognized as an important factor in relation to angiogenesis and regulates the function of the endothelium in terms of the proliferation and the migration of blood endothelial cells, the formation of tubular structures, and an increase in the vascular permeability. Many studies have examined the relationship of VEGF expression with the determining factors of prognosis and with the degree of direct angiogenesis in different tumors. According to many studies, the expression rate of VEGF has been identified to be associated with histological differentiation, a high density of micro-blood vessels, lymphatic metastasis, clinical stages, recurrence and survival rates, and others [3-5]. In addition to VEGF, VEGF-B, C, D, E, placenta growth factors (PlGFs), etc. have been recently clarified as other member of the VEGF family that are similar to VEGF in exhibiting diverse degrees of direct angiogenesis [6].
PlGF is a dimeric glycoprotein regulating the activities of VEGF by combining with the VEGF receptor type 1 and associating with VEGF structurally and functionally [7]. The PlGF level in blood plasma and the PlGF expression in tumors are reported to be profoundly related to the progress of the disease and to the survival of patients with various forms of tumors, including gastric cancer, breast cancer, and pulmonary cancer [8-10]. In this study, the authors aimed to examine the presence of PlGF expression in colorectal cancer tissues and to identify the relationship of that expression with different clinicopathological factors and with the prognosis for patients with colorectal cancer.
METHODS
Study subjects
Study subjects comprised patients who were diagnosed with colorectal cancer and underwent a resection for primary colorectal cancer at Soonchunhyang University Cheonan Hospital from January 2002 to July 2003. The study included resected tissue samples as paraffin-embedded blocks that were sufficient in amount and stored under favorable conditions. A total of 83 subjects were included: 46 males and 37 females with a mean age of 65.4 years old. According to the TNM staging system of the AJCC, 8 cases involved stage I cancer (10.8%), 27 cases stage II cancer (36.5%), 38 cases stage III cancer (40.5%), and 10 cases stage IV cancer (12.2%). The subjects were compared by dividing them into lymphatic metastasis and nonlymphatic metastasis groups.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded blocks were cut into a 4-µm-thick pieces and placed on snowcoat X-tra slides (Surgipath, Richmond, IL, USA). Subsequently, the slides were put into an oven at 60℃ for 15 minutes, and paraffin was eliminated by repeated treatment with xylene for four times in two minutes at room temperature. Xylene was completely removed by treating the slides with 100% alcohol three times for 10 seconds. Each slide was treated with alcohol twice each in 95%, 80%, and 70% alcohol solutions, in that order, and was hydrated in distilled water. Afterwards, the slides were put into a 0.01-M citric-acid buffer solution (pH 6.0) and treated in a microwave oven for 20 minutes to recover antigens. After the slides had been cooled, they were washed three times with phosphate-buffered saline for five minutes. The following procedures were carried out with the Bond-X Autostainer (Leica Biosystems Pty Ltd., Mount Waverley, Australia), and the primary antigen was used by diluting in a ratio of 1:80. After staining, all slides were washed in distilled water and then treated in 95% and 100% alcohol solutions, respectively, twice for one minute each time. Subsequently, they were treated with xylene three times for one minute each, followed by a transparent procedure three times for one minute each and were then sealed with non-aqueous inclusion bodies. Finally, the slides were examined using an optical microscope.
The slides were interpreted by two pathologists. PlGF was judged to be positive when a nucleus was stained. The positive levels were classified from strong (4 points) to weak (1 point) depending on the degree of staining intensity. Among these, cases with no or weak (1 point) staining were categorized as the negative group, and the rest of the cases stained at higher intensity were judged as the positive group (Fig. 1).
Fig. 1.
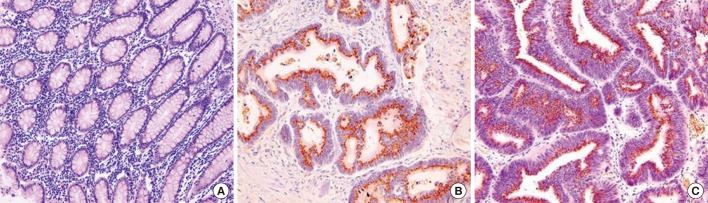
Expression of placenta growth factor (PlGF) in colorectal cancer cells. (A) Immunohistochemical stain of PlGF in the normal colonic mucosa (×40). (B) Immunohistochemical stain with antibody for PlGF protein shows a mild (×400) nuclear expression at tumor cells. (C) Strong nuclear immunoreactivity in colorectal cancer cells (immunohistochemical stain with antibody for PlGF protein, ×400).
Statistical analysis
Statistical analyses were performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA), and the correlations between PlGF and the clinicopathological variables were examined using a chi-square test. P-values of less than 0.05 were defined as statistically significant. Differences in survival rate were analyzed using a Kaplan-Meier analysis, and the significance was evaluated with the log-rank test. Variables with P < 0.05 from univariate analyses were chosen as the variables for the multivariate analyses.
RESULTS
Relationship between PlGF expression and clinicopathologic variables
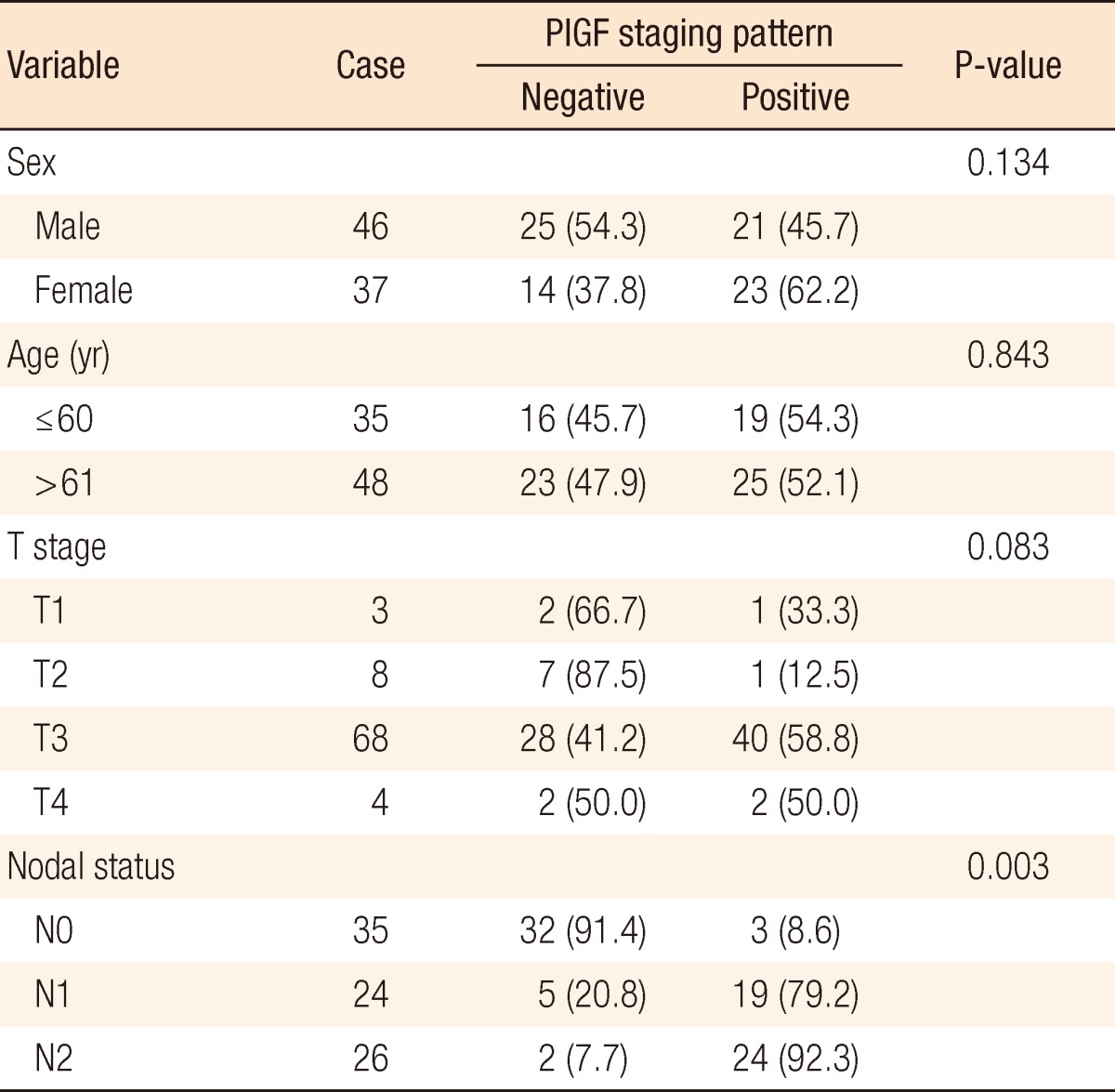
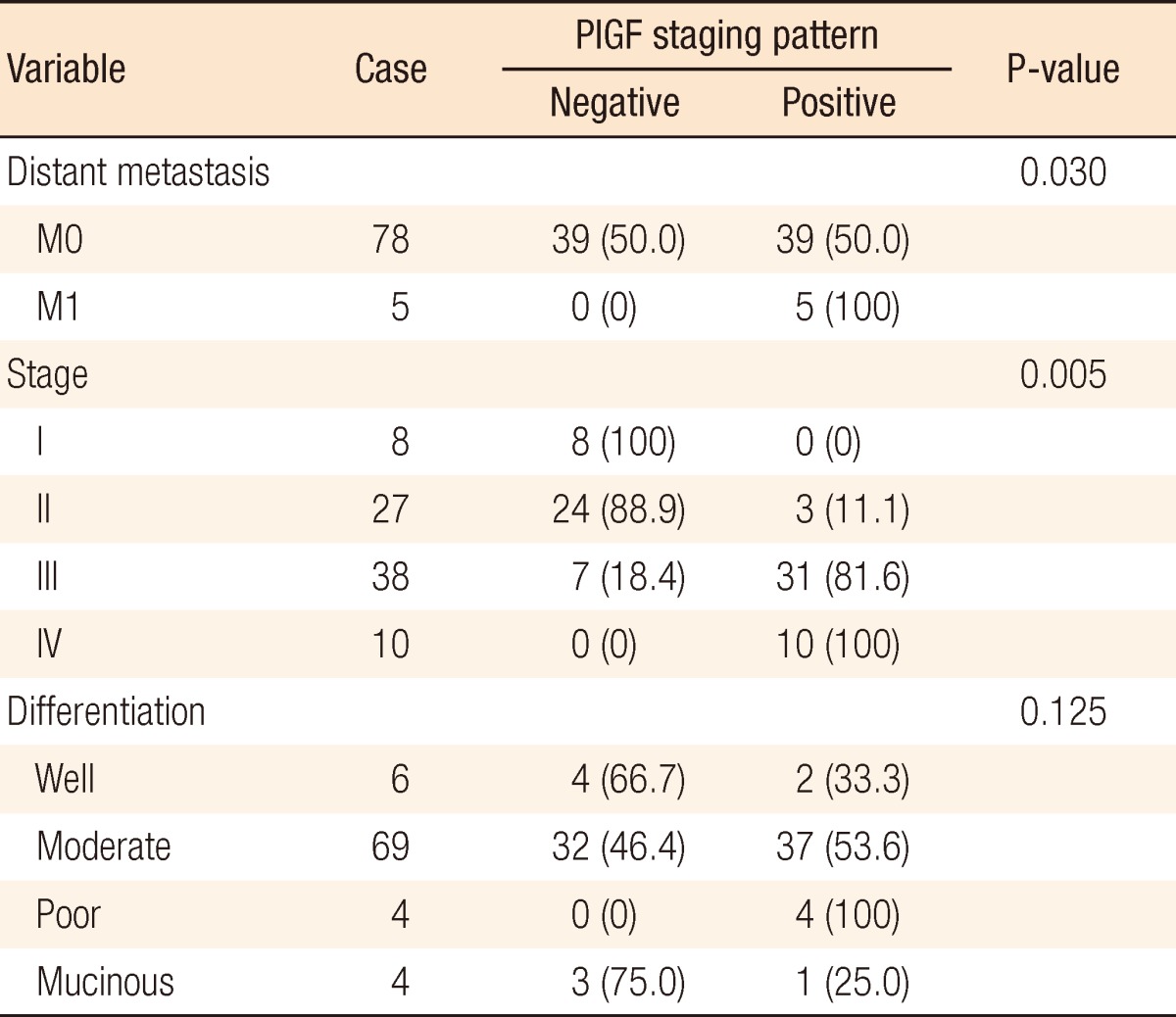
Among the total of 83 colorectal cancer patients, PlGF was highly expressed in 21 (45.7%) of the 46 males and in 23 (62.2%) of the 37 females. However, there were no significant differences in terms of sex (P = 0.134) and age (P = 0.843). According to the TNM classification, PlGF expression levels were high in one (33.3%) among 3 T1 patients, one (12.5%) among 8 T2 patients, 40 (58.8%) among 68 T3 patients, and 2 (50%) among 4 T4 patients. However, no differences were detected based on the TNM staging system (P = 0.083) (Table 1). Among the entire TNM classification, no case of PlGF expression was found in stage I (0%) patients. PlGF was expressed in 3 (11.1%) among 27 stage II patients, 31 (81.6%) among 38 stage III patients, and all 10 of the stage IV patients. Our results indicated that PlGF was more powerfully expressed as T stages advanced (P = 0.005) (Table 2).
Table 1.
Correlation between placenta growth factor (PlGF) expression and clinical factors
Values are presented as number (%).
Table 2.
Correlation between placenta growth factor (PlGF) expression and clinical pathological factors
Values are presented as number (%).
According to the presence of lymph node metastasis, PlGF level was highly expressed in 3 (8.6%) among the 32 patients without lymphatic metastasis. In contrast, PlGF level was highly expressed in 24 (92.3%) among the 26 patients with N2 lymph node metastasis, exhibiting a stronger expression of PlGF as lymphatic metastasis advanced (P = 0.003) (Table 1).
Our study was able to verify that PlGF was more expressed in patients with distant metastasis (P = 0.03). Moreover, PlGF expression in colorectal cancer tissues exhibited statistical correlations with the presence of vascular invasion in colorectal cancer tissues and lymphatic invasion. In addition, a significant correlation was shown with microvessel density (Table 3).
Table 3.
Correlation between placenta growth factor (PlGF) expression and pathological factors
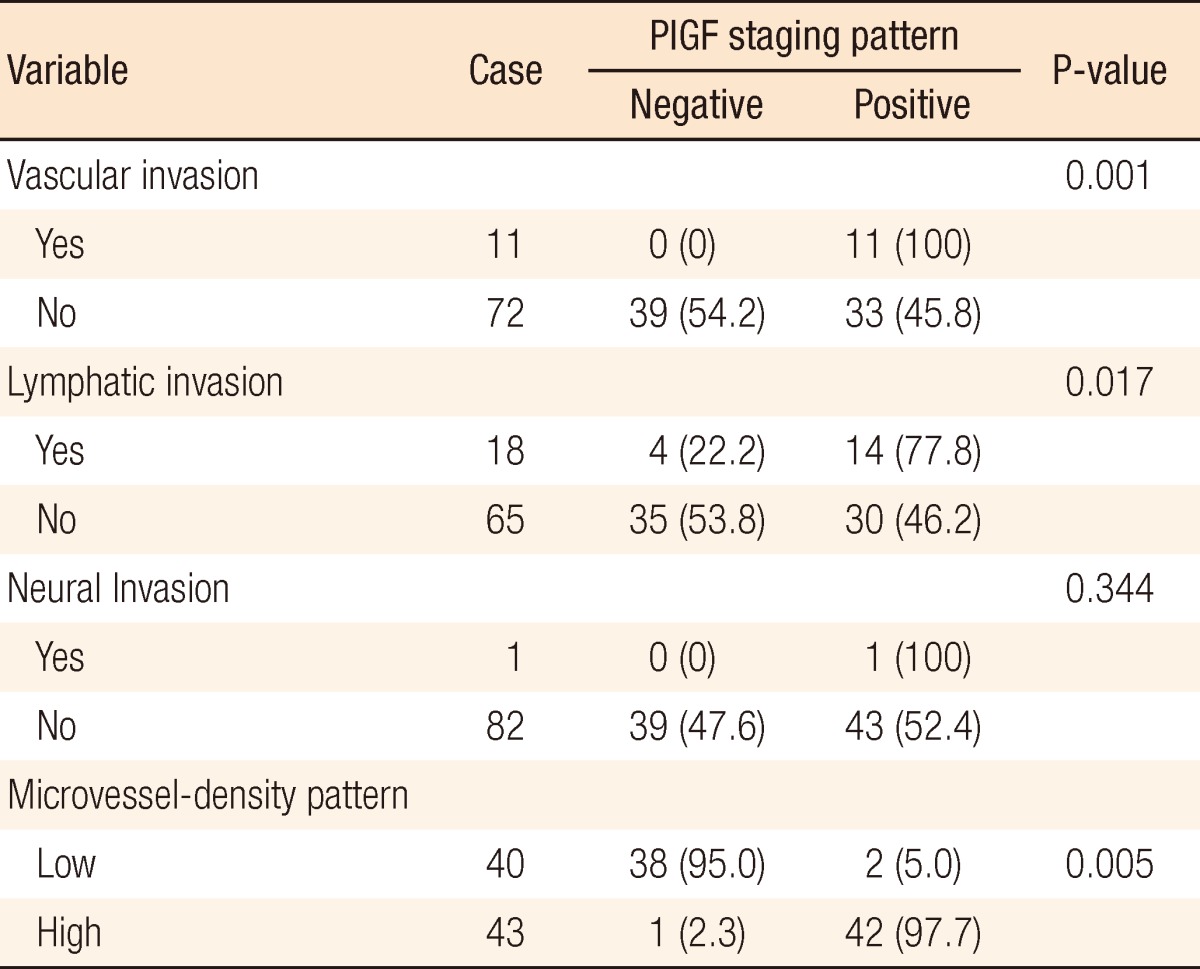
Values are presented as number (%).
Survival analysis based on PlGF expression
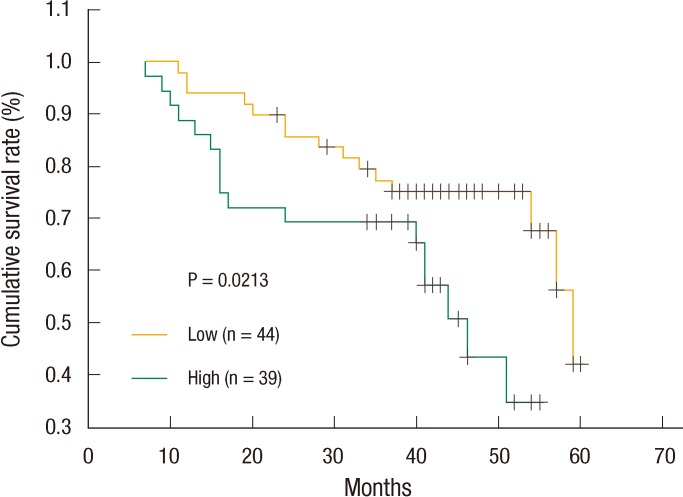
The survivals of 83 subjects were observed from the shortest follow-up period of 5 months to the longest follow-up period of 107 months. The median survival time was 81 months in the PlGF-expressed group. On the other hand, the median survival time was 59 months in non-PlGF-expressed group. The survival rate was significantly higher (P = 0.021) in the PlGF-expressed group than in the non-PlGF-expressed group according to the Kaplan-Meier survival curve, and PlGF expression levels exhibited significant differences with TNM staging, the presence of lymphatic metastasis, and survival rate. The presence of lymphatic metastasis and the TNM staging showed statistical significance in the multivariate analyses, and differences were found in the survival of patients depending on the presence of PlGF expression (P = 0.04) (Fig. 2).
Fig. 2.
Kaplan-Meier survival curves of colorectal cancer regarding placenta growth factor expression (log-rank test).
DISCUSSION
Angiogenesis is one of the critical processes in tumor growth and metastasis [2, 11-13]. PlGF has recently drawn attention as the main molecule involved in angiogenesis under various pathologic conditions including cancer [7]. PlGF is originally cloned from a human placental cDNA library and is 46% identical in amino acid composition to that of VEGF. A low degree of PlGF mRNA is occasionally detected in thyroid glands or lung tissues. The alternative exon splicing of the PlGF gene generates PlGF-1 (PlGF129) and PlGF-2 (PlGF152). PlGF homodimers are composed of PlGF subunits while heterodimers are composed of both VEGF165 and PlGF subunits. PlGF is a selective ligand for VEGFR-1. An interesting fact is that PlGF/VEGF165 heterodimers bind with high affinity to a soluble VEGFR-2. Purified PlGF-1 promotes blood vessel formation according to rabbit cornea assessments or chick chorioallantoic membrane assessments, and the process is induced mainly through promoted movement instead of proliferation in the endothelium [13].
This study examined PlGF expression in colorectal carcinomas by means of immunohistochemical staining. As a result, in cases of colorectal carcinomas, PlGF was more highly expressed in patients with lymph-node metastasis than in those without metastasis. Statistically significant differences were present depending on the stage. Furthermore, PlGF expression in colorectal carcinomas was found to be related to the prognosis, including survival rate, for patients.
Different hypotheses have been proposed regarding the mechanisms through which PlGF promotes angiogenesis. One hypothesis suggests that angiogenesis is promoted by more VEGF combining with VEGFR-2 through the combination of VEGFR-1, another receptor of VEGF, and by more PlGF allowing VEGFR-2 to efficiently bind to VEGF, generally known as the most powerful factor in angiogenesis [7]. Because PlGF solely has its own signal transmission path independent of the signal transmission path of VEGF expression, it is involved in cell death or proliferation and the formation of new blood vessels by binding to VEGFR-1 [14]. Moreover, the loss of PlGF activity impairs angiogenesis under pathological conditions such as ischemia, inflammation, tumors, and others [7]. These results suggest that PlGF may be a molecule regulating an angiogenic switch under pathological conditions. These data aligned with our results that PlGF expression levels were significantly higher in tumor tissues than in nontumor tissues depending on lymphatic metastasis and colon cancer stage. Many previous studies have demonstrated that VEGF and PlGF expressions correlate with tumor growth and angiogenesis [15-17].
Although our study carried out research on PlGF expression and colorectal cancer advancement, several studies have also verified that VEGF is associated with colorectal cancer advancement and angiogenesis. The findings support the theory suggesting that PlGF has a complementary relation with VEGF function in angiogenesis [7].
Although VEGF receptors, as they are known, stimulate angiogenesis, new blood vessels formed through this process are fragile, leaky, and prone to regression. PlGF promotes vessel maturation and stabilization by being involved in the formation of such new vessels [18]. Therefore, elevated PlGF and VEGF levels contribute in maintaining mature nonleaky vessels during angiogenesis in tumor tissues. PlGF expression could be regulated by oxygen tension and cytokines [19]. However, the mechanism of increased PlGF expression in colorectal cancer remains unknown. Elevated expression of VEGF in colorectal cancer patients was already identified by many previous studies. VEGF expression and patient survival are generally known not to be correlated [20-23]. Our studies also showed that PlGF expression, unlike VEGF expression, levels not only were increased in tumor tissues of colorectal cancer but also were correlated with cancer stage and the prognosis for patients with colorectal cancer. This implies that PlGF may have an independent role in colorectal cancer besides its supplementary role in VEGF function in angiogenesis. Thus, PlGF may be useful as a prognostic indicator in terms of clinical implications.
Based on recent studies, bevacizumab, a VEGF specific antibody, has been utilized for treating colorectal cancer patients, studies and many reports have suggested that the medication has promoted survival in patients with advanced colorectal cancer [24]. However, VEGF and VEGFR-2 are not only involved in pathological angiogenesis but also in normal vessel growth and maintenance. Many studies have also suggested that other signal transmission paths may exist in addition to identified paths [25]. Moreover, VEGF influences motor neuron survival, lung maturation, liver regeneration, blood pressure regulation, and glomerular development [26, 27]. This indicates that long-term inhibition of VEGF may also affect these processes [28]. Compared to VEGF, PlGF affects blood vessel formation only under pathological, but not physiological, conditions [7]. Therefore, as shown in the above results of our study, PlGF may be a therapeutic target for inhibiting angiogenesis in colorectal cancer with the over-expression of PlGF being an alternative method to overcome various limitations of VEGF.
In conclusion, we were able to demonstrate that PlGF expression levels involved in angiogenesis were mainly observed in tumor tissues rather than in normal tissues in patients with colorectal cancer. Moreover, PlGF was expressed more in advanced colorectal cancer than in early colorectal cancer, indicating that PlGF expression plays a definite role in colorectal cancer. The study was able to verify that PlGF expression correlates with disease progression, which suggests its potential use as a therapeutic target, in addition to VEGF, for inhibiting angiogenesis in colorectal cancer. Our study had some limitations. Our results were limited to a small sample size, and a definite effect of PlGF is still unclear in the process of colorectal cancer incidence. Thus, further studies are thought to be crucial in overcoming these limitations by clarifying definite mechanisms related to PlGF from the aspects of molecular biology.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Fontanini G, Vignati S, Boldrini L, Chine S, Silvestri V, Lucchi M, et al. Vascular endothelial growth factor is associated with neovascularization and influences progression of nonsmall cell lung carcinoma. Clin Cancer Res. 1997;3:861–865. [PubMed] [Google Scholar]
- 4.Ohta Y, Watanabe Y, Murakami S, Oda M, Hayashi Y, Nonomura A, et al. Vascular endothelial growth factor and lymph node metastasis in primary lung cancer. Br J Cancer. 1997;76:1041–1045. doi: 10.1038/bjc.1997.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibusa T, Shijubo N, Abe S. Tumor angiogenesis and vascular endothelial growth factor expression in stage I lung adenocarcinoma. Clin Cancer Res. 1998;4:1483–1487. [PubMed] [Google Scholar]
- 6.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 8.Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, et al. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73–82. doi: 10.1016/j.canlet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Parr C, Watkins G, Boulton M, Cai J, Jiang WG. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41:2819–2827. doi: 10.1016/j.ejca.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of Placenta growth factor (PlGF) in non-small cell lung cancer (NSCLC) and the clinical and prognostic significance. World J Surg Oncol. 2005;3:68. doi: 10.1186/1477-7819-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997;273(5 Pt 2):H2091–H2104. doi: 10.1152/ajpheart.1997.273.5.H2091. [DOI] [PubMed] [Google Scholar]
- 14.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 16.Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–2752. [PubMed] [Google Scholar]
- 17.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 18.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 19.Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: implications for abnormal placentation. J Soc Gynecol Investig. 2003;10:178–188. doi: 10.1016/s1071-5576(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 20.Wong MP, Cheung N, Yuen ST, Leung SY, Chung LP. Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer. 1999;81:845–850. doi: 10.1002/(sici)1097-0215(19990611)81:6<845::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 22.Landriscina M, Cassano A, Ratto C, Longo R, Ippoliti M, Palazzotti B, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. Br J Cancer. 1998;78:765–770. doi: 10.1038/bjc.1998.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960–968. doi: 10.1002/cncr.11152. [DOI] [PubMed] [Google Scholar]
- 24.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 27.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 28.Luttun A, Autiero M, Tjwa M, Carmeliet P. Genetic dissection of tumor angiogenesis: are PlGF and VEGFR-1 novel anti-cancer targets? Biochim Biophys Acta. 2004;1654:79–94. doi: 10.1016/j.bbcan.2003.09.002. [DOI] [PubMed] [Google Scholar]