Abstract
Several lines of evidence, including the recent discovery of novel susceptibility genes, point out an important role for the mammalian target of rapamycin (mTOR) signaling pathway in the development of pheochromocytoma. Analyzing a set of pheochromocytomas from patients with different genetic backgrounds, we observed and confirmed a significant overexpression of key mTOR complex (mTORC) signaling mediators. Using selective ATP-competitive inhibitors targeting both mTORC1 and mTORC2, we significantly arrested the in vitro cell proliferation and blocked migration of pheochromocytoma cells as a result of the pharmacological suppression of the Akt/mTOR signaling pathway. Moreover, AZD8055, a selective ATP-competitive dual mTORC1/2 small molecular inhibitor, significantly reduced the tumor burden in a model of metastatic pheochromocytoma using female athymic nude mice. This study suggests that targeting both mTORC1 and mTORC2 is a potentially rewarding strategy and supports the application of selective inhibitors in combinatorial drug regimens for metastatic pheochromocytoma.
Pheochromocytoma is a neuroendocrine tumor arising from the chromaffin cells of the adrenal medulla; extra-adrenal pheochromocytomas are related to the sympathetic and parasympathetic ganglia and are usually referred to as paragangliomas (1). Most are benign and surgically curable, but when malignant, there are few effective therapies (2, 3). After pharmacological treatment for hypertension and other catecholamine-dependent symptoms (using α- and β-adrenergic receptor antagonists), surgery is the main therapeutic option. For persisting disease, radiolabeled meta-iodobenzylguanidine therapy, peptide receptor radionucleotide therapy with radiolabeled somatostatin analogs (4), and certain types of chemotherapy may be helpful, but in advanced disease, in particular in patients carrying succinate dehydrogenase subunit B (SDH-B) mutations, surgical resection is frequently ineffective and recurrence is frequent and eventually lethal (2).
As a part of larger clinical trials for the evaluation of novel targeted therapies in neuroendocrine tumors or as single case reports, a small number of patients with malignant pheochromocytomas and paragangliomas have shown at least temporary responses to the multiple tyrosine kinase inhibitor sunitinib (5). Other specific targeted therapies, including the tyrosine kinase inhibitor imatinib, were not found to be of significant benefit for these patients (6). Thus, there is an ongoing and urgent need for specific targeted therapies for such patients.
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that is a master regulator of cell proliferation and survival (7), integrating complex upstream pathways and signals, including insulin, growth factors, and nutrient sensing, from the surrounding environment. The role of mTOR in cancer is well established (8), and it represents a rational molecular target in oncology (9). Two major mTOR complexes (mTORCs) regulate its activity: mTORC1, which is allosterically inhibited by the macrolide antibiotic rapamycin (sirolimus) and contains the regulatory-associated protein raptor, and mTORC2 including the rapamycin-insensitive mTOR companion protein rictor (10). mTORC1 is mostly involved in growth factor-stimulated cellular proliferation and cellular homeostasis through phosphorylation of the ribosomal protein S6 kinase 1 (S6K1) and the eukaryotic translation initiation factor 4E-binding protein 1. It is allosterically inhibited by rapamycin, but the downstream substrate 4E-binding protein 1 is only partially dephosphorylated by rapamycin. This explains the limited effect of rapalogs on protein synthesis. Rapamycin-resistant mTORC2 plays a prominent role in the regulation of the actin cytoskeleton and cellular motility. mTORC2 directly phosphorylates the serine/threonine protein kinase Akt/protein kinase B at S473, linking this complex to the activation of the mTORC1 pathway. Activation of mTORC2 leads to Akt phosphorylation and thus feeds forward in a positive fashion (11).
Accumulating evidence has supported that the phosphoinositide 3-kinase (PI3K)/AKT/mTOR signaling pathway plays an important role in the pathogenesis of several neuroendocrine tumors, including pheochromocytoma (3, 12). For instance, S6K1, as a downstream target of the pathway, has been shown to be overexpressed in human pheochromocytoma, suggesting the potential use of inhibitors of this pathway in this disease (13). Recent reports also link the mTOR pathway to mutations in the TMEM127 gene, which predisposes to the development of pheochromocytoma (14), emphasizing the importance of studying familial syndromes of pheochromocytoma to understand the pathogenic mechanisms involved in both sporadic and familial forms of the disease.
Unfortunately, studies using mTOR inhibitors in patients with pheochromocytoma have not clearly shown any therapeutic benefit. The mTOR inhibitor everolimus (RAD001; Novartis, Basel, Switzerland) failed to demonstrate a major clinical benefit in a small group of patients with pheochromocytoma (15). However, the inhibitors used in this study target only partially mTORC1, and in some solid tumors, treatment with these drugs has been associated with elevated Akt phosphorylation (16). These unpromising clinical studies were consistent with early experimental work showing that rapamycin inhibited proliferation of normal rat chromaffin cells stimulated by exogenous mitogens but was relatively ineffective against spontaneously proliferating PC12 rat pheochromocytoma cells (17).
Recent data have identified mTORC2 as the major kinase that phosphorylates Akt on Ser-473 (18, 19), and we have previously reported that levels of phospho-Akt are increased in pheochromocytoma compared with normal adrenal tissue (20). Moreover, there are several lines of evidence emphasizing a prominent role for mTORC2 in development of pheochromocytoma. For example, hypoxia-inducible factor 2α, which is downstream of the mTORC2 pathway (21), is particularly overexpressed in some subtypes of pheochromocytoma (22–25). This suggests that drugs that would target both mTORC1 and mTORC2 might be of a greater benefit and demonstrate antitumor activity where agents targeting only the mTORC1 have failed.
Novel inhibitors targeting both mTORC1 and mTORC2 have been recently developed, including AZD8055 and Torin-1 (26–28). Compared with rapamycin and everolimus, they have high activity against both mTORC1 and mTORC2. In addition to their antiproliferative effects, these drugs can potentially inhibit tumor cell invasion and metastatic spread (specifically through inhibition of mTORC2) (29), which is the most lethal complication, especially in patients with SDH-B mutations (30). In the current study, using mTORC1- and mTORC2-selective dual inhibitors, we now report inhibition of both cell proliferation and migration of pheochromocytoma cells in vitro; the inhibitory effect was associated with a potent inhibition of the Akt/mTOR signaling pathway. Moreover, we have demonstrated the ability of AZD8055 to significantly reduce tumor burden in an animal model of metastatic pheochromocytoma. These results argue in favor of the importance of pursuing selective targeting of mTORC1 and mTORC2 as a potential novel approach for patients with malignant pheochromocytoma.
Materials and Methods
Cell lines and reagents
The mouse pheochromocytoma cell line MTT was maintained in DMEM supplemented with 10% fetal bovine serum, 5% horse serum, and antibiotic/antimycotic (Gibco-Life Technologies, Grand Island, New York). Cells were grown until 80% confluence and then detached using 0.05% trypsin/EDTA, incubated for 3 minutes at 37°C, resuspended, and counted to obtain the desired concentration. AZD8055 was provided by AstraZeneca (London, United Kingdom). Torin-1 was developed by the Dana-Farber Cancer Institute, Biological Chemistry and Molecular Pharmacology Section, Harvard Medical School (Boston, Massachusetts). All of the compounds were dissolved in dimethylsulfoxide (DMSO); stock solutions were stored at −20°C and thawed prior to use. Controls were treated with the highest concentration of DMSO in the panel.
Human samples
Pheochromocytoma tumor tissue for the real-time PCR and for drug testing was obtained from patients visiting our clinic (Institutional Review Board [IRB] protocol 00-CH-0093); the study was approved by the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), and patients gave written informed consent. Tables 1 and Table 2 summarize patient clinical information. Guidelines for genetic testing were previously described (31). Normal human adrenal medullas (n = 4) were obtained from anonymous organ donors with absent adrenal tumor or dysfunction and collected within 2 to 5 hours after confirmed brain death at the Department of Urology, School of Medicine, Comenius University, Bratislava, Slovakia. Separation of cortex from adrenal medulla was ascertained as previously described (32).
Table 1.
Patient Information Relative to the Samples Used for the Gene Expression Study
| Patient | Sex | Age, y | Type | Location | Genetic Background | Biochemistry |
|---|---|---|---|---|---|---|
| 1 | M | 26 | Met | Retroperitoneal | SDHB | NE, NMN |
| 2 | F | 36 | Met | Peri-iliac | SDHB | NE |
| 3 | M | 31 | P | Left peri-aortic mass | SDHB | DA |
| 4 | M | 53 | Mlt | Nasopharyngeal | SDHB | NE, DA |
| 5 | F | 9 | Mlt | Left iliac bifurcation | SDHB | Epi, NE, DA |
| 6 | M | 31 | Mlt | Left adrenal | SDHD | NE, DA |
| 7 | M | 62 | P | Left adrenal | SDHD | |
| 8 | F | 37 | P | Adrenal | SDHD | |
| 9 | F | 26 | Mlt | Right carotid body | SDHD HNP | NE |
| 10 | M | 67 | Bi | Carotid body | SDHD HNP | |
| 11 | F | 47 | Mlt | Carotid body | SDHD HNP | |
| 12 | F | 64 | P | Right glomus jugular tumor | SDHD HNP | |
| 13 | M | 33 | Bi and Mlt | Bilateral adrenal | VHL | |
| 14 | F | 42 | Bi | Right adrenal | VHL | |
| 15 | M | 25 | P | Right adrenal | VHL | |
| 16 | M | 7 | P | Left adrenal | VHL | NE, Epi |
| 17 | M | 45 | Met | Liver | SDHB Met | NE, NMN, DA |
| 18 | M | 39 | Met | Right lung | SDHB Met | NE |
| 19 | M | 48 | Met | Liver | SDHB Met | NE, DA |
| 20 | F | 37 | Met | Parietal bone mass | SDHB Met | Epi, NE |
Abbreviations: Bi, bilateral; DA, dopamine; Epi, epinephrine; F, female; HNP, head and neck paragangliomas; M, male; Met, metastatic; Mlt, multiple; NE, norepinephrine; NMN, normetanephrine; P, primary.
Table 2.
Patient Information of the Samples Used for Primary Cell Cultures and Treatmentsa
| Patient | Sex | Age, y | Type | Location | Genetic Background | Biochemistry | Inhibition at 1μM AZD8055, % | Inhibition at 3μM AZD8055, % |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | P | Right adrenal | Sporadic | NE, DA, NMN | 27.1 | 48.5 (Torin-1, 48.2) |
| 2 | F | 28 | P | Right adrenal | SDHB | NE, DA, NMN, CgA | 22.6 | 68.9 |
| 3 | F | 47 | P | Right carotid body | Sporadic | CgA | 23.2 | |
| 4 | M | 5 | P | Left adrenal | VHL | MN, NMN | 17.1 | 51.9 (Torin-1, 52.4) |
Abbreviations: CgA, chromogranin A; DA, dopamine; F, female; M, male; MN, metanephrine; NE, norepinephrine; NMN, normetanephrine; P, primary.
Included are the percent inhibition with 1 μM and 3 μM AZD8055 and 3 μM Torin-1.
Cell proliferation assays
Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT cells (15 × 103) were incubated in 96-well plates for 24 hours before serum deprivation and treatment with the indicated concentrations of rapamycin, AZD8055, or Torin-1 in the presence of serum (10%) for 24 hours. An MTT solution (1 mg/mL; Sigma Chemical Co, St. Louis, Missouri) was added and plates were incubated at 37°C for 3 hours before measuring absorbance at 562 nm (Bio-TEK Instruments, Winooski, Vermont).
Cell migration assay
Cell migration was measured using modified Boyden chambers (Transwell assay). MTT cells were seeded at 150 000 cells per chamber, and cell migration was stimulated with serum (10%) in presence of the drugs (AZD8055 and Torin-1) or vehicle alone (control). At the end of the experiment, nonmigrating MTT cells were removed from the upper chamber with cotton swabs, and migrated MTT cells were fixed and stained with Diff-Quick reagents. Bright light images were digitally acquired. Mean values from four fields (1 × 1.4 mm) were calculated for each of triplicate wells per condition. IC50 values were determined using GraphPad Prism software.
Real-time PCR
Real-time PCR was performed on a 7500 real-time PCR system (Applied Biosystems, Foster City, California) using detector TaqMan, reporter FAM (6-carboxyfluorescein) and quencher TAMRA (tetramethylrhodamine) for the genes and detector VIC (4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein) for 18S rRNA. Primer sequence TaqMan gene expression assays were from Applied Biosystems as recommended for the genes. Primers were premixed to a concentration of 18 μM for each primer and 5μM for the probe, which represented a 20× mix. Final reaction volume was 25 μl; amounts of templates for 18S rRNA and for other genes were 5 and 25 ng, respectively. PCR was performed as follows: 50°C for 2 minutes, 95°C for 10 minutes, and 50 cycles of two-step PCR (95°C for 15 seconds and 60°C for 1 minute). The results were analyzed by 7500 system software, version 1.3 (Applied Biosystems), calculated based on the Δ−Δ Ct (threshold cycle) method and then by Microsoft Excel. Each result was correlated to the housekeeping gene 18S rRNA.
Drug treatment and Western blotting
MTT cells were grown to log phase (∼70% confluent) before treatment with the indicated concentrations of rapamycin, AZD8055, or Torin-1 for 6 hours at 37°C and 5% CO2. Control cells were incubated with the vehicle for the same period of time. The cells were washed twice with ice-cold PBS and lysed in cell lysis buffer (Cell Signaling Technology, Danvers, Massachusetts) supplemented with Complete protease inhibitor cocktail (Roche, Indianapolis, Indiana) and 1mM phenylmethylsulfonyl fluoride. Protein lysates were denatured by boiling with 4× sodium dodecyl sulfate sample buffer for 5 minutes. Proteins were separated by 4%–20% SDS-PAGE and transferred to polyvinylidene difluoride membrane. The membrane was incubated with total Akt, phospho-Akt (Ser473), phospho-S6 (Ser235/236), phospho-S6 (Ser240/244), phospho-eukaryotic elongation factor-2 kinase (Ser366), phospho-eukaryotic translation initiation factor 4B (eIF4B) (Ser422), and actin primary antibodies (Cell Signaling) overnight at 4°C, which was followed by washing and incubation with the horseradish peroxidase-conjugated secondary antibody at room temperature for 1 hour. The blots were visualized using Amersham ECL Plus Western blotting reagents.
Animal experiments and bioluminescence imaging
All animal studies were conducted in accordance to the principles and procedures outlined in the NIH Guide for the Care and Use of Animals and approved by the NIH Animal Care and Use Committee. For the spontaneous metastasis model, 1.5 × 106 MTT-luc cells were injected sc in the right flank of female athymic nude mice (Taconic, Germantown, Maryland). The experimental group consisted of 10-week-old mice (n = 7) housed in a pathogen-free facility. After 10 days, allowing for tumor cells to engraft, we started a treatment with 20 mg/kg AZD8055 in one group of animals (n = 7). Animals in the control group (n = 7) were treated with the same volume of vehicle instead, on a daily, 7 days a week schedule. The animals were imaged weekly by Bioluminescence (described below). All bioluminescent data were collected and analyzed with a Xenogen IVIS system. For in vivo imaging, luciferase activity was performed on anesthetized animals (1%–2% isoflurane) 15 minutes after ip administration of 150 mg/kg luciferin in PBS. The mice were then placed inside a camera box under continuous exposure to 1%–2% isoflurane. The experiments were performed in the NIH Mouse Image Facility in accordance to ACUC regulations.
All imaging variables were equal, and photographic and bioluminescent images at different time points were collected for each sample. The bioluminescence data are presented visually as a color overlay on the photographic image. Using the Living Image software (Xenogen), a region of interest was drawn around tumor sites of interest and the total photon count or photons per second was quantified.
Tumor dissociation and tyrosine hydroxylase immunocytochemistry in primary cell culture
Pheochromocytoma tumor tissue was obtained from patients visiting our clinic (IRB protocol 00-CH-0093); the study was approved by the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, and patients gave written informed consent. Dissociated cells were plated at low density (∼5 × 103 cells/35-mm dish) in RPMI 1640 medium with 15% fetal bovine serum plus penicillin/streptomycin. Cultures in control medium or dosed with 1 μM or 3 μM AZD8055 or 3 μM Torin-1 were maintained for 1 week with media replaced twice. Cultures were then fixed and stained for tyrosine hydroxylase (TH), a marker of catecholamine-synthesizing ability, to discriminate tumor cells from non-neoplastic fibroblasts and other cell types in primary cultures (33). To measure drug-induced cytotoxicity, surviving TH-positive cells were counted in an area of the culture dish defined by a randomly placed 22 × 22-mm2 coverslip.
Statistical analysis
Tumor volume and mean bioluminescence were determined for each experiment together with the SEM. Statistical analyses were performed using GraphPad Prism software, including unpaired t test and nonparametric Mann-Whitney U test (GraphPad Software, San Diego, California).
Results
mTOR, raptor, and rictor are overexpressed in subsets of pheochromocytomas
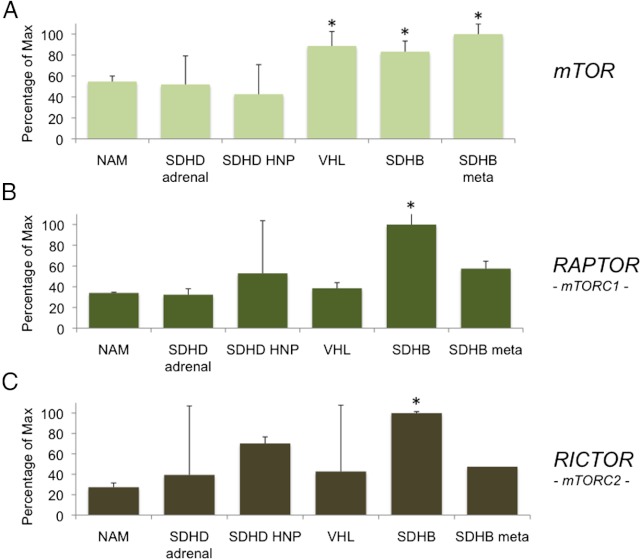
First, we wanted to verify the expression of mTOR, raptor, and rictor in several tumors from our collection of pheochromocytomas/paragangliomas from patients with different genetic backgrounds. Expression analysis was performed with 20 tumor samples from patients with von Hippel-Lindau (VHL), SDH-B, SDH-B metastases, SDH-D adrenal, SDH-D head and neck paraganglioma mutations, and 4 normal adrenal medulla. Table 1 summarizes the clinical information of the patients used for these studies. Expression of the three genes in these groups was analyzed by real-time PCR (Figure 1). As shown in Figure 1, patients with SDH-B and VHL mutations showed a significant increase (compared with normal adrenal medulla) in mTOR expression when compared with the other samples analyzed in this panel. Interestingly, both SDH-B and metastatic SDH-B pheochromocytoma showed a significant increase in expression of mTOR compared with normal adrenal medulla (P < .05). Analysis of both raptor and rictor expression demonstrated a significant increase in SDH-B pheochromocytoma tissue when compared with patients in other groups as well as the normal adrenal medulla.
Figure 1.
Levels of mTOR, raptor, and rictor are elevated in tumors with SDHB gene mutations. Pheochromocytoma/paraganglioma tumor samples from patients with different genetic backgrounds were analyzed by real-time PCR. SDH-B, metastatic SDH-B, and VHL-related pheochromocytoma exhibit a significant increase in expression of mTOR (*P < .05) than in normal adrenal when compared with samples of other genetic background tumors. Raptor and rictor expression is significantly increased in SDH-B pheochromocytoma patients when compared with normal adrenal medulla. Results are presented as percentage of the maximal value. Abbreviations: HNP, head and neck paraganglioma; NAM, normal adrenal medulla; SDHB meta, SDH-B metastatic.
mTORC1/2 inhibitors inhibit pheochromocytoma cell proliferation and migration
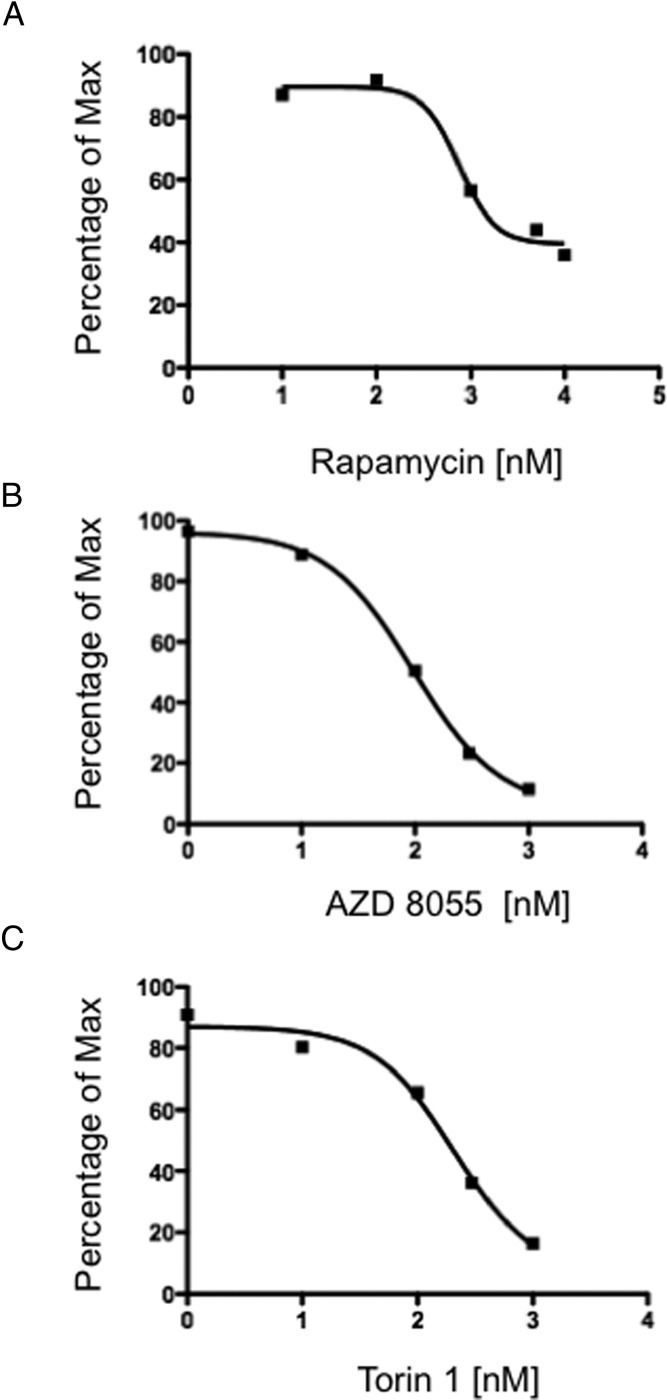
Based on the importance of the mTOR pathway in pheochromocytomas and paragangliomas, we tested mTOR inhibitors in a proliferation assay using the metastatic mouse pheochromocytoma-derived MTT cell line (34). As shown in Figure 2, the original mTOR inhibitor rapamycin was able to inhibit MTT cell proliferation with an IC50 of 756nM (Figure 2A). In comparison, the novel mTORC1/2 inhibitors AZD-8055 and Torin-1 were able to significantly inhibit cellular proliferation in a dose-dependent manner in MTT cells over a range of concentrations (1nM to 1μM) with an IC50 of 96nM (Figure 2B) and 207nM (Figure 2C), respectively. These data show that rapamycin induces only partial growth inhibition, whereas the dual inhibitors have a much greater suppression of proliferation.
Figure 2.
Rapamycin, AZD8055, and Torin-1 inhibit proliferation of MTT cells in vitro. The MTT proliferation assay was performed using MTT cells, and 15 000 cells per well were plated on a 96-well plate. The next day, MTT cells were treated for 48 hours with rapamycin (A), AZD8055 (B), or Torin-1 (C). Drug concentrations used were as follows: rapamycin, 10 nM, 100 nM, 1000 nM, 5000 nM, and 10 000 nM; AZD8055 and Torin-1, 1 nM, 10 nM, 100 nM, 300 nM, and 1000 nM. The graph represents the dose response of log10 concentrations of rapamycin, AZD8055, or Torin-1 vs signal intensity in a thiazolyl blue formazan (MTT)-based assay. IC50 for each compound was calculated using GraphPad Prism.
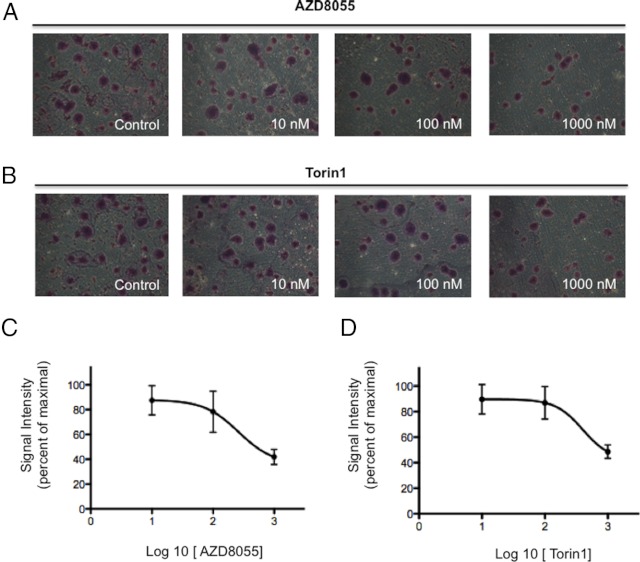
The effect of mTORC1/2 inhibitors treatment on cell migration was tested in the metastatic pheochromocytoma cell line MTT; we found that AZD8055 reduced serum-stimulated migration (Figure 3, A and C), with an IC50 of 260nM. Torin-1 was also able to significantly inhibit MTT cell migration, with an IC50 of 400 nM (Figure 3, B and D). Interestingly, MTT cells tended to become confluent in clusters, reminiscent of the zellballen nested arrangement characteristic of pheochromocytoma histopathology. As shown in the micrograph images in Figure 3, inhibition with either mTOR inhibitors reduced the formation of such clusters.
Figure 3.
Incubation with AZD8055 or Torin-1 inhibits cell migration of pheochromocytoma MTT cells. MTT cells plated in a Transwell chamber treated with different concentrations (10 nM, 100 nM, and 1000 nM) of AZD8055 (A) or Torin-1 (B) in the presence of serum for 24 hours show a decreased ability to migrate when compared with the control cells. Inhibition of migration caused by the drugs is accompanied by a lowered ability of the cells to form clusters. Mean numbers of migrated cells per microscopic field (at ×10 magnification) were counted and are represented in the graph: AZD8055 (C) and Torin-1 (D) dose-response curves; error bars represent SDs.
AZD8055 and Torin-1 treatment affects mTOR downstream signaling
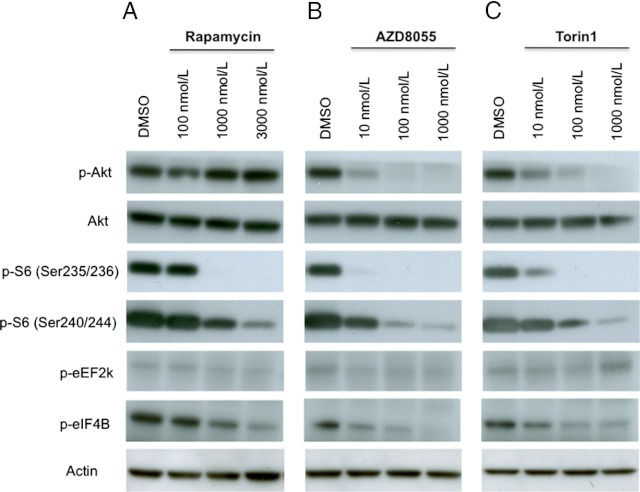
As part of the assessment of mTORC1/2 inhibitor activity in pheochromocytoma cells, MTT cells were exposed to different concentrations of rapamycin, AZD8055, and Torin-1 for 6 hours to assess downstream markers of mTOR kinase activity. Both AZD8055 and Torin-1 decreased phosphorylation of S6 ribosomal protein on Ser235/236 and Ser240/244 as well as phosphorylation of eukaryotic translation initiation factor 4B on Ser422. Unlike the allosteric mTOR inhibitor rapamycin, the dual inhibitors were also able to significantly decrease phosphorylation of Akt on Ser473. Moreover, lower concentrations of AZD8055 and Torin-1 (Figure 4, B and C) were necessary to obtain the same degree of biomarker modulation compared with rapamycin (Figure 4A).
Figure 4.
mTOR inhibition by rapamycin, AZD8055, and Torin-1 influences several protein in the mTOR pathway. MTT cells were treated with rapamycin (A), AZD8055 (B), or Torin-1 (C) at different concentrations (100 nM, 1000 nM, and 3000 nM for rapamycin; 10 nM, 100 nM, and 1000 nM for AZD8055 and Torin-1). The cells were incubated in the presence of the drugs for 6 hours. Total cell lysates were prepared and analyzed by Western blotting. Control cells were treated with DMSO alone for the same period of time. The levels of phosphorylated forms of proteins (phospho [p]-Akt[Ser473], p-S6[Ser235/236], p-S6[Ser240/244], p-eEF2k, and p-eIF4B) decrease with increasing concentrations of the drugs. Actin was used as a loading control.
AZD8055 reduces pheochromocytoma tumor and metastatic burden in vivo
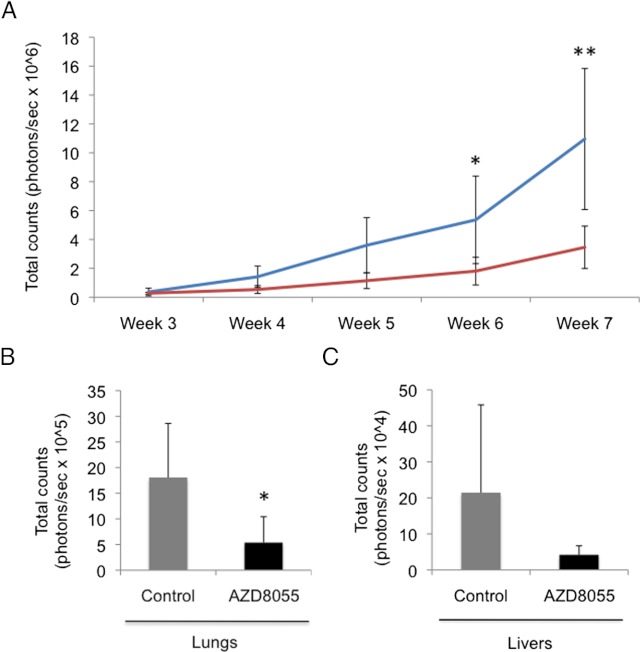
The antitumor effect of AZD8055 was assessed in vivo in a recently developed spontaneous metastasis model of pheochromocytoma (35). From week 3 through week 7, a significant reduction of tumor burden was observed in the AZD8055-treated animals (n = 7) compared with vehicle-treated animals (n = 7; Figure 5A). As previously described (35) in the MTT pheochromocytoma model, the two main organs where spontaneous metastases develop are the lungs and liver. On day 49, after in vivo bioluminescence measurements had been taken, the animals were euthanized and both lungs and livers were excised and luciferase activity was measured by Xenogen imaging. A trend of reduced metastatic burden was observed in lungs (Figure 5B) and livers (Figure 5C) of the treated animals compared with the control group, although only differences in lung metastasis were statistically significant using nonparametric statistics in the time frame of our experiment.
Figure 5.
AZD8055 reduces pheochromocytoma tumor and metastatic burden in mice. MTT-luc cells (1.5 × 106) were injected sc in the flank of nude mice. After 7 weeks of treatment with 20 mg/kg AZD8055, or the same volume of vehicle in the control group, the animals were euthanized and lungs and livers were excised for comparison. A, Tumor burden as a function of tumor cell bioluminescence signal is graphed: AZD8055-treated animals (red line) is compared with the control animals (blue line). **P = .0021; *P = .012 (t test). B and C, Sizes of metastatic tumors in lungs (B) (*P < .05, Mann-Whitney U test) and livers (C) were compared in treated mice compared with animals treated with vehicle (control).
AZD8055 and Torin-1 inhibits proliferation of primary cells from patients with pheochromocytoma
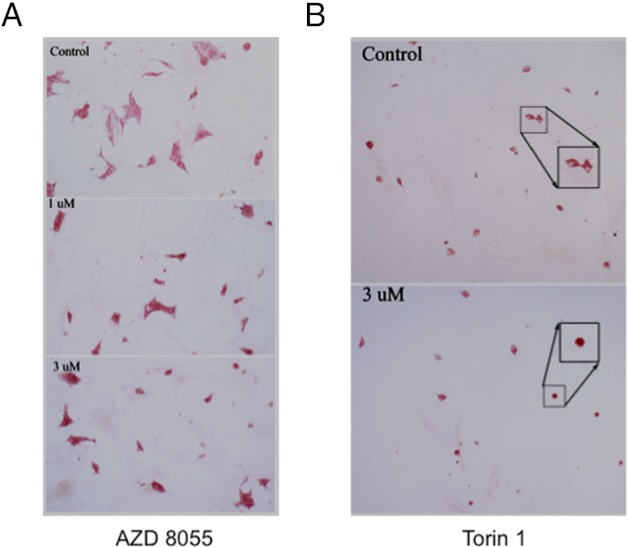
The growth-inhibitory effect of AZD8055 and Torin-1 was evaluated in human primary cells derived from donated tumor tissue collected from patients suffering from pheochromocytoma, including one SDH-B tumor. Immunohistochemical staining for TH, the rate-limiting step in the biosynthesis of catecholamines, was used as a marker of pheochromocytoma cells to discriminate them from other cell types that are inevitably present in primary cultures. As shown in Figure 6, AZD8055 (Figure 6A) as well as Torin-1 (Figure 6B) decreased the number of TH-positive cells to 50% of control cells, confirming their cytotoxic effect also on human pheochromocytoma cells. Table 2 summarizes the clinical information on the patients used for these studies.
Figure 6.
AZD8055 and Torin-1 show an inhibitory effect on survival of primary human pheochromocytoma cells. Dissociated cells from human tumor samples were plated at low density and treated with 1μM and 3μM AZD8055 (A) or 1μM Torin-1 (B) for 1 week. Control cells were incubated with DMSO for the same period of time. The cells were then fixed and stained for TH to discriminate tumor cells from other cell types in primary cultures. TH-positive cells were then counted to compare the number of treated cells with control cells. In the drug-containing culture, neoplastic chromaffin cells (red cytoplasm) are decreased in number and some are rounded (inset). Original magnification, ×20; expanded boxes show selected cells at higher magnification.
Discussion
The treatment of rare tumors represents a major challenge in oncology. In particular, neuroendocrine tumors, such as pheochromocytomas and paragangliomas, are rare tumors for which there are very limited therapeutic options (36, 37). Malignant pheochromocytomas have been historically very difficult to treat, because traditional chemotherapy has been mostly unsuccessful and data from clinical trials are difficult to interpret due to the low prevalence of the disease and the inability to recruit in the same trial an adequate number of patients (38).
However, the study of the molecular determinants of pheochromocytoma tumorigenesis has suggested a handful of potential targets to explore with novel targeted therapies, which have already demonstrated successful treatment for other forms of cancer (39). Currently, there is increasing interest in the pheochromocytoma research community to seek novel targeted therapies that have predominantly a cytostatic effect by interfering with a specific molecular target needed for carcinogenesis and sustained tumor growth (3, 38, 40).
A significant number of tumors is associated with familial etiology and can be included in familial syndromes such as VHL, multiple endocrine neoplasia type 2, neurofibromatosis type 1 (NF1), and SDH mutation-related tumors (4). VHL and SDH-related tumors in these cases seem to share the same tumorigenic pseudohypoxia/angiogenesis pathways. There are currently no human cell lines available for these tumors, hindering the testing of novel drugs in vitro.
Hereditary pheochromocytomas and paragangliomas can be divided into two clusters based on the transcription profile revealed by microarray analysis. Cluster 1 includes the tumors with VHL and SDHx mutant genes, cluster 2 involves tumors with mutations in RET and NF1 genes (25, 41). Sporadic tumors were surprisingly represented in both clusters (42). As further genes were discovered, additional microarray studies were performed to classify them into these two clusters. Mutations in KIF1Bb, TMEM127, and MAX genes were clustered with RET/NF1 (43). RET and NF1 mutations lead to activation of the rat sarcoma/rapidly accelerated fibrosarcoma/MAPK and the PI3K/Akt/mTOR signaling pathways. TMEM127 mutant tumors cluster with the RET/NF1 group, and they enhance mTOR activity independent of the above two kinase pathways. Microarray expression analysis of KIF1Bb mutant tumors also groups them with tumors associated with RET/NF1 mutations, although a potential role of the mutation in kinase pathways is not yet known (43). On the other hand, a recently discovered MAX gene mutation, which leads to dysregulation of the myelocytomatosis–myc-associated factor X–MAX dimerization protein 1 network, is grouped with this cluster for its connection with mTOR pathway (44). Conversely, SDHAF2 and SDHA mutations, recently described, can be clustered with SDHx/VHL (43).
Identification of a unifying player in tumor cells with deregulated energy metabolism and altered redox balance is required to elucidate molecular mechanisms associated with these discrepancies. Several pieces of experimental evidence indicate a potential role for the PI3K/Akt/mTOR pathway. Recently identified mutations in the TMEM127 gene have confirmed the importance of this pathway in the pathogenesis of pheochromocytoma (45) and further stress the importance of studying the molecular pathways downstream of susceptibility genes in expanding our understanding of the disease (37, 46). Elevated levels of the phosphorylated form of S6K1 were observed in both cluster 1 and cluster 2 tumor samples with more significant increases in cluster 2 tumors. Such results further support an important role for the mTOR pathway in pheochromocytomas/paragangliomas (47).
The present work has shown that the dual mTORC1 and mTORC2 inhibitors AZD8055 and Torin-1 were able to block proliferation in our mouse pheochromocytoma cell line and were more effective than the pure mTORC1 inhibitor rapamycin. The Western blotting studies confirmed inhibition of the targets downstream to mTOR, but additionally, the dual inhibitors decrease Akt phosphorylation. We have previously demonstrated increased levels of activated Akt in a model of pheochromocytoma induced by ectopic expression of ErbB-2 (20), in conjunction with reduction of the phosphatase and tensin homolog protein, consistent with other experimental work (48). These experimental studies reinforce the importance of the pathway.
The dual inhibitors also blocked cell migration. These results translated to the in vivo situation in our transplanted athymic mice. Finally, we were able to use a small number of human tumors to show cell loss induced by the inhibitors. Thus, it would appear that the dual TORC1/2 inhibitors may offer a novel and effective therapy for pheochromocytomas and paragangliomas with invasive and/or malignant characteristics, and it is worth considering their use in the clinical situation.
Of note, all efforts to establish cell lines from human pheochromocytomas and paragangliomas have been unsuccessful because the cells survive but do not proliferate in vitro (49). For this reason, we used first-passage primary cell cultures from patients with pheochromocytoma to access the effect of AZD8055 on human samples. TH, a marker of catecholamine-producing cells, was used to distinguish pheochromocytoma cells from non-neoplastic fibroblast and other cell types in the primary cell culture.
Our results suggest that dual inhibition of mTORC1 and mTORC2 (29) is a potential novel therapeutic approach for patients with pheochromocytoma and may overcome the problem encountered with the use of mTORC1 inhibitors alone (15, 50). Future efforts will be directed toward using these compounds in combinations with other chemotherapeutic drugs or novel targeted therapies.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. J.F.P. and A.S.T. were supported by Grant W81XWH-11-1-0670 from the Department of Defense.
Disclosure Summary: S.G. is a current employee and shareholder of AstraZeneca. All other authors declare no competing financial interests.
Footnotes
- DMSO
- dimethylsulfoxide
- eIF4B
- eukaryotic translation initiation factor 4B
- IRB
- Institutional Review Board
- mTOR
- mammalian target of rapamycin
- mTORC
- mTOR complex
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF1
- neurofibromatosis type 1
- PI3K
- phosphoinositide 3-kinase
- SDH-B
- succinate dehydrogenase subunit B
- S6K1
- S6 kinase 1
- VHL
- von Hippel-Lindau.
References
- 1. Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med. 2008;132:1272–1284 [DOI] [PubMed] [Google Scholar]
- 2. Adjalle R, Plouin PF, Pacak K, Lehnert H. Treatment of malignant pheochromocytoma. Horm Metab Res. 2009;41:687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nolting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23:21–33 [DOI] [PubMed] [Google Scholar]
- 4. Kaltsas GA, Papadogias D, Makras P, Grossman AB. Treatment of advanced neuroendocrine tumours with radiolabelled somatostatin analogues. Endocr Relat Cancer. 2005;12:683–699 [DOI] [PubMed] [Google Scholar]
- 5. Jimenez C, Cabanillas ME, Santarpia L, et al. Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. J Clin Endocrinol Metab. 2009;94:386–391 [DOI] [PubMed] [Google Scholar]
- 6. Gross DJ, Munter G, Bitan M, et al. The role of imatinib mesylate (Glivec) for treatment of patients with malignant endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer. 2006;13:535–540 [DOI] [PubMed] [Google Scholar]
- 7. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484 [DOI] [PubMed] [Google Scholar]
- 8. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734 [DOI] [PubMed] [Google Scholar]
- 9. Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22 [DOI] [PubMed] [Google Scholar]
- 11. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horsch D, Grabowski P, Schneider CP, et al. Current treatment options for neuroendocrine tumors. Drugs Today (Barc). 2011;47:773–786 [DOI] [PubMed] [Google Scholar]
- 13. Nardella C, Lunardi A, Fedele G, et al. Differential expression of S6K2 dictates tissue-specific requirement for S6K1 in mediating aberrant mTORC1 signaling and tumorigenesis. Cancer Res. 2011;71:3669–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qin Y, Yao L, King EE, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Druce MR, Kaltsas GA, Fraenkel M, Gross DJ, Grossman AB. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001). Horm Metab Res. 2009;41:697–702 [DOI] [PubMed] [Google Scholar]
- 16. Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219 [DOI] [PubMed] [Google Scholar]
- 17. Powers JF, Tischler AS, Cherington V. Discordant effects of rapamycin on proliferation and p70S6 kinase phosphorylation in normal and neoplastic rat chromaffin cells. Neurosci Lett. 1999;259:137–140 [DOI] [PubMed] [Google Scholar]
- 18. Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168 [DOI] [PubMed] [Google Scholar]
- 19. Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416 [DOI] [PubMed] [Google Scholar]
- 20. Lai EW, Rodriguez OC, Aventian M, et al. ErbB-2 induces bilateral adrenal pheochromocytoma formation in mice. Cell Cycle. 2007;6:1946–1950 [DOI] [PubMed] [Google Scholar]
- 21. Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1α and 2α on mTORC1 and mTORC2. J Biol Chem. 2008;283:34495–34499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pollard PJ, El-Bahrawy M, Poulsom R, et al. Expression of HIF-1α, HIF-2α (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91:4593–4598 [DOI] [PubMed] [Google Scholar]
- 23. Cervera AM, Apostolova N, Crespo FL, Mata M, McCreath KJ. Cells silenced for SDHB expression display characteristic features of the tumor phenotype. Cancer Res. 2008;68:4058–4067 [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Jimenez E, Gomez-Lopez G, Leandro-Garcia LJ, et al. Research resource: transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24:2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Favier J, Briere JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4:e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Q, Chang JW, Wang J, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benz o[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem. 2010;53:7146–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chresta CM, Davies BR, Hickson I, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298 [DOI] [PubMed] [Google Scholar]
- 29. Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kantorovich V, King KS, Pacak K. SDH-related pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2010;24:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Erlic Z, Rybicki L, Peczkowska M, et al. Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res. 2009;15:6378–6385 [DOI] [PubMed] [Google Scholar]
- 32. Fliedner SM, Breza J, Kvetnansky R, et al. Tyrosine hydroxylase, chromogranin A, and steroidogenic acute regulator as markers for successful separation of human adrenal medulla. Cell Tissue Res. 2010;340:607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tischler AS, Ruzicka LA, Riseberg JC. Immunocytochemical analysis of chromaffin cell proliferation in vitro. J Histochem Cytochem. 1992;40:1043–1045 [DOI] [PubMed] [Google Scholar]
- 34. Martiniova L, Lai EW, Elkahloun AG, et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin Exp Metastasis. 2009;26:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giubellino A, Woldemichael GM, Sourbier C, et al. Characterization of two mouse models of metastatic pheochromocytoma using bioluminescence imaging. Cancer Lett. 2012;316:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grogan RH, Mitmaker EJ, Duh QY. Changing paradigms in the treatment of malignant pheochromocytoma. Cancer Control. 2011;18:104–112 [DOI] [PubMed] [Google Scholar]
- 37. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Santarpia L, Habra MA, Jimenez C. Malignant pheochromocytomas and paragangliomas: molecular signaling pathways and emerging therapies. Horm Metab Res. 2009;41:680–686 [DOI] [PubMed] [Google Scholar]
- 39. Schilsky RL. Personalized medicine in oncology: the future is now. Nat Rev Drug Discov. 2010;9:363–366 [DOI] [PubMed] [Google Scholar]
- 40. Ye L, Santarpia L, Gagel RF. The evolving field of tyrosine kinase inhibitors in the treatment of endocrine tumors. Endocr Rev. 2010;31:578–599 [DOI] [PubMed] [Google Scholar]
- 41. Waldmann J, Fendrich V, Holler J, et al. Microarray analysis reveals differential expression of benign and malignant pheochromocytoma. Endocr Relat Cancer. 2010;17:743–756 [DOI] [PubMed] [Google Scholar]
- 42. Dahia PL, Ross KN, Wright ME, et al. A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Welander J, Soderkvist P, Gimm O. Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:R253–R276 [DOI] [PubMed] [Google Scholar]
- 44. Jafri M, Maher ER. The genetics of phaeochromocytoma: using clinical features to guide genetic testing. Eur J Endocrinol. 2012;166:151–158 [DOI] [PubMed] [Google Scholar]
- 45. Jiang S, Dahia PL. The busy road to pheochromocytomas and paragangliomas has a new member, TMEM127. Endocrinology. 2011;152:2133–2140 [DOI] [PubMed] [Google Scholar]
- 46. Qin Y, Buddavarapu K, Dahia PL. Pheochromocytomas: from genetic diversity to new paradigms. Horm Metab Res. 2009;41:664–671 [DOI] [PubMed] [Google Scholar]
- 47. Favier J, Igaz P, Burnichon N, et al. Rationale for anti-angiogenic therapy in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23:34–42 [DOI] [PubMed] [Google Scholar]
- 48. Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224 [DOI] [PubMed] [Google Scholar]
- 49. Tischler AS, Powers JF, Alroy J. Animal models of pheochromocytoma. Histol Histopathol. 2004;19:883–895 [DOI] [PubMed] [Google Scholar]
- 50. Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14:569–585 [DOI] [PubMed] [Google Scholar]