Abstract
Notch1 to Notch4 transmembrane receptors determine cell fate, and release of the Notch intracellular domain (NICD) in the cytoplasm induces gene expression. Notch regulates endochondral ossification, but it is not clear whether Notch interacts with signals controlling chondrocyte differentiation. Nuclear factor of activated T cells (Nfatc) transcription factors regulate chondrogenesis, and we asked whether Notch modifies Nfat signaling in chondrocytes. Notch was induced in teratocarcinoma ATDC5 chondrogenic cells infected with a retroviral vector, where the cytomegalovirus (CMV) promoter directs NICD expression. NICD suppressed chondrocyte differentiation and inhibited Nfat transactivation and Nfatc1 expression. Notch was activated in chondrocytes from RosaNotch mice, where the Rosa26 promoter is upstream of a loxP-flanked STOP cassette and NICD. To excise the STOP cassette and express NICD, RosaNotch chondrocytes were infected with an adenoviral vector where the CMV promoter directs Cre expression (Ad-CMV-Cre). Notch1 and Notch2 mediate the effects of Notch in skeletal cells, and to inhibit Notch signaling, chondrocytes from mice homozygous for Notch1 and Notch2 alleles targeted with loxP sites were infected with Ad-CMV-Cre. NICD suppressed chondrogenic nodules formation and expression of selected chondrocyte gene markers, induced Col10a1 and Mmp13, and suppressed Nfat transactivation and Nfatc1 expression, whereas inactivation of Notch1 and Notch2 did not affect chondrocyte differentiation. To investigate Nfatc1 function in chondrocytes, Nfatc1 was induced in RosaNotch chondrocytes overexpressing NICD or controls. Nfatc1 suppressed chondrocyte differentiation and opposed Col10a1 induction by Notch. In conclusion, Notch suppresses Nfat transactivation in chondrocytes and Notch and Nfatc1 regulate chondrocyte differentiation.
Skeletal elements formed by endochondral bone formation are preceded by a template of hyaline cartilage, which is generated during embryonic life by the condensation and chondrogenic differentiation of mesenchymal cells. Chondrocytes inside the hyaline cartilage proliferate, undergo hypertrophic differentiation, and induce the mineralization of the surrounding matrix before becoming apoptotic. These events lead to vascularization of the cartilage scaffold and colonization by precursor cells that replace the hyaline cartilage with bone (1).
The Notch receptors (Notch1 to Notch4) and Jagged and Delta-like ligands, are transmembrane proteins that regulate developmental processes and the renewal of differentiated tissues by determining cell fate (2–4). Interactions of Notch with ligands expressed by neighboring cells result in the proteolytic cleavage and release of the Notch intracellular domain (NICD) in the cytoplasm (5). In the Notch canonical signaling pathway, NICD translocates to the nucleus and interacts with Epstein-Barr virus latency C promoter binding factor 1, Suppressor of hairless and Lag-1 (Csl), also known as Rbpjκ in mice, a DNA-binding protein that suppresses gene expression by recruiting repressors of transcription. Association of NICD with Csl induces the formation of a ternary complex with Mastermind-like proteins, displaces transcriptional repressors, and recruits activators of transcription (6). These events lead to the expression of Hairy enhancer of split (Hes) and Hairy/Hes related with YRPW motif (Hey) transcription factors (7–10). Overexpression of NICD in vitro suppresses the differentiation of chondrogenic cells, and Notch signaling inhibition in limb bud cell cultures enhances chondrogenesis (11, 12). Accordingly, inactivation of Notch1 and Notch2 in the limb bud causes an accumulation of hypertrophic chondrocytes, whereas NICD overexpression in mesenchymal cells suppresses chondrogenesis (13, 14). The inhibitory effects of Notch are not observed in the absence of Csl, suggesting that Notch canonical signaling suppresses chondrogenesis (14). Constitutive NICD overexpression in chondrocytes prevents hypertrophic differentiation, and inactivation of Csl in chondrocytes causes elongation of the hypertrophic zone (15). In contrast, inducible overexpression of NICD in chondrocytes causes a shortening of the hypertrophic zone, and this discrepancy may be due to differences in the experimental model employed to induce Notch signaling (16).
Nuclear factor of activated T cells (Nfatc) are transcription factors (Nfatc1 to Nfatc4) that regulate the differentiation and function of multiple cell types. The phosphatase calcineurin induces Nfat transactivation by dephosphorylating specific serine residues in the SRR and SPXX repeat motifs of the regulatory domain of Nfat (17). Dephosphorylated Nfat translocates to the nucleus and induces expression of Nfat target genes, such as the isoform of Regulator of calcineurin 1 transcribed from the promoter region upstream of exon 4 (Rcan1.4) (17, 18). Phosphorylation of the SRR and SPXX repeat motifs prevents association to DNA and induces Nfat nuclear export, resulting in inhibition of Nfat transactivation (17). Nfatc1 to Nfatc4 are expressed in murine chondrocytes, and Nfatc2-null mice display ectopic chondrogenesis and joint abnormalities due to hypertrophic differentiation of articular chondrocytes (19, 20). Accordingly, inhibition of calcineurin prevents the differentiation of ATDC5 chondrogenic cells to a mature chondrocyte phenotype, indicating that Nfat signaling suppresses chondrocyte differentiation (21). In contrast, a constitutively active Nfatc3 mutant promoted chondrogenic differentiation of mesenchymal cells in vitro (22). However, activation of Nfatc2 during early chondrogenesis of limb bud cell cultures leads to enhanced chondrocyte differentiation, whereas Nfatc2 activation in more mature cells prevented further differentiation, indicating that the effects of Nfat in the chondrocyte lineage are determined by the degree of cellular maturation (23).
It is not clear whether Notch regulates other signaling pathways that determine the differentiation of cells of the chondrocyte lineage, but we demonstrated that Notch suppresses Nfat transactivation and Nfatc1 expression in cells of the osteoblast lineage to regulate osteoblast differentiation and function (24). However, it is not known whether this regulatory mechanism exists in other cellular environments, and we postulated that Notch and Nfat signaling interact in cells of the chondrocyte lineage (25). To verify this hypothesis, the effects of Notch on Nfat transactivation, Nfatc1 expression, and chondrocyte differentiation were explored in teratocarcinoma ATDC5 cells, an in vitro model of chondrogenesis, and in primary chondrocytes misexpressing Notch (26–29). In addition, the effects of Nfatc1 constitutive activation on the differentiation of RosaNotch chondrocytes were assessed either in the context of NICD overexpression or under basal conditions.
Materials and Methods
Expression vectors
A 2.4-kilobase (kb) cDNA containing the coding sequence of the NICD was obtained from J. S. Nye (Johnson and Johnson, New Brunswick, New Jersey) (30). The NICD cDNA was cloned into the retroviral vector pLPCX (Clontech, Mountain View, California) to generate a pLPCX-NICD vector where the cytomegalovirus (CMV) promoter directs the expression of NICD. A 2.1-kb cDNA containing the coding sequence of murine Nfatc1, where multiple serine to alanine mutations in the SRR and SPXX repeat motifs of the regulatory domain render it constitutively active (caNfatc1), was obtained from A. Rao (Addgene plasmid 11793; Harvard Medical School, Boston, Massachusetts) (31). This construct was used to create an adenoviral vector where the CMV promoter directs the expression of caNfatc1 (Ad-CMV-caNFATc1; Vector Biolabs, Philadelphia, PA).
ATDC5 cell culture and retroviral infection
ATDC5 cells, derived from a murine teratocarcinoma cell line (Cell Bank, Riken BioResource Center, Tsukuba, Japan), were grown in a humidified 5% CO2 incubator at 37°C in a Ham's F12/α-MEM 1:1 mixture (Life Technologies, Carlsbad, California), supplemented with 5% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, Georgia) (32). The retroviral vectors pLPCX and pLPCX-NICD were transfected into Phoenix packaging cells (American Type Culture Collection, Manassas, Virgnia) with TransFast transfection reagent, according to manufacturer's instructions (Promega Corporation, Madison, Wisconsin) and cells selected in 2 μg/ml puromycin (Sigma-Aldrich, St. Louis, Missouri), as described (33). Conditioned medium containing retroviral particles was harvested, filtered through a 0.45-μm membrane, and used to infect ATDC5 cells in the presence of 8 μg/ml polybrene (Sigma-Aldrich) (33). ATDC5 cells were grown, trypsinized, and selected in the presence of 2 μg/ml puromycin (Sigma-Aldrich). After selection, ATDC5 cells were cultured in F12/α-MEM supplemented with 5% FBS, and chondrogenic differentiation was induced by exposing confluent cells to 10 μg/ml bovine insulin (Sigma-Aldrich) (32).
Mice models of Notch misexpression
To induce Notch signaling in chondrocytes, RosaNotch mice were obtained from D. A. Melton (Harvard University, Cambridge, Massachusetts) (27). In these mice, the Rosa26 locus was targeted by homologous recombination with a DNA construct encoding the NICD coding sequence, preceded by a STOP cassette flanked by loxP sites, cloned downstream of the Rosa26 promoter. Expression of NICD from the Rosa26 locus occurs after the excision of the STOP cassette by Cre recombination of the loxP sequences.
Notch1 and Notch2 possibly mediate the effects of Notch signaling in the skeleton, and their inactivation in chondrocytes would allow investigating the consequences of Notch signaling inhibition in chondrocytes (4). To this end, Notch1loxP/loxP and Notch2loxP/loxP mice were obtained from F. Radtke (École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland), and T. Gridley (The Jackson Laboratory, Bar Harbor, Maine), respectively, and bred to generate Notch1loxP/loxP;Notch2loxP/loxP mice (28, 29). In these mice, inactivation of Notch1 and Notch2 occurs after the excision of loxP sequences targeted to the Notch1 and Notch2 loci, as described (28, 29). Experimental protocols were approved by the Animal Care and Use Committee of Saint Francis Hospital and Medical Center.
Chondrocyte culture, adenoviral infection, and cytochemical assays
Cells enriched in chondrocytes were isolated from 3- to 4-day-old old RosaNotch or Notch1loxP/loxP;Notch2loxP/loxP mice, and a mixed population of cells from male and female mice was used in the subsequent studies. Tissues surrounding the distal epiphyses of both femur and tibia and the proximal epiphysis of the tibia were dissected under a Unitron Z850 stereo microscope (Unitron, Commack, NY), and epiphyseal cartilage was collected in high-glucose DMEM (Life Technologies). Cartilage was exposed to 0.25% trypsin/0.9mM EDTA for 40 minutes (Life Technologies) and subsequently incubated with 200 U/ml of collagenase type II (Worthington Biochemical Corporation, Lakewood, New Jersey) for 2 hours at 37°C with continuous mixing. Tissue debris was removed by straining through a 70-μm membrane; cells were collected by centrifugation at 500g for 5 minutes and cultured in DMEM supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator (34). Experimental protocols were approved by the Animal Care and Use Committee of Saint Francis Hospital and Medical Center.
At 70% confluence, chondrocyte-enriched cultures were transferred to DMEM containing 2% FBS for 1 hour and exposed overnight to 100 multiplicity of infection of replication-defective recombinant adenoviruses. RosaNotch or Notch1loxP/loxP;Notch2loxP/loxP chondrocytes were infected with an adenoviral vector expressing Cre recombinase under the control of the CMV promoter (Ad-CMV-Cre; Vector Biolabs) to induce recombination of the loxP sequences and NICD expression, or Notch1 and Notch2 inactivation, respectively. An adenoviral vector expressing green fluorescent protein (GFP) under the control of the CMV promoter (Ad-CMV-GFP; Vector Biolabs) was used as control. In selected experiments, RosaNotch chondrocytes were co-infected with Ad-CMV-caNfatc1, or Ad-CMV-GFP as control, and expression of chondrocyte gene markers was analyzed. Cells were allowed to recover for 24 hours and cultured in the presence of DMEM containing 10% FBS, and confluent cultures were exposed to 100 μg/ml ascorbic acid (Sigma-Aldrich) to promote chondrocyte differentiation (35).
Morphology of primary chondrocytes was assessed by phase-contrast microscopy of live cells, and to determine formation of chondrogenic and mineralized nodules, cells were fixed for 10 minutes at room temperature in 3.7% formaldehyde in PBS and stained with 1% alcian blue in 3% acetic acid or 2% alizarin red in H2O (all from Sigma-Aldrich). Images were acquired with a Coolpix 995 digital camera mounted on an Eclipse TS100 inverted microscope equipped with contrast-phase lenses (all from Nikon Inc, Melville, New York).
Transient transfections
Activation of Notch signaling was assessed in ATDC5 cells infected with pLPCX-NICD or control pLPCX, and RosaNotch chondrocytes were infected with Ad-CMV-Cre or with Ad-CMV-GFP. Cells were transiently transfected with a construct containing 6 multimerized dimeric CSL binding sites, linked to the β-globin basal promoter (12xCSL-Luc; L. J. Strobl, Munich, Germany) or with 354-base-pair (bp), 2.9-kb, and 2.0-kb fragments of the Hes1 (Hes1-Luc; U. Lendahl, Stockholm, Sweden), Hey1 (Hey1-Luc; M. M. Maier, Wurzburg, Germany), and Hey2 (Hey2-Luc; T. Iso, University of Southern California, Los Angeles, California) promoters, respectively, all cloned upstream of luciferase (36–39). To test the effects of Notch signaling on Nfat transactivation, ATDC5 cells and RosaNotch chondrocytes overexpressing NICD and controls were transiently transfected with a construct containing 9 copies of an Nfat consensus sequence, linked to a minimal α-myosin heavy chain promoter and cloned into pGL3 basic (9xNFAT-Luc; J. D. Molkentin, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio) (40). To confirm activation of Nfat, cells were transiently transfected with a 0.9-kb fragment of the Rcan1.4 promoter (Rcan1.4-Luc; B. Rothermel, University of Texas, Dallas, Texas), which contains 15 Nfat consensus binding sites controlling luciferase expression (18, 41). To correct for transfection efficiency, cells were cotransfected with a construct where β-galactosidase is expressed under the direction of the CMV promoter (Clontech). At 70% confluence, cells were exposed to a FuGENE6-DNA mix (3 μl FuGene6/2 μg DNA) for 16 hours, transferred to fresh medium for 24 hours, and harvested according to manufacturer's instructions (Roche, Indianapolis, Indiana). Luciferase and β-galactosidase activities were measured using an Optocomp luminometer (MGM Instruments, Hamden, Connecticut), and luciferase activity was corrected for β-galactosidase activity.
Quantitative RT-PCR
Total RNA was extracted with the RNeasy mini kit, according to manufacturer's instructions (QIAGEN, Valencia, California), and changes in mRNA levels were determined by quantitative RT-PCR (qRT-PCR). One microgram of total RNA was reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, California) according to manufacturer's instructions, and amplified in the presence of specific primers (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) and iQ SYBR Green Supermix (Bio-Rad) at 60°C for 45 cycles. mRNA copy number was estimated by comparison with a 10-fold dilution series of cDNA for Alpl, Col1a1, Col2a1, Hes1, Rpl38, and Vegf (all from American Type Culture Collection); Col10a1, Ihh, Mmp13, Notch1, Notch2, and Sox9 (all from Open Biosystems, Huntsville, Alabama); Hey1 and Hey2 (both from T. Iso); Nfatc1 (from A. Rao); and Gapdh (from R. Wu, Cornell University, Ithaca, New York) (31, 42–44). Reactions were conducted in a CFX96 real-time PCR detection system (Bio-Rad), fluorescence monitored during every PCR cycle at the annealing step, and specificity of the reaction was assessed by melting curve analysis.
Western blot analysis
The layer of RosaNotch chondrocytes was washed in PBS (Amresco, Solon, Ohio) and extracted in cell lysis buffer (Cell Signaling Technology, Beverly, Massachusetts) in the presence of protease and phosphatase inhibitors and 1mM dithiothreitol (all from Sigma-Aldrich) at 4°C for 30 min. Protein concentrations were determined using a DC protein assay kit (Bio-Rad) and 20 μg of total protein were fractionated by gel electrophoresis in 10% polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Billerica, Massachusetts). Membranes were blocked with 3% BSA in PBS and exposed to a 1:1000 dilution of murine monoclonal antibody to Nfatc1 (7A6; Santa Cruz Biotechnology, Santa Cruz, California), and to assess even loading of the samples, membranes were exposed to a 1:1000 dilution of a goat polyclonal antibody against actin (I-19; Santa Cruz Biotechnology). All blots were exposed to either antimouse or antigoat IgG conjugated to horseradish peroxidase, incubated with a chemiluminescence detection reagent (Bio-Rad), and acquired with a cooled charge-coupled device camera mounted on a Chemidoc XSR molecular imager (Bio-Rad).
Statistical analysis
Data are expressed as mean ± SEM. Statistical differences were determined by Student's t test for pairwise comparisons or ANOVA with Schaeffé's post hoc analysis for multiple comparisons (45).
Results
NICD inhibits chondrocyte differentiation of ATDC5 cells
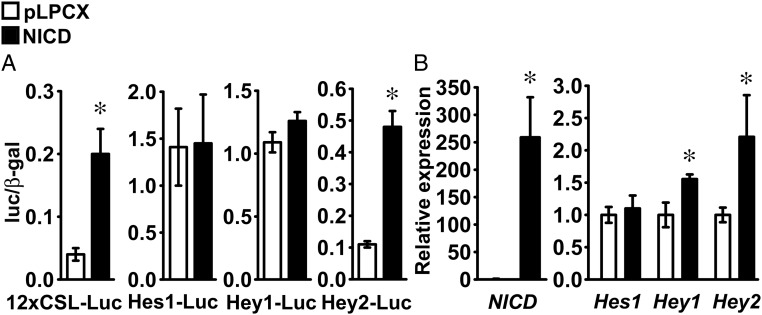
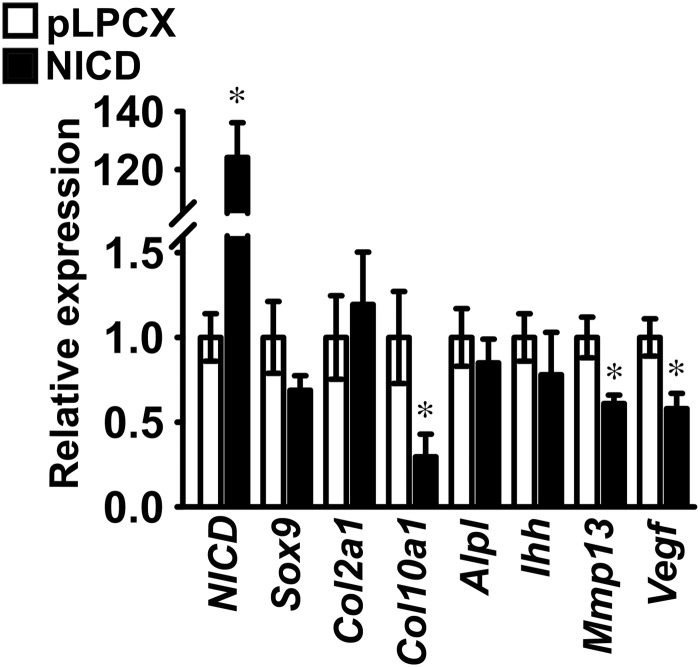
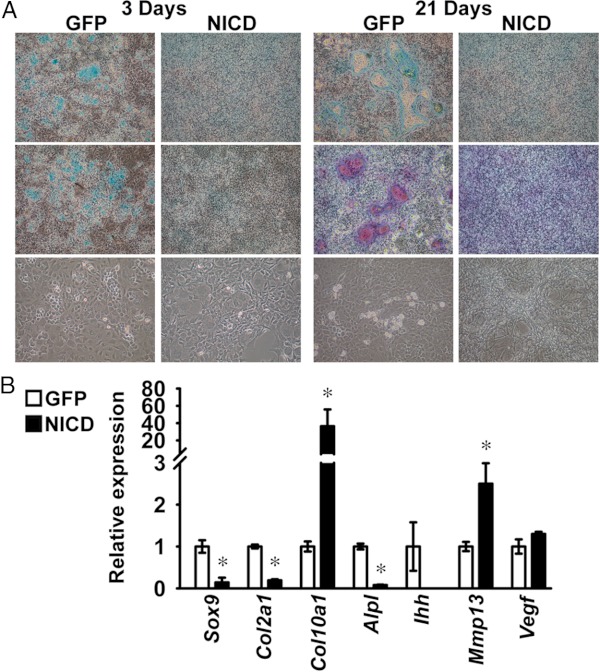
Induction of Notch signaling was assessed in ATDC5 cells infected with pLPCX-NICD or in control cells infected with the pLPCX vector. Proliferating ATDC5 cells were transiently transfected with the 12xCSL-Luc reporter construct or fragments of the Hes1 (Hes1-Luc), Hey1 (Hey1-Luc), or Hey2 (Hey2-Luc) promoters. The 12xCSL-Luc reporter and the Hey2-Luc promoter constructs were transactivated in ATDC5 cells infected with pLPCX-NICD, indicating Notch activation (Figure 1A). In parallel experiments, qRT-PCR analysis demonstrated induction of NICD and Hey2 mRNA, confirming expression of NICD after retroviral infection, and activation of Notch signaling (Figure 1B). Although NICD did not transactivate the Hey1-Luc promoter construct, it increased Hey1 transcript levels, suggesting that the promoter fragment cloned in Hey1-Luc is insufficient to replicate the conditions required for Hey1 induction by NICD in ATDC5 cells (Figure 1, A and B). Expression of Hes1 and the activity of its promoter were not changed by NICD, suggesting that Hes1 is not a target of Notch signaling in ATDC5 cells (Figure 1, A and B). Confluent ATDC5 cells were exposed to 10 μg/ml bovine insulin to induce chondrogenic differentiation, and the effects of NICD on the expression of chondrocyte gene markers were investigated. NICD had no effect on Sox9 and Col2a1 mRNA levels, indicating that Notch did not inhibit early chondrogenic differentiation of ATDC5 cells. Conversely, NICD suppressed Col10a1, Mmp13, and Vegf transcripts, although expression of Alpl and Ihh was not changed, indicating that Notch prevents terminal differentiation to a hypertrophic chondrocyte phenotype by ATDC5 cells (Figure 2).
Figure 1.
Notch canonical signaling is activated in ATDC5 chondrogenic cells. ATDC5 cells were infected with a pLPCX-NICD vector (NICD, black bars) to induce Notch, or with a pLPCX vector (white bars), as control. A, Cells were transfected with a Notch-responsive 12xCSL-Luc reporter or with fragments of the Hes1 (Hes1-Luc), Hey1 (Hey1-Luc), or Hey2 (Hey2-Luc) promoters and a β-galactosidase expression vector and harvested after 48 hours. Data shown represent luciferase/β-galactosidase (luc/β-gal) activity. Bars represent means ± SEM for 6 technical replicates. B, Total RNA was extracted from confluent cells, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of NICD, Hes1, Hey1, and Hey2 copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between NICD and pLPCX, P < .05.
Figure 2.
Notch suppresses chondrocyte differentiation of ATDC5 chondrogenic cells. ATDC5 cells were infected with a pLPCX-NICD vector (NICD, black bars) to induce Notch or with a pLPCX vector (white bars) as control. ATDC5 cells were exposed to 10 μg/ml insulin to induce chondrogenic differentiation. Total RNA was extracted from cells after 2 weeks of culture, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of NICD, Sox9, Col2a1, Col10a1, Alpl, Ihh, Mmp13, and Vegf copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between NICD and pLPCX, P < .05.
NICD suppresses Nfat transactivation in ATDC5 cells
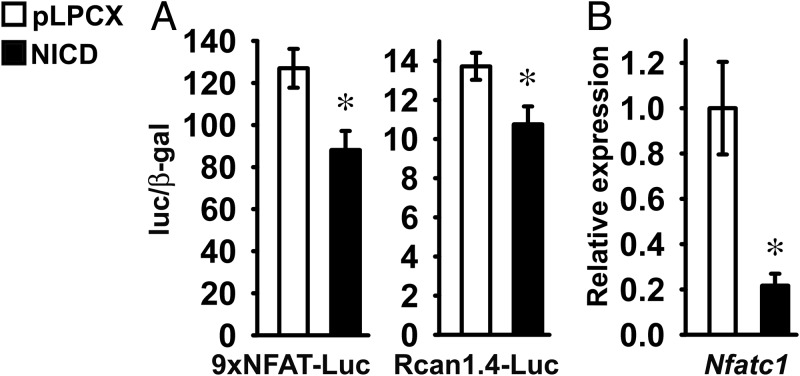
To test whether Notch regulates Nfat transactivation in chondrogenic cells, ATDC5 cells were transfected with a 9xNFAT-Luc reporter or a fragment of the Rcan1.4 promoter (Rcan1.4-Luc), either in the context of NICD overexpression or under basal conditions. NICD suppressed the transactivation of 9xNFAT-Luc and Rcan1.4-Luc, indicating that Notch suppresses Nfat signaling in chondrogenic cells (Figure 3A). To investigate the mechanisms of the inhibitory effect of NICD on Nfat transactivation, we measured Nfatc1 mRNA expression. NICD suppressed Nfatc1 mRNA levels, suggesting that in ATDC5 cells, Notch inhibits Nfat transactivation by repressing Nfatc1 expression (Figure 3B).
Figure 3.
Notch inhibits Nfat transactivation in ATDC5 chondrogenic cells. ATDC5 cells were infected with a pLPCX-NICD vector (NICD, black bars) to induce Notch or with a pLPCX vector (white bars) as control. A, Cells at 70% confluence were transfected with an Nfat-responsive 9xNFAT-Luc reporter or with a fragment of the Rcan1.4 (Rcan1.4-Luc) promoter and a β-galactosidase expression vector and harvested after 48 hours. Data shown represent luciferase/β-galactosidase (luc/β-gal) activity. Bars represent means ± SEM for 6 technical replicates. B, Total RNA was extracted from confluent cells, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of Nfatc1 copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between NICD and pLPCX, P < .05.
NICD regulates the differentiation of RosaNotch chondrocytes
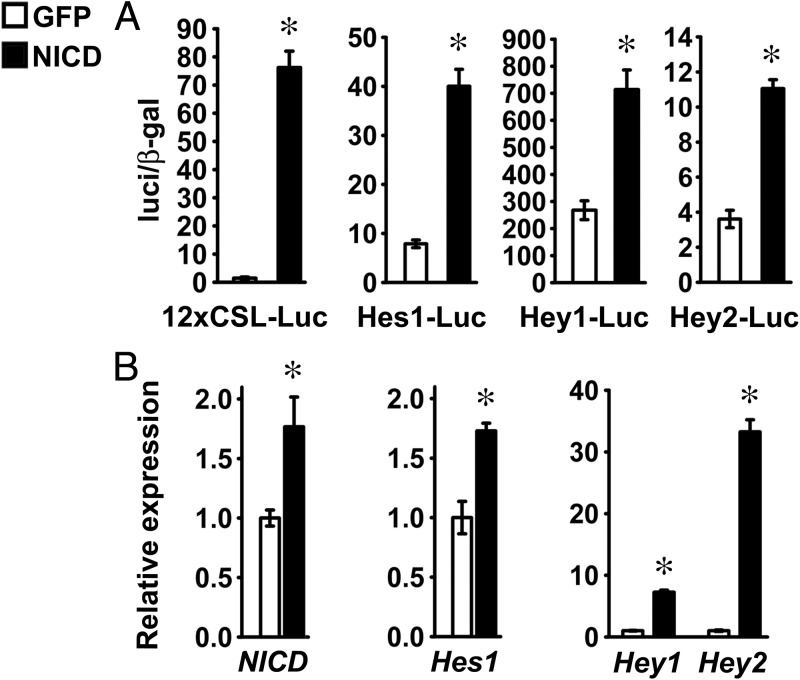
The consequences of Notch activation were investigated also in primary cells enriched in chondrocytes harvested from RosaNotch mice. NICD was induced after adenoviral infection with Ad-CMV-Cre, and cells infected with Ad-CMV-GFP were used as controls. To document activation of Notch signaling, chondrocytes were transfected with the 12xCSL-Luc reporter, and fragments of the Hes1 (Hes1-Luc), Hey1, and Hey2 promoters. NICD transactivated the transfected reporter and promoter fragments, and qRT-PCR revealed increased Hes1, Hey1, and Hey2 transcripts in cells infected with Ad-CMV-Cre, demonstrating activation of Notch signaling in RosaNotch chondrocytes (Figure 4, A and B).
Figure 4.
Notch signaling is activated in chondrocytes. Primary RosaNotch chondrocytes were infected with Ad-CMV-Cre (NICD, black bars) or with Ad-CMV-GFP (GFP, white bars) as control. A, Cells at 70% confluence were transfected with a Notch-responsive 12xCSL-Luc reporter or with fragments of the Hes1 (Hes1-Luc), Hey1 (Hey1-Luc), or Hey2 (Hey2-Luc) promoters and a β-galactosidase expression vector and harvested after 48 hours. Data shown represent luciferase/β-galactosidase (luc/β-gal) activity. Bars represent means ± SEM for 6 technical replicates. B, Total RNA was extracted from confluent cells, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of NICD, Hes1, Hey1, and Hey2 copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between NICD and GFP, P < .05.
The effects of NICD on the deposition of extracellular matrix, cellular morphology, and on the expression of chondrocyte gene markers, were studied in RosaNotch chondrocytes exposed to 100 μg/ml ascorbic acid to promote acquisition of a hypertrophic phenotype. Alcian blue-positive nodules were observed in control cells exposed to ascorbic acid for 3 days, and alizarin red-positive nodules were detected at 21 days, indicating deposition of a mineralized matrix. Accordingly, rounded and enlarged cells were detected in nodules and in the surrounding cell monolayer, suggesting differentiation of hypertrophic chondrocytes. The expression of Col1a1 transcripts increased in control cells 8.1 ± 0.7-fold (P < .05) from 3 to 14 days of culture (data not shown), indicating the possible presence of osteoblastic or fibroblastic cells in the primary culture. Cells overexpressing NICD did not form alcian blue-positive and alizarin red-positive nodules, exhibited a fibroblastic appearance, and displayed suppressed Sox9, Col2a1, Ihh, and Alpl mRNA levels, suggesting inhibition of chondrocyte differentiation by Notch (Figure 5, A and B). In contrast, NICD induced Col10a1 and Mmp13 mRNA and did not affect Vegf transcript levels, suggesting that regulation of Col10a1 and Mmp13 expression are effects of Notch independent from the inhibition of chondrocyte differentiation (Figure 5B).
Figure 5.
NICD regulates chondrocyte differentiation. Primary RosaNotch chondrocytes were infected with Ad-CMV-Cre (NICD, black bars) or with Ad-CMV-GFP (GFP, white bars) as control. At confluence, RosaNotch chondrocytes were exposed to 100 μg/ml ascorbic acid to induce differentiation. A, Representative images from 6 technical replicates of cells fixed and stained with alcian blue (top) or alcian blue and alizarin red (middle) and from phase interference microscopy of live cells (bottom). B, Total RNA was extracted from cells after 14 days of culture, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of Sox9, Col2a1, Col10a1, Alpl, Ihh, Mmp13, and Vegf copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between NICD and GFP, P < .05.
Notch1 and Notch2 are dispensable for chondrocyte differentiation in vitro
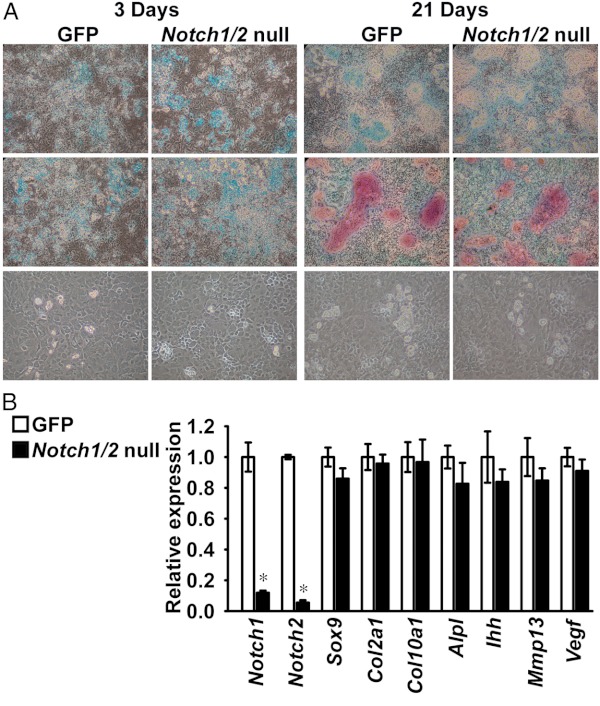
We investigated whether Notch signaling is required for the differentiation of primary chondrocyte-enriched cells from Notch1loxP/loxP;Notch2loxP/loxP mice. Cells were infected with Ad-CMV-Cre to inactivate Notch1 and Notch2 or with Ad-CMV-GFP as a control. Notch1 and Notch2 transcripts were suppressed up to 95% in cells infected with Ad-CMV-Cre in comparison with controls, indicating inactivation of Notch1 and Notch2 by Cre recombination in chondrocytes (Figure 6B). Notch1loxP/loxP;Notch2loxP/loxP chondrocytes exposed to 100 μg/ml ascorbic acid formed alcian blue-positive nodules after 3 days of culture, and alcian blue/alizarin red-positive nodules were detected at 21 days. Accordingly, cells with a rounded and enlarged morphology, indicating hypertrophic differentiation, were observed (Figure 6A). This was confirmed by changes in Col10a1 mRNA levels, which were increased 2.2 ± 0.2-fold (P < .05) from 3 to 21 days of culture. Expression of Col1a1 increased 3.4 ± 0.2-fold (P < .05) from 3 to 14 days of culture, indicating the presence of collagen type I synthesizing cells (data not shown). Progression to a mature phenotype proceeded to a similar extent in the context of Notch1 and Notch2 inactivation or under control conditions, and Notch1 and Notch2 inhibition did not affect Sox9, Col2a1, Col10a1, Ihh, Alpl, Mmp13, and Vegf mRNA levels, suggesting that Notch1 and Notch2 are dispensable for chondrocyte differentiation in vitro (Figure 6, A and B).
Figure 6.
Notch1 and Notch2 are dispensable for chondrocyte differentiation in vitro. Primary Notch1loxP/loxP;Notch2loxP/loxP chondrocytes were infected with Ad-CMV-Cre (Notch1/2-null, black bars) or with Ad-CMV-GFP (GFP, white bars) as control. At confluence, cells were exposed to 100 μg/ml ascorbic acid to induce differentiation. A, Representative images from six technical replicates of cells fixed and stained with alcian blue (top) or alcian blue and alizarin red (middle) and from phase interference microscopy of live cells (bottom). B, Total RNA was extracted from chondrocytes after 14 days of culture, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of Notch1, Notch2, Sox9, Col2a1, Col10a1, Alpl, Ihh, Mmp13, and Vegf copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between Notch1/2-null and GFP, P < .05.
NICD suppresses Nfat transactivation in RosaNotch chondrocytes
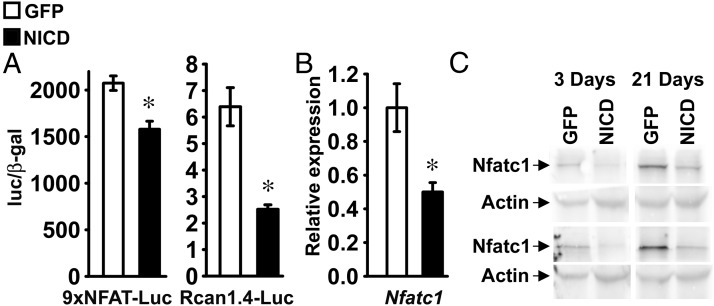
To assess whether Notch signaling regulates Nfat transactivation in RosaNotch chondrocytes, cells were transfected with 9xNFAT-Luc or Rcan1.4-Luc, either in the context of NICD overexpression or under basal conditions. Confirming the inhibitory effects of NICD on Nfat transactivation in ATDC5 cells, NICD suppressed the activity of 9xNFAT-Luc and Rcan1.4-Luc in RosaNotch chondrocytes (Figure 7A). Accordingly, NICD suppressed Nfatc1 mRNA and protein levels, indicating that Notch suppresses Nfat transactivation by inhibiting Nfatc1 expression in chondrocytes (Figure 7, B and C).
Figure 7.
Notch inhibits Nfat transactivation and Nfatc1 expression in chondrocytes. Primary RosaNotch chondrocytes were infected with Ad-CMV-Cre (NICD, black bars) or with Ad-CMV-GFP (GFP, white bars) as control. A, Subconfluent cells were transfected with an Nfat-responsive 9xNFAT-Luc reporter or a fragment of the Rcan1.4 promoter (Rcan1.4-Luc) and a β-galactosidase expression vector and harvested after 48 hours. Data shown represent luciferase/β-galactosidase (luc/β-gal) activity. Bars represent means ± SEM for 6 observations. B, Total RNA was extracted from confluent cells, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of Nfatc1 copy number corrected for Rpl38 expression, relative to corrected expression in control cells. Bars represent means ± SEM for 4 technical replicates. * Significantly different between NICD and GFP, P < .05. C, Cellular extracts were obtained from RosaNotch chondrocytes exposed to 100 μg/ml ascorbic acid for 3 or 21 days. Total proteins were fractioned by gel electrophoresis and transferred to an Immobilon-P membrane, which was incubated with an antibody against Nfatc1 or with an antibody against actin. Results from 2 independent experiments are shown in the upper and lower sets.
Notch and Nfatc1 regulate chondrocyte differentiation
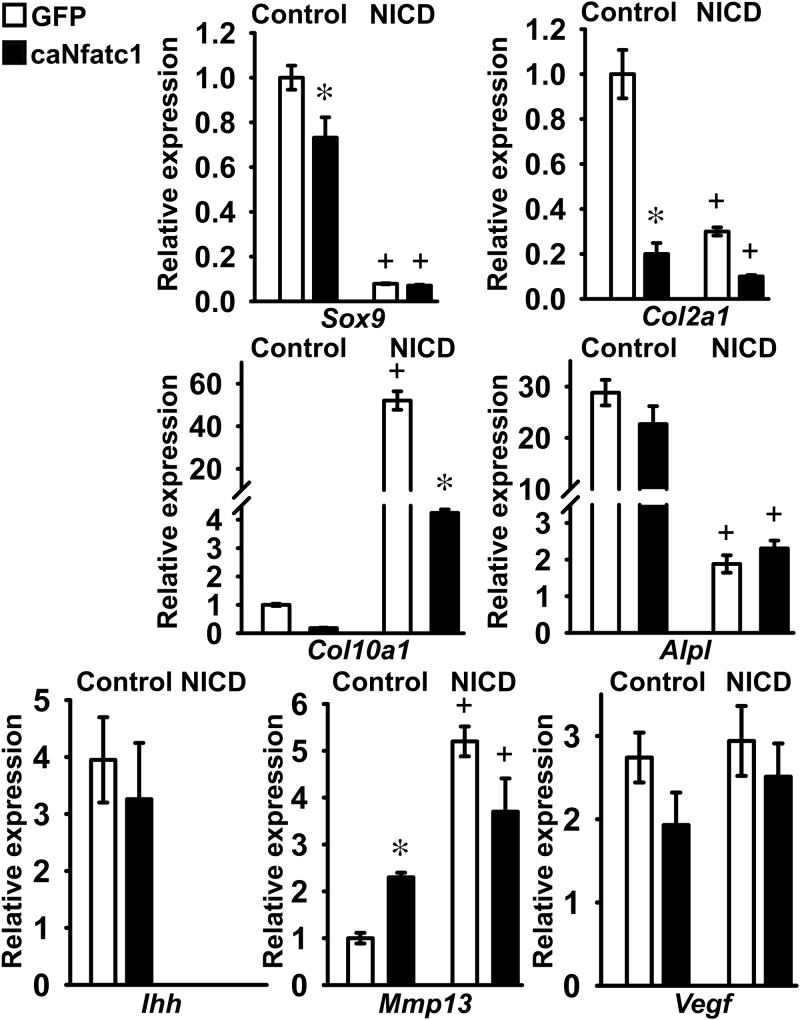
The functional interactions of Nfatc1 and Notch were examined in RosaNotch chondrocytes infected with Ad-CMV-caNfatc1 or Ad-CMV-GFP as control, either in the context, or not, of NICD overexpression. Changes in gene expression of early or late markers of chondrocyte differentiation were assessed after 21 days of exposure to 100 μg/ml ascorbic acid. Suppression of Sox9, Col2a1, Alpl, and Ihh mRNA levels, and induction of Col10a1 and Mmp13 transcripts by NICD were confirmed, and in agreement with the previous results, activation of NICD did not affect Vegf expression (Figure 8). After 3 days of culture, Nfatc1 caused a modest decrease in the level of Sox9 expression and did not modify the inhibitory effect of NICD on Sox9 mRNA. Nfatc1 and NICD suppressed Col2a1 expression to a similar extent, and no additive inhibition was observed when the two proteins were coexpressed (Figure 8). Therefore, Nfatc1, like Notch, suppresses expression of Sox9 and Col2a1 in RosaNotch chondrocytes. At 21 days, Nfatc1 caused a modest induction of Mmp13 expression that was not observed under conditions of Notch activation. Nfatc1 inhibited Col10a1 mRNA levels and did not affect Alpl, Ihh, and Vegf expression, either under control conditions or in the context of NICD overexpression. These results indicate that Nfatc1 phenocopies only partially the effects of Notch in chondrocytes (Figure 8).
Figure 8.
Notch and Nfatc1 regulate chondrocyte differentiation. Primary RosaNotch chondrocytes were infected with Ad-CMV-caNfatc1 (Nfatc1, black bars) or with Ad-CMV-GFP (GFP, white bars) as control, either in the context of NICD overexpression (NICD) or under basal conditions (control). RosaNotch chondrocytes were exposed to 100 μg/ml ascorbic acid for 3 days (upper panel) or 21 days (middle and lower panels), and total RNA was extracted, reverse-transcribed, and amplified by qPCR. Data are expressed as ratio of Sox9, Col2a1, Col10a1, Alpl, Ihh, Mmp13, and Vegf copy number corrected for Gapdh or Rpl38 expression, relative to corrected gene expression in control cells at 3 days for Sox9, Col2a1, Alpl, Ihh, and Vegf and at 21 days for Col10a1 and Mmp13. Bars represent means ± SEM for 4 technical replicates. * Significantly different between Nfatc1 and GFP, P < .05; +, significantly different between NICD and control, P < .01
Discussion
In the present study, we examined the regulation of Nfat signaling by Notch during chondrogenesis and chondrocyte differentiation. ATDC5 cells stably overexpressing NICD were used as a model for chondrogenesis, and in these cells, we demonstrated activation of Notch canonical signaling. NICD did not change the expression levels of Hes3 (data not shown), a target of Notch noncanonical signaling (46), confirming the notion that the effects of Notch in chondrogenesis are mediated by the canonical signaling pathway (14). Continuous Notch induction in ATDC5 cells did not prevent chondrogenic differentiation, but inhibited the acquisition of a terminally differentiated chondrocyte phenotype. In previous studies, induction of Notch signaling in ATDC5 cells transiently infected with an adenoviral vector expressing NICD, or cocultured with cells expressing a Notch ligand to activate Notch signaling, suppressed chondrogenesis (11). Accordingly, overexpression of NICD targeted to the developing limb suppresses endochondral bone formation in mice (14). The discrepancy between our results and those published in these reports may relate to differences in the duration of Notch induction, because duration of Notch signaling determines the effects of Notch on cell differentiation (4). Transient overexpression of NICD in ATDC5 induced Hes1 transcripts, whereas in the present studies, we detected induction of Hey1 and Hey2, but not of Hes1 (11). Therefore, an alternate explanation for this discrepancy relates to the differential expression of Notch target genes, because Hes1 and Hey transcription factors may not have identical effects on the differentiation of ATDC5 cells.
In agreement with data from other laboratories, our results demonstrated that overexpression of NICD in RosaNotch chondrocytes suppressed Sox9 and Col2a1 expression (14, 15, 47). We reported that activation of Notch canonical signaling in chondrocytes induces Hes1 and Hey1 expression, and both transcription factors are known to inhibit the activity of the Col2a1 promoter, suggesting that Hes1 and Hey1 mediate the effects of NICD on Col2a1 expression in RosaNotch chondrocytes (48). Our findings are in agreement with elongation of the hypertrophic zone caused by conditional inactivation of Csl in chondrocytes and confirm that Notch canonical signaling suppresses chondrocyte differentiation (15). Conversely, we report that inactivation of Notch1 and Notch2 had no effect on chondrocyte maturation in vitro, and genetic compensation by Notch3 and Notch4 is not likely because the latter two paralogs are expressed at minimal levels in chondrocytes (14). This is not consistent with the expansion of the hypertrophic zone caused by preferential inactivation of Notch1 and Notch2 in the limb bud (13). However, inactivation of Notch1 and Notch2 in the present study occurred in cells committed to the chondrocyte lineage, suggesting that Notch receptors are dispensable for later stages of chondrocyte differentiation. Alternatively, Csl may regulate chondrocyte differentiation by a mechanism that is independent from activation of the Notch receptors (15, 16).
In contrast with the inhibitory effects of Notch on chondrocyte differentiation, overexpression of NICD in RosaNotch cells induced expression of Col10a1 and Mmp13, gene markers of hypertrophic chondrocyte differentiation (1, 13–15, 47). RosaNotch chondrocytes overexpressing NICD displayed a fibroblastic appearance, were unable to deposit a mineralized matrix, and exhibited reduced Alpl mRNA levels in comparison with controls. This phenotype is not consistent with chondrocyte hypertrophy, suggesting that induction of Col10a1 is a direct effect of Notch activation on gene expression in chondrocytes. Induction of Col10a1 and Mmp13 by NICD overexpression confirms work from other laboratories demonstrating that under selected conditions, NICD overexpression induces expression of both genes in chondrocytes (16). In addition, suppression of Notch signaling in murine articular chondrocytes inhibits Mmp13 expression and activity, and induction of Mmp13 mRNA by Notch is consistent with these results (49). The discordant effects of NICD overexpression in ATDC5 chondrogenic cells and RosaNotch chondrocytes might be related to the different degree of chondrocytic differentiation of the two cell systems. In ATDC5 cells, suppression of Col10a1 and Mmp13 expression is a consequence of the inhibitory effect of Notch on chondrocyte differentiation. Conversely, induction of Col10a1 and Mmp13 by NICD overexpression in RosaNotch chondrocytes is due to activation of Notch signaling in differentiated cells, suggesting that regulation of Col10a1 and Mmp13 mRNA levels by Notch is dependent on the degree of cell maturation. Accordingly, the consequences of Notch activation on the differentiation and function of cells of the osteoblastic lineage, which share a mesenchymal origin with chondrocytes, are determined by the degree of osteoblastic maturation (50, 51). Induction of Col10a1 and Mmp13, and suppression of Sox9 and Col2a1 transcripts by Notch is reminiscent of the changes in gene expression observed in osteoarthritic chondrocytes (52). This is congruent with the induction of Notch signaling reported in chondrocytes from human osteoarthritic cartilage, and investigation of the effects of Notch in articular chondrocytes may contribute to the understanding of cellular processes that lead to osteoarthritis (53, 54).
Notch inhibited Nfat transactivation and Nfatc1 expression in cells of the chondrocyte lineage. Although Nfat activity is determined by nuclear or cytoplasmic localization, availability of Nfatc1 provides an additional level of regulation in skeletal cells and may determine Nfat transactivation in cells of the chondrocyte lineage (17, 55). A similar effect of Notch was described in C2C12 myoblastic cells and primary calvarial osteoblasts, suggesting that inhibition of Nfat transactivation by Notch is a common control mechanism of Nfat activity in mesenchymal cells (24, 56). Conversely, association of the intracellular domain of Notch2 with the transcription factor nuclear factor-κB induces Nfatc1 expression in osteoclasts (57). These observations indicate that Notch1 and Notch2 have divergent mechanisms of action on Nfatc1 expression or that these effects of Notch are cell context dependent. Nfatc1 phenocopied the inhibitory effects of Notch on gene markers of early chondrocyte differentiation, and both Notch and Nfatc1 induced Mmp13 transcript levels, indicating that suppression of Nfatc1 expression by Notch represents a regulatory mechanism to temper the activity of factors that oppose chondrocyte differentiation. Conversely, Nfatc1 opposed the induction of Col10a1 by NICD, suggesting that expression of Col10a1 is determined in part by the balance of Notch and Nfatc1 activity and that the pronounced stimulatory effect of Notch on Col10a1 expression is due to concurrent suppression of Nfat transactivation.
In conclusion, Notch and Nfatc1 regulate chondrocyte differentiation, and inhibition of Nfatc1 expression by Notch may represent a mechanism to regulate selected effects of Notch on gene expression in chondrocytes.
Acknowledgments
We thank Drs. D. A. Melton for RosaNotch mice, F. Radtke for Notch1loxP/loxP mice, T. Gridley for Notch2loxP/loxP mice, J. S. Nye for NICD cDNA, A. Rao for caNfatc1 cDNA, R. Wu for Gapdh cDNA, L. J. Strobl for 12xCSL-Luc, U. Lendahl for Hes1-Luc, M. M. Maier for Hey1-Luc, T. Iso for Hey2-Luc and Hey1 and Hey2 cDNAs, J. D. Molkentin for 9xNFAT-Luc, and B. Rothermel for Rcan1.4-Luc. We also thank M. Monarca, D. Bridgewater, and L. Kranz for technical assistance and M. Yurczak for secretarial assistance.
This work was supported by Grant DK045227 from the National Institute of Diabetes and Digestive and Kidney Diseases (to E.C.) and Research Fellowship 5371 from the Arthritis Foundation (to S.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ca
- constitutively active
- CMV
- cytomegalovirus
- CSL
- Epstein-Barr virus latency C promoter binding factor 1/suppressor of hairless/Lag 1
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- Hes
- hairy enhancer of split
- Hey
- hairy/hes related with YRPW motif
- Nfat
- nuclear factor of activated T cells
- NICD
- Notch intracellular domain
- qRT-PCR
- quantitative RT-PCR.
References
- 1. Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220 [DOI] [PubMed] [Google Scholar]
- 2. Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647 [DOI] [PubMed] [Google Scholar]
- 3. D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zanotti S, Canalis E. Notch and the Skeleton. Mol Cell Biol. 2010;30:886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehebauer M, Hayward P, Martinez-Arias A. 2006 Notch signaling pathway. Sci STKE. 2006:cm7. [DOI] [PubMed] [Google Scholar]
- 6. Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–5109 [DOI] [PubMed] [Google Scholar]
- 7. McElhinny AS, Li JL, Wu L. Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways. Oncogene. 2008;27:5138–5147 [DOI] [PubMed] [Google Scholar]
- 8. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776 [DOI] [PubMed] [Google Scholar]
- 9. Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iso T, Sartorelli V, Poizat C, et al. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol Cell Biol. 2001;21:6080–6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe N, Tezuka Y, Matsuno K, et al. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Miner Metab. 2003;21:344–352 [DOI] [PubMed] [Google Scholar]
- 12. Fujimaki R, Toyama Y, Hozumi N, Tezuka K. Involvement of Notch signaling in initiation of prechondrogenic condensation and nodule formation in limb bud micromass cultures. J Bone Miner Metab. 2006;24:191–198 [DOI] [PubMed] [Google Scholar]
- 13. Hilton MJ, Tu X, Wu X, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong Y, Jesse AM, Kohn A, et al. RBPjκ-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137:1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mead TJ, Yutzey KE. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc Natl Acad Sci U S A. 2009;106:14420–14425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kohn A, Dong Y, Mirando AJ, et al. Cartilage-specific RBPjκ-dependent and -independent Notch signals regulate cartilage and bone development. Development. 2012;139:1198–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656 [DOI] [PubMed] [Google Scholar]
- 18. Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med. 2003;13:15–21 [DOI] [PubMed] [Google Scholar]
- 19. Ranger AM, Gerstenfeld LC, Wang J, et al. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med. 2000;191:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Gardner BM, Lu Q, et al. Transcription factor Nfat1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J Pathol. 2009;219:163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishigaki F, Sakuma S, Ogawa T, Miyata S, Ohkubo T, Goto T. FK506 induces chondrogenic differentiation of clonal mouse embryonic carcinoma cells, ATDC5. Eur J Pharmacol. 2002;437:123–128 [DOI] [PubMed] [Google Scholar]
- 22. Tomita M, Reinhold MI, Molkentin JD, Naski MC. Calcineurin and NFAT4 induce chondrogenesis. J Biol Chem. 2002;277:42214–42218 [DOI] [PubMed] [Google Scholar]
- 23. Bradley EW, Drissi MH. WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-κB pathways. Mol Endocrinol. 2010;24:1581–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zanotti S, Smerdel-Ramoya A, Canalis E. Reciprocal regulation of notch and nuclear factor of activated T-cells (NFAT)c1 transactivation in osteoblasts. J Biol Chem. 2011;286:4576–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canalis E. The fate of circulating osteoblasts. N Engl J Med. 2005;352:2014–2016 [DOI] [PubMed] [Google Scholar]
- 26. Shukunami C, Ishizeki K, Atsumi T, Ohta Y, Suzuki F, Hiraki Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J Bone Miner Res. 1997;12:1174–1188 [DOI] [PubMed] [Google Scholar]
- 27. Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558 [DOI] [PubMed] [Google Scholar]
- 29. McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33 [DOI] [PubMed] [Google Scholar]
- 30. Nye JS, Kopan R, Axel R. An activated Notch suppresses neurogenesis and myogenesis but not gliogenesis in mammalian cells. Development. 1994;120:2421–2430 [DOI] [PubMed] [Google Scholar]
- 31. Monticelli S, Rao A. NFAT1 and NFAT2 are positive regulators of IL-4 gene transcription. Eur J Immunol. 2002;32:2971–2978 [DOI] [PubMed] [Google Scholar]
- 32. Atsumi T, Miwa Y, Kimata K, Ikawa Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ Dev. 1990;30:109–116 [DOI] [PubMed] [Google Scholar]
- 33. Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E. Notch 1 impairs osteoblastic cell differentiation. Endocrinology. 2003;144:5631–5639 [DOI] [PubMed] [Google Scholar]
- 34. Pfander D, Cramer T, Schipani E, Johnson RS. HIF-1α controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci. 2003;116:1819–1826 [DOI] [PubMed] [Google Scholar]
- 35. Lefebvre V, Garofalo S, Zhou G, Metsaranta M, Vuorio E, de CB. Characterization of primary cultures of chondrocytes from type II collagen/β-galactosidase transgenic mice. Matrix Biol. 1994;14:329–335 [DOI] [PubMed] [Google Scholar]
- 36. Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358 [DOI] [PubMed] [Google Scholar]
- 37. Strobl LJ, Hofelmayr H, Stein C, et al. Both Epstein-Barr viral nuclear antigen 2 (EBNA2) and activated Notch1 transactivate genes by interacting with the cellular protein RBP-Jκ. Immunobiology. 1997;198:299–306 [DOI] [PubMed] [Google Scholar]
- 38. Maier MM, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem Biophys Res Commun. 2000;275:652–660 [DOI] [PubMed] [Google Scholar]
- 39. Iso T, Chung G, Hamamori Y, Kedes L. HERP1 is a cell type-specific primary target of Notch. J Biol Chem. 2002;277:6598–6607 [DOI] [PubMed] [Google Scholar]
- 40. Dai YS, Xu J, Molkentin JD. The DnaJ-related factor Mrj interacts with nuclear factor of activated T cells c3 and mediates transcriptional repression through class II histone deacetylase recruitment. Mol Cell Biol. 2005;25:9936–9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang J, Rothermel B, Vega RB, et al. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68 [DOI] [PubMed] [Google Scholar]
- 42. Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol. 2001;21:6071–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kouadjo KE, Nishida Y, Cadrin-Girard JF, Yoshioka M, St-Amand J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genomics. 2007;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sokal RR, Rohlf FJ. 1981 Biometry. 2nd ed San Francisco, CA: WH Freeman; 1981 [Google Scholar]
- 46. Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255 [DOI] [PubMed] [Google Scholar]
- 47. Chen S, Tao J, Bae Y, et al. Notch gain of function inhibits chondrocyte differentiation via Rbpj-dependent suppression of Sox9. J Bone Miner Res. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grogan SP, Olee T, Hiraoka K, Lotz MK. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis Rheum. 2008;58:2754–2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blaise R, Mahjoub M, Salvat C, et al. Involvement of the Notch pathway in the regulation of matrix metalloproteinase 13 and the dedifferentiation of articular chondrocytes in murine cartilage. Arthritis Rheum. 2009;60:428–439 [DOI] [PubMed] [Google Scholar]
- 50. Engin F, Yao Z, Yang T, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149:3890–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther. 2010;12:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karlsson C, Brantsing C, Egell S, Lindahl A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs. 2008;188:287–298 [DOI] [PubMed] [Google Scholar]
- 54. Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mammucari C, Tommasi di Vagnano A, Sharov AA, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8:665–676 [DOI] [PubMed] [Google Scholar]
- 57. Fukushima H, Nakao A, Okamoto F, et al. The association of Notch2 and NF-κB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol. 2008;28:6402–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]