Abstract
Extracellular ATP plays a critical role in regulating insulin secretion in pancreatic β cells. The ATP released from insulin secretory vesicles has been proposed to be a major source of extracellular ATP. Currently, the mechanism by which ATP accumulates into insulin secretory granules remains elusive. In this study, the authors identified the expression of a vesicular nucleotide transporter (VNUT) in mouse pancreas, isolated mouse islets, and MIN6 cells, a mouse β cell line. Immunohistochemistry and immunofluorescence revealed that VNUT colocalized extensively with insulin secretory granules. Functional studies showed that suppressing endogenous VNUT expression in β cells by small hairpin RNA knockdown greatly reduced basal- and glucose-induced ATP release. Importantly, knocking down VNUT expression by VNUT small hairpin RNA in MIN6 cells and isolated mouse islets dramatically suppressed basal insulin release and glucose-stimulated insulin secretion (GSIS). Moreover, acute pharmacologic blockade of VNUT with Evans blue, a VNUT antagonist, greatly attenuated GSIS in a dose-dependent manner. Exogenous ATP treatment effectively reversed the insulin secretion defect induced by both VNUT knockdown and functional inhibition, indicating that VNUT-mediated ATP release is essential for maintaining normal insulin secretion. In contrast to VNUT knockdown, overexpression of VNUT in β cells resulted in excessive ATP release and enhanced basal insulin secretion and GSIS. Elevated insulin secretion induced by VNUT overexpression was reversed by pharmacologic inhibition of P2X but not P2Y purinergic receptors. This study reveals VNUT is expressed in pancreatic β cells and plays an essential and novel role in regulating insulin secretion through vesicular ATP release and extracellular purinergic signaling.
In addition to serving as a major cellular energy source, ATP plays a unique role in insulin secretion in the pancreatic β cell. When plasma glucose levels rise, extracellular glucose enters β cells through the glucose transporter 2, where it is phosphorylated by glucokinase and metabolized to produce ATP (1–3). An elevated cytosolic ATP/ADP ratio closes ATP-sensitive potassium channels (KATP), triggering calcium (Ca2+) influx through voltage-dependent Ca2+ channels (VDCCs), which consequently stimulates the exocytosis of insulin secretory vesicles and the release of insulin (4, 5).
Accumulating evidence has also shown that ATP can serve as an extracellular signal to regulate insulin secretion in addition to its intracellular effect on KATP channels (6–8). A number of purinergic receptors with high affinity for ATP have been identified on β cells (6, 9–11) and functional studies have shown these receptors are involved in modulating insulin secretion (6). Moreover, significant quantities of ATP are present in insulin secretory vesicles and are released into the local environment during exocytosis (12–14). Currently, the mechanism by which ATP accumulates in insulin secretory vesicles is unknown. Furthermore, the physiologic significance of extracellular ATP in insulin secretion remains to be clarified.
Vesicular nucleotide transporter (VNUT) is a vesicular membrane protein first identified in adrenal chromaffin cells (15). Sequence analysis has determined VNUT is a member of the solute carrier 17 (SLC17) phosphate transporter family, which also includes vesicular transporters for glutamate and sialin (16). Functional studies demonstrate VNUT is responsible for ATP uptake into secretory granules using membrane potential as the driving force (15). In addition to adrenal chromaffin cells, VNUT has been identified in a number of different cell types, including neurons, biliary epithelial cells, and T lymphocytes (17–19). VNUT has also been shown to mediate ATP uptake into secretory vesicles in each of these cells types, which proves to be crucial for the vesicular release of ATP. The role of VNUT as a transporter that accumulates ATP into secretory vesicles raised the possibility that VNUT may also be present in pancreatic β cells and thus may be responsible for transporting ATP into insulin secretory vesicles for its subsequent release.
In the current study, we show VNUT is expressed in pancreatic β cells and is colocalized with insulin secretory granules. Functional studies demonstrate that VNUT mediates both basal- and glucose-induced ATP release in β cells. More importantly, we show VNUT is involved in regulating insulin secretion by modulating vesicular ATP release and consequently extracellular purinergic signaling. These results thus demonstrate that VNUT-mediated ATP release from insulin secretory granules is critical for insulin secretion in β cells.
Materials and Methods
Cell culture
MIN6 cells, a mouse clonal β cell line, were cultured in high glucose DMEM supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 1% penicillin/streptomycin and 86 μM β-mercaptoethanol. MIN6 cells were transduced with lentiviral particles and cultured for 72 hours before use in experiments. All cell culture reagents were purchased from Invitrogen (Carlsbad, California).
Islet isolation
Islets were isolated from male C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) via intraductal collagenase digestion and density centrifugation, as described previously (20). After isolation, islets were hand-picked, size-matched, and maintained in RPMI media supplemented with 10% (v/v) fetal bovine serum, and 1% penicillin/streptomycin. Islets were immediately transduced with lentiviral particles and cultured for 72 hours before use in experiments. All animal studies were approved by the University of Virginia Animal Care and Use Committee.
RNA isolation, cDNA synthesis, and semiquantitative RT-PCR
Semiquantitative RT-PCR analysis was carried out on mouse pancreas, isolated mouse islets, and MIN6 cells. Total RNA was extracted with an RNeasy kit (QIAGEN, Inc, Hilden, Germany) following the manufacturer's protocol. RNA samples (1 μg) were reverse transcribed with an iScript cDNA Synthesis kit (Bio-Rad Laboratories, Berkeley, California) using the conditions: 25°C for 5 minutes, 32°C for 30 minutes, and 85°C for 5 minutes to generate cDNA. PCR for VNUT was performed with a 25μl PCR reaction mixture consisting of 175 ng of the cDNA samples, 2.5 μl ThermoPol Buffer (New England Biolabs, Ipswich, Massachusetts),10 mM deoxyribonucleotide triphosphates, 2 μM primers (Invitrogen), and 5 U Bio-X-Act Long polymerase (New England Biolabs). The following specific VNUT primers were used for PCR: 5′-CCCTGTCAGTGAAACGGAAT-3′ and 5′-ACCTTGTTCTGGGGTCTGTG-3′ corresponding to nucleotides 1420-1439 (sense) and 1999-2018 (antisense); the predicted band size is 598 bp. The following specific β-actin primers were used for PCR and served as an internal control: 5′-GTGGGCCGCCCTAGGCACCAG-3′ and 5′-CTCTTTGATGTCACGCACGATTTC-3′ corresponding to nucleotides 146-166 (sense) and 665-688 (antisense); the predicted band size is 538 bp. The PCR reaction consisted of 32 cycles: denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, extension at 68 °C for 40 seconds, and a final extension at 68°C for 7 minutes. PCR products were resolved on a 1% agarose gel and sequenced to confirm the expression of VNUT.
Real-time PCR
Total RNA isolation and RT were performed as described in the preceding paragraph. VNUT expression was determined using the following primers: 5′- GTGCGTAAGTTCATGCAGGT-3′ and 5′-GGAAGCTGATGCAAAGATCA-3′ corresponding to nucleotides 982-1001 (sense) and 1232-1251 (antisense). β-actin expression was determined using the following primers: 5′-AACCGTGAGAAAATGACCCAGATCATGTTT-3′ and 5′- AGCAGCCGTGGCCATCTCTTGCTCGAACTG-3′ corresponding to nucleotides 422-451 (sense) and 743-772 (antisense). Each real-time PCR reaction consisted of: 12.5 μL iQ SYBR Green Supermix (Bio-Rad Laboratories), 80 nM primers, and 100 ng cDNA for a final reaction volume of 25 μl. PCR reactions were performed in iCycler iQ 96-well PCR plates (Bio-Rad Laboratories) on an MyIQ Single-Color Real-Time Detection System (Bio-Rad Laboratories) with sequence detection software. Each reaction was performed in duplicate or triplicate, and VNUT expression was normalized to β-actin.
Immunohistochemistry
Pancreata were removed from male C57BL/6J mice, fixed in 4% paraformaldehyde, embedded in paraffin, sliced 6 μm thick, and mounted on slides. Paraffin was removed and slides were incubated in rabbit anti-VNUT antibodies (1:1,000) for 48 hours at 4°C. Sections were washed and incubated in biotinylated donkey antirabbit IgG (1:600, Jackson ImmunoResearch Laboratories, Inc, West Grove, Pennsylvania) for 1 hour. Sections were washed and incubated in A/B solution (Vector Laboratories, Inc, Burlingame, California) for 1 hour. The antibody-peroxidase complex was visualized with a mixture of 3′, 3′-diaminobenzidine (DAB, 0.25 mg/ml) and 3% H2O2 in 0.05 M Tris-saline solution. After color development, slides were counterstained, dehydrated, and coverslipped with Permount (Fisher Scientific, Fair Lawn, New Jersey). Results were examined with a Nikon light microscope. Images were acquired through a QImaging digital camera controlled by iVision imaging software (BioVision, Milpitas, California). To double-label VNUT and insulin, mouse pancreatic sections were incubated in rabbit anti-VNUT antibodies (1:1,000) and guinea pig anti-insulin antibodies (1:5,000, Linco Research, Inc, St. Charles, Missouri) at 4°C for 48 hours. Sections were then incubated in a mixture of biotinylated donkey antirabbit IgG (1:600) and Cy3-conjugated donkey antiguinea pig IgG (1:350, Jackson ImmunoResearch Laboratories, Inc) for 1 hour, followed by Cy2-conjugated streptavidin (1:1,000, Jackson ImmunoResearch Laboratories, Inc) for 1 hour. Slides were coverslipped, and images were acquired by confocal microscopy.
Immunocytochemistry
MIN6 cells infected with either control or small hairpin RNA (shRNA) lentiviral vectors were seeded onto glass coverslips, fixed with 4% paraformaldehyde, and incubated in primary antibodies (rabbit anti-VNUT antibody [1:1,000]; guinea pig anti-insulin antibody lsqb]1:5,000]) at 4°C for 48 hours. The cells were then incubated in fluorophore-conjugated secondary antibodies for 1 hour at room temperature. For quinacrine and insulin double-labeling, cells were stained with quinacrine (5 μM) for 20 minutes in the dark. DRAQ5 (1:1,000, Cell Signaling Technology, Inc, Danvers, Massachusetts) was used for nuclear staining. Coverslips were mounted onto slides with Aqua Poly/Mount (Polysciences, Inc, Warrington, Pennsylvania) and images were acquired by confocal microscopy. VNUT and insulin expression were quantified by densitometry using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Production of VNUT antibodies
Polyclonal antibodies were raised in rabbits against synthetic peptides of mouse VNUT containing residues IPEETRKTPSAAAEDTRWSRPC or CGLPVTKPSKVPWRQLFRKASVWA (LifeTein, South Plainfield, New Jersey) conjugated to keyhole limpet hemocyanin (Cocalico Biologicals, Reamstown, Pennsylvania). Serum samples were collected at appropriate intervals to test for specificity.
Lentiviral vector production
A sequence to knockdown mouse VNUT expression was designed based on previous reports (15, 18). A lentiviral vector expressing shRNA against mouse VNUT was generated as described previously (21). Briefly, a VNUT shRNA expressing cassette driven by a U6 promoter was constructed and cloned into a lentiviral packaging vector (p156RRLsinPPtCMV-GFP-PREU3Nhe, kindly provided by Dr. Inder Verma, The Salk Institute, San Diego, California). The viral vector expresses green fluorescent protein under the control of a cytomegalovirus promoter, allowing visual identification of cells infected by the viral vector. The shRNA VNUT vector and lentiviral packaging plasmids were cotransfected into HEK239FT cells. After transfection, the supernatant containing viral particles was harvested, clarified, and concentrated by centrifugation. In addition to the shRNA VNUT lentiviral vectors, a control vector expressing a noncoding scrambled sequence was generated using the same method. To produce an overexpressing VNUT (o/e VNUT) lentiviral vector, mouse VNUT cDNA was cloned into a lentiviral packaging vector (pCSC-SP-PW, kindly provided by Dr. Inder Verma) and generated as described above.
Static culture ATP assay
MIN6 cells (80,000-120,000 cells per well) were plated in a 48-well plate. The cells were washed twice with Krebs-Ringer bicarbonate buffer (KRB) [129 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO6, 10 mM HEPES, 5 mM NaHCO3, 2.8 mM glucose] and incubated for 10 minutes at 37°C in KRB containing 100 μM ARL67156 (Sigma, St. Louis, Missouri), a nucleotidase inhibitor, for an additional 10 minutes. The pretreatment was followed immediately by stimulation with testing agents for 20 minutes. After the incubation, KRB was collected and ATP content was measured in the KRB with an ATP Bioluminescence Assay Kit (Roche Diagnostics, Branchburg, New Jersey) using an Optocomp II Luminometer (MGM Instruments, Inc, Hamden, Connecticut). Cellular protein content was determined using a Quick Start Bradford Protein Assay (Bio-Rad) following manufacturer's protocol to normalize results. ATP assays were performed in triplicate and repeated at least three times.
Quinacrine staining
Quinacrine staining was performed as described previously (18). Briefly, MIN6 cells (40,000 per well) were cultured on a glass-bottom, 8-well, imaging cover glass. Immediately before imaging, the cells were incubated for 10 minutes with 4 μM FM 4-64 (Molecular Probes, Eugene, Oregon) to stain the plasma membrane and 5 μM quinacrine (Sigma) to label vesicles rich in ATP. Imaging was performed using spinning disk confocal microscopy. ATP-containing vesicles were quantified using densitometry by measuring the area of quinacrine fluorescence in single slices from the z-stack of each image with ImageJ. Quinacrine staining was performed in quadruplicate for each condition.
Insulin secretion
Ten islets were incubated in 250 μL of KRB per well in a 48-well plate, or 80,000 MIN6 cells were seeded per well in a 48-well plate. Both the cells and islets were equilibrated for 15 minutes at 37°C in KRB. The preincubation was immediately followed by a 30-minute pretreatment with testing agents, then stimulated with high glucose (16.8 mM) for 60 min. Insulin concentrations in KRB were determined with an insulin ELISA kit (Linco Research, Inc). Glucose-stimulated insulin secretion (GSIS) assays were performed in triplicate, and each experiment was repeated two to three times.
Insulin content
Lentiviral vector-infected MIN6 cells were seeded in 24-well plates in triplicate. After 48 hours, the cells were washed twice with ice-cold PBS at 4°C and extracted with acidified ethanol (0.15 M HCl in 75% ethanol in H2O) for 16 hours at 4°C. Supernatants were collected, and insulin content was determined by ELISA. The results were normalized to the total cell number. Insulin ELISAs were averaged in duplicate.
Statistics
Results are expressed as the mean ± SEM. Statistical analyses were performed using repeated measures, two-way ANOVA with post hoc tests, or Student t tests when appropriate using Prism 5 software (GraphPad Software Inc, La Jolla, California).
Results
VNUT is expressed in pancreatic β cells
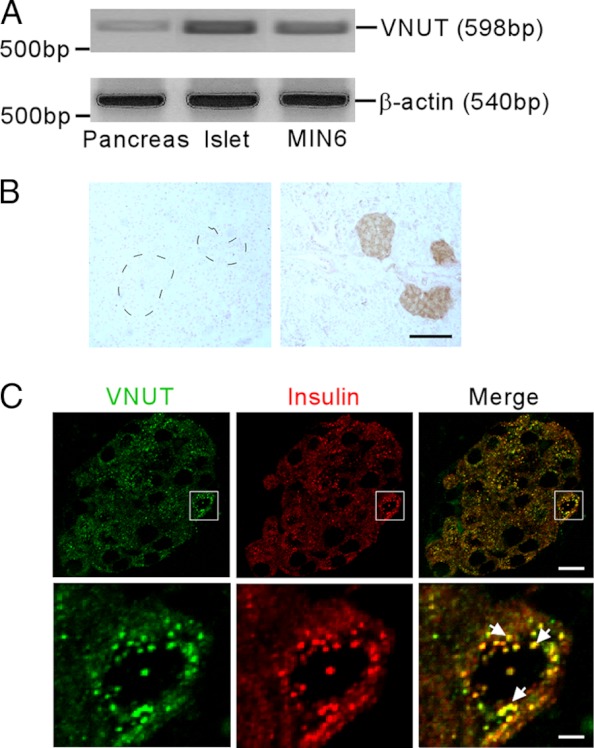
VNUT expression was first examined by RT-PCR. VNUT transcripts were expressed in the mouse pancreas as well as in isolated mouse islets and MIN6 cells, a mouse clonal β cell line (Figure 1A). Immunohistochemical analysis confirmed that specific VNUT immunoreactivity is localized predominantly to mouse pancreatic islets (Figure 1B). Furthermore, double-label immunofluorescence indicated VNUT is highly colocalized with insulin, confirming β cell specificity. Under high magnification, we revealed extensive colocalization of VNUT and insulin in insulin secretory granules (Figure 1C).
Figure 1.
Expression of VNUT in the pancreas. A, Representative agarose gel electrophoresis showing the expression of VNUT and β-actin mRNA in total RNA samples isolated from mouse pancreas, isolated mouse islets, and MIN6 cells. B, Representative photomicrographs showing VNUT immunostaining in mouse pancreatic sections with (left) or without (right) preabsorption of the anti-VNUT serum with 30 μM mouse VNUT peptide. VNUT-positive cells (dark brown) were observed predominantly in the islets. Mouse islets in the left panel are indicated by a dashed line. Scale bar, 100 μm. C, Upper panels: Stacked serial confocal images of VNUT (upper left, green) and insulin (upper middle, red) staining in the islet. Upper right: Visualization of both VNUT and insulin simultaneously showing most VNUT and insulin colocalize in the same cells. Bottom panels: High magnification of the confocal images in the boxed area showing VNUT (lower left, green) and insulin (lower middle, red) in a single β cell. Bottom right: Merged image visualizing VNUT and insulin simultaneously. Representative VNUT/insulin double-labeled granules are indicated by white arrows. Scale bar: Top panel, 10 μm; Bottom panel, 2.5 μm.
Endogenous VNUT mediates ATP secretion in β cells
The prominent expression of VNUT on insulin granules is consistent with its function in secretory vesicles. Before probing the function of VNUT in β cells, we first confirmed glucose stimulates ATP release from ΜΙΝ6 cells in a dose-dependent manner (Figure 2A). We also used quinacrine as a marker to visualize vesicular ATP content and release. Similar to an earlier report on biliary epithelial cells (18, 22), we identified pronounced quinacrine-positive vesicles in MIN6 cells (Figure 2B), which colocalized extensively with insulin-secretory granules. Please see Supplemental Figure 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Consistent with our ATP release results, high glucose treatment also diminished quinacrine fluorescence (Figure 2, B and C), suggestive of glucose-induced ATP secretion. A lentiviral vector expressing VNUT-specific shRNA was used to knock down endogenous VNUT expression in MIN6 cells (Figure 2D, Supplemental Figure 2). Reducing VNUT expression not only significantly diminished basal quinacrine staining (Figure 2, E and F), but also greatly suppressed glucose-induced ATP release (Figure 2G). On the other hand, knocking down VNUT in MIN6 cells has no significant impact on the number of insulin granules and total insulin content (Supplemental Figure 3).
Figure 2.
Endogenous VNUT is critical for ATP release from pancreatic β cells. A, Measurement of ATP release from MIN6 cells stimulated with various concentrations of glucose (G) or KCl (50 mM). B, Representative confocal images showing MIN6 cells stained with quinacrine and FM4–64 under basal conditions (2.8 mM) and after a 10-minute challenge with high glucose (25 mM). C, Summary of quinacrine staining in MIN6 cells treated with 2.8 mM or 25 mM glucose. D, VNUT mRNA expression measured by real-time PCR from MIN6 cells infected with either control or shRNA VNUT lentiviral vectors for 72 hours. E, MIN6 cells were infected with control or shRNA VNUT lentiviral vectors 72 hours before being stained with quinacrine. Representative monolayers were imaged using confocal microscopy under basal conditions (2.8 mM). F, Summary of quinacrine staining in MIN6 cells infected with control or shRNA VNUT lentiviral vectors. G, ATP release from control or VNUT shRNA-infected MIN6 cells under basal conditions (2.8 mM glucose) or after 20 minutes of glucose stimulation (25 mM glucose). Scale bar: 20 μm. * P < .05, ** P < .01.
VNUT-mediated ATP release is essential for insulin secretion
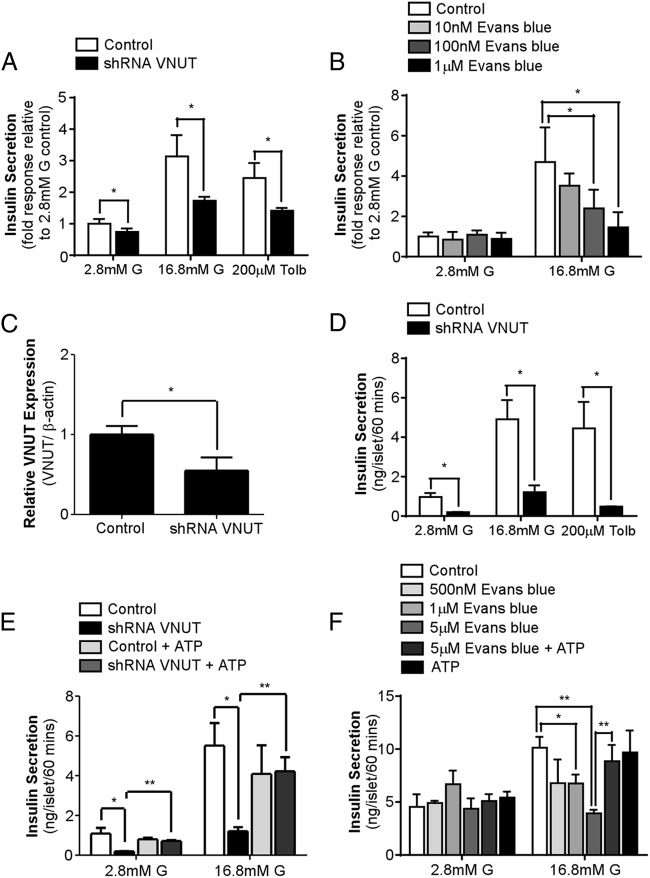
To determine the physiological role of VNUT in β cells, we assessed insulin secretion in MIN6 cells infected with VNUT shRNA lentiviral vectors or treated with Evans blue, a VNUT inhibitor (15). Insulin secretion in VNUT shRNA-infected MIN6 cells was significantly reduced under basal conditions and during high glucose or tolbutamide stimulation (Figure 3A). Likewise, Evans blue treatment also attenuated GSIS in a dose-dependent manner (Figure 3B).
Figure 3.
VNUT-mediated ATP release is essential for insulin secretion. A, Insulin secretion from MIN6 cells infected with either control or VNUT shRNA lentiviral vectors under basal conditions (2.8 mM G), stimulated with glucose (16.8 mM G) or tolbutamide (200 μM) for 60 minutes. B, Insulin secretion from MIN6 cells pretreated with various concentrations of Evans blue for 30 minutes before stimulation with high glucose (16.8 mM G) for 60 minutes. C, VNUT mRNA expression measured by real-time PCR from isolated mouse islets infected with either control or shRNA VNUT lentiviral vector for 72 hours. D, Insulin secretion from isolated mouse islets infected with either control or VNUT shRNA lentiviral vectors under basal conditions (2.8 mM G), stimulated with glucose (16.8 mM G) or tolbutamide (200 μM) for 60 minutes. E, Insulin secretion from control and VNUT shRNA-infected islets stimulated with glucose (16.8 mM) in the presence or absence of exogenous ATP (200 μM) for 60 minutes. F, Insulin secretion from islets pretreated with various concentrations of Evans blue for 30 minutes before stimulation with glucose (16.8 mM) with or without exogenous ATP (200 μM) for 60 minutes. * P < .05, ** P < .01.
To further confirm the physiologic importance of VNUT in insulin secretion, the effect of blocking VNUT function on insulin secretion was determined in isolated mouse islets. Isolated islets were infected with either control or shRNA VNUT lentiviral vectors to knock down VNUT expression (Figure 3C). Similar to the MIN6 cell study, knocking down VNUT expression in islets significantly decreased basal insulin release, as well as GSIS and tolbutamide-stimulated insulin secretion (Figure 3D). Importantly, exogenous ATP treatment effectively restored basal and glucose-induced insulin secretion in VNUT shRNA-infected islets (Figure 3E). Similar to shRNA knockdown, pretreating islets with Evans blue dose-dependently attenuated GSIS (Figure 3F), which was also recovered by the addition of ATP. Taken together, our data strongly suggest that endogenous VNUT-mediated ATP release is critical in regulating insulin secretion.
Overexpressing VNUT in β cells increases vesicular ATP uptake and release
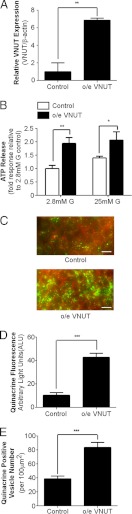
A gain-of-function approach was used to further evaluate the functional role of VNUT in β cells. Enhancing VNUT expression with an o/e VNUT lentiviral vector in MIN6 cells (Figure 4A) significantly elevated ATP release under both basal- and glucose-stimulated conditions (Figure 4B). Consistent with this observation, o/e VNUT-infected MIN6 cells also exhibited a significant increase in vesicular quinacrine staining under basal conditions, indicating increased ATP accumulation into vesicles (Figure 4, C and D). Interestingly, more quinacrine-positive vesicles were observed in the o/e VNUT ΜΙΝ6 cells than in the control cells (Figure 4E). On the other hand, no differences were found in total insulin content or total number of insulin vesicles between control MIN6 cells and cells infected with o/e VNUT lentiviral vector (Supplemental Figure 4). This indicates that modulating VNUT expression alters vesicular ATP uptake without having a significant impact on vesicular insulin content.
Figure 4.
Overexpression of VNUT enhances ATP release from β cells. A, VNUT mRNA expression measured by real-time PCR from MIN6 cells infected with either control or o/e VNUT lentiviral vectors for 72 hours. B, ATP release was measured from control and o/e VNUT MIN6 cells under basal conditions (2.8 mM glucose) or after 20 minutes of glucose stimulation (25 mM glucose). C, Representative confocal images showing quinacrine (green) and FM4–64 (red) staining in MIN6 cells infected with either control (upper panel) or o/e VNUT lentiviral vectors (lower panel) in basal media (2.8 mM glucose). D, Summary of quinacrine staining in control and o/e VNUT MIN6 cells. E, Quantification of the average number of quinacrine positive vesicles per 100 μm2. Scale bar: 20 μm. * P < .05, ** P < .01, *** P < .001.
VNUT overexpression enhances insulin secretion by activating P2X purinergic receptors
To determine the effect of VNUT overexpression on insulin secretion, isolated mouse islets were infected with o/e VNUT lentiviral vector, resulting in a 3-fold increase in VNUT expression (Figure 5A). Islets overexpressing VNUT showed significantly elevated basal- and glucose- or tolbutamide-induced insulin secretion as compared with control islets (Figure 5B). Pretreating o/e VNUT islets with iso-pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid, a selective P2X receptor antagonist, effectively suppressed basal insulin secretion and GSIS in o/e VNUT islets (Figure 5C). It is important to note that antagonizing P2X receptors also significantly attenuated GSIS in control islets. On the other hand, pretreating islets with MRS 2179, a P2Y-specific antagonist, failed to modify insulin secretion in control or o/e VNUT islets. Taken together, these results indicate P2X receptors mediate the effect of vesicular ATP release on insulin secretion.
Figure 5.

Overexpressing VNUT potentiates insulin secretion. A, VNUT mRNA expression measured by real-time PCR from isolated mouse islets infected with either control or o/e VNUT lentiviral vectors for 72 hours. B, Insulin secretion from isolated mouse islets infected with either control or o/e VNUT lentiviral vectors under basal conditions (2.8 mM G) or glucose stimulation (16.8 mM G) for 60 minutes. C and D, Insulin secretion from control and o/e VNUT-infected islets under basal conditions or stimulated with glucose (16.8 mM) for 60 minutes in the presence or absence of iso-pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (iso-PPADS; 50 μM) (C) or MRS 2179 (0.5 μM) (D). * P < .05, *** P < .001.
Discussion
It is well documented that ATP is accumulated and stored within insulin secretory vesicles in pancreatic β cells (12, 13), yet the mechanism of ATP transport into insulin granules has remained unknown. Recently, Sawada et al. (15) identified VNUT as the transporter responsible for accumulating ATP into secretory vesicles in adrenal chromaffin cells. In the current study, we show that VNUT is expressed in pancreatic β cells and is colocalized with insulin secretory vesicles. Our VNUT knockdown studies demonstrate endogenous VNUT is required for vesicular ATP uptake and release, as well as insulin secretion in β cells under basal conditions and during glucose stimulation. The addition of exogenous ATP completely restores insulin secretion under both of these circumstances when VNUT expression is knocked down, reinforcing the notion that vesicular ATP release is critical for regulating insulin secretion. It is worth noting that exogenous ATP treatment restored both basal and glucose-induced insulin secretion in VNUT knockdown islets comparable with that of control islets. This indicates that exogenous ATP-induced insulin secretion in VNUT knockdown islets is also glucose dependent, indicating that VNUT-mediated extracellular ATP release is crucial for appropriate insulin secretion. In addition to the involvement of VNUT in GSIS, the current study shows that VNUT participates in the insulin release induced by tolbutamide, a sulfonylurea agonist (23). Tolbutamide stimulates insulin secretion by closing KATP channels, which raises the membrane potential, eliciting an influx of Ca2+ through VDCCs and stimulating the exocytosis of insulin secretory granules. We reveal that β cell depolarization by tolbutamide-induced KATP channel closure is insufficient to induce insulin secretion in VNUT shRNA lentiviral vector-infected MIN6 cells or mouse islets, indicating that vesicular ATP release is necessary to attain the full effect of tolbutamide as an insulin secretagogue.
In addition to knocking down VNUT expression with shRNA, we also used Evans blue, a pharmacologic inhibitor of VNUT (15), to assess the function of VNUT in insulin secretion in vitro. Similar to VNUT shRNA knockdown, Evans blue treatment significantly inhibited GSIS in MIN6 cells and isolated islets in a dose-dependent manner, thus reinforcing the notion that endogenous VNUT plays a critical role in regulating GSIS. Exogenous ATP treatment effectively recovered GSIS inhibited by Evans blue, indicating that the inhibitory effect of Evans blue on GSIS is likely due to suppression of ATP release from β cells. Evans blue has been shown to antagonize the function of a number of molecules, including epithelial sodium channels, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and kainate receptors (24, 25). Nonetheless, the fact that exogenous ATP reverses the inhibitory effect of Evans blue on GSIS strongly argues that in our experimental conditions Evans blue primarily targets VNUT to impair GSIS. Taken together, both the VNUT knockdown and Evans blue results support the hypothesis that VNUT-mediated ATP release is crucial for glucose-induced insulin secretion.
In the current study, we observed an interesting discrepancy in basal insulin secretion between the two different approaches we used to block VNUT function. Knocking down VNUT expression by shRNA infection in MIN6 cells and islets greatly suppressed basal insulin release and GSIS. On the other hand, pharmacologic blockade of VNUT function with Evans blue similarly suppressed GSIS but had no effect on basal insulin secretion in either MIN6 cells or islets. The source of the apparent discrepancy is unclear. With VNUT shRNA knockdown, MIN6 cells or islets were infected with lentiviral vectors for 72 hours before the glucose challenge. Our results show VNUT shRNA lentiviral infection led to reduced vesicular ATP uptake (Figure 2, E and F) and consequently basal ATP secretion (Figure 2G). Conversely, in the pharmacologic approach, MIN6 cells and islets were treated with Evans blue acutely for 30 minutes before glucose stimulation. Thus, it is reasonable to assume that before the glucose challenge, vesicular ATP levels in MIN6 cells and islets infected with VNUT shRNA are greatly reduced relative to those acutely treated with Evans blue. This difference in basal vesicular ATP levels may account, at least in part, for the apparent disparity in basal insulin release between the two different approaches. It is also possible that chronically reducing VNUT expression may lead to alterations in extracellular purinergic tone because of reduced basal ATP release, which may also modulate basal insulin secretion. More studies are needed to further resolve this issue.
It has been shown that ATP is coreleased with insulin from pancreatic β cells during GSIS (12–14). Our results are consistent with the notion that vesicular ATP release participates in a feed-forward mechanism to further facilitate insulin secretion in an autocrine or paracrine manner (7, 8, 26). In other words, the ATP released from insulin secretory vesicles likely plays an important role in the second phase of GSIS. Interestingly, a number of studies have demonstrated that insulin vesicles in β cell can undergo kiss-and-run exocytosis (27, 28). During this process the vesicle lumen temporarily connects to the extracellular space, allowing for extrusion of small molecules, such as ATP, through the transient fusion pore (27–30). However, insulin molecules are retained in the vesicles during kiss-and-run exocytosis because of size limitations (27, 31). The vesicular ATP released before insulin secretion may function as a precursor to “prime” the β cell for glucose-induced insulin secretion (27). Therefore, it is conceivable that the ATP released from vesicles is critical in both the first and second phases of GSIS. Indeed, this notion is supported by the complete suppression of GSIS in VNUT knockdown cells.
In the current study, quinacrine was used to visualize ATP-containing vesicles. Quinacrine is an acridine derivative with a high affinity for intracellular ATP that fluoresces when accumulated at high concentrations (32–34). Consistent with the findings of a previous report, we show reduced VNUT expression by shRNA knockdown resulted in significantly decreased quinacrine staining (18) and VNUT overexpression greatly enhanced quinacrine fluorescence. Moreover, high glucose stimulation significantly lowered quinacrine intensity in MIN6 cells, indicative of ATP secretion; this is congruent with our ATP release results. In addition to serving as an ATP indicator, quinacrine has been shown to stain cellular compartments with an acidic pH (35). It has been demonstrated previously that the expression of VNUT on vesicles has no significant effect on intravesicular pH (15). Moreover, pH within insulin secretory vesicles is primarily regulated by a vacuolar type H+-ATPase expressed on the vesicle (36). Although it cannot be ruled out that the quinacrine staining observed in the current study may reflect pH levels in insulin secretory vesicles, our results strongly argue that quinacrine staining is indicative of changes in vesicular ATP content in light of its agreement with ATP release.
A gain-of-function approach was also used to determine the role of VNUT in β cells. Consistent with its function in vesicular ATP accumulation, we determined VNUT overexpression greatly enhanced both ATP accumulation into vesicles and its consequent secretion. We also found o/e VNUT MIN6 cells have significantly more ATP-positive vesicles as revealed by quinacrine staining when compared with control cells. Importantly, modulating VNUT expression in MIN6 cells has no significant effect on insulin content or number of insulin secretory vesicles (Supplemental Figures 3 and 4). It is unclear how overexpression of VNUT results in more ATP-containing vesicles. This may simply be due to increased vesicular ATP uptake resulting in enhanced detection of quinacrine staining. However, we cannot rule out the possibility that VNUT overexpression promotes increased formation of ATP-containing vesicles. Nonetheless, enhanced ATP accumulation or an increase in vesicle number augments the probability of kiss-and-run events (27), which likely explains why overexpressing VNUT leads to increased ATP release in β cells.
Along with elevated ATP secretion resulting from the overexpression of VNUT, we also show that chronically overexpressing VNUT for 72 hours results in enhanced basal and GSIS from isolated islets. These results suggest that the augmented ATP released from o/e VNUT islets facilitates insulin secretion. Moreover, our pharmacologic study indicates the effect of o/e VNUT on insulin secretion is mediated primarily by P2X receptors, but not by P2Y receptors. On the other hand, it has been shown that acute exogenous ATP treatment stimulates insulin secretion in human and monkey islets, but not in murine or pig islets (8, 37–39). Consistent with these earlier reports, we show acute exogenous ATP treatment in control mouse islets has no effect on insulin secretion (Figure 3, E and F). Currently the discrepancy in insulin secretion between acute ATP treatment and chronic extracellular ATP stimulation due to VNUT overexpression is not clear. It has been shown that chronic treatment of ATP analogs significantly increases the expression of P2X receptors in INS-1 cells, a rat clonal β cell line (40). Thus, it is conceivable that elevated ATP secretion in β cells due to chronic VNUT overexpression leads to changes in purinergic receptor expression and activity in the islets to further facilitate insulin secretion. Similar to glucose stimulation, o/e VNUT also potentiates tolbutamide-induced insulin secretion. As mentioned, the effect of o/e VNUT on insulin secretion is mainly mediated by P2X receptors, which are ligand-gated cation channels (32). It has been shown in human β cells that activation of P2X receptors by ATP induces Ca2+ influx (8). Thus, it is reasonable to assume that along with tolbutamide-induced Ca2+ entry through VDCCs, enhanced extracellular ATP stimulation results in additional Ca2+ influx through the P2X receptor to potentiate tolbutamide-stimulated insulin secretion.
In conclusion, we show VNUT is expressed in pancreatic β cells and that VNUT-mediated ATP release is an integral part of the mechanism that governs glucose-induced insulin secretion in β cells. Moreover, we showed that P2X receptors are critical in mediating the effect of ATP on insulin secretion when VNUT is overexpressed. This study provides new insight into the overall mechanism of insulin release, particularly regarding the role of vesicular ATP and purinergic signaling, which exposes yet another level of regulation in the complex mechanism of insulin secretion.
Acknowledgments
We thank Connor Wang for providing technical assistance in RNA extraction and real-time PCR analysis, Charles Kresge for assistance in cell culture maintenance, and Drs. Ed Perez-Reyes and Iulia Vitko for assistance in quinacrine imaging. We also thank Drs. Peilin Chen, Michael Thorner, Thurl Harris, Norbert Leitinger, Eugene Barrett, and Zhen Yan for providing comments on the manuscript.
This work was supported by the National Institute of Diabetes, Digestive and Kidney Diseases Grants R01 DK078049 (to C.L.), DK078587 (to A.P.F.), and R01 DK089182 (to C.S.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- KATP
- ATP-sensitive potassium channels
- KRB
- Krebs-Ringer bicarbonate buffer
- o/e VNUT
- overexpressing VNUT
- shRNA
- small hairpin RNA
- VNUT
- vesicular nucleotide transporter
- VDCC
- voltage-dependent Ca2+ channel.
References
- 1. Thorens B. Molecular and cellular physiology of GLUT-2, a high-Km facilitated diffusion glucose transporter. Int Review Cytol. 1992;137:209-238 [DOI] [PubMed] [Google Scholar]
- 2. German MS. Glucose sensing in pancreatic islet beta cells: the key role of glucokinase and the glycolytic intermediates. Proc Natl Acad Sci U S A. 1993;90:1781-1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2:163-214 [DOI] [PubMed] [Google Scholar]
- 4. Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271-273 [DOI] [PubMed] [Google Scholar]
- 5. Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev. 1981;61:914-973 [DOI] [PubMed] [Google Scholar]
- 6. Petit P, Lajoix AD, Gross R. P2 purinergic signalling in the pancreatic beta-cell: control of insulin secretion and pharmacology. Eur J Pharm Sci. 2009;37:67-75 [DOI] [PubMed] [Google Scholar]
- 7. Richards-Williams C, Contreras JL, Berecek KH, Schwiebert EM. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic Signal. 2008;4:393-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacques-Silva MC, Correa-Medina M, Cabrera O, et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A. 2010;107:6465-6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertrand G, Chapal J, Loubatieres-Mariani MM, Roye M. Evidence for two different P2-purinoceptors on beta cell and pancreatic vascular bed. Br J Pharmacol. 1987;91:783-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coutinho-Silva R, Parsons M, Robson T, Lincoln J, Burnstock G. P2X and P2Y purinoceptor expression in pancreas from streptozotocin-diabetic rats. Mol Cell Endocrinol. 2003;204:141-154 [DOI] [PubMed] [Google Scholar]
- 12. Leitner JW, Sussman KE, Vatter AE, Schneider FH. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975;96:662-677 [DOI] [PubMed] [Google Scholar]
- 13. Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671-4676 [DOI] [PubMed] [Google Scholar]
- 14. Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437:31-35 [DOI] [PubMed] [Google Scholar]
- 15. Sawada K, Echigo N, Juge N, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683-5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sreedharan S, Shaik JH, Olszewski PK, Levine AS, Schioth HB, Fredriksson R. Glutamate, aspartate and nucleotide transporters in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics. 2010;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larsson M, Sawada K, Morland C, et al. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2011;22:1203-1214 [DOI] [PubMed] [Google Scholar]
- 18. Sathe MN, Woo K, Kresge C, et al. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. J Biol Chem. 2011;286:25363-25376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406-17416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online. 2009;11:3-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao H, Digruccio M, Chen P, Li C. Type 2 corticotropin-releasing factor receptor in the ventromedial nucleus of hypothalamus is critical in regulating feeding and lipid metabolism in white adipose tissue. Endocrinology. 2012;153:166-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feranchak AP, Lewis MA, Kresge C, et al. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138-8147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51 Suppl 3:S368–376 [DOI] [PubMed] [Google Scholar]
- 24. Yamamura H, Ugawa S, Ueda T, Shimada S. Evans blue is a specific antagonist of the human epithelial Na+ channel delta-subunit. J Pharmacol Exp Ther. 2005;315:965-969 [DOI] [PubMed] [Google Scholar]
- 25. Price CJ, Raymond LA. Evans blue antagonizes both alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and kainate receptors and modulates receptor desensitization. Mol Pharmacol. 1996;50:1665-1671 [PubMed] [Google Scholar]
- 26. Petit P, Bertrand G, Schmeer W, Henquin JC. Effects of extracellular adenine nucleotides on the electrical, ionic and secretory events in mouse pancreatic beta-cells. Br J Pharmacol. 1989;98:875-882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacDonald PE, Braun M, Galvanovskis J, Rorsman P. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic beta cells. Cell Metab. 2006;4:283-290 [DOI] [PubMed] [Google Scholar]
- 28. MacDonald PE, Obermuller S, Vikman J, Galvanovskis J, Rorsman P, Eliasson L. Regulated exocytosis and kiss-and-run of synaptic-like microvesicles in INS-1 and primary rat beta-cells. Diabetes. 2005;54:736-743 [DOI] [PubMed] [Google Scholar]
- 29. Rutter GA, Tsuboi T. Kiss and run exocytosis of dense core secretory vesicles. Neuroreport. 2004;15:79-81 [DOI] [PubMed] [Google Scholar]
- 30. Fernandez-Peruchena C, Navas S, Montes MA, Alvarez de Toledo G. Fusion pore regulation of transmitter release. Brain Res Brain Res Rev. 2005;49:406-415 [DOI] [PubMed] [Google Scholar]
- 31. Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271-4282 [DOI] [PubMed] [Google Scholar]
- 32. Alund M, Olson L. Quinacrine affinity of endocrine cell systems containing dense core vesicles as visualized by fluorescence microscopy. Cell Tissue Res. 1979;204:171-186 [DOI] [PubMed] [Google Scholar]
- 33. Belai A, Burnstock G. Pattern of distribution and co-localization of NOS and ATP in the myenteric plexus of human fetal stomach and intestine. Neuroreport. 2000;11:5-8 [DOI] [PubMed] [Google Scholar]
- 34. Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900-908 [DOI] [PubMed] [Google Scholar]
- 35. Roberts CJ, Raymond CK, Yamashiro CT, Stevens TH. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644-661 [DOI] [PubMed] [Google Scholar]
- 36. Ohta T, Yamamoto M, Numata M, et al. Differential expression of vacuolar-type H+-ATPase between normal human pancreatic islet B-cells and insulinoma cells. Int J Oncol. 1997;11:597-601 [DOI] [PubMed] [Google Scholar]
- 37. Petit P, Hillaire-Buys D, Manteghetti M, Debrus S, Chapal J, Loubatieres-Mariani MM. Evidence for two different types of P2 receptors stimulating insulin secretion from pancreatic B cell. Br J Pharmacol. 1998;125:1368-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leon C, Freund M, Latchoumanin O, et al. The P2Y(1) receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signal. 2005;1:145-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poulsen CR, Bokvist K, Olsen HL, et al. Multiple sites of purinergic control of insulin secretion in mouse pancreatic beta-cells. Diabetes. 1999;48:2171-2181 [DOI] [PubMed] [Google Scholar]
- 40. Santini E, Cuccato S, Madec S, Chimenti D, Ferrannini E, Solini A. Extracellular adenosine 5′-triphosphate modulates insulin secretion via functionally active purinergic receptors of X and Y subtype. Endocrinology. 2009;150:2596-2602 [DOI] [PubMed] [Google Scholar]