Abstract
Combined pituitary hormone deficiency (CPHD) diseases result in severe outcomes for patients including short stature, developmental delays, and reproductive deficiencies. Little is known about their etiology, especially the developmental profiles and the influences of genetic background on disease progression. Animal models for CPHD provide valuable tools to investigate disease mechanisms and inform diagnostic and treatment protocols. Here we examined hormone production during pituitary development and the influence of genetic background on phenotypic severity in the Lhx3W227ter/W227ter mouse model. Lhx3W227ter/W227ter embryos have deficiencies of ACTH, α-glycoprotein subunit, GH, PRL, TSHβ, and LHβ during prenatal development. Furthermore, mutant mice have significant reduction in the critical pituitary transcriptional activator-1 (PIT1). Through breeding, the Lhx3W227ter/W227ter genotype was placed onto the 129/Sv and C57BL/6 backgrounds. Intriguingly, the genetic background significantly affected viability: whereas Lhx3W227ter/W227ter animals were found in the expected frequencies in C57BL/6, homozygous animals were not viable in the 129/Sv genetic environment. The hormone marker and PIT1 reductions observed in Lhx3W227ter/W227ter mice on a mixed background were also seen in the separate strains but in some cases were more severe in 129/Sv. To further characterize the molecular changes in diseased mice, we conducted a quantitative proteomic analysis of pituitary proteins. This showed significantly lower levels of PRL, pro-opiomelanocortin (ACTH), and α-glycoprotein subunit proteins in Lhx3W227ter/W227ter mice. Together, these data show that hormone deficiency disease is apparent in early prenatal stages in this CPHD model system. Furthermore, as is noted in human disease, genetic background significantly impacts the phenotypic outcome of these monogenic endocrine diseases.
The pituitary gland is critically important for growth, reproduction, and the maintenance of physiological homeostasis in mammals. The anterior pituitary (AP) secretes polypeptide hormones from five cell types: corticotropes (releasing ACTH), gonadotropes (FSH and LH), thyrotropes (TSH), somatotropes (GH), and lactotropes (prolactin [PRL]). Hormones produced by the AP regulate growth and metabolism (GH and TSH), the stress response (ACTH), reproduction (FSH, LH, and PRL), the thyroid (TSH), and lactation (PRL). During development, after inductive events between the pituitary primordium (Rathke's pouch) and the diencephalon, the actions of transcription factors such as HESX1, PITX1/2, LIM (lin-1, Isl-1, and mec-3)-homeodomain 3 (LHX3), LHX4, PIT1, and PROP1 are key to establishment of the hormone-secreting cell types of the AP (1–4).
The LHX3 transcription factor is expressed during the early stages of pituitary and nervous system development and plays essential roles in establishment of the differentiated cells of the AP and specialized neurons in the brain and spinal cord (5–10). Mice lacking function of the entire Lhx3 gene exhibit structural arrest of pituitary gland development, failed differentiation of the lactotrope, gonadotrope, somatotrope, and thyrotrope AP lineages, and die shortly after birth presumably due to nervous system deficits (5–7). Pituitary target genes of LHX3 include those encoding the β-subunits of FSH and TSH (FSHβ and TSHβ), α-glycoprotein subunit (αGSU, the common subunit of the FSH, LH, and TSH glycoprotein hormones), PRL, GnRH receptor, and the later-stage PIT1 transcription factor (eg, 9, 11–14).
Mutations in the human LHX3 gene are associated with severe combined pituitary hormone deficiency (CPHD) diseases. Affected patients have insufficiencies in GH, TSH, LH, FSH, and PRL hormones and also frequently have associated syndromic symptoms such as ACTH deficiency, mental retardation, and sensorineural hearing loss (15–23). In addition to CPHD, most characterized patients with LHX3 mutations also display a rigid cervical spine resulting in limited head rotation, a symptom that is considered to result from loss of LHX3 function during early spinal cord neuron development. Described LHX3 gene mutations in humans, mice, and dogs act in recessive fashion (eg, 6, 18, 19, 24, 25); however, a recent report indicates that symptoms in the heterozygous condition are possible (26).
One form of LHX3 gene mutation (W224ter) introduces a stop codon resulting in a predicted truncated form of the LHX3 protein lacking the transcriptional activation domains required for pituitary gene activation (19, 27). Patients with the W224ter mutation have symptoms associated with CPHD, but they do not have the cervical spine rigidity, suggesting that the nervous system functions of LHX3 are preserved (19). To test the hypothesis that the pituitary and nervous system functions of LHX3 are separable, a knock-in mouse strain was created carrying an equivalent mutation (Lhx3W227ter) (24). The resulting Lhx3W227ter/W227ter homozygous mice are viable and provide a useful animal model for CPHD, having deficiencies of ACTH, GH, PRL, TSH, LH, and FSH (24). The mice display dwarfism, lethargy (due to thyroid insufficiency), and reproductive problems but no nervous system deficits (24).
Pediatric CPHD diseases associated with genomic mutations in transcription factor genes such as LHX3 are developmental in nature. However, because cases are typically diagnosed postnatally and due to the inaccessible location of the pituitary gland, little is known about the nature and time course of disease onset in human patients, especially at the molecular and cellular levels. Furthermore, a range of disease symptoms and severity of hormone deficiencies has been observed in patients with LHX3 mutations (15–23). This phenotypic disease variation may be due in part to differences in genetic background and the actions of modifier genes. The viable Lhx3W227ter/W227ter mouse model has hormone deficiencies that model the human disease, providing a valuable reagent to analyze the molecular nature and progression of the human disease condition. We therefore examined the expression of pituitary hormones during development and the influence of genetic background in Lhx3W227ter/W227ter mice. Additionally, we conducted a proteomic analysis to identify and quantify proteins that might be affected by the CPHD-causing mutation.
Materials and Methods
Animals and tissue harvesting
Lhx3W227ter/W227ter knock-in mice were housed in standard conditions with access to food and water provided ad libitum and genotyped using PCR as reported (24). Animals were fed 2018SX Teklad Global 18% protein rodent diet. All procedures were approved by the Indiana University Committee on Use and Care of Animals and followed National Institutes of Health Guidelines for the Care and Use of Experimental Animals. Embryos were dissected from females of heterozygous pairs at embryonic day 13.5 (E13.5), E15.5, and E17.5. The day after conception was set as E0.5. Embryos were genotyped by PCR. Tissues were fixed in 4% paraformaldehyde in PBS (137mM NaCl, 5.4mM KCl, 16.2mM Na2HPO4, 2.9mM KH2PO4). After fixation, all tissues were rinsed in PBS and incubated in 20% sucrose in PBS at 4°C overnight. Tissues were rapidly frozen in Peel-A-Way disposable embedding molds (Polysciences, Warrington, PA) using Tissue-Tek O.C.T. Compound (Sakura, Torrance, California), oriented for sagittal sectioning and stored at −80°C until cryosection.
Cryosections and immunohistochemistry
Frozen tissue blocks were sectioned in 7-μm sections using a CM 1950 cryostat (Leica Microsystems, Buffalo Grove, Illinois). Procedures for fluorescent immunohistochemistry (IHC) using pituitary hormone antibodies from the National Hormone and Pituitary Program (NHPP, Torrance, California) were as reported (24) except the recently generated rabbit antirecombinant PRL antibody (NHPP AFP10712402Rb) (1:100) and rat anti-FSHβ (NHPP 85GP9691bFSHb) (1:500) were used for hormone staining. Similar procedures were used with the anti-PIT1 antibody (28) at 1:1000. At least 2 sections per animal type were examined.
Genetic background analyses
Lhx3W227ter/+ (129/Sv + C57BL/6 mixed background) (24) were separately backcrossed into 129/Sv or C57BL/6 strains for 6 generations to obtain a 98.4% pure background in each strain. Lhx3W227ter/+ from generation 6 within each strain were separately intercrossed to obtain wild-type (WT), Lhx3W227ter/+, and Lhx3W227ter/W227ter progeny. Animals from 129/Sv litters (n = 18) and C57BL/6 litters (n = 11) were genotyped and analyzed in comparison with observations on a mixed background (24). E17.5 embryos were dissected from females of heterozygous pairs for IHC as described above.
Quantitative PCR analyses
Tissue harvest and cDNA preparation using triplicate samples were as described (24). Parallel negative control reactions contained no reverse transcriptase enzyme. The relative abundance of the genes of interest was determined using real-time quantitative PCR in triplicate reactions as described (24). Mouse Pit1 (Pou1f1) gene (Mm00476852_m1) primers and probes were from Applied Biosystems (Carlsbad, California). Data were normalized by determining the relative abundance of 36B4 mRNA. The mouse 36B4 (also known as Rplp0) gene encodes an acidic ribosomal phosphoprotein P0. WT data were normalized to 1, and heterozygote and homozygote data were subsequently adjusted.
Proteomic analyses
WT and Lhx3W227ter/W227ter female adults (n = 5 in each group) were rapidly killed and perfused with ice-cold saline (0.9%). The age of mice ranged from 3 to 6 months (2 WT and 3 Lhx3W227ter/W227ter were 3 months, 2 WT and 1 Lhx3W227ter/W227ter were 4 months, and 1 WT and 1 Lhx3W227ter/W227ter were 6 months). Pituitaries were frozen in liquid nitrogen and then solubilized in lysis buffer (8M urea, 10mM dithiothreitol). Protein concentration was determined by the Bradford method (Bio-Rad, Hercules, California). Samples were reduced and alkylated by triethylphosphine and iodoethanol and subjected to trypsin digestion as described (29). Digested samples were analyzed using a Thermo-Finnigan linear ion-trap (linear trap quadrupole) mass spectrometer coupled with a Surveyor autosampler and mass spectrometry HPLC system (Thermo-Finnigan, San Jose, California). Tryptic peptides were analyzed using a C18 RP column as described (29). Data were searched against the International Protein Index mouse database (IPI Mouse version 3.77 FASTA format) using SEQUEST (version 28 revision 12) algorithms in Bioworks (version 3.3). General parameters were set to: peptide tolerance 2.0 atomic mass units, fragment ion tolerance 1.0 amu, enzyme limits set as “fully enzymatic–cleaves at both ends,” and missed cleavage sites set at 2. Searched peptides and proteins were validated by PeptideProphet (30) and ProteinProphet (31) in the Trans-Proteomic Pipeline (TPP version 3.3.0) (http://tools.proteomecenter.org/software.php). Only proteins with P ≥ .90 and peptides with P ≥ .80 were reported. Label-free protein quantification was performed using IdentiQuantXL software as described (32). The retention time of peptide for its intensity extraction was carried out with an experiment-based algorithm, RetentionTimeXL. A Student's t test was performed to determine the significance of differences between the two groups. False discovery rate (33) was estimated using Q-value software. Data were further analyzed using Interactive Pathway Analysis software (Ingenuity Systems, Redwood City, California).
Results
W227ter knock-in mice have decreased expression of AP hormones during development
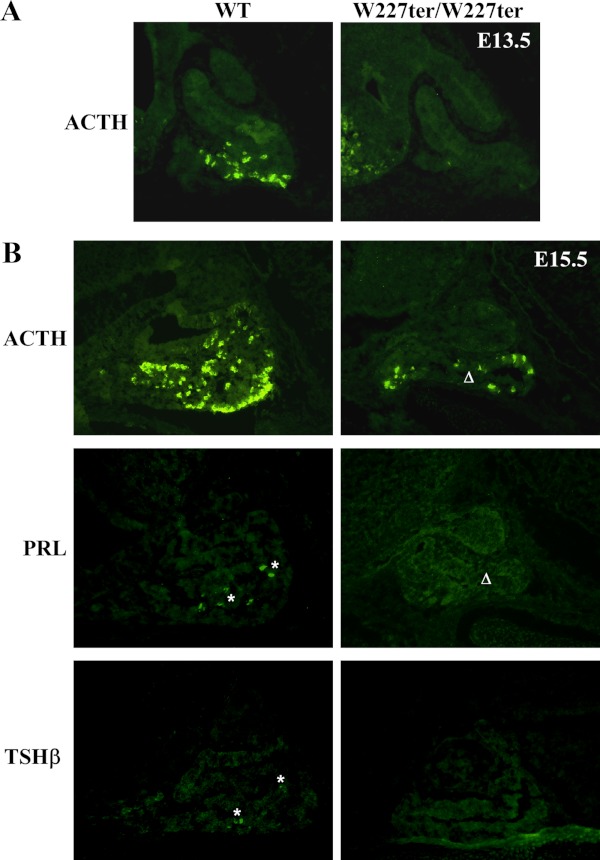
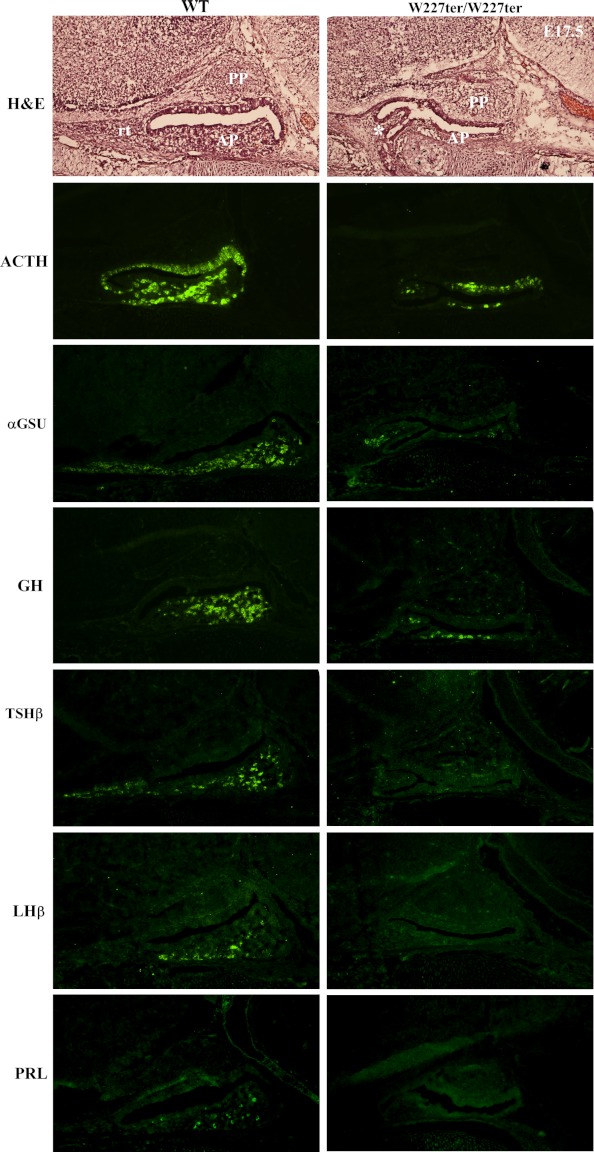
LHX3 is critical for the development of the hormone-producing cells of the AP. Therefore, we hypothesized that Lhx3W227ter/W227ter animals would have decreased expression of AP hormones during developmental stages. Using IHC, AP hormone expression was assessed using sections of embryonic pituitary glands in Lhx3W227ter/W227ter and littermate WT embryos from timed pregnancies. ACTH was the only hormone readily detectable at E13.5, and it was notably decreased in the AP of Lhx3W227ter/W227ter animals (Figure 1A) and also later at E15.5 (Figure 1B). Whereas the αGSU, PRL, and TSHβ were detectable at low levels in WT animals at E15.5, these hormones were not observed in the AP glands of Lhx3W227ter/W227ter animals (Figure 1B and data not shown). At both E15.5 and E17.5, the pituitary glands of Lhx3W227ter/W227ter animals often had morphological abnormalities involving hypoplastic AP lobes and sometimes forked/bifurcated structures (eg, Figures 1B and 2–5). At E17.5, ACTH, αGSU, and GH had reduced expression in Lhx3W227ter/W227ter animals, and PRL, TSHβ, and LHβ were not detected in the knock-in mice (Figure 2).
Figure 1.
Delayed onset of AP hormone expression in Lhx3W227ter/W227ter mice during development. ACTH, PRL, and TSHβ hormones were labeled using fluorescent IHC in pituitary sections of homozygote Lhx3W227ter/W227ter and WT mice at E15.5 (n = 5 per group). ACTH was also labeled in E13.5 sections (n = 5 per group). A and B, No specific ACTH expression was observed in Lhx3W227ter/W227ter animals at E13.5, and ACTH expression was decreased at E15.5. B, Low levels of PRL and TSHβ (asterisks) were detectable at E15.5, but no specific staining was seen in Lhx3W227ter/W227ter mutants. Lhx3W227ter/W227ter anterior pituitary lobes often were hypoplastic compared with WT controls (eg, Δ).
Figure 2.
The expression of AP hormones is reduced or absent in embryonic Lhx3W227ter/W227ter mice. ACTH, αGSU, GH, TSHβ, LHβ, and PRL pituitary hormone markers were labeled using IHC in E17.5 pituitary sections of Lhx3W227ter/W227ter homozygote and WT mice. Decreased expression of ACTH, αGSU, and GH was observed for Lhx3W227ter/W227ter animals, and no labeling was detected for TSHβ, LHβ, and PRL (n = 5 per group). Hematoxylin and eosin (H&E) staining (top panels) revealed that Lhx3W227ter/W227ter APs often were hypoplastic with structural abnormalities such as bifurcations (asterisk). PP indicates the posterior lobe; rt, rostral tip.
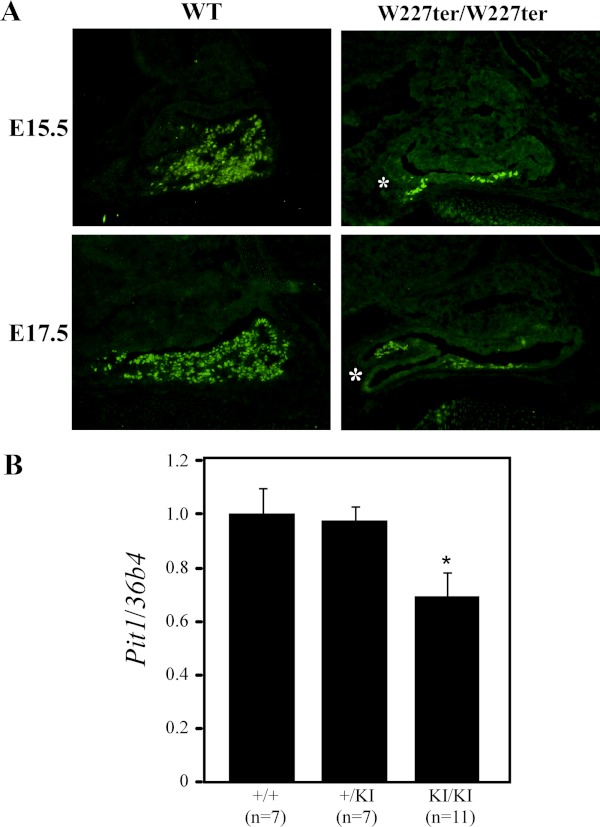
Figure 3.
Expression of the PIT1 pituitary transcription factor is reduced in Lhx3W227ter/W227ter animals. A, PIT1 protein was labeled using IHC in pituitary sections of Lhx3W227ter/W227ter homozygote and WT mice at E15.5 and E17.5. Lhx3W227ter/W227ter animals had decreased amounts of nuclear staining in PIT1-positive cells (n = 5 per group). Examples of a hypoplastic, bifurcated AP lobe also are noted (asterisks). B, Measurement of Pit1/Pou1f1 transcript levels in pituitary glands of 12-week-old adult mice using real-time quantitative PCR revealed a significant decrease in levels in the Lhx3W227ter/W227ter homozygote knock-in (KI/KI) mice. The asterisk indicates significance when compared with WT controls with P < .05. Data were normalized by determining the relative abundance of 36B4 (acidic ribosomal phosphoprotein P0 [Rplp0 gene]) mRNA. Examples of hypoplastic, bifurcated anterior pituitary lobes are noted (*, asterisks).
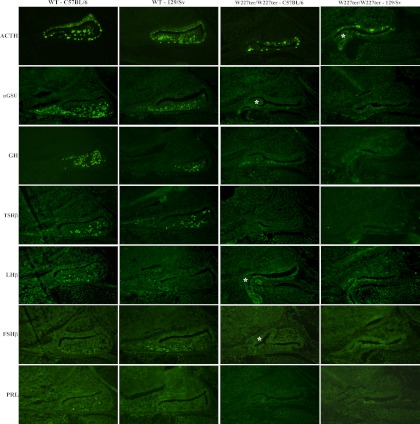
Figure 4.
Genetic background impacts the expression of AP hormones in the Lhx3W227ter/W227ter mouse. Pituitary hormone markers were analyzed by IHC in animals on C57BL/6 and 129/Sv backgrounds. AP hormone expression in Lhx3W227ter/W227ter homozygote knock-in and WT mice at E17.5 on a 98.4% C57BL/6 or 129/Sv genetic background. Lhx3W227ter/W227ter animals had decreased expression of ACTH and αGSU and no expression of PRL, FSHβ, TSHβ, or LHβ in both strains. Neither Lhx3W227ter/W227ter nor WT animals on a 129/Sv background had any PRL labeling. Lhx3W227ter/W227ter animals on a 129/Sv background had no GH expression, whereas those on a C57BL/6 background had decreased expression of GH compared with WT. Examples of hypoplastic, bifurcated AP lobes are noted (asterisks).
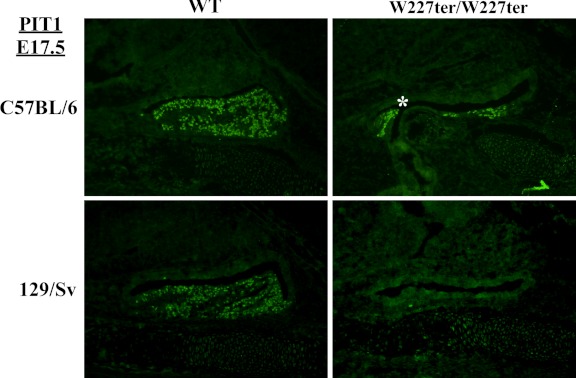
Figure 5.
Reduced expression of PIT1 protein in Lhx3W227ter/W227ter animals on C57BL/6 and 129/Sv backgrounds. PIT1 protein was detected using IHC in pituitary sections of Lhx3W227ter/W227ter homozygote and WT mice at E17.5 on a 98.4% C57BL/6 or 129/Sv background. Decreased expression was seen in Lhx3W227ter/W227ter animals on a C57BL/6 background, and no expression was seen in Lhx3W227ter/W227ter mice on a 129/Sv background. An example of a hypoplastic, bifurcated AP lobe is labeled (asterisk).
Lhx3W227ter/W227ter mice have decreased expression of PIT1
The PIT1 transcription factor is essential for the development of differentiated somatotropes, lactotropes, and thyrotropes and acts at later stages of pituitary development than LHX3 (4). PIT1 also is known to synergize with LHX3 to activate transcription of other pituitary genes (9). Using IHC, we saw decreased expression of PIT1 protein in Lhx3W227ter/W227ter knock-in embryos at E15.5 and E17.5 (Figure 3A). Additionally, 12-week-old adults had significantly lower expression of Pit1/Pou1f1 mRNA measured by quantitative RT-PCR (Figure 3B).
Genetic background significantly influences the effect of the Lhx3W227ter/W227ter mutation
The W227ter mutation was originally assessed in mice of a mixed background (129/Sv + C57BL/6) (24). To assess the influence of genetic background on the W227ter mutation, we bred Lhx3W227ter/W227ter mice into the individual 129/Sv and C57BL/6 strains for six generations (98.4% pure background). We counted the number of WT, Lhx3W227ter/+, and Lhx3W227ter/W227ter animals from heterozygous matings that survived to weaning at 21 days. From 18 litters on the 129/Sv background, there were 18 WT and 50 heterozygous animals born and weaned; however, no Lhx3W227ter/W227ter animals survived to weaning. This indicates that W227ter homozygotes are not viable on this mouse background (Table 1). By contrast, animals born on the C57BL/6 background from 11 litters followed the expected 1:2:1 Mendelian ratio, with 22 WT, 43 Lhx3W227ter/+, and 21 Lhx3W227ter/W227ter knock-ins generated and surviving to weaning (Table 1).
Table 1.
Lhx3W227ter/W227ter Mice Are Not Viable on the 129/Sv Genetic Background
| C57BL/6 | 129/Sv | |
|---|---|---|
| WT | 22 | 18 |
| Lhx3W227ter/+ | 43 | 50 |
| Lhx3W227ter/W227ter | 21 | 0 |
| Total | 86 | 68 |
Pups born to Lhx3W227ter/+ (heterozygous) parents from generation 6 backcrosses (98.4% pure) of C57BL/6 or 129/Sv strains were weaned and tallied for 11 and 18 litters, respectively. The table lists numbers of weaned (viable) pups of each genotype (WT, heterozygous [Lhx3W227ter/+], and homozygous [Lhx3W227ter/W227ter]) observed on either a separate C56BL/6 or 129/Sv background. C57BL/6 pups followed an approximately 1:2:1 expected Mendelian ratio, whereas no Lhx3W227ter/W227ter pups survived to weaning from 129/Sv animals.
To further examine the effects of genetic background on the W227ter mutation, we used IHC to detect hormone proteins and PIT1 in 129/Sv and C57BL/6 embryos at E17.5. Overall, hormone expression profiles in the individual strains were similar to those observed during the development of animals on a mixed-strain background (Figures 2 and 4). Addition of IHC experiments detecting FSHβ (as a further marker of gonadotrope cells) revealed comparable expression profiles to LHβ staining (Figure 4). Although GH was detected at a low level in Lhx3W227ter/W227ter animals on either the mixed background or C57BL/6, it was not detected in Lhx3W227ter/W227ter mice on the 129/Sv background (Figures 2 and 4). PRL was not detected in WT 129/Sv animals but was detected in WT controls on the mixed and C57BL/6 backgrounds (Figures 2 and 4). PIT1 transcription factor protein was not detected in Lhx3W227ter/W227ter knock-ins on the 129/Sv background, but it was detected in Lhx3W227ter/W227ter mice on the mixed background or the C57BL/6 background (Figures 3 and 5).
Proteomic analysis of pituitary hormone markers in adult WT and Lhx3W227ter/W227ter animals
To investigate molecular differences between WT and Lhx3W227ter/W227ter knock-in animals, we conducted a quantitative proteomic analysis of the proteins expressed by adult pituitary glands. A total of 2416 unique proteins were identified and quantified in the screen. Differential expression in 425 of these was observed between knock-in and WT animals at P < .00876 (corresponding to q < 0.05). Within this group, 58 proteins were down-regulated in the knock-in, and 367 proteins were up-regulated (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
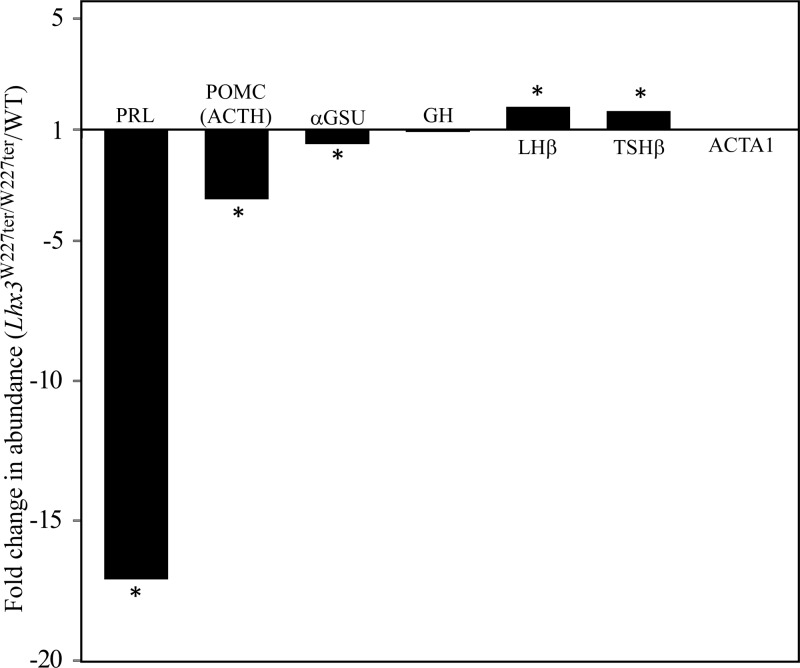
Of the AP hormones, PRL was 17.1-fold lower in the Lhx3W227ter/W227ter animals when compared with WT (Figure 6). Pro-opiomelanocortin (POMC, the precursor peptide containing ACTH) and αGSU also were significantly lower in the Lhx3W227ter/W227ter mice, at 3.5-fold and 1.6-fold, respectively (Figure 6). These results correlate with quantitative PCR measurements of POMC (mouse Pomc gene) and αGSU (Cga gene) mRNA levels that showed modest reductions in Pomc and Cga mRNA levels in homozygous pituitary mRNA levels (ref. 24; and (S.C.C. and S.J.R., data not shown). LHβ and TSHβ were significantly higher in the Lhx3W227ter/W227ter mice, at 1.8- and 1.7-fold. GH, the most abundant protein detected, was not significantly different between Lhx3W227ter/W227ter and WT animals at this age (Figure 6). A negative control, ACTA1 (alpha actin) showed no significant change between the two groups. Unfortunately, pituitary transcription factors such as PIT1, ISL1, LHX3, and LHX4 were not detectable by this approach. The whole proteomic data set was further analyzed using Interactive Pathway Analysis software to investigate whether proteins that significantly changed between Lhx3W227ter/W227ter and WT pituitaries were associated with specific cellular functions or pathways. The highest scoring affected network functions were cell cycle, cellular assembly and organization, cellular function and maintenance, molecular transport, and RNA modification and trafficking.
Figure 6.
Proteomic analysis of pituitary hormone markers in Lhx3W227ter/W227ter animals. Pituitaries were harvested from adult female Lhx3W227ter/W227ter or WT animals, and protein extracts were analyzed by label-free quantitative liquid chromatography tandem mass spectroscopy (see Materials and Methods). Fold changes in relative abundances of PRL, POMC (representing ACTH), αGSU (from the mouse Cga gene), LHβ, TSHβ, GH, and ACTA1 (α-actin, as a comparator negative control) proteins/peptides were calculated (Lhx3W227ter/W227ter homozygote/WT). PRL, POMC, and αGSU were significantly lower in Lhx3W227ter/W227ter animals at −17.1-fold, −3.5-fold, and −1.6-fold respectively. (Note that in proteomic method development, the PRL result depicted here was shown as an isolated example for the technique.) (32). GH showed no significant change. LHβ and TSHβ were increased in Lhx3W227ter/W227ter animals at 1.8-fold and 1.7-fold, respectively. The control ACTA1 showed no significant change. Asterisks indicate significance at P < .00876 (q < 0.05) (n = 5 per group).
Discussion
This study reveals that the Lhx3W227ter/W227ter mouse model of CPHD disease has pituitary hormone deficiencies that start during embryonic development (Figures 1, 2, and 4), consistent with an important role for LHX3 in organogenesis of the pituitary gland (6, 7, 34, 35) and observations that the carboxy terminus of the protein (removed due to the mutation) is important for pituitary gene regulation (9, 27). These findings add to the profile of hormone deficiencies also observed in juvenile and adult Lhx3W227ter/W227ter animals (24). Together, these observations establish the Lhx3W227ter/W227ter mouse as a useful model for pituitary hormone deficiency diseases that will facilitate future molecular and cellular studies of disease onset and mechanisms to obtain data that cannot be practically obtained from human patients.
Structural abnormalities were noted in many of the analyzed sections of Lhx3W227ter/W227ter embryonic pituitary glands. A hypoplastic AP region was evident in many cases as early as E15.5 (Figure 1) and in all cases by E17.5 (Figure 2). As seen in juvenile and adult Lhx3W227ter/W227ter mice (24), the posterior pituitary was a similar size in WT and mutant animals, indicating that it is unaffected by loss of LHX3 carboxyl-terminal function. Bifurcated or forked pituitary structures were observed in some Lhx3W227ter/W227ter embryonic pituitaries, but not in every case. Figures 1B and 2–5 show labeled examples of these abnormal structures within the developing AP. Structural deformities including bifurcations have been seen during pituitary development in other mouse models with mutations in pituitary transcription factor and regulatory genes, such as the Aes (36), Prop1 (37, 38), Wnt5a (39), Tcf4 (40), Sox2 (41), and Hesx1 genes (42). It is postulated that such structural defects reflect loss of signaling pathway specificity or abnormalities in signaling protein gradients. These dysmorphologies appear at different time points depending upon the regulatory gene that is impacted, suggesting that multiple pathways may be disrupted to form these unusual structures.
Studies of mice with complete Lhx3 gene ablation or with Lhx3 gene action disrupted by Cre insertion, suggest roles for LHX3 in preventing cell death and cellular proliferation. The developing pituitaries of these animals display reduced proliferation and differentiation and excessive cell death appears to be a major factor in the morphological defects (eg, 6, 7, 35, 43). Lhx3−/− mice were first analyzed by in situ hybridization of hormone marker mRNAs. These experiments demonstrated almost complete absence of AP hormone transcripts except for a few Pomc-staining corticotrope cells (6). Immunostaining of developing Lhx3−/− mouse pituitaries also indicates very few corticotrope cells (35). Lhx3W227ter/W227ter mice seem to have less severe structural and differentiation defects during pituitary development than Lhx3−/− mice. Our observations here of hypoplastic AP lobes and reduced differentiation in developing Lhx3W227ter/W227ter mouse pituitaries are consistent with roles for LHX3 in AP cellular survival, proliferation, and specification. They indicate that the knock-in mutation causing loss of the LHX3 carboxyl terminus compromises these roles but does not overall result in as severe a pituitary phenotype. The carboxyl terminus contains the most potent pituitary activation domain in the LHX3 protein (27), but other regions of the protein have transcription modulatory activities (27, 44, 45) and may be able to maintain some functions. It is also possible that in some genetic environments, compensatory changes in other genes may assist LHX3W227ter function. Lhx4 might be a candidate for such roles, but preliminary experiments measuring Lhx4 mRNA levels in Lhx3W227ter/W227ter pituitaries have not shown any significant changes in Lhx4 gene expression (S.C.C. and S.J.R., data not shown).
PIT1 is an essential pituitary transcription factor located downstream of LHX3 in the transcription factor cascade that is associated with anterior lobe development (reviewed in Refs. 1, 2, and 4). We observe that PIT1 expression is decreased in the Lhx3W227ter/W227ter animals (Figures 3 and 5). Proper expression of PIT1 is crucial to ensure differentiation of somatotropes, lactotropes, and thyrotropes. Mutations in the human PIT1/POU1F1 gene are associated with CPHD diseases featuring loss of GH, PRL, and TSH hormones, a more restricted outcome compared with LHX3-based diseases (1). The observed decreases in PIT1 (Pou1f1 gene) expression in the Lhx3W227ter/W227ter mouse model are consistent with prior suggestions that PIT1 lineage-specific actions are downstream of LHX3 transcription factor action (9). The Snell and Jackson mouse models, which carry mutations in the mouse Pit1 (Pou1f1) gene, are dwarfed and have hypoplastic pituitary glands in which little or no GH, PRL, and TSH are detected (46). Studies of the Snell mouse have shown that in the developing AP, two separate populations of thyrotropes appear at different times. First, a small cluster of cells that are not dependent upon PIT1 appears rostrally, and later, cells that are PIT1 dependent appear more centrally (47). We did not detect cells producing TSHβ in the Lhx3W227ter/W227ter mice at E15.5 or E17.5 (Figures 1, 2, and 4), suggesting that the PIT1-independent population is perhaps affected by the loss of LHX3 function in the homozygous mutants.
Using immunostaining to detect hormone and regulatory factor proteins, we noted that Lhx3W227ter/W227ter 129/Sv embryos had less staining for ACTH than those on the C57BL/6 background and had no detectable GH or PIT1 protein, but we did observe low levels of GH and PIT1 in the C57BL/6 animals (Figures 4 and 5). Consistent with our observations of viability, the 129/Sv background appears to perhaps be more sensitive to loss of LHX3 functions, reflected in the poor development of AP hormone-producing cells. Wild-type 129/Sv animals did not have any PRL or PIT1 staining at E17.5, whereas both were present in the C57BL/6 WT sections. This may indicate that those genes activated slightly later in the 129/Sv strain, or the levels were so low that they were undetectable by IHC.
Our genetic backcross study suggests that Lhx3W227ter/W227ter mice are not viable when they are on the 129/Sv background, but on the C57BL/6 or original mixed backgrounds, they are born and weaned in the expected Mendelian ratios. We chose weaning as the time point of measurement for this study, but only one live pup genotyped as an Lhx3W227ter/W227ter mouse was born on the 129/Sv background. This animal died shortly before the weaning date of unknown causes. Additionally, three stillborn 129/Sv knock-in pups were identified. These four homozygous knock-in pups were born to three different 129/Sv breeder pairs. None of these animals had obvious problems such as neural tube closure defects. It is also possible that there may have been additional stillborn pups that were cannibalized by parents. Consistent with this idea, analysis of embryo frequencies at E17.5 suggests that genotype ratios are roughly as predicted at that time. Loss of viability has been seen before in mouse models of pituitary disease. In the case of Prop1 gene knockout mice, those on a 129S1 background were less viable when compared with animals on a mixed background or other strains such as C56BL/6 (48). Differences in phenotypic outcomes in different strains also have been reported involving mouse models of other diseases, such as kidney diseases and stress-related disorders, including noted differences between 129/Sv-based and C57BL/6 animals (49–51). Some strain differences are specific to treatments, but in some cases, anatomical or physiological variations in pituitary biology are observed between untreated WT animals. For example, WT 129/Sv animals have a larger intermediate lobe than C57BL/6 animals (52). Together, our observations in this study are consistent with described variations in human patients with LHX3 gene alterations (15–23), and in other diseases such as cystic fibrosis (53) and Huntington's disease (54), suggesting that the actions of modifier genes in different genetic backgrounds influence disease onset and character and in human patients may sometimes result in nonviable pregnancies.
It is postulated that the Lhx3 whole gene knockout mice die due to nervous system deficits (5–7). By contrast, the viability of Lhx3W227ter/W227ter animals in mixed (24) and C57BL/6 genetic backgrounds (this study) suggests that LHX3 functions in the pituitary and nervous systems can be separated, thereby impacting viability. In the nervous system, LHX3 synergizes with partner basic helix-loop-helix class transcription factors and the LIM-homeodomain factor ISL1 to specify motor neuron subtypes during development (55). It is possible, for example, that there is a threshold environment for effective function of the truncated W227ter LHX3 protein in the nervous system and that the levels of partner proteins (ie, the actions of modifier genes) in 129/Sv animals are below the levels required to permit appropriate nervous system development and viability. Other models might involve higher levels of proteins with possible compensatory functions (such as LHX4) in the mixed and C57BL/6 backgrounds.
The proteomic analysis of pituitary hormone proteins is consistent with previous initial characterization of juvenile and adult Lhx3W227ter/W227ter animals using other approaches (24), except in the detected levels of the TSHβ and LHβ glycoprotein hormone subunits. The observed GH levels are as expected because, although the Lhx3W227ter/W227ter dwarf mice have hypoplastic pituitaries, they do express GH (24). Our proteomic results indicate that Lhx3W227ter/W227ter adult females have slightly increased levels of TSHβ and LHβ proteins, whereas IHC using anti-β-subunit antibodies indicates a reduced number of cells producing those markers in adults (24) and in development (this study). Intriguingly, quantitative RT-PCR experiments also show increases in TSHβ-encoding (mouse Tshb gene) and LHβ subunit (Lhb) mRNAs in adult animals (S.C.C. and S.J.R., unpublished). This discrepancy may have several explanations. One possibility may be sex differences; all 10 of the animals used in the proteomic analysis here were females, whereas the IHC data previously reported (24) was for a combination of males and females. Regulation of LH is a complex process, occurring at multiple levels of the hypothalamic-pituitary-gonadal axis. Estrogen affects the synthesis of the LHβ subunit at the hypothalamic level through its effect on GnRH, and there are reports of direct action in gonadotropes (reviewed in Ref. 56). Although the Lhx3W227ter/W227ter females are infertile, evidence suggests that they have mostly normal ovarian function (24). Differences in estrogen levels of the females used may have contributed to the slight increase seen in LHβ during the comparison in our proteomic analysis. TSH regulation also is complex, occurring at the level of the hypothalamus and pituitary (reviewed in Ref. 57). Thyroid hormone has been shown to regulate transcription of the α- and β-subunits at different levels (58). It is therefore possible that complex feedback mechanisms may have contributed to the modestly increased TSHβ (and LHβ) subunits detected in the Lhx3W227ter/W227ter female adults with the complete hormone action still impaired.
The proteomic analysis indicated that affected protein networks included cell cycle, cellular maintenance, and protein translation networks. Intriguingly, some proteins with functions in aspects of the cell cycle (integrin β1, metadherin, small G protein signaling modulator 1, and spectrin α) and elongation of actin filaments (profilin 1 and vasodilator-stimulated phosphoprotein) were up-regulated (Supplemental Table 1). For protein translation components, there were several up-regulated translation initiation-related proteins along with a number of ribosomal proteins, including eukaryotic translation initiation factors 2S, 3H, 3M, and 4G1, and 18 ribosomal proteins including S18, L7, L27, S11, L21, L13A, S24, and S5, among others. For cellular assembly and organization proteins, there was up-regulation of proteins involved in microtubule dynamics and cytoskeletal organization such as β-tubulin, structure-specific recognition protein 1, N-myc downstream regulated 1, major prion protein, dihydropyrimidinase-like 3, hepatoma-derived growth factor, and catalytic cAMP-dependent protein kinase α. For apoptosis-related proteins, overall cell death-related processes were decreased and viability and survival were increased in the dwarf. Seventeen proteins associated with apoptosis were all down-regulated: semaphorin 3B, PRL, polycystic kidney disease 1, peptidyl-tRNA hydrolase 2, and nucleolar and coiled-body phosphoprotein 1 among others. Some apoptosis-related proteins were up-regulated: major prion protein, low-density lipoprotein receptor-related protein-associated protein 1, and glycogen synthase kinase 3β. The observation that many apoptosis-related proteins are down-regulated is interesting considering that apoptosis in increased in Lhx3−/− mouse developing pituitaries (34, 35) and that developing Lhx3W227ter/W227ter pituitaries are hypoplastic. However, it is important to note that the proteomic studies were done on the pituitaries of adult mice; a similar analysis with developing pituitary tissue (although technically challenging to perform) might reveal a different physiological status for developing normal and Lhx3W227ter/W227ter dwarf pituitary tissue.
Lhx3W227ter/W227ter animals have striking deficiencies in PRL hormone (Figures 1, 2, 4, and 6) (24). In these experiments, we used an anti-PRL antibody that is optimized for developmental studies and is different from the one used by Colvin et al (24) to evaluate the adult and P1 animals but gives consistent data. Our results demonstrate that PRL deficiency begins as early as E15.5, indicating that PRL loss is likely a failure to establish PRL production rather than a later onset of deficiency. POMC is also decreased during development (Figures 1, 2, and 5), consistent with observed low levels in P1 and adult Lhx3W227ter/W227ter animals and reduced Pomc mRNA transcripts in adult mice (24). Supplemental Table 1 provides a complete listing and quantitation of all detected pituitary proteins from the proteomic analysis of the mutant and WT animals. This data set provides a resource for others investigating pituitary physiology and may serve to suggest biomarkers for future studies.
Acknowledgments
We are grateful to Dr Richard Day for advice, Dr Glenn Bohlen for guidance on perfusion, Dr Sally Camper for advice on anti-PRL antibodies, and Dr Kyle Sloop and Aaron Showalter for assistance with real-time PCR assays.
This work was supported by Grants NIH R01-HD42024 (to S.J.R.), NIH F32HD068113 (to K.L.P.), and NIH ES018810 and GM085218 (to F.A.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP
- anterior pituitary
- CPHD
- combined pituitary hormone deficiency
- E13.5
- embryonic day 13.5
- αGSU
- α-glycoprotein subunit
- IHC
- immunohistochemistry
- LIM
- lin-1, Isl-1, and mec-3
- LHX3
- LIM-homeodomain 3
- POMC
- pro-opiomelanacortin
- PRL
- prolactin
- WT
- wild-type.
References
- 1. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30:790–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis SW, Castinetti F, Carvalho LR, et al. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323:4–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J. A tridimensional view of pituitary development and function. Trends Endocrinol Metab. 2012;23:261–269 [DOI] [PubMed] [Google Scholar]
- 4. Prince KL, Walvoord EC, Rhodes SJ. The role of homeodomain transcription factors in heritable pituitary disease. Nat Rev Endocrinol. 2011;7:727–737 [DOI] [PubMed] [Google Scholar]
- 5. Sharma K, Sheng HZ, Lettieri K, et al. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828 [DOI] [PubMed] [Google Scholar]
- 6. Sheng HZ, Zhadanov AB, Mosinger B, Jr, et al. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science. 1996;272:1004–1007 [DOI] [PubMed] [Google Scholar]
- 7. Sheng HZ, Moriyama K, Yamashita T, et al. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812 [DOI] [PubMed] [Google Scholar]
- 8. Seidah NG, Barale JC, Marcinkiewicz M, Mattei MG, Day R, Chretien M. The mouse homeoprotein mLIM-3 is expressed early in cells derived from the neuroepithelium and persists in adult pituitary. DNA Cell Biol. 1994;13:1163–1180 [DOI] [PubMed] [Google Scholar]
- 9. Bach I, Rhodes SJ, Pearse RV, 2nd, et al. P-Lim, a LIM homeodomain factor, is expressed during pituitary organ and cell commitment and synergizes with Pit-1. Proc Natl Acad Sci U S A. 1995;92:2720–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhadanov AB, Bertuzzi S, Taira M, Dawid IB, Westphal H. Expression pattern of the murine LIM class homeobox gene Lhx3 in subsets of neural and neuroendocrine tissues. Dev Dynam. 1995;202:354–364 [DOI] [PubMed] [Google Scholar]
- 11. Granger A, Bleux C, Kottler ML, Rhodes SJ, Counis R, Laverriere JN. The LIM-homeodomain proteins Isl-1 and Lhx3 act with steroidogenic factor 1 to enhance gonadotrope-specific activity of the gonadotropin-releasing hormone receptor gene promoter. Mol Endocrinol. 2006;20:2093–2108 [DOI] [PubMed] [Google Scholar]
- 12. McGillivray SM, Bailey JS, Ramezani R, Kirkwood BJ, Mellon PL. Mouse GnRH receptor gene expression is mediated by the LHX3 homeodomain protein. Endocrinology. 2005;146:2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sloop KW, Meier BC, Bridwell JL, Parker GE, Schiller AM, Rhodes SJ. Differential activation of pituitary hormone genes by human Lhx3 isoforms with distinct DNA binding properties. Mol Endocrinol. 1999;13:2212–2225 [DOI] [PubMed] [Google Scholar]
- 14. West BE, Parker GE, Savage JJ, et al. Regulation of the follicle-stimulating hormone β gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology. 2004;145:4866–4879 [DOI] [PubMed] [Google Scholar]
- 15. Bonfig W, Krude H, Schmidt H. A novel mutation of LHX3 is associated with combined pituitary hormone deficiency including ACTH deficiency, sensorineural hearing loss, and short neck-a case report and review of the literature. Eur J Pediatr. 2011;170:1017–1021 [DOI] [PubMed] [Google Scholar]
- 16. Bhangoo AP, Hunter CS, Savage JJ, et al. Clinical case seminar: a novel LHX3 mutation presenting as combined pituitary hormonal deficiency. J Clin Endocrinol Metab. 2006;91:747–753 [DOI] [PubMed] [Google Scholar]
- 17. Kristrom B, Zdunek AM, Rydh A, Jonsson H, Sehlin P, Escher SA. A novel mutation in the LIM homeobox 3 gene is responsible for combined pituitary hormone deficiency, hearing impairment, and vertebral malformations. J Clin Endocrinol Metab. 2009;94:1154–1161 [DOI] [PubMed] [Google Scholar]
- 18. Netchine I, Sobrier ML, Krude H, et al. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet. 2000;25:182–186 [DOI] [PubMed] [Google Scholar]
- 19. Pfaeffle RW, Savage JJ, Hunter CS, et al. Four novel mutations of the LHX3 gene cause combined pituitary hormone deficiencies with or without limited neck rotation. J Clin Endocrinol Metab. 2007;92:1909–1919 [DOI] [PubMed] [Google Scholar]
- 20. Rajab A, Kelberman D, de Castro SC, et al. Novel mutations in LHX3 are associated with hypopituitarism and sensorineural hearing loss. Hum Mol Genet. 2008;17:2150–2159 [DOI] [PubMed] [Google Scholar]
- 21. Savage JJ, Hunter CS, Clark-Sturm SL, Jacob TM, Pfaeffle RW, Rhodes SJ. Mutations in the LHX3 gene cause dysregulation of pituitary and neural target genes that reflect patient phenotypes. Gene. 2007;400:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sobrier ML, Attie-Bitach T, Netchine I, Encha-Razavi F, Vekemans M, Amselem S. Pathophysiology of syndromic combined pituitary hormone deficiency due to a LHX3 defect in light of LHX3 and LHX4 expression during early human development. Gene Expr Patterns. 2004;5:279–284 [DOI] [PubMed] [Google Scholar]
- 23. Bechtold-Dalla Pozza S, Hiedl S, Roeb J, et al. A recessive mutation resulting in a disabling amino acid substitution (T194R) in the LHX3 homeodomain causes combined pituitary hormone deficiency. Horm Res Paediatr. 2012;77:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colvin SC, Malik RE, Showalter AD, Sloop KW, Rhodes SJ. Model of pediatric pituitary hormone deficiency separates the endocrine and neural functions of the LHX3 transcription factor in vivo. Proc Natl Acad Sci U S A. 2011;108:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voorbij AM, van Steenbeek FG, Vos-Loohuis M, M, et al. A contracted DNA repeat in LHX3 intron 5 is associated with aberrant splicing and pituitary dwarfism in German shepherd dogs. PloS one. 2011;6:e27940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sobrier ML, Brachet C, Vie-Luton MP, et al. Symptomatic heterozygotes and prenatal diagnoses in a nonconsanguineous family with syndromic combined pituitary hormone deficiency resulting from two novel LHX3 mutations. J Clin Endocrinol Metab. 2012;97:E503–E509 [DOI] [PubMed] [Google Scholar]
- 27. Sloop KW, Dwyer CJ, Rhodes SJ. An isoform-specific inhibitory domain regulates the LHX3 LIM homeodomain factor holoprotein and the production of a functional alternate translation form. J Biol Chem. 2001;276:36311–36319 [DOI] [PubMed] [Google Scholar]
- 28. Voss JW, Yao TP, Rosenfeld MG. Alternative translation initiation site usage results in two structurally distinct forms of Pit-1. J Biol Chem. 1991;266:12832–12835 [PubMed] [Google Scholar]
- 29. Lai X, Bacallao RL, Blazer-Yost BL, Hong D, Mason SB, Witzmann FA. Characterization of the renal cyst fluid proteome in autosomal dominant polycystic kidney disease (ADPKD) patients. Proteomics Clin Appl. 2008;2:1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392 [DOI] [PubMed] [Google Scholar]
- 31. Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658 [DOI] [PubMed] [Google Scholar]
- 32. Lai X, Wang L, Tang H, Witzmann FA. A novel alignment method and multiple filters for exclusion of unqualified peptides to enhance label-free quantification using peptide intensity in LC-MS/MS. J Proteome Res. 2011;10:4799–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol. 2002;64:479–498 [Google Scholar]
- 34. Zhao Y, Mailloux CM, Hermesz E, Palkovits M, Westphal H. A role of the LIM-homeobox gene Lhx2 in the regulation of pituitary development. Dev Biol. 2010;337:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ellsworth BS, Butts DL, Camper SA. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol. 2008;313:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brinkmeier ML, Potok MA, Cha KB, et al. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–2161 [DOI] [PubMed] [Google Scholar]
- 37. Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239 [DOI] [PubMed] [Google Scholar]
- 38. Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–1390 [DOI] [PubMed] [Google Scholar]
- 39. Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–194 [DOI] [PubMed] [Google Scholar]
- 40. Brinkmeier ML, Potok MA, Davis SW, Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311:396–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kelberman D, Rizzoti K, Avilion A, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sajedi E, Gaston-Massuet C, Signore M, et al. Analysis of mouse models carrying the I26T and R160C substitutions in the transcriptional repressor HESX1 as models for septo-optic dysplasia and hypopituitarism. Dis Model Mech. 2008;1:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, Morales DC, Hermesz E, Lee WK, Pfaff SL, Westphal H. Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development. Mech Dev. 2006;123:605–613 [DOI] [PubMed] [Google Scholar]
- 44. Parker GE, Sandoval RM, Feister HA, Bidwell JP, Rhodes SJ. The homeodomain coordinates nuclear entry of the Lhx3 neuroendocrine transcription factor and association with the nuclear matrix. J Biol Chem. 2000;275:23891–23898 [DOI] [PubMed] [Google Scholar]
- 45. Parker GE, West BE, Witzmann FA, Rhodes SJ. Serine/threonine/tyrosine phosphorylation of the LHX3 LIM-homeodomain transcription factor. J Cell Biochem. 2005;94:67–80 [DOI] [PubMed] [Google Scholar]
- 46. Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533 [DOI] [PubMed] [Google Scholar]
- 47. Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development. 1994;120:515–522 [DOI] [PubMed] [Google Scholar]
- 48. Nasonkin IO, Ward RD, Raetzman LT, et al. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13:2727–2735 [DOI] [PubMed] [Google Scholar]
- 49. Ishola DA, Jr, van der Giezen DM, Hahnel B, et al. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrol Dial Transplant. 2006;21:591–597 [DOI] [PubMed] [Google Scholar]
- 50. Robson MG, Cook HT, Pusey CD, Walport MJ, Davies KA. Antibody-mediated glomerulonephritis in mice: the role of endotoxin, complement and genetic background. Clin Exp Immunol. 2003;133:326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McCutcheon JE, Fisher AS, Guzdar E, Wood SA, Lightman SL, Hunt SP. Genetic background influences the behavioural and molecular consequences of neurokinin-1 receptor knockout. Eur J Neurosci. 2008;27:683–690 [DOI] [PubMed] [Google Scholar]
- 52. Kelly MA, Rubinstein M, Asa SL, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–113 [DOI] [PubMed] [Google Scholar]
- 53. Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci. 2010;1214:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gusella JF, MacDonald ME. Huntington's disease: the case for genetic modifiers. Genome Med. 2009;1:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee SK, Pfaff SL. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745 [DOI] [PubMed] [Google Scholar]
- 56. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chiamolera MI, Wondisford FE. Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–1096 [DOI] [PubMed] [Google Scholar]
- 58. Gurr JA, Kourides IA. Regulation of thyrotropin biosynthesis. Discordant effect of thyroid hormone on alpha and beta subunit mRNA levels. J Biol Chem. 1983;258:10208–10211 [PubMed] [Google Scholar]