Abstract
Animals experiencing major invasive surgery during biomedical research must receive appropriate and sufficient analgesia. The concept of pain management in veterinary medicine has evolved over the past several decades, and a multimodal, preemptive approach to postoperative analgesia is the current standard of care. Here, the pathophysiology of pain and a multimodal approach to analgesia for neurosurgical procedures is discussed, with emphasis on those involving nonhuman primates.
Abbreviation: COX, cyclooxygenase; NSAID, nonsteroidal antiinflammatory drugs
The concept of pain management in veterinary medicine, especially in laboratory animals, has evolved dramatically over the past decade.36 Although little attention had previously been given to such concepts as preemptive analgesia, ‘wind-up’ of nociceptors, and chronic pain, new knowledge of the important physiologic and behavioral changes associated with pain has prompted the veterinary community to carefully examine provision of analgesia to animals. In addition, regulations require researchers to address pain management in protocols and to provide appropriate analgesia for procedures expected to cause pain.42 Importantly, the Animal Welfare Act requires the consultation of a veterinarian in determining the most appropriate analgesic regimen for a procedure, in accordance with standard veterinary practice.4 This type of performance standard allows the specific requirement for analgesics to evolve with the current standard of veterinary care. However, as these standards change, this practice can lead to variation in provision of analgesics for similar procedures even within the same institution and to legitimate differences of opinion regarding the choice of appropriate analgesics. Although protocols and procedures implemented years ago may continue to produce acceptable outcomes, a veterinary care program must “foster and support enhancement of the program through the identification and adoption of techniques, procedures, and policies that improve laboratory animal health and wellbeing.”2
Nonhuman primates are used often as research models for neuroanatomy and neurophysiology studies that require the implantation of cranial devices, including head posts and electrode recording chambers. The implantation of these devices is classified as major invasive surgery, because screws are placed into the skull, thereby exposing the cranium. Accordingly, appropriate analgesia is required for these procedures. The current report briefly addresses the evolution of the concept of pain management in veterinary medicine and the pathophysiology of pain in neurosurgical procedures and concisely reviews multimodal analgesic therapy for neurosurgical procedures, particularly those involving nonhuman primates.
The Pathophysiology of Pain in Neurosurgery
In humans, pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage” and is recognized as being “always subjective.”43 Humans and animals share similar anatomic structures and neurophysiologic mechanisms leading to pain perception, suggesting that any stimulus considered painful in people is painful to animals.91 This conserved mechanism of nociception is the basis for the requirements for appropriate analgesia in laboratory animals, even when they may not appear to be experiencing pain. The recognition of pain in animals is complicated by the lack of a commonly accepted objective scoring system and the innate instinct of animals to disguise pain.69 In particular, nonhuman primates display few signs of pain after surgical procedures or injuries, such that animals that actually demonstrate signs of pain likely are experiencing severe pain.41 In addition, the increased responsiveness of pain receptors as a result of repeat surgeries (the ‘wind-up’ effect) may heighten the pain response.36 Nonhuman primates on neuroscience protocols may be at particular risk for this escalation, given that these animals potentially experience numerous surgeries for serial implantations of head posts and multiple recording chambers.
Further complicating the provision of analgesics to nonhuman primates undergoing neurosurgical procedures is the issue of pain and analgesic therapy in humans that experience similar surgical procedures. Historically, pain associated with neurosurgical procedures such as craniotomies was considered neither severe nor necessary to treat.31 Surveys of people undergoing craniotomies demonstrate that these patients experience moderate to severe pain in the postoperative period and that this pain is often undertreated.27,47,52,68 A recent editorial regarding pain in people after intracranial surgery concludes that “perioperative pain is meaningful and may persist, but relatively simple and familiar tools are effective” in managing this pain.31 The same conclusion should be made for nonhuman primates experiencing similar procedures.
Human patients undergoing craniotomies describe a superficial pain, suggesting a somatic origin rather than visceral.19 Severity of pain is associated with the surgical approach, with surgeries at the base of the skull being the most painful.19 Because the scalp is densely innervated with type C fibers, pain arises from disruption of temporal muscles and soft tissues.73 These type C fibers are small, unmyelinated nerve fibers that are responsible for so-called ‘secondary pain,’ described as a poorly localized, burning sensation that persists beyond the painful stimulus.57 Tissue injury results in cell damage and the recruitment of inflammatory cells that modify nociceptor responses in a process called ‘peripheral sensitization.’ If uncontrolled, inflammatory mediators such as prostaglandins can cause permanent changes in nociceptor function, resulting in states of chronic pain.57 Inadequate pain management after craniotomies can lead to significant postoperative complications including agitation, hypertension, and vomiting, which can result in intracranial bleeding.95 Because many nonhuman primates that experience these procedures also must participate in behavioral experiments, these complications not only are detrimental to the animal but also can delay data collection and increase the duration and cost of research projects.
Neurosurgeons are becoming more aware of the need for appropriate analgesics in human patients, but the lack of controlled trials assessing analgesic regimens prevents the adoption of uniform standards.34 Surveys have indicated that opioids tend to be the first-choice analgesic for these patients, but various reports of efficacy indicate the need for continued refinement of analgesic regimens.34,68,70 The growing attention to the need for improved analgesia in neurosurgical patients has led to several recent trials to identify optimal analgesic regimens, but these efforts are still relatively few.6,8,47,52,58,67,72,76,95 Similarly, few controlled studies address optimal postoperative analgesic regimens in nonhuman primates undergoing neurosurgery. In addition, authors may not describe the anesthetic and analgesic regimens that were used in studies involving neurosurgery of nonhuman primates. However, veterinarians should consider that the moderate to severe pain expressed by human neurosurgical patients likely is similar in nonhuman primates, and analgesics should be provided accordingly.
Analgesic Agents
Preemptive, multimodal pain management is the current standard of practice in veterinary medicine36 and therefore is required by the Animal Welfare Act.4 The benefits of this approach to analgesia are numerous. Multimodal analgesia targets multiple points along the pain pathway optimizing analgesia through synergistic effects while also reducing the required doses of individual drugs.57 Preemptive analgesic use treats pain prior to its onset to modulate the pain response and prevent maladaptive pain.36,49 During surgical procedures, this approach may be anesthetic-sparing, so that animals have reduced requirements for maintenance of anesthesia.84 This decreased reliance on maintenance drugs can improve recovery time and minimize side effects from high doses of volatile anesthetics. These advantages can be particularly important during intracranial surgery, given that many anesthetic and analgesic agents have the potential to alter intracranial physiology. One group of researchers has noted that “the use of balanced anesthesia that utilizes combination of high doses of opioid analgesic, low inspired concentration of volatile anesthetic, and muscle relaxant provides physiologically stable anesthesia that is important for neurosurgical procedures.”84
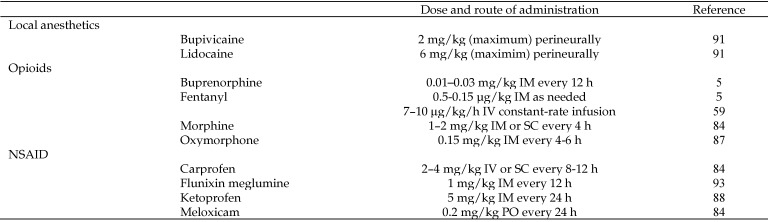
The importance of appropriate analgesia is highlighted by the availability of a wide variety of analgesic drugs that differ in potency, method of administration, and duration and mechanism of action. Many drug classes useful for the induction and maintenance of anesthesia, such as dissociative anesthetics (namely, ketamine) and α2 agonists, also provide analgesia. A discussion of all these drugs is beyond the scope of this review. Researchers and veterinarians are encouraged to consider incorporating these drugs into their anesthetic protocols as part of a multimodal and preemptive approach to pain management. The current discussion is limited to local anesthetics, opioids, and NSAID because these drug classes are the current mainstay of pain prevention and treatment in veterinary patients. Figure 1 summarizes specific agents in these drug classes and provides dose ranges and routes of administration for macaques, given that this species is most frequently used in neuroscience studies.
Figure 1.
A summary of suggested drugs, doses, and routes of administration for macaques.
Local anesthetics.
Local anesthetic agents provide analgesia through the inhibition of sodium channels essential for neural conduction.7 This drug class includes commonly used veterinary drugs such as lidocaine and bupivacaine. Because lipid solubility is correlated with increased diffusion through nerve sheaths, agents that are highly lipid soluble (for example, bupivacaine) are more potent than those that are less soluble (for example, lidocaine). In addition, bupivacaine has a greater protein-binding capacity—and thus a longer halflife (90 min compared with 300 min) and duration of action—than does lidocaine.7 To prolong the duration of anesthesia, vasopressors, usually epinephrine, can be combined with local anesthetics. In addition, the α- and β-agonistic activities of epinephrine provide local hemostasis and reduce the risk of systemic toxicity by delaying absorption of the local anesthetic. Because local anesthetics are CNS depressants, large doses can result in hypotension and seizures (due to loss of central inhibitory tracts) and can potentiate respiratory depression secondary to opioid administration.7 However, at clinical doses, adverse effects of local anesthetics are rare.
Local anesthetics have been used to control neurosurgical pain in humans for more than a century.75 Recent clinical trials have demonstrated the efficacy of either local scalp infiltration or a more regional scalp-block approach in reducing the postoperative pain associated with craniotomies.6,8,72 Although one trial found less pain but not reduced opioid requirements in patients,72 local anesthetic use in another trial decreased patients’ requirements for postoperative analgesics.58 Two other trials demonstrated an analgesic effect of the local anesthetic long after the drug's duration of action, thus highlighting the importance of preemptive analgesia.6,72 In addition to reducing postoperative pain, local anesthetics modulate the hemodynamic effects of surgery and improve circulatory stability in human craniotomy patients.8,67,77
Opioids.
Opioids provide the foundation of analgesic therapy in both human and veterinary medicine, especially in patients experiencing moderate to severe pain.9,76,81 Three opioid receptors have been identified in the brain and peripheral tissues of animals—μ, κ, and δ. μ receptors were the first discovered and are associated with the classic effects of morphine and related drugs—supraspinal analgesia, euphoria, sedation, and physical dependence. μ receptors can be subdivided further, and differential binding to these subtypes is responsible for the differing effects of various μ agonists, including degree of analgesia, respiratory depression, pruritus, dependence, and sedation.92 In addition, κ receptors are associated with analgesia, but little is known regarding δ receptors, which are largely restricted to the brain.71 Opioid drugs are classified based on their activity at these receptors. Pure μ agonists, including morphine, hydromorphone, and fentanyl, are the most potent analgesics, but these agents have short durations of action and the greatest potential for adverse side effects. In the practice of laboratory animal medicine, buprenorphine is used the most frequently, because of its relatively long duration of effect with potential analgesia for 8 to 12 h.24 Buprenorphine has been classified as a partial opioid agonist–antagonist, but at the dosages used clinically, buprenorphine acts a pure μ agonist.73 Buprenorphine is as much as 40 times more potent than is morphine, with a rapid onset and long duration of action.16 Buprenorphine produces analgesia at low plasma concentrations with a relatively long duration, because of its high affinity for μ receptors and slow dissociation.79 This high affinity is responsible for the inability of opioid antagonists such as naloxone to reverse the effects of buprenorphine.
Opioids have been investigated extensively for the control of postcraniotomy pain in humans.70 These studies conclude that opioids are effective and safe in the management of these patients and that the choice of opioid affects the level of postoperative pain. The relatively weak opioid codeine is the predominant first-choice drug, but more potent μ agonists such as morphine and fentanyl have been used also.47 Patients in which codeine is used initially often require rescue analgesia with more potent opioids.27 Although not described as an analgesic agent for human neurosurgery, buprenorphine has been used as the sole agent for perioperative pain after orthopedic and abdominal surgery in people, with adequate analgesia and minimal side effects.30 In children, buprenorphine provided equal postoperative analgesic activity to morphine after thoracotomy.66
Buprenorphine alone or in combination with NSAID has been used in veterinary species for various procedures. In dogs undergoing ovariohysterectomies, preemptive administration of buprenorphine provided lasting analgesia attributed to suppressing pain before it occurred and preventing wind-up pain.55 In cats, buprenorphine significantly decreased the need for inhalant anesthetics, although to a lesser degree than did other opioids.40 In olive baboons (Papio anubis), buprenorphine alone did not provide sufficient analgesia after laparotomy for all animals within the buprenorphine-only treatment group; however, a combination of buprenorphine and carprofen did provide adequate analgesia, thus highlighting the synergistic effects of a multimodal analgesic regimen.1
Many reports of nonhuman primate neuroscience experiments do not include details regarding the analgesic regimens that were used or their relative safety or effectiveness. However, several authors have described the use of opioids alone or in combination with NSAID for neurosurgery of nonhuman primates. Intravenous fentanyl followed postoperatively by intramuscular oxymorphone has been used in rhesus macaques experiencing bilateral craniotomies.59 Another author reports the use of intravenous fentanyl that continued after neurosurgery in sedated animals.84 However, buprenorphine is the most frequently reported opioid in neuroscience procedures involving nonhuman primates.13,18,28,45 Buprenorphine alone has been used for craniotomies involving imaging studies and intracerebral injections as well as for placement of deep-brain stimulation electrodes and fudicial pegs in nonhuman primates.28,29,56 In addition, buprenorphine has been combined with NSAID for the implantation of head posts18 and microelectrode arrays.45
Excluding the potential for respiratory depression, potential adverse effects of opioids are generally not clinically significant and are easily controlled. Hypotension and bradycardia during anesthesia are dose-dependent and can be avoided at therapeutic doses.84 Reduction in heart rate but not blood pressure has occurred secondary to buprenorphine dosing in dogs and rats, but no alterations in hemodynamics were observed in anesthetized cats.15 In another study, the intravenous administration of clinical doses of buprenorphine was not associated with any adverse effects in dogs.3 Typical intraoperative supportive care including intravenous fluid administration is usually sufficient to prevent the occurrence of opioid-induced hypotension, when it does occur. Anticholinergics may be indicated to increase heart rate if bradycardia is significant, given that the buprenorphine-reduced heart rate is due to increased vagal tone.80 Additional potential adverse effects of opioid use include sedation, vomiting, constipation, and urinary retention.80 Long-term opioid use is associated with tolerance and dependence, but this potential is less with buprenorphine than with pure μ agonists.80
Considerable attention has been given to the potential respiratory depression associated with opioid drugs. Overall, opioid-associated respiratory depression has been documented infrequently in veterinary medicine and apparently occurs rarely in animals compared with humans.79 Respiratory depression associated with buprenorphine has been described but is generally not clinically significant in humans.46,73 In animals, buprenorphine alone can produce respiratory depression, but buprenorphine has the least respiratory depressant potential of all opioids and typically is not associated with respiratory depression at the low doses that produce analgesia.17,73,74 Due to its partial agonist–antagonist activity, buprenorphine actually has been shown to antagonize the respiratory effects of pure μ agonists in rhesus monkeys.62 This effect to reverse respiratory depression has also been used clinically in rhesus monkeys.25 Another study in rhesus monkeys demonstrated that administration of 10 mg/kg buprenorphine (that is, 1000 times greater than the analgesic dose) suppressed respiration for more than 24 h, but none of the monkeys affected required respiratory support.51 No respiratory depression (as defined by increased pCO2) was observed in rhesus macaques administered oxymorphone, a pure μ agonist with a potential for respiratory depression greater than that of buprenorphine; however, the respiratory rate did decrease.50
The risk of significant respiratory depression is increased by the coadministration of sedative and anesthetic drugs, especially benzodiazepines.46,97 Because diazepam and midazolam are commonly used in the induction of anesthesia of nonhuman primates, laboratory animal veterinarians should be aware of the potential for this interaction. However, human cases of respiratory depression associated with the coadministration of opioids and benzodiazepines typically are associated with abuse of either or both of these drugs, resulting in overdosing, or with repeated dosing of the opioid.23,89 Similar to the typical practice in nonhuman primates, single doses of diazepam in human patients treated with buprenorphine had minimal effects on physiologic parameters including respiratory rate.63 Although CO2 levels were increased in anesthetized patients that received multiple doses of buprenorphine in addition to a volatile anesthetic, respiratory depression was not clinically significant even when patients had been pretreated with a long-acting benzodiazepine.53
In animals, combinations of buprenorphine and midazolam or diazepam have either not caused clinically significant respiratory depression14,82 or have resulted in respiratory depression only when doses several orders of magnitude higher than clinically effective doses are used.33 The combination of fentanyl, a more potent opioid and respiratory depressant than buprenorphine, and midazolam had no significant respiratory depressant effect in rabbits.39 The combination of ketamine, midazolam, and buprenorphine has been used as a premedication in a rhesus macaque model of myocardial occlusion–reperfusion without impairment of cardiorespiratory parameters.85 The preanesthetic regimen in the cited study85 included the anticholinergic drug atropine, which likely contributed to the observed cardiorespiratory stability. Clinically, at least one study has shown a relative absence of respiratory depression in nonhuman primates treated with buprenorphine.84 Veterinarians should be aware that naloxone is not effective at antagonizing the respiratory effects of buprenorphine and that doxapram should be used in the rare case when therapeutic intervention is required.35 In conclusion, respiratory depression associated with buprenorphine occurs only infrequently in veterinary patients and is rarely clinically significant even when therapeutic doses of both buprenorphine and a benzodiazepine are used simultaneously.
NSAID.
NSAID prevent the formation of prostanoids, a subgroup of prostaglandins that mediates inflammation, pain, and pyrexia, through the inhibition of cyclooxygenase, an enzyme required for the metabolism of arachidonic acid to prostaglandins.86 Two isoforms of cyclooxygenase have been identified. COX1 is constitutively expressed in many tissues and has important roles in the maintenance of normal gastrointestinal, renal, and cardiovascular function. Although COX2 is constitutively expressed to some degree, its expression increases markedly in response to inflammatory mediators, leading to increased production of proinflammatory prostaglandins.86 Side effects associated with NSAID are predominantly secondary to the inhibition of COX1. Loss of prostaglandins leads to a loss of vascular tone, cytoprotective mechanisms in the gastrointestinal mucosa, normal blood flow to the kidneys, and thromboxane formation by platelets. This alteration in normal physiologic function can result in gastrointestinal ulceration and hemorrhage, reduced glomerular filtration rate and kidney disease, and coagulopathies.
Several NSAID are commonly used in nonhuman primates in biomedical research, including flunixin meglumine, carprofen, ketoprofen, and meloxicam.25 Flunixin meglumine is the oldest of these drugs and represented a breakthrough in the development of potent nonnarcotic analgesics. In the tail-shock test in rhesus macaques, flunixin meglumine was comparable to morphine, leading to the incorporation of flunixin meglumine in a wide variety of laboratory animal analgesic protocols,11 and the use of this agent during nonhuman primate neurosurgery has been described.93 However, flunixin is a more potent inhibitor of COX1 than COX2 in various species and therefore is more likely to have adverse effects than are newer NSAID.10 In contrast, carprofen was the first COX2-selective NSAID approved for use in veterinary medicine12 and is the only commonly used veterinary NSAID specifically approved for alleviation of postsurgical pain,83 although its selectivity is weaker than those of ketoprofen and meloxicam.10 Although not specifically labeled for the treatment of postoperative pain, both ketoprofen and meloxicam have been used successfully to manage pain in veterinary orthopedic surgery patients.20 In addition, ketoprofen and carprofen provide comparable postoperative analgesia in dogs undergoing orthopedic surgery,32 and meloxicam and carprofen provide similar analgesic activity after ovariohysterectomy.61 In macaques, both meloxicam and ketoprofen have been used to provide analgesia after orthopedic surgery60 and neurosurgery,88 although these agents often are used in combination with other classes of drugs (especially opioids).1,18,44,100
Because NSAID inhibit only the inflammatory mediators that are products of the cyclooxygenase pathway, a ceiling effect limits their analgesic potential.57 NSAID alone are insufficient to provide adequate analgesia after major surgery.96 By combining NSAID with drugs from other classes, most frequently opioids, reliable and effective analgesia with minimal side effects can be achieved. This combination is used frequently in laboratory animal medicine and should be considered the standard for surgical procedures that are expected to cause moderate to severe pain. However, at least one clinical trial in humans has found no benefit to adding an NSAID to local anesthetics combined with patient-controlled morphine after craniotomy.99 Concern regarding an increased risk of bleeding with subsequent intracranial hematoma has limited the widespread use of NSAID in neurosurgical patients.96 One large study of human craniotomy patients reported that NSAID use may have been the cause of the 1.1% incidence of postoperative hematomas, in that all but one patient with hematomas received an NSAID during the 2 wk preceding surgery.78 NSAID vary in their effects on platelet function, with COX2-specific NSAID less likely to clinically alter hemostasis in the postoperative period. Most trials demonstrating the adverse effects of NSAID have focused on long-term treatment for chronic pain disorders. Little attention has been given to trials to determine the relative benefits and risks of short-term administration in the perioperative setting. However, 7 d of treatment with flunixin, ketoprofen, and meloxicam increased bleeding time in dogs.64 In another study, ketoprofen had a more significant effect on hemostasis in dogs than did carprofen.32 However, NSAID vary among species in their potency and selectivity against COX1 and COX2.12 The effect of short-term, low-dose perioperative treatment with NSAID is unknown, but in view of the wide use of this class of analgesics, noteworthy adverse effects apparently are unlikely in normal, healthy nonhuman primates.
Adjunct treatments.
Dexamethasone frequently is used for treatment or prevention of cerebral edema during neurosurgery.37 The antiinflammatory properties of dexamethasone were recently investigated for use in reducing perioperative pain in humans.31 Although the dexamethasone-associated modulation of the immune response in reducing inflammation via suppression of prostaglandin synthesis has been thoroughly described, the analgesic mechanism of glucocorticoids is not understood. Dexamethasone has been demonstrated to suppress bradykinin and the release of other neuropeptides from nerve endings and to have a direct inhibitory effect on C fibers in injured nerves.38 Clinical trials have found variable effects of single-dose dexamethasone on pain depending on the type of surgery.48 Because the effect seems to be strongest in dental patients, the major analgesic effect is presumed to be related to reduction of local swelling and edema.38 Caution should be exercised when dexamethasone is added to a preoperative regimen including minimally selective NSAIDs. One safety study in dogs demonstrated decreased gastrointestinal health in as little as 3 d when therapeutic doses of dexamethasone and meloxicam were coadministered.12 When using poorly specific NSAID such as flunixin meglumine, these adverse effects can reasonably be anticipated to occur earlier. The lack of demonstrated analgesic effect of dexamethasone in neurosurgery prevents the recommendation of its use as an analgesic, but single-dose dexamethasone may be effective in other applications, including reduction of brain swelling, in nonhuman primates on neurosurgical protocols.21,65,93
Because craniotomies are associated with a high risk of subsequent seizures, prophylactic treatment with anticonvulsants is recommended in human patients.90 Anticonvulsants have been shown to reduce the risk of postoperative seizures in humans by 40% to 50%.90 Some researchers have reported anecdotally that they incorporate these drugs into their neurosurgery protocols in nonhuman primates. Because anticonvulsants reduce neuronal excitability, there is increasing interest in their use in the treatment of neuropathic pain.54 Phenytoin is a commonly used anticonvulsant in human and nonhuman primate medicine that is not practical for seizure control in small animals.80 Because phenytoin depresses nerve conduction, its analgesic activity has been investigated in people. Healthy volunteers reported a reduction in pain after a cold-pressor test with the use of phenytoin.98 However, another study found that phenytoin did not reduce postoperative pain or analgesic consumption;95 the authors also investigated the analgesic activity of gabapentin, an analog of the inhibitor neurotransmitter GABA, in surgical patients. Although gabapentin originally was developed as an anticonvulsive agent, its use in the treatment of chronic pain recently has expanded in both human and veterinary medicine.80,95 In healthy human volunteers, gabapentin augmented the analgesic activity of morphine,22 and in surgical patients, gabapentin has been shown to reduce both postoperative pain and morphine consumption.94,95 Although promising as adjunct agents, more evidence is needed for a recommendation to standardize the use of anticonvulsants in analgesic regimens for neurosurgical patients.
Conclusion
The concept of appropriate analgesia has evolved over the last several decades. Preemptive, multimodal analgesic therapy for procedures that are expected to cause pain is the standard of care in veterinary medicine and is required for laboratory animals. Combinations of local anesthetics, opioids, and NSAID provide adequate analgesia without significant adverse effects. Adjunct treatments such as glucocorticoids and anticonvulsants provide considerable benefits to human neurosurgical patients and may supplement analgesic regimens in animals. Researchers and veterinarians should work together to provide appropriate analgesia to nonhuman primates that undergo neurosurgery that is consistent with currently accepted standards.
References
- 1.Allison SO, Halliday LC, French JA, Novikov DD, Fortman JD. 2007. Assessment of buprenorphine, carprofen, and their combination for postoperative analgesia in olive baboons (Papio anubis). J Am Assoc Lab Anim Sci 46:24–31 [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Laboratory Animal Medicine. [Internet]. Position statement: adequate veterinary care. [Cited 23 March 2012]. Available at: http://www.aclam.org/Content/files/files/Public/Active/position_adeqvetcare.pdf.
- 3.Andaluz A, Moll X, Abellan R, Ventura R, Carbo M, Fresno L, Garcia F. 2009. Pharmacokinetics of buprenorphine after intravenous administration of clinical doses to dogs. Vet J 181:299–304 [DOI] [PubMed] [Google Scholar]
- 4.Animal Welfare Regulations. 2008. 9 CFR §2.33.
- 5.Association of Primate Veterinarians. [Internet]. Formulary. Lee DR, Doane CJ. [Cited 1 June 2012]. Available at: http://www.primatevets.org/content/files/public/education/nonhuman%20primate%20formulary.xls.
- 6.Bala I, Gupta B, Bhardwaj N, Ghai B, Khosla VK. 2006. Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesth Intensive Care 34:224–227 [DOI] [PubMed] [Google Scholar]
- 7.Becker DE, Reed KL. 2006. Essentials of local anesthetic pharmacology. Anesth Prog 53:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. 1998. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg 87:579–582 [DOI] [PubMed] [Google Scholar]
- 9.Bowdle TA. 1998. Adverse effects of opioid agonists and agonist–antagonists in anaesthesia. Drug Saf 19:173–189 [DOI] [PubMed] [Google Scholar]
- 10.Brideau C, Van Staden C, Chan CC. 2001. In vitro effects of cyclooxygenase inhibitors in whole blood of horses, dogs, and cats. Am J Vet Res 62:1755–1760 [DOI] [PubMed] [Google Scholar]
- 11.Ciofalo VB, Latranyi MB, Patel JB, Taber RI. 1977. Flunixin meglumine: a nonnarcotic analgesic. J Pharmacol Exp Ther 200:501–507 [PubMed] [Google Scholar]
- 12.Clark TP. 2006. The clinical pharmacology of cyclooxygenase-2–selective and dual inhibitors. Vet Clin North Am Small Anim Pract 36:1061–1085 [DOI] [PubMed] [Google Scholar]
- 13.Conner JM, Darracq MA, Roberts J, Tuszynski MH. 2001. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Natl Acad Sci USA 98:1941–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conzemius MG, Brockman DJ, King LG, Perkowsi SZ. 1994. Analgesia in dogs after intercostal thoracotomy: a clinical trial comparing intravenous buprenorphine and interpleural bupivacine. Vet Surg 23:291–298 [DOI] [PubMed] [Google Scholar]
- 15.Cowan A, Doxey JC, Harry EJ. 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol 60:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowan A, Lewis JW, Macfarlane IR. 1977. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis TS, Torab K, House P, Greger B. 2009. A minimally invasive approach to long-term head fixation in behaving nonhuman primates. J Neurosci Methods 181:106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. 2005. Comparison of the respiratory effects of intravenous buprenoprhine and fentanyl in humans and rats. Br J Anaesth 94:825–834 [DOI] [PubMed] [Google Scholar]
- 19.deGray LC, Matta BF. 2005. Acute and chronic pain following craniotomy: a review. Anaesthesia 60:693–704 [DOI] [PubMed] [Google Scholar]
- 20.Deneuche AJ, Dufayet C, Goby L, Fayolle P, Desbois C. 2004. Analgesic comparison of meloxicam or ketoprofen for orthopedic surgery in dogs. Vet Surg 33:650–660 [DOI] [PubMed] [Google Scholar]
- 21.Driesse MJ, Vincent AJ, Smitt PA, Hoogerbrugge PM, Avezaat CJ, Valerio D, Bout A. 1998. Intracerebral injection of adenovirus harboring the HSVtk gene combined with ganciclovir administration: toxicity study in nonhuman primates. Gene Ther 5:1122–1129 [DOI] [PubMed] [Google Scholar]
- 22.Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. 2000. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg 91:185–191 [DOI] [PubMed] [Google Scholar]
- 23.Faroqui MH, Cole M, Curran J. 1983. Buprenorphine, benzodiazepines, and respiratory depression. Anaesthesia 38:1002–1003 [DOI] [PubMed] [Google Scholar]
- 24.Flecknell PA. 1984. The relief of pain in laboratory animals. Lab Anim 18:147–160 [DOI] [PubMed] [Google Scholar]
- 25.Flecknell P. 2005. Clinical experience with NSAIDs in macaques. Lab Prim Newsl 44:4 [Google Scholar]
- 26.Flecknell P. 2009. Laboratory animal anesthesia, 3rd ed. London (UK): Academic Press. [Google Scholar]
- 27.Flexman AM, Ng JL, Gelb AW. 2010. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol 23:551–557 [DOI] [PubMed] [Google Scholar]
- 28.Fremont JJ, Marini RP, Fox JG, Rogers AB. 2008. Acute respiratory distress syndrome in 2 rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 47:61–66 [PMC free article] [PubMed] [Google Scholar]
- 29.Frey S, Comeau R, Hynes B, Mackey S, Petrides M. 2004. Frameless sterotaxy in the nonhuman primate. Neuroimage 23:1226–1234 [DOI] [PubMed] [Google Scholar]
- 30.Germain JL, Hermant JL, Hasnaoui AB, Casteran R, Lamotte N, Pouurriat JL. 1992. The use of buprenorphine for preoperative analgesia. Cah Anesthesiol 40:9–13 [PubMed] [Google Scholar]
- 31.Gottschalk A. 2009. Craniotomy pain: trying to do better. Anesth Analg 109:1379–1381 [DOI] [PubMed] [Google Scholar]
- 32.Grisneaux E, Pibarot P, Dupuis J, Blais D. 1999. Comparison of ketoprofen and carprofen administered prior to orthopedic surgery for control of postoperative pain in dogs. J Am Vet Med Assoc 215:1105–1110 [PubMed] [Google Scholar]
- 33.Gueye PN, Borron SW, Risede P, Monier C, Buneaux F, Debray M, Baud FJ. 2002. Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol Sci 65:107–114 [DOI] [PubMed] [Google Scholar]
- 34.Hassouneh B, Centofanti JE, Reddy K. 2011. Pain management in postcraniotomy patients: a survey of Canadian neurosurgeons. Can J Neurol Sci 38:456–460 [DOI] [PubMed] [Google Scholar]
- 35.Heel RC, Brogden RN, Speight TM, Avery GS. 1979. Buprenorphine: a review of its pharmacologic properties and therapeutic efficacy. Drugs 17:81–110 [DOI] [PubMed] [Google Scholar]
- 36.Hellyer P, Rodan I, Downing R, Hagedorn JE, Robertson SA. 2007. AAHA–AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc 43:235–248 [DOI] [PubMed] [Google Scholar]
- 37.Hockey B, Leslie K, Williams D. 2009. Dexamethasone for intracranial neurosurgery and anaesthesia. J Clin Neurosci 16:1389–1393 [DOI] [PubMed] [Google Scholar]
- 38.Holte K, Kehlet H. 2002. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 195:694–712 [DOI] [PubMed] [Google Scholar]
- 39.Hyatt J, Coro C, Bergman SA, Wynn RL. 1989. Effects of midazolam on fentanyl antinociception and respiration in a rabbit model. J Oral Maxillofac Surg 47:1298–1302 [DOI] [PubMed] [Google Scholar]
- 40.Ilkiw JE, Pascoe PJ, Tripp LD. 2002. Effects of morphine, butorphanol, buprenorphine, and U50588H on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 63:1198–1202 [DOI] [PubMed] [Google Scholar]
- 41.Institute for Laboratory Animal Research. 1992. Recognition and alleviation of pain and distress in laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 42.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 43.International Association for the Study of Pain. [Internet]. 1994. Part III: pain terms, a current list with definitions and notes on usage. In: Merskey H, Bogduk N. Classification of chronic pain, 2nd ed. IASP Task Force on Taxonomy, Seattle (WA): IASP Press. [Cited 19 March 2012]. Available at: http://www.iasp-pain.org/Content/NavigationMenu/GeneralResourceLinks/PainDefinitions/default.htm.
- 44.Izquierdo A, Murray EA. 2005. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci 22:2341–2346 [DOI] [PubMed] [Google Scholar]
- 45.Jackson A, Fetz EE. 2007. Compact movable microwire array for long-term chronic unit recording in cerebral cortex of primates. J Neurophysiol 98:3109–3118 [DOI] [PubMed] [Google Scholar]
- 46.Johnson RE, Fudala PJ, Payne R. 2005. Buprenorphine: considerations for pain management. J Pain Symptom Manage 29:297–326 [DOI] [PubMed] [Google Scholar]
- 47.Jones SJ, Cormack J, Murphy MA, Scott DA. 2009. Parecoxib for analgesia after craniotomy. Br J Anaesth 102:76–79 [DOI] [PubMed] [Google Scholar]
- 48.Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. 2008. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg 106:1253–1257 [DOI] [PubMed] [Google Scholar]
- 49.Kelly DJ, Ahmad M, Brull SJ. 2001. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth 48:1000–1010 [DOI] [PubMed] [Google Scholar]
- 50.Kelly KR, Pypendop BH, Grayson JK, Stanley SD, Christe KL, Summers LM, Lerche NW. 2011. Pharmacokinetics of oxymorphone in titi monkeys (Callicebus spp.) and rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 50:212–220 [PMC free article] [PubMed] [Google Scholar]
- 51.Kishioka S, Paronis CA, Lewis JW, Woods JH. 2000. Buprenorphine and methoclocinnamox: agonist and antagonist effects on respiratory function in rhesus monkeys. Eur J Pharmacol 391:289–297 [DOI] [PubMed] [Google Scholar]
- 52.Klimek M, Ubben JF, Ammann J, Borner U, Klein J, Verbrugge SJ. 2006. Pain in neurosurgically treated patients: a prospective observational study. J Neurosurg 104:350–359 [DOI] [PubMed] [Google Scholar]
- 53.Knoche E, Dick W, Rummel C, Koneitzke D. 1988. Quality of buprenorphine and morphine as components of combined anesthesia. Anaesthesist 37:57–64 [PubMed] [Google Scholar]
- 54.Knotkova H, Pappagallo M. 2007. Adjuvant analgesics. Anesthesiol Clin 25:775–786 [DOI] [PubMed] [Google Scholar]
- 55.Ko JC, Freeman LJ, Barletta M, Weil AB, Payton ME, Johnson BM, Inoue T. 2011. Efficacy of oral transmucosal and intravenous administration of buprenorphine before surgery for postoperative analgesia in dogs undergoing ovariohysterectomy. J Am Vet Med Assoc 238:318–328 [DOI] [PubMed] [Google Scholar]
- 56.Lacan G, DeSalles AAF, Gorgulho AA, Krahl SE, Frighetto L, Behnke EJ, Melega WP. 2008. Modulation of food intake following deep brain stimulation of the ventromedial hypothalamus in the vervet monkey: laboratory investigation. J Neurosurg 108:336–342 [DOI] [PubMed] [Google Scholar]
- 57.Lamont LA. 2008. Multimodal pain management in veterinary medicine: the physiologic basis of pharmacologic therapies. Vet Clin North Am Small Anim Pract 38:1173–1186 [DOI] [PubMed] [Google Scholar]
- 58.Law-Koune JD, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. 2005. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J Neurosurg Anesthesiol 17:139–143 [DOI] [PubMed] [Google Scholar]
- 59.Lavenex PB, Amaral DG, Lavenex P. 2006. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci 26:4546–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JI, Kim Y, Kim M, Hong SH. 2008. Idiopathic new bone formation in the femoral shafts of a cynomolgus monkey (Macaca fasciularis). J Am Assoc Lab Anim Sci 47:68–71 [PMC free article] [PubMed] [Google Scholar]
- 61.Leece EA, Brearley JC, Harding EF. 2005. Comparison of carprofen and meloxicam for 72 hours following ovariohysterectomy in dogs. Vet Anaesth Analg 32:184–192 [DOI] [PubMed] [Google Scholar]
- 62.Liguori A, Morse WH, Bergman J. 1996. Respiratory effects of opioid full and partial agonists in rhesus monkeys. J Pharmacol Exp Ther 277:462–472 [PubMed] [Google Scholar]
- 63.Lintzeris N, Mitchell TB, Bond A, Nestor L, Strang J. 2006. Interactions on mixing diazepam with methadone or buprenorphine in maintenance patients. J Clin Psychopharmacol 26:274–283 [DOI] [PubMed] [Google Scholar]
- 64.Luna SP, Basilio AC, Steagall PV, Machado LP, Moutinho FQ, Takahira RK, Brandao CV. 2007. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res 68:258–264 [DOI] [PubMed] [Google Scholar]
- 65.Machado CJ, Bachevalier J. 2007. The effects of selective amygdale, orbital frontal cortex, or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci 25:2885–2904 [DOI] [PubMed] [Google Scholar]
- 66.Maunuksela EL, Korpela R, Olkkola KT. 1988. Double-blind, multiple-dose comparison of buprenorphine and morphine in postoperative pain of children. Br J Anaesth 60:48–55 [DOI] [PubMed] [Google Scholar]
- 67.Mohammadi SS, Shahbazian E, Shoeibi G, Almassi F. 2009. Effect of scalp infiltration with bupivacaine on early hemodynamic responses during craniotomy under general anesthesia. Pak J Biol Sci 12:603–606 [DOI] [PubMed] [Google Scholar]
- 68.Mordhorst C, Latz B, Kerz T, Wisser G, Schmidt A, Schneider A, Jahn-Eimermacher A, Werner C, Engelhard K. 2010. Prospective assessment of postoperative pain after craniotomy. J Neurosurg Anesthesiol 22:202–206 [DOI] [PubMed] [Google Scholar]
- 69.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress, and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116:431–436 [DOI] [PubMed] [Google Scholar]
- 70.Na HS, An SB, Park HP, Lim YJ, Hwang JW, Jeon TY, Min SW. 2011. Intravenous patient-controlled analgesia to manage the postoperative pain in patients undergoing craniotomy. Korean J Anesthesiol 60:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Negus SS, Bidlack JM, Mellow NK, Furness MS, Rice KC, Brandt MR. 2002. Delta-opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol 13:557–570 [DOI] [PubMed] [Google Scholar]
- 72.Nguyen A, Girard F, Boudreault D, Furgere F, Ruel M, Moumdjian R, Bouthilier A, Caron JL, Bojanowski MW, Girard DC. 2001. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg 93:1272–1276 [DOI] [PubMed] [Google Scholar]
- 73.Ohtani M. 2007. Basic pharmacology of buprenorphine. Eur J Pain Suppl 1:69–73 [Google Scholar]
- 74.Ohtani M, Kotaki H, Nishitateno K, Sawada Y, Iga T. 1997. Kinetics of respiratory depression in rats induced by buprenorphine and its metabolite, norbuprenorphine. J Pharmacol Exp Ther 281:428–433 [PubMed] [Google Scholar]
- 75.Osborn I, Sebeo J. 2010. ‘Scalp block’ during craniotomy: a classic technique revisted. J Neurosurg Anesthesiol 22:187–194 [DOI] [PubMed] [Google Scholar]
- 76.Ortiz-Cardona J, Bendo AA. 2007. Perioperative pain management in the neurosurgical patient. Anesthesiol Clin 25:655–674 [DOI] [PubMed] [Google Scholar]
- 77.Pakulski C, Nowicki R, Badowicz B, Bak P, Mikulski K, Wojnarska B. 2001. Effect of scalp infiltration with lidocaine on the circulatory response to craniotomy. Med Sci Monit 7:725–728 [PubMed] [Google Scholar]
- 78.Palmer JD, Sparrow OC, Ianotti F. 1994. Postoperative haematoma: a 5-year survey and identification of avoidable risk factors. Neurosurgery 35:1061–1065 [DOI] [PubMed] [Google Scholar]
- 79.Papich MG. 2000. Pharmacologic considerations for opiate analgesic and nonsteroidal antiinflammatory drugs. Vet Clin North Am Small Anim Pract 30:815–837 [DOI] [PubMed] [Google Scholar]
- 80.Papich MG. 2007. Saunders handbook of veterinary drugs, 2nd ed. St Louis (MO): Saunders. [Google Scholar]
- 81.Pascoe PJ. 2000. Opioid analgesics. Vet Clin North Am Small Anim Pract 30:757–772 [DOI] [PubMed] [Google Scholar]
- 82.Pirnay SO, Megarbane B, Borron SW, Risede P, Monier C, Ricordel I, Baud FJ. 2008. Effects of various combinations of benzodiazepines with buprenorphine on arterial blood gases in rats. Basic Clin Pharmacol Toxicol 103:228–239 [DOI] [PubMed] [Google Scholar]
- 83.Plumb DC. 2005. Plumb's veterinary drug handbook, 5th ed. Ames (IA): Blackwell. [Google Scholar]
- 84.Popilskis SJ, Lee DR, Elmore DB. 2008. Anesthesia and analgesia in nonhuman primates, p 335–363. In: Fish RE, Brown MJ, Danneman PJ, Kara AZ. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 85.Portier KG, Broillet A, Rioful G, Lepage OM, Depecker M, Taborik F, Contamin H. 2012. A novel minimal invasive closed-chest myocardial ischemia reperfusion model in rhesus monkeys (Macaca mulatta): improved stability of cardiorespiratory parameters. Lab Anim 46:129–135 [DOI] [PubMed] [Google Scholar]
- 86.Rao P, Knaus EE. 2008. Evolution of nonsteroidal antiinflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci 11:81s–110s [DOI] [PubMed] [Google Scholar]
- 87.Rosenberg DP. 1991. Nonhuman primate analgesia. Lab Anim 20:22–32 [Google Scholar]
- 88.Saleem KS, Tanaka K. 1996. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci 16:4757–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sekar M, Mimpriss TJ. 1987. Buprenorphine, benzodiazepines, and prolonged respiratory depression. Anaesthesia 42:567–568 [DOI] [PubMed] [Google Scholar]
- 90.Temkin NR. 2002. Prophylactic anticonvulsants after neurosurgery. Epilepsy Curr 2:105–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tranquilli WJ, Grimm KA, Lamont LA. 2000. Pain management for the small animal practitioner, 2nd ed. Jackson (WY): Teton. [DOI] [PubMed] [Google Scholar]
- 92.Trescot AM, Datta S, Lee M, Hansen H. 2008. Opioid pharmacology. Pain Physician 11:S133–S153 [PubMed] [Google Scholar]
- 93.Tu HW, Hampton RR, Murray EA. 2011. Perirhinal cortex removal dissociates 2 memory systems in matching-to-sample performance in rhesus monkeys. J Neurosci 31:16336–16343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turan A, Karamanlioglu B, Memis D, Hamamcioglu MK, Tukenmez B, Pamukcu Z, Kurt I. 2004. Analgesic effects of gabapentin after spinal surgery. Anesthesiology 100:935–938 [DOI] [PubMed] [Google Scholar]
- 95.Ture H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Ture U. 2009. The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: a prospective randomized study. Anesth Analg 109:1625–1631 [DOI] [PubMed] [Google Scholar]
- 96.Umamaheswara Rao GS, Gelb AW. 2009. To use or not to use: the dilemma of NSAIDs and craniotomy. Eur J Anaesthesiol 26:625–626 [DOI] [PubMed] [Google Scholar]
- 97.Vadivelu N, Anwar M. 2010. Buprenorphine in postoperative pain management. Anesthesiol Clin 28:601–609 [DOI] [PubMed] [Google Scholar]
- 98.Webb J, Kamali F. 1998. Analgesic effects of lamotrigine and phenytoin on cold-induced pain: a crossover placebo-controlled study in healthy volunteers. Pain 76:357–363 [DOI] [PubMed] [Google Scholar]
- 99.Williams DL, Pemberton E, Leslie K. 2011. Effect of intravenous parecoxib on postcraniotomy pain. Br J Anaesth 107:398–403 [DOI] [PubMed] [Google Scholar]
- 100.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. 2012. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135:2277–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]