Abstract
The decrease with age of the adrenal-secreted dehydroepiandrosterone sulfate (DHEAS) in serum has suggested that it may be causally related to longevity. For the PAQUID [People (Personnes) Aged (Agées) About What (Quid, in Latin)] cohort of elderly subjects, we have previously reported higher DHEAS in men than in women, a decrease with age and, among men, a negative correlation between the DHEAS level and mortality at 2 and 4 years. Here, with an 8-year followup in 290 subjects, we show a global decrease of 2.3% per year for men and 3.9% per year for women. However, in approximately 30% of cases, there was an increase of DHEAS. We observed no relationship between the evolution of DHEAS level and functional, psychological, and mental status, possibly because of selection by death. In women, no association was found between mortality and DHEAS level. In men, the relative risk (RR) of death was higher for the lowest levels of DHEAS (RR = 1.9, P = 0.007), with RR = 6.5, P = 0.003 for those under 70 years old, a result indicating heterogeneity of the population. There was an effect of subjective health on mortality that disappeared after adjustment of DHEAS levels, suggesting its relation with these DHEAS levels. Death RR was much higher in smokers with a low DHEAS level than in nonsmokers with high DHEAS (RR = 6.7, P = 0.001). We submit that the involvement of DHEAS is possibly different according to gender, that association between low DHEAS level and mortality only for men under 70 years old possibly reflects heterogeneity of the population, and that DHEAS level is a reliable predictor of death in male smokers.
In human beings, dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) are secreted adrenal steroids whose role is still poorly understood. Except in the nervous system, no receptor for those steroids has been described, and they likely operate in part by a transformation into androgens and estrogens (1–5) and probably also directly as neuroactive steroids (6). Furthermore, DHEAS concentration declines with age (7, 8) and remains 10–20% higher in men than in women (9), despite an important interindividual range.
The decline of DHEAS concentrations with aging has led to the suggestion that DHEAS could play a role in itself and be implicated in longevity. Thus, DHEAS has been studied with controversial results in several processes that occur during aging (10, 11). In particular with reference to the mortality issue studied in this paper, we note that DHEAS has been inversely related to cardiovascular diseases in men (12–16), but this relation was not found in women (17).
To clarify the role of DHEAS, our strategy within the PAQUID‖ program (a prospective cohort study of elderly subjects from the southwest of France) was to relate DHEAS level with several health parameters and mortality. Among the 3,777 elderly subjects of this cohort, 622 volunteers agreed to have blood sampling after 1 year of followup. The measurement of DHEAS concentrations at this time allowed us to confirm the already known decrease of DHEAS level with age and the difference according to sex with a higher DHEAS level in men. Significantly lower values of DHEAS were recorded in women in cases of functional limitation, confinement, dyspnea, depressive symptomatology, and frequent use of medications. No relation was found with cases of incident dementia in the following 4 years. In men, but not in women, lower DHEAS concentrations were significantly associated with an increased short-term mortality at 2 and 4 years after DHEAS measurement (18). Among these 622 subjects, 346 had a second measurement of DHEAS level 7 years after the first.
Intraindividual changes of DHEAS level over adulthood are insufficiently documented in longitudinal studies (8, 19–24). The knowledge of intraindividual evolution of DHEAS level would be of importance to determine the possible pathological role of DHEAS. Here the evolution of the DHEAS level over 7 years is described in a relatively large population of old men and women We also studied the relation between changes in DHEAS level and several health parameters. Finally, we examined mortality at 10 years of followup as a function of DHEAS level.
Materials and Methods
Samples.
The PAQUID study is a prospective cohort of elderly subjects living in the southwest of France. This study began in 1988 and included 3,777 subjects (2,792 subjects in the Gironde area, 985 in the Dordogne area). To participate in this study, subjects had to be over 65 years old on December 31, 1987, living at home, and on the local electoral list.
All participants had a standardized interview that included sociodemographic items, several health parameters, and current medications. Furthermore, a course of psychometric tests was undertaken. Subjects were re-evaluated 1, 3, 5, 8, and 10 years after the baseline visit, following the same method.
Among the 2,792 subjects from the Gironde, 622 volunteers agreed to have blood sampling at a 1-year followup. At an 8-year followup, 346 of them (56%) accepted a new blood sampling, and the others subjects refused (124 subjects, 20%) or were dead (152 subjects, 24%). The mean time between the two measurements was 6.3 years (SD = 6 months).
Serum DHEAS Measurement.
Blood samples were kept in liquid nitrogen until analysis [serum DHEAS is not affected by freezing (8)]. DHEAS concentrations were measured directly in serum by an automated immunoenzymatic assay on a Serono (Geneva) SR1 analyzer. Correlation with standard radioimmunoassay was assessed (r = 0.98) (18). The lower limit of detection of the assay was 16 ng/ml. The intraassay coefficient of variation was 6%, and the interassay coefficient of variation ranged from 3% (concentrations lower than 100 ng/ml) to 20% (concentrations higher than 1,000 ng/ml).
The evolution of the DHEAS level during the longitudinal study is calculated by subtracting the DHEAS concentration at an 8-year followup (DHEAS2) from the first measurement (DHEAS1).
Not all participants who had blood sampled were interviewed in the same year as the assay. So, for the statistical analysis, only those subjects having had the followup and measurement of DHEAS level at the same time were retained, that is, 595 subjects for the first measurement (253 men, 342 women) and 290 subjects for the measurement at 8-year followup (119 men, 171 women).
Health Parameters.
The health parameters were classified as follows:
(i) Sociodemographic items such as age, sex and education with two categories: no schooling or primary school level for one category, secondary school level and over for the other.
(ii) Lifestyle such as cigarette smoking (nonsmoker, smoker, and former smoker); wine consumption with three levels: never drinking, 0.25 or more than 0.25 liters per day; and current physical activity.
(iii) Medical parameters such as weight, height, current medication with three classes (no or only one, two to four, or more than four medications), personal history of heart disease, stroke, or peripheral artery disease, dyspnea defined by Vestbo et al. (25). The physical functional disability was measured by four scales, which are the Activities of Daily Living (ADL) of Katz et al. (26), the Instrumental Activities of Daily Living (IADL) of Lawton and Brody (27), the scale of Rosow and Breslau for mobility (28), and confinement when subjects were limited to home or bed. Subjective health was assessed by the following formulation: Do you presently rate your health status very good, good, fair, bad, or very bad? Subjects were considered in poor condition if they rated themselves as fair, bad, or very bad. Depressive symptomatology was measured by the Center for Epidemiological Studies–Depression (CES–D) scale (29). The presence of a depressive symptomatology was defined by a CES–D scale under 16 for men and under 22 for women. Global cognitive functions were assessed by the Mini Mental State Examination (MMSE) of Folstein et al. (30), with scores ranging from 0 to 30. A low MMSE score was defined by a score under 24 for low educational level, less than 26 for high educational level.
Global mortality of the cohort which was determined for each followup.
Statistical Analysis.
Analysis was conducted by using sas* software (SAS Institute, Cary, NC). Because DHEAS levels are different in men and women, with possibly different biological effects, all analyses were performed as a function of sex. Means (m) of DHEAS are given with SD.
First, we studied the evolution of DHEAS level over 7 years. We examined the variability of DHEAS level by using the same approach as Orentreich et al. (22), who determined cutoff values for the long-term change of the DHEAS level. In their study, these authors suggested that a true long-term change in DHEAS concentrations is indicated by a variability of DHEAS level of more than 19%, because they have previously shown a mean short-term variability of 19% in normal men. Second, a relationship between the evolution of DHEAS level and several health parameters was investigated. These analyses were performed by ANOVA, single or multifactorial. Next, we compared the initial DHEAS level with respect to the situation of the subjects at an 8-year followup (second measurement of DHEAS level, refusal, or death before the second measurement) by using a Student's t test. Finally, we studied the cumulative mortality at an 8-year followup according to the first measurement of DHEAS and several health parameters collected at a 1-year followup, by using a Cox model with delayed entry (31) (age considered as time scale) to adjust for several covariates. We defined a population with a low level of DHEAS at the first assay when this level was inferior to the lowest quartile of DHEAS concentrations, taking age and gender into account.
Results
Cross-Sectional Study: Sampling.
The 595 subjects who had the first DHEAS measurement have been compared with other subjects of the PAQUID cohort whose DHEAS was not measured. These 595 subjects did not differ from the others in term of sex, presence or absence of dyspnea or depressive symptomatology, subjective health, cognitive functions, and medication consumption. However, they were younger (75.2 vs. 76.5, P = 0.0001), had a higher educational level (P = 0.05), were less dependent in terms of ADL (P = 0.05) and IADL (P = 0.001), and had better mobility (P = 0.01) (18).
The subjects whose DHEAS level was measured at an 8-year followup were compared with those whose DHEAS was not measured, because they were dead or refused the followup (Tables 1 and 2). For men as for women, the health parameters at 1-year followup were better for subjects who had the second DHEAS measurement than for those who refused the analysis; these parameters in turn were better for those who had died (see Tables 1 and 2 for age, educational level, dependence, cognitive functions, and medication consumption). The men who agreed to the second DHEAS measurement were less depressed (P = 0.004) and had shown better subjective health (P = 0.001) at the time of the first assay than those who refused or were dead, but these differences were not significant in women.
Table 1.
Health parameters at 1-year followup according to the outcome for men at 8-year followup
| Health parameters at 1 year | Second assay n = 130 | Refused n = 54 | Dead n = 69 | P |
|---|---|---|---|---|
| Age at 1 year: m, SD | 73 (5) | 75 (6) | 77 (5) | 0.0001 |
| No schooling or primary school level, % | 15 | 19 | 30 | 0.03 |
| ADL limitation, % | 8 | 4 | 20 | 0.004 |
| IADL limitation, % | 9 | 11 | 35 | 0.001 |
| Confinement, % | — | 2 | 10 | 0.0003 |
| Rosow limitation, % | 46 | 70 | 84 | 0.001 |
| Dyspnea, % | 9 | 24 | 26 | 0.004 |
| Poor subjective health, % | 25 | 46 | 49 | 0.001 |
| Depressive symptomatology, % | 2 | 7 | 15 | 0.004 |
| MMS score: m, SD | 27 (3) | 27 (3) | 26 (5) | 0.02 |
| Number of medications: m, SD | 3 (2) | 4 (2) | 6 (3) | 0.0001 |
n = 253.
Table 2.
Health parameters at 1-year followup, according to the outcome for women at 8-year followup
| Health parameters at 1 year | Second assay n = 189 | Refused n = 70 | Dead n = 83 | P |
|---|---|---|---|---|
| Age at 1 year: m, SD | 74 (5) | 76 (7) | 82 (8) | 0.0001 |
| No schooling or primary school level, % | 30 | 26 | 43 | 0.04 |
| ADL limitation, % | 10 | 9 | 33 | 0.001 |
| IADL limitation, % | 16 | 29 | 60 | 0.001 |
| Confinement, % | 1 | 3 | 25 | 0.001 |
| Rosow limitation, % | 77 | 84 | 93 | 0.006 |
| Dyspnea, % | 22 | 28 | 49 | 0.001 |
| Poor subjective health, % | 39 | 47 | 52 | NS |
| Depressive symptomatology, % | 9 | 16 | 15 | NS |
| MMS score: m, SD | 27 (3) | 27 (3) | 22 (9) | 0.0001 |
| Number of medications: m, SD | 4 (3) | 5 (3) | 5 (3) | 0.002 |
n = 342. NS, not significant.
Serum DHEAS Level.
As for the first measurement of DHEAS, the second measurement of DHEAS at 8-year followup showed higher values in men than in women and in younger than in older subjects, and remarkably the same levels of DHEAS were observed for each age class (Table 3).
Table 3.
DHEAS level at 1-year followup (DHEAS1) and at 8-year followup (DHEAS2), according to age and gender
| Age at time of measurement of DHEAS level | Men
|
Women
|
|||
|---|---|---|---|---|---|
| DHEAS1 | DHEAS2 | DHEAS1 | DHEAS2 | ||
| <70 years | n | 71 | 80 | ||
| m (SD) | 1001 (678) | 660 (452) | |||
| 70–74 years | n | 66 | 21 | 71 | 28 |
| m (SD) | 903 (620) | 1048 (591) | 522 (325) | 558 (484) | |
| 75–79 years | n | 68 | 46 | 90 | 55 |
| m (SD) | 761 (499) | 777 (415) | 499 (363) | 563 (362) | |
| ≥80 years | n | 48 | 52 | 101 | 88 |
| m (SD) | 692 (383) | 731 (446) | 486 (386) | 427 (286) | |
| P | 0,02 | 0,03 | 0,02 | 0,05 | |
n, number of subjects; m, mean (ng/ml); DHEAS1, DHEAS2, DHEAS level at 1-year and 8-year followup, respectively.
Correlations Between the Second DHEAS Measurement and Health Parameters at the Same Time.
Previously, we reported a correlation between DHEAS level at 1 year and subjective health in men and women, and among women only, an association with dependence, dyspnea, depressive symptomatology, and use of medications. At the 8-year followup, we observed higher DHEAS level in both sexes only when subjects used less than two medications, when men had better mobility, and when women were less dependent by IADL. Thus we did not confirm several associations between DHEAS level and health parameters, including subjective health observed at the time of the first measurement. No association was found between DHEAS level and MMSE score.
Furthermore, reanalyzing the relationship between the first DHEAS measurement and health parameters observed in men and women whose DHEAS level was reassayed at the 8-year followup, we found in men the same relationship with subjective health as in the whole initial sample, but in women, a relation only with depressive symptomatology and not with the other parameters was found to be associated with DHEAS level.
Longitudinal Study: Evolution of DHEAS Level.
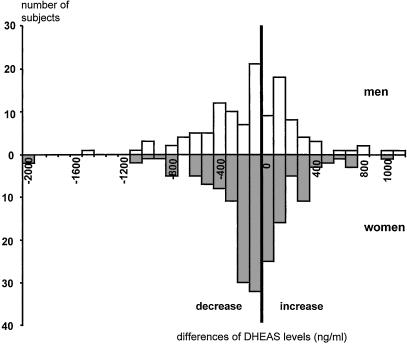
There was a trend to decline of DHEAS from 2.3% per year among men (P = 0.5) and from 3.9% per year among women (P = 0.08). However, 40% of men and 39% of women showed an increase of DHEAS level. Differences between the two measurements of DHEAS are reported in Fig. 1.
Figure 1.
Evolution of DHEAS levels over 7 years, as shown by the difference between DHEAS levels at 8- and 1-year followup. A positive difference means an increase of DHEAS levels; a negative difference means a decrease.
Lack of Correlation Between Evolution of DHEAS Level and Health Parameters.
To explain the variability of DHEAS levels over 7 years, we studied the relationship between the evolution of DHEAS level and several health parameters. We found a negative correlation between the evolution of DHEAS levels and the initial DHEAS levels (P = 0.0001), and thus a lower initial DHEAS level tended to lead to an increase in DHEAS level; conversely, higher initial DHEAS level led to a more pronounced decrease over 7 years. However, no association was found with health parameters collected initially even after adjustment for initial DHEAS level and age. Furthermore, we examined changes over 7 years regarding the MMSE score, depressive symptomatology, and subjective health: no association was found with the evolution of DHEAS level, adjusted to the initial DHEAS level, except for an increase in men of the MMS score which was correlated to an increase of 179 ng/ml of DHEAS level (P = 0.01).
Heterogeneity as an Explanation?
To explain these negative results, we studied the cohort attrition as a function of the first DHEAS level. We found higher DHEAS at the 1-year followup for men who accepted a second DHEAS assay at the 8-year followup, in comparison to those who died before this second measurement [m = 929 ng/ml (559) vs. m = 724 ng/ml (724), P = 0.02]. A similar but not significant trend was found for women. There was no difference in initial DHEAS level between subjects agreeing to or refusing the second DHEAS measurement.
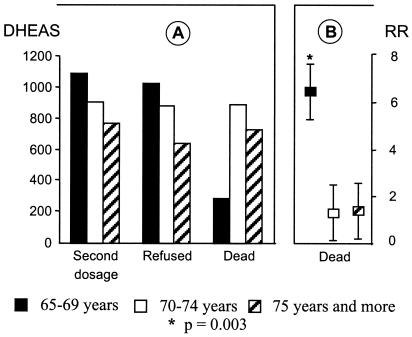
When studying initial levels of DHEAS according to three age classes (65–69, 70–74, ≥75 years), it was lower for older than for younger subjects both in men (Fig. 2) and in women and whatever their future status, except for men less than 70 years old who died before the second measurement. Indeed, in this latter group of seven men, the DHEAS level was particularly low (m = 289 ng/ml, SD = 252), in comparison with the older men who also died before the second measurement. This result suggests a strong selection by death and a heterogeneity of the initial sample because low DHEAS may predict death only for the 65- to 69-year-old group of men.
Figure 2.
Relationship between initial DHEAS level and mortality among men. (A) Initial DHEAS level according to age and outcome of men at 8 years. (B) RR of death at 10 years according to age, in men with low DHEAS level.
Survival According to Initial DHEAS Level.
In men, the relative risk of death at 10-year followup was doubled when the first DHEAS level was low (RR = 1.9 [1.2–2.8], P = 0.003) but not in women (RR = 0.8 [0.5–1.2], P = 0.3). When we studied mortality among men within the three age groups, we noticed a much higher relative risk of death associated with low DHEAS level only for the younger group (RR = 6.5, P = 0.003 for men of 65–69 years; RR = 1.3, P = 0.6 for men of 70–74 years; and RR = 1.5, P = 0.2 for men of over 75 years). For women, there was no effect on DHEAS level on the relative risk of death for any of the three age groups.
As a second step, for men only, we studied several health parameters that may potentially confound or mediate the effect of DHEAS level on survival (Table 4). There was a higher risk of death in smokers, subjects with a poor subjective health, and subjects consuming more than five medications. After adjustment of all predictors of survival (associated with mortality in the univariate analysis with P < 0.25, that is DHEAS level, tobacco consumption, subjective health, medication consumption, physical activity, history of peripheral arterial disease, and history of heart disease), only two factors remain associated with mortality: the initial DHEAS level and tobacco consumption. There was no significant difference of DHEAS level related to smoking habits.
Table 4.
RR of death at 10-year followup among men, according to health parameters at 1-year followup (univariate analysis)
| Health parameters | RR | IC 95% | P |
|---|---|---|---|
| DHEAS level | |||
| Low vs. high | 1.9 | (1.2–2.8) | 0.003 |
| Tobacco consumption | |||
| Smoker vs. nonsmoker | 2.1 | (1.1–4.1) | 0.03 |
| Former smoker vs. nonsmoker | 1.7 | (1.0–2.7) | 0.04 |
| Subjective health | |||
| Poor vs. good | 1.5 | (1.0–2.3) | 0.04 |
| Medications | |||
| 2–4 vs. 0–1 medications | 1.5 | (0.7–3.4) | 0.3 |
| 5 and more vs. 0–1 medications | 3.0 | (1.4–6.3) | 0.005 |
| Current physical activity | |||
| Yes vs. no | 0.6 | (0.4–1.1) | 0.09 |
| History of peripheral arterial disease | |||
| Yes vs. no | 1.6 | (1.0–2.8) | 0.08 |
| History of heart disease | |||
| Yes vs. no | 1.4 | (0.9–2.1) | 0.2 |
| Blood pressure | |||
| >16/9.5 vs. <16/9.5 | 1.2 | (0.6–2.4) | 0.6 |
| History of stroke | |||
| Yes vs. no | 1.1 | (0.6–2.0) | 0.7 |
| Wine consumption | |||
| 0.25 liters vs. nondrinking | 0.8 | (0.5–1.3) | 0.4 |
| More than 0.25 liters vs. nondrinking | 1.0 | (0.6–1.7) | 1.0 |
| Depressive symptomatology | |||
| Yes vs. no | 1.5 | (0.7–2.8) | 0.3 |
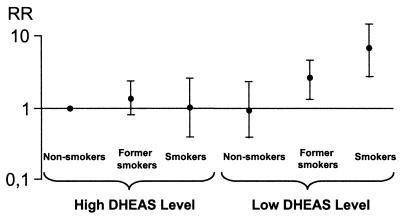
Furthermore, there was a strong interaction between smoking habits and DHEAS level (P < 0.02) as far as effect on mortality is concerned.
Taking into account this interaction (Fig. 3), we noticed only a slight nonsignificant increase of the risk of death in current or former smokers when DHEAS level was high (RR = 1.0 and RR = 1.3, respectively). Conversely, when DHEAS level was low, we observed a higher risk of death in smokers (RR = 6.7, P = 0.001) and in former smokers (RR = 2.5, P = 0.006), taking nonsmokers with high DHEAS levels as the reference.
Figure 3.
RR of death at 10 years, in men, according to smoking habits and initial DHEAS level (multiple regression analysis).
Discussion
Our study indicates three main points: First, the longitudinal study of DHEAS level showed a global trend to decline, but an increase of DHEAS level in one-third of our subjects. However, no health parameter could explain this evolution except the initial DHEAS level. Second, several results suggested selection by death (heterogeneity of the population) in men but not in women. Third, we found a synergetic effect of tobacco consumption and low initial DHEAS level on 10-year mortality in men.
The population studied here is not exactly representative of the entire cohort of the PAQUID program, even if they are very similar. Only two measurements of DHEAS serum concentration were obtained in 290 subjects at 7-year intervals, thus limiting the power of longitudinal analysis. However, the remarkable stability of levels recorded for the same ages after a 7-year interval indicates confidence in the reliability of DHEAS level assessment. Recorded health information was obtained only by self report, not systematically checked by a physician, and causes of death were not documented.
Longitudinal Study.
A subsample of the PAQUID cohort has allowed us to investigate a second assay of DHEAS level at an 8-year followup in a large cohort of elder subjects living at home and to compare their survival with the initial DHEAS level. We found a global trend to decline of DHEAS level over the 7 years at 2.3% per year among men and 3.9% per year among women. This decline is consistent with those found by several (20, 21, 23, 24) but not all authors (19). Despite this global decline, we found an increase of DHEAS level in approximately one-third of our subjects (Fig. 1). This phenomenon was already observed by Orentreich et al. (22), who found an increase of more than 10% in DHEAS level with 19.6% of 97 men aged 32–83 years old followed over 10 years. Thus, even if classically there is a lower DHEAS level in older people (cross-sectional studies), a decrease of DHEAS level is not observed in each subject (longitudinal studies). Indeed, the evolution of the DHEAS level was inversely correlated with the baseline DHEAS level, leading to a decrease of DHEAS level when the initial DHEAS concentration was high and to an increase when the initial DHEAS level was low. This relationship was probably because of a regression toward the mean and has been found in other longitudinal studies (19, 20). We found no significant relationship between the evolution of DHEAS level and several health parameters, even those known to be cross-sectionally associated with this level (18). The only significant relationship was found in eight subjects with an increase in MMSE performance over 7 years. But this fact is difficult to interpret because of the multiplicity of the tests and the low number of subjects. Furthermore, in others studies (19, 20), there was no relation between longitudinal change of DHEAS sulfate and the cognitive status. The global negative results could be explained by a lack of validity in data collection or by confounders not taken into account in our analysis, but the main explanation is certainly selection by death, evoking a possible population heterogeneity.
Population Heterogeneity?
Several results suggest heterogeneity of the studied population:
(i) In women, cross-sectional relationships between DHEAS level and health parameters observed initially were not found at the 8-year followup.
(ii) Among men who died during the interval, we observed very low initial DHEAS levels only in subjects less than 70 years old, suggesting that this deficiency in DHEAS serum concentration could predict mortality. In fact, there is a relative risk of death twice higher in men with low initial DHEAS level, actually because of the very high risk of death (RR = 6.5, P = 0.003) of men under 70 years old. Therefore, by de facto self selection of more robust subjects, low DHEAS level no longer predicted mortality in older men. Thus, the surviving sample differs from the initial sample because the initial sample represents a heterogeneous population composed of various homogeneous subgroups. This observation of heterogeneity is well known in aging studies, and by leading to selection, it may interfere with the correlation among variables (32). For example, Kähönen et al. (19) failed to correlate initial DHEAS levels and 10-year survival, possibly because their 271 subjects were too old (75 years and over).
Gender and DHEAS.
The absence of effect of a low DHEAS level on mortality in women is consistent with the concept that DHEAS plays different roles according to gender. First, DHEAS levels are higher in men than in women. Second, the metabolism of DHEA and DHEAS is somewhat different in men and women (33–36), but we are unable to draw any deduction as to the role of these observed differences. For cardiovascular mortality, Barret-Connor et al. have suggested a lack of predictive effect of DHEAS level in women (17), contrary to men (15). As indicated previously (18), differences between men and women were observed when studying the relation between DHEAS level and health parameters. Furthermore, we did not find any relation between DHEAS level and smoking habits in women, as was found in men. However, in our sample, only 5% of women were smokers. If our hypothesis (see later), that mortality in men is associated with DHEAS level through smoking habits, is reliable, it is not surprising that no association between survival and DHEAS level was found in women.
Subjective Health.
Both subjective health and the use of five or more medications are often associated with a low DHEAS level and, in men, with mortality according to univariate analysis, even if this association statistically disappears after adjustment on other predictor factors of mortality.
Subjective health appears to be related to mortality in men, as is DHEAS level, and thus there is a possibility of relation between subjective health and DHEAS. The effect of subjective health on mortality disappears after adjustment, suggesting that it may be partially explained by DHEAS level, a concept consistent with the neuroactivity of the steroid (6).
Tobacco Consumption and DHEAS.
After adjustment for possible survival predictors, only two factors remain associated with mortality in men: a low initial DHEAS level and current or past tobacco consumption. Furthermore, there was an interaction between smoking habits and DHEAS levels. This means that the predictive value of the DHEAS level for survival is different according to smoking habits. The DHEAS level seems to be a strong predictor of survival in current smokers and, to a lesser degree, in past smokers, although it seems to have no predictive value in nonsmokers. The effect of the interaction between low DHEAS level and tobacco consumption on mortality suggests that DHEAS may be involved in cardiovascular mortality. Indeed, Barrett-Connor et al. (15) have shown a negative correlation between DHEAS level and mortality from cardiovascular disease in men followed for 12 years, but to a lesser degree in the 19-year followup of those men (37). In the same way, other authors (14, 16) found a negative correlation between DHEAS level and cardiovascular morbidity.
Tobacco consumption may have a direct effect on DHEAS level, even if results are controversial: several authors found a higher level of DHEAS in smokers (15, 16, 38–40), whereas others did not (41, 42), or even found the opposite (22, 43). Another explanation for the interaction is that a low DHEAS level in men could be an indicator and/or a potentiator of cardiovascular disease related to smoking habits, or reciprocally that a low DHEAS level could lead to cardiovascular dysfunction that would be worsened by tobacco consumption. Finally, it could be that DHEAS counteracts the toxic effect of tobacco. This hypothesis could be tested by an appropriate randomized intervention trial in elderly smokers with low DHEAS level.
In conclusion, our findings underline the heterogeneity that exists in a cohort sample of old subjects, needing cautious interpretation. We also confirm a different role of DHEAS according to sex: The effect of DHEAS level seems to be less important in women than in men, possibly because of different hormonal metabolism. Finally, DHEAS appears to be a predictor of death among men, even if the pathological mechanism remains unclear. The strong interaction between smoking habits and DHEAS level leads us to think that the DHEAS level is implicated in mortality from cardiovascular or other smoking-related disease in men. Whatever the explanation, if these results are confirmed by further studies, it would be of great interest from a public health point of view to detect smokers with high risk of death, i.e., with low levels of DHEAS. In all good sense, cessation of smoking and DHEA supplementation would be recommended.
Acknowledgments
We thank Dr. C. Verret, Dr. K. Rajkowski, and D. Diop for help in writing the manuscript. This work has been supported by the Institut National de la Santé et de la Recherche Médicale, the Fondation pour la Recherche Médicale, the Fondation Nationale de Gérontologie, the Fondation de France, the Conseil Général de Dordogne, the Conseil Général de Gironde, the Assurances Scor Vie, Novartis Pharma, and Artémis.
Abbreviations
- RR
relative risk
- DHEA
dehydroepiandrosterone
- DHEAS
DHEA sulfate
- MMSE
Mini Mental State Examination
- ADL
Activities of Daily Living
- IADL
Instrumental Activities of Daily Living
- m
mean
Footnotes
PAQUID: Personnes Agées Quid, i.e., People (Personnes) Aged (Agées) About What (Quid, in Latin).
References
- 1.Vande Wiele R L, MacDonald P C, Gurpide E, Lieberman S. Recent Prog Horm Res. 1963;19:275–290. [PubMed] [Google Scholar]
- 2.Baulieu E-E, Corpechot C, Dray F, Emiliozzi R, Lebeau M-C, Mauvais-Jarvis P, Robel P. Recent Prog Horm Res. 1965;21:411–500. [PubMed] [Google Scholar]
- 3.Arlt W, Justl H-G, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte H-M, Allolio B. J Clin Endocrinol Metab. 1998;83:1928–1934. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- 4.Labrie F, Bélanger A, Cusan L, Candas B. J Clin Endocrinol Metab. 1997;82:2403–2409. doi: 10.1210/jcem.82.8.4161. [DOI] [PubMed] [Google Scholar]
- 5.Young J, Couzinet B, Nahoul K, Brailly S, Chanson P, Baulieu E-E, Schaison G. J Clin Endocrinol Metab. 1997;82:2578–2585. doi: 10.1210/jcem.82.8.4157. [DOI] [PubMed] [Google Scholar]
- 6.Baulieu E-E, Robel P. Proc Natl Acad Sci USA. 1998;95:4089–4091. doi: 10.1073/pnas.95.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migeon C J, Keller A R, Lawrence B, Shepard T H. J Clin Endocrinol Metab. 1957;17:1051–1062. doi: 10.1210/jcem-17-9-1051. [DOI] [PubMed] [Google Scholar]
- 8.Orentreich N, Brind J L, Rizer R L, Vogelman J H. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 9.Zumoff B, Rosenfeld R S, Strain G W, Levin J, Fukushima D K. J Clin Endocrinol Metab. 1980;51:330–333. doi: 10.1210/jcem-51-2-330. [DOI] [PubMed] [Google Scholar]
- 10.Svec F, Porter J R. Proc Soc Exp Biol Med. 1998;218:174–191. doi: 10.3181/00379727-218-44285. [DOI] [PubMed] [Google Scholar]
- 11.Thijssen J H H, Nieuwenhuyse H, editors. DHEAS: A Comprehensive Review. New York: Parthenon; 1999. [Google Scholar]
- 12.Hautanen A, Mänttäri M, Manninen V, Tenkanen L, Huttunen J K, Frick M H, Adlercreutz H. Atherosclerosis. 1994;105:191–200. doi: 10.1016/0021-9150(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 13.Moriyama Y, Yasue H, Yoshimura M, Mizuno Y, Nishiyama K, Tsunoda R, Kawano H, Kugiyama K, Ogawa H, Saito Y, et al. J Clin Endocrinol Metab. 2000;85:1834–1840. doi: 10.1210/jcem.85.5.6568. [DOI] [PubMed] [Google Scholar]
- 14.Herrington D M, Gordon G B, Achuff S C, Trejo J F, Weisman H F, Kwiterovich P O J, Pearson T A. J Am Coll Cardiol. 1990;16:862–870. doi: 10.1016/s0735-1097(10)80334-1. [DOI] [PubMed] [Google Scholar]
- 15.Barrett-Connor E, Khaw K-T, Yen S S C. N Engl J Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 16.Feldman H A, Johannes C B, McKinlay J B, Longcope C. Ann Epidemiol. 1998;8:217–228. doi: 10.1016/s1047-2797(97)00199-3. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor E, Goodman-Gruen D. Circulation. 1995;91:1757–1760. doi: 10.1161/01.cir.91.6.1757. [DOI] [PubMed] [Google Scholar]
- 18.Berr C, Lafont S, Debuire B, Dartigues J-F, Baulieu E-E. Proc Natl Acad Sci USA. 1996;93:13410–13415. doi: 10.1073/pnas.93.23.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kähönen M H, Tilvis R S, Jolkkonen J, Pitkälä K, Härkönen M. Aging. 2000;12:308–314. doi: 10.1007/BF03339852. [DOI] [PubMed] [Google Scholar]
- 20.Moffat S D, Zonderman A B, Mitchell Harman S, Blackman M R, Kawas C, Resnick S M. Arch Intern Med. 2000;160:2193–2198. doi: 10.1001/archinte.160.14.2193. [DOI] [PubMed] [Google Scholar]
- 21.Rannevik G, Carlström K, Jeppsson S, Bjerre B, Svanberg L. Maturitas. 1986;8:297–307. doi: 10.1016/0378-5122(86)90038-1. [DOI] [PubMed] [Google Scholar]
- 22.Orentreich N, Brind J L, Vogelman J H, Andres R, Baldwin H. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 23.Thomas G, Frenoy N, Legrain S, Sebag-Lanoe R, Baulieu E-E, Debuire B. J Clin Endocrinol Metab. 1994;79:1273–1276. doi: 10.1210/jcem.79.5.7962319. [DOI] [PubMed] [Google Scholar]
- 24.Nafziger A N, Bowlin S J, Jenkins P L, Pearson T A. J Lab Clin Med. 1998;131:316–323. doi: 10.1016/s0022-2143(98)90181-0. [DOI] [PubMed] [Google Scholar]
- 25.Vestbo J, Knudsen K M, Rasmussen F V. Am Rev Respir Dis. 1988;137:1114–1118. doi: 10.1164/ajrccm/137.5.1114. [DOI] [PubMed] [Google Scholar]
- 26.Katz S, Downs T D, Cash H R, Grotz R C. Gerontology. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 27.Lawton M P, Brody E M. Gerontology. 1969;9:179–186. [PubMed] [Google Scholar]
- 28.Rosow I, Breslau N. J Gerontol. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 29.Fuhrer R, Rouillon F. Psychiatr Psychobiol. 1989;4:163–166. [Google Scholar]
- 30.Folstein M F, Folstein S E, McHugh P R. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Cnaan A, Ryan L. Stat Med. 1989;8:1255–1268. doi: 10.1002/sim.4780081009. [DOI] [PubMed] [Google Scholar]
- 32.Vaupel J W, Yashin A I. Am Stat. 1985;39:176–185. [PubMed] [Google Scholar]
- 33.Baulieu E E, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy M P, Latour F, et al. Proc Natl Acad Sci USA. 2000;97:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn M A, Weaver-Osterholtz D, Sharpe-Timms K L, Allen S, Krause G. J Clin Endocrinol Metab. 1999;84:1527–1533. doi: 10.1210/jcem.84.5.5672. [DOI] [PubMed] [Google Scholar]
- 35.Morales A J, Nolan J J, Nelson J C, Yen S S C. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 36.Zumoff B, Bradlow H L. J Clin Endocrinol Metab. 1980;51:334–336. doi: 10.1210/jcem-51-2-334. [DOI] [PubMed] [Google Scholar]
- 37.Barrett-Connor E, Goodman-Gruen D. Ann NY Acad Sci. 1995;29:259–270. doi: 10.1111/j.1749-6632.1995.tb17386.x-i1. [DOI] [PubMed] [Google Scholar]
- 38.Field A E, Colditz G A, Willett W C, Longcope C, McKinlay J B. J Clin Endocrinol Metab. 1994;79:1310–1316. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- 39.Khaw K-T, Tazuke S, Barrett-Connor E. N Engl J Med. 1988;318:1705–1709. doi: 10.1056/NEJM198806303182601. [DOI] [PubMed] [Google Scholar]
- 40.Salvini S, Stampfer M J, Barbieri R L, Hennekens C H. J Clin Endocrinol Metab. 1992;74:139–143. doi: 10.1210/jcem.74.1.1530789. [DOI] [PubMed] [Google Scholar]
- 41.Key T J A, Pike M C, Baron J A, Moore J W, Wang D Y, Thomas B S, Bulbrook R D. J Steroid Biochem Mol Biol. 1991;39:529–534. doi: 10.1016/0960-0760(91)90247-3. [DOI] [PubMed] [Google Scholar]
- 42.Küpeli B, Soygür T, Aydos K, Özdiler E, Küpeli S. Br J Urol. 1997;80:201–204. doi: 10.1046/j.1464-410x.1997.00299.x. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh C-C, Signorello L B, Lipworth L, Lagiou P, Mantzoros C S, Trichopoulos D. J Clin Epidemiol. 1998;51:837–841. doi: 10.1016/s0895-4356(98)00069-9. [DOI] [PubMed] [Google Scholar]