Abstract
Buprenorphine is the cornerstone of pain management in nonhuman primates, but the pharmacokinetics of this widely used drug are unknown. The purpose of this study was to evaluate the pharmacokinetic profiles of buprenorphine (0.01 and 0.03 mg/kg IM) and sustained-release buprenorphine (0.2 mg/kg SC) in 2 macaque species (M. mulatta and M. fascicularis) by using mass spectrometry. The pharmacokinetics did not differ significantly between species, and buprenorphine was dose-proportional at the tested doses. The low and high doses of buprenorphine had elimination half-lives of 2.6 ± 0.7 and 5.3 ± 2.0 h, respectively, but the low-dose data were constrained by the sensitivity of the analytical method. Sustained-release buprenorphine had an elimination half-life of 42.6 ± 26.2 h. The AUC0-Tlast of buprenorphine were 9.1 ± 4.3 and 39.0 ± 25.1 ng×h/mL for the low and high doses, respectively, and sustained-release buprenorphine had an AUC0-Tlast of 177 ± 74 ng×h/mL. Assuming a hypothesized therapeutic buprenorphine plasma concentration threshold of 0.1 ng/mL in macaques, these results suggest that buprenorphine doses of 0.01 mg/kg IM should be administered every 6 to 8 h, whereas doses of 0.03 mg/kg IM can be administered every 12 h. These results further demonstrate that a single 0.2-mg/kg SC injection of sustained-release buprenorphine maintains plasma concentrations above 0.1 ng/mL for 5 d in macaques. These findings support a new dosing strategy using sustained-release buprenorphine to improve pain management, decrease animal stress, improve animal welfare, and simplify the postoperative management of nonhuman primates in laboratory animal and zoological settings.
Abbreviation: λz, elimination constant; Cmax, maximal observed plasma concentration; HDB, high-dose buprenorphine; LDB, low-dose buprenorphine; MRT, mean residence time; SRB, sustained-release buprenorphine; Tlast, time of last quantifiable plasma analyte concentration; Tmax, time to Cmax; V, volume of distribution
Buprenorphine is a key component of veterinary multimodal pain management, especially in nonhuman primates. The long duration of action, low risk of respiratory depression, and negligible cardiovascular effects in healthy animals make it an advantageous opioid analgesic agent.43 The widely accepted dosage range for buprenorphine in nonhuman primates is 0.01 to 0.03 mg/kg IM twice daily.13,14,20 This dosage is based on the canine dose and anecdotal evidence, because few studies in the primary literature address therapeutic dosages in laboratory animal species.24,38,42 The premise that the current recommended dosing regimen of buprenorphine provides appropriate analgesia is unsubstantiated, introducing the possibility that nonhuman primates do not gain sufficient pain control from the opioid component of the pain management plan.
A new formulation of buprenorphine is reported to have analgesic activity for up to 72 h in cats and rats.7,15 The manufacturer reports that, when administered at 0.27 mg/kg SC, this sustained-release buprenorphine (SRB; ZooPharm, Fort Collins, CO) reaches maximal plasma concentration within 1 h and remains above 1.0 ng/mL for 72 h after injection in dogs. In light of the prolonged duration attained in dogs, this new formulation warrants further evaluation in nonhuman primates.
Drugs with prolonged durations of action are preferred in veterinary medicine and are typically developed for either production or companion animals rather than laboratory animal species. Recently, 2 studies evaluated cefovecin sodium in nonhuman primates, with the expectation that this third-generation cephalosporin antibiotic would have an extended duration of activity in nonhuman primates as it does in dogs and cats. Unfortunately, both studies concluded that the plasma clearance of the antibiotic was 20-fold higher in nonhuman primates than in dogs, providing only 12 to 24 h of antibiotic activity and therefore no dosing advantage over other cephalosporin antibiotics in nonhuman primates.37,39 One study further demonstrated differences in the metabolism of the drug between nonhuman primates species.39 Collectively, these findings highlight the importance of determining optimal dosing strategies for any drug in targeted nonhuman primates species, rather than simply using a published dose for a different species without further evaluation.
The purpose of this study was to evaluate the plasma concentrations and elimination kinetics of buprenorphine and SRB at clinically relevant dosages and administration routes in the 2 most common Old World nonhuman primate species used in research. Specifically, we used liquid chromatography–electrospray ionization–tandem mass spectrometry to confirm that buprenorphine and SRB achieved quantifiable plasma concentrations after injection and to verify how long buprenorphine and individual metabolites remained detectable in the plasma.
Materials and Methods
Animals.
Five adult male cynomolgus macaques (age, 7.8 ± 1.7 y; weight, 6.1 ± 1.8 kg) and 5 adult male rhesus macaques (age, 8.7 ± 0.9 y; weight, 9.4 ± 1.1 kg) were used to complete this study. All procedures were performed under approval from the University of Illinois at Chicago Animal Care Committee. All animals were housed in accordance with the Guide for the Care and Use of Laboratory Animals,22 Public Health Service Policy,36 and Animal Welfare Act4 and Regulations5 in an AAALAC-accredited facility. Macaques were housed in visual and auditory contact with conspecifics or were pair-housed whenever possible; they received 15% Monkey Diet (8714, Harlan-Teklad, Madison, WI) once daily and municipal tap water ad libitum. Fresh produce or foraging materials were provided once daily. Rooms were maintained at 22 ± 2 °C and 30% to 70% relative humidity with 100% conditioned air at 15 to 20 changes hourly. Fluorescent lighting was provided on a 12:12-h light:dark cycle (lights on, 0600 to 1800). Macaques were provided toys and manipulanda placed directly in the cage, and speakers in the animal rooms provided auditory enrichment. All animals were tuberculosis-free as determined by semiannual skin testing and healthy as assessed by regular physical examination, CBC, serum chemistry analysis, and fecal flotation.
Drugs.
Macaques received 0.1 to 1 mL buprenorphine HCl (American Regent, Shirley, NY) and 0.1 to 0.3 mL SRB (Buprenorphine SR, ZooPharm, Fort Collins, CO) with a minimum of 10 d between injections. Animals were injected with buprenorphine dosed at 0.01 mg/kg IM (low-dose buprenorphine [LDB]), followed by 0.03 mg/kg IM (high-dose buprenorphine [HDB]) after a 10-d washout period. These doses represent the lower and upper limits of the range commonly used in nonhuman primates.13,14 After an additional 10-d washout period, the macaques were injected with SRB (0.2 mg/kg SC); this dose was based on discussions with small animal veterinarians, who had observed side effects of sedation, anorexia, and injection site reactions at 0.27 mg/kg SC in healthy, postoperative dogs.
All macaques were weighed before each experiment to ensure accurate drug dosing. Drugs were administered in accordance with the manufacturer's recommendations, under ketamine sedation (10 mg/kg IM). The quadriceps region was shaved prior to drug administration, and the injection site was circled by using a permanent marker for visual monitoring of injection site reaction. The injection sites were monitored throughout the study for erythema, swelling, and pruritis; the macaques were monitored for excessive sedation and cardiovascular and respiratory depression.
Sample collection.
Blood samples (approximately 2 mL each) were collected at the designated time points under ketamine sedation (5 to 10 mg/kg IM) into 3-mL sodium heparin tubes. For the first dose, an initial blood sample was collected at baseline (time 0) followed by a single dose of buprenorphine (0.01 mg/kg IM). Macaques were maintained under sedation, and blood samples were collected at 15, 30, and 60 min after injection. The macaques were resedated for further blood collections at 2, 4, 6, 8, 12, 16, 20, and 24 h. After a 10-d washout period, the dosing and blood collection procedure was repeated by using buprenorphine at 0.03 mg/kg IM. After an additional 10-d wash out period, all macaques were sedated for another baseline blood sample followed by dosing with SRB (0.2 mg/kg SC). Macaques were maintained under sedation for blood collection at 30 and 60 min after injection; the animals were resedated for additional sample collections at 2, 4, 6, 12, 18, 24, 36, 48, 60, 72, 84, 96, and 120 h. At the time of the last blood draw in each series, macaques received iron dextran (50 mg IM) and vitamin B12 (500 μg SC) to provide sufficient substrate for erythrocyte maturation and to mitigate potential effects of the blood volume lost through repeated collection.
During the sample collection process, tubes were placed immediately on ice after blood collection. The tubes were centrifuged at 1000 × g for 10 min within 15 min of collection. The plasma was collected and stored at −80 °C until shipment on dry ice for analysis (University of Utah, Salt Lake City, UT).
Evaluation of adverse effects of SRB.
At 14 d after the last blood collection for the pharmacokinetics study, all macaques received an injection of SRB (0.2 mg/kg SC) without ketamine to evaluate the potential for adverse effects due to SRB in nonhuman primates. The macaques were observed 3 times daily for 3 d for sedation, anorexia, respiratory depression, and injection site reactions. No ketamine was administered to any animal over this observation period to avoid masking potential sedative effects of the SRB formulation.
Health assessment.
The health of the macaques was monitored throughout the course of the study. All macaques were evaluated 1 wk prior to initiation of the study and underwent a complete physical exam, CBC, and serum chemistry analysis. Similar evaluations were repeated 1 wk after each period of dosing and blood collection. Macaques were evaluated daily by cage-side observation to monitor food consumption, injection site reaction, and level of sedation. Weight was monitored weekly to ensure that macaques maintained body weight.
Sample analysis.
All plasma samples were analyzed at the Center for Human Toxicology (University of Utah) by using a validated liquid chromatography–electrospray ionization–tandem mass spectrometry method described previously.19 This method allowed for simultaneous detection of buprenorphine and its metabolites norbuprenorphine, buprenorphine-3-glucuronide, and nobuprenorphine-3-glucuronide in the plasma sample. Briefly, 1-mL aliquots of plasma were extracted with methanol over a C18 solid-phase extraction column, reconstituted to 75 µL, centrifuged, and transferred to an autosampler vial. The autosampler (Surveyor, Thermo-Finnigan, San Jose, CA) injected the sample into a YMC 50 × 2 mm, 3S ODS-AQ column (Waters, Milford, MA) for analysis by the TSQ-quantum triple-stage quandrupole mass spectrometer (Thermo-Finnigan). Calibration curves with known peak-area ratios were used to determine the concentration of all analytes in each sample. The lower limit of quantitation of the assay for all analytes was 0.1 ng/mL for a 1-mL sample of plasma.19 This quantitative assay has been validated at the University of Utah and used in both human and veterinary studies.1,19
Data analysis: pharmacokinetics and statistical analysis.
Pharmacokinetic analysis was performed on plasma concentration–time data obtained after intramuscular (LDB and HDB) or subcutaneous (SRB) administration. Data were analyzed for normal distribution, and outliers were removed from analysis if found to be significant according to the Grubb test. Peak plasma concentration (Cmax) and the time of Cmax (Tmax) were determined directly from the individual observed concentration–time data. Pharmacokinetic parameters (AUC0-Tlast, t1/2; and mean residence time [MRT]) were derived by using a model-independent approach (noncompartmental analysis) according to a uniform weighting scheme (WinNonlin version 6.2, Pharsight, Cary, NC). AUC0-Tlast was determined by using the linear up–log down trapezoidal rule. The area was extrapolated to infinity (AUC0-∞) using the rate constant of the terminal elimination phase (λz), which was determined from the slope of the terminal log-linear portion of the concentration–time curve by using a minimum of 3 measureable time points after Cmax was achieved. In addition, t1/2 was calculated by dividing λz into the natural logarithm of 2. Clearance and volume of distribution (V) were calculated by using the following equations:
Elimination phase parameters were not estimated if 3 time points were not available for estimation or if the R2 value was less than 0.8. Macaques were excluded from the calculated elimination-phase parameter means for this reason, and the numbers of animals included in the analyses of LDB, HDB, and SRB for buprenorphine and all metabolites are reported. The dose proportionality of buprenorphine was verified by comparing the dose-normalized AUC0-Tlast for significant differences between the 2 doses (0.01 and 0.03 mg/kg) for each species and between species (OriginPro 8.6, OriginLab Corporation, Northampton, MA). AUC0-Tlast from the 2 buprenorphine doses for both macaque species was normalized to the dose and compared by using paired t tests, with significance set at a P value of less than 0.05. AUC0-Tlast for LDB, HDB and SRB for both macaque species was normalized to the dose and compared by using unpaired t tests, with significance set at a P value of less than 0.05.
Results
Animal health.
All macaques remained healthy over the course of the study. Body weight fluctuated within normal colony variance. Hct and Hgb both decreased during the initial phase of the study with LDB, but no further drop was seen with subsequent blood draws for HDB or SRB (Table 1). No macaques were pale or tachycardic on physical exam or demonstrated excessive sedation or respiratory depression on cageside observation. No significant changes were noted in the CBC or serum chemistry analysis at any time point.
Table 1.
Hct (%; mean ± 1 SD, n= 5) and Hgb (g/dL; mean ± 1 SD, n= 5) 1 wk after administration of LDB, HDB, and SRB (10 to 14 d between treatments) to macaques
| Rhesus |
Cynomolgus |
|||
| Hct | Hgb | Hct | Hgb | |
| Baseline | 42.2 ± 5.2 | 13.2 ± 1.4 | 44.6 ± 2.8 | 13.3 ± 1.0 |
| LDB | 35.4 ± 1.9 | 11.3 ± 0.7 | 37.9 ± 2.0 | 11.1 ± 1.0 |
| HDB | 37.4 ± 3.7 | 11.6 ± 0.8 | 38.1 ± 2.1 | 11.1 ± 1.3 |
| SRB | 40.0 ± 4.6 | 12.1 ± 1.1 | 39.6 ± 2.5 | 11.3 ± 1.1 |
Adverse effects.
The adverse effects detected over the course of the study were all related to the administration of SRB. Specifically, 4 macaques (40%) had injection site reactions to SRB that varied from a mild erythema that resolved over 5 d (1 cynomolgus macaque) to a 5-mm raised pink plaque that resolved over 1 mo (2 rhesus and 1 cynomolgus macaques). No macaque was observed to scratch at the injection site. Administration of 0.2 mg/kg SRB to conscious macaques with no subsequent ketamine sedation was not associated with observed sedative effects, respiratory depression, or changes in appetite during the 72-h period after injection.
Pharmacokinetics.
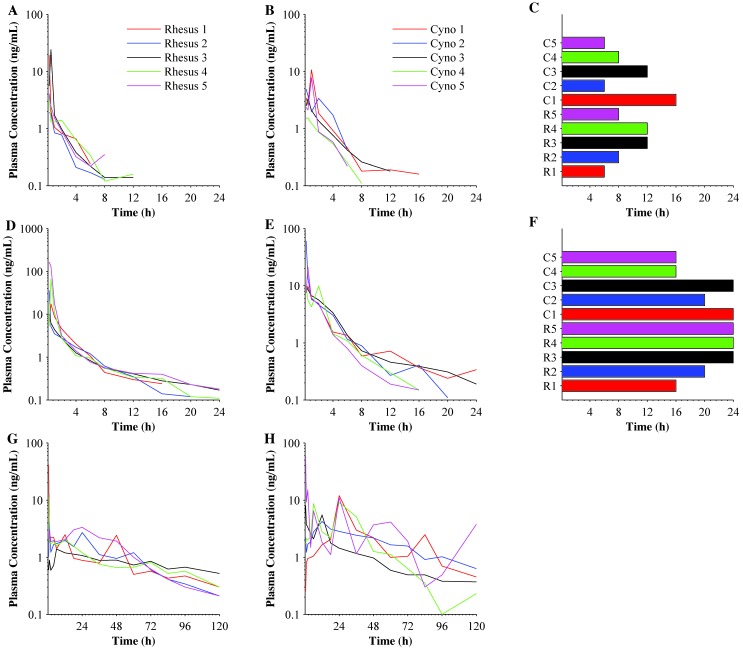
The buprenorphine plasma concentrations for LDB, HDB, and SRB varied between animals over the evaluation period (Figure 1). The last quantifiable buprenorphine plasma concentration was as early as 6 h post-injection for LDB and 16 h for HDB (Figure 1 C and F). In addition, 5 macaques had quantifiable plasma concentrations of buprenorphine at the last sample collection time point (24 h post-injection) for HDB. SRB plasma buprenorphine concentrations remained above the lower limit of quantitation (0.1 ng/mL) for the entire 120-h postadministration sample period in all 10 animals.
Figure 1.
The duration of plasma buprenorphine concentrations after a single injection of LDB, HDB, or SRB. LDB plasma buprenorphine concentrations for (A) rhesus (n = 5) and (B) cynomolgus (n = 5) macaques; (C) Tlast for LDB in all (n = 10) macaques. HDB plasma buprenorphine concentrations in (D) rhesus and (E) cynomolgous macaques; (F) Tlast for LDB in all macaques. SRB plasma buprenorphine concentrations in (G) rhesus and (H) cynomolgus macaques. The last time point in each curve represents the last quantifiable plasma concentration in a specific animal.
Buprenorphine was dose proportional at both the 0.01- (P = 0.27) and 0.03-mg/kg (P = 0.94) doses in both rhesus and cynomolgus monkeys. There was no statistical difference between the dose-normalized AUC0-Tlast for rhesus and cynomolgus monkeys for LDB (P = 0.25) or HDB (P = 0.45). There was a statistical difference between the dose-normalized AUC0-Tlast for rhesus and cynomolgus monkeys for SRB (P < 0.01), but when the animals with R2 < 0.8 were removed from pharmacokinetic analysis, the difference between species was no longer significant (P = 0.06). Therefore, the plasma concentrations and pharmacokinetic analysis for both species were combined to compare LDB, HDB, and SRB. Due to outlier status, one cynomolgus monkey was eliminated from the HDB buprenorphine-3-glucuronide analysis and another from the 120-h time point of SRB buprenorphine. The pharmacokinetic parameters for the metabolites and the number of animals included in the calculated pharmacokinetic parameters are reported in Tables 2 through 5.
Table 2.
Buprenorphine pharmacokinetics (mean ± 1 SD) after a single injection of LDB, HDB, or SRB in macaques
| Parameter | LDB | HDB | SRB |
| Cmax (ng/mL) | 8.1 ± 7.7 | 40.7 ± 48.7 | 15.3 ± 19.1 |
| Tmax (h) | 0.5 ± 0.3 | 0.5 ± 0.5 | 9.3 ± 10.8 |
| Tlast (h) | 10.8 ± 4.5 | 20.8 ± 3.7 | 120a |
| λz (1/h) | 0.28 ± 0.07 | 0.15 ± 0.05 | 0.02 ± 0.01 |
| t1/2 (h) | 2.6 ± 0.7 | 5.3 ± 2.0 | 42.6 ± 26.2 |
| AUC0-Tlast (ng×h/mL) | 9.1 ± 4.3 | 39.0 ± 25.1 | 177.0 ± 74.0 |
| AUC0-∞ (ng×h/mL) | 9.3 ± 4.0 | 40.4 ± 25.3 | 180.0 ± 55.4 |
| MRT (h) | 2.5 ± 1.0 | 3.4 ± 1.2 | 40.1 ± 7.4 |
| V (L/kg) | 4.5 ± 1.5 | 3.4 ± 4.4 | 74.8 ± 48.6 |
| Clearance (L/h/kg) | 1.3 ± 0.6 | 0.4 ± 0.5 | 1.2 ± 0.3 |
For most parameters, n = 10 macaques; for λz, t1/2, AUC0-∞, MRT, V, and clearance, n = 8 for LDB and n = 7 for SRB.
The last sample collection occurred at 120 h, and all macaques had measurable plasma concentrations. This value therefore would have been greater had sample collection been extended beyond this point.
Table 5.
Norbuprenorphine-3-glucuronide pharmacokinetics (mean ± 1 SD) after a single injection of LDB, HDB, or SRB in macaques
| Parameter | LDB | HDB | SRB |
| Cmax (ng/mL) | 0.5 ± 0.2 | 1.0 ± 0.6 | 1.6 ± 1.5 |
| Tmax (h) | 9.5 ± 9.5 | 4.8 ± 6.4 | 39.4 ± 23.8 |
| Tlast (h) | 20.6 ± 6.8 | 24.0 ± 0.0 | 120.0 ± 0.0 |
| λz (1/h) | — | — | 0.02 ± 0.00 |
| t1/2 (h) | — | — | 37.5 ± 10.3 |
| AUC0–Tlast (ng×h/mL) | 4.8 ± 2.2 | 10.9 ± 5.7 | 86.4 ± 47.3 |
| AUC0–∞ (ng×h/mL) | — | — | 114.7 ± 62.9 |
| MRT (h) | — | — | 47.7 ± 4.0 |
| V (L/kg) | — | — | 125.5 ± 87.7 |
| Clearance (L/h/kg) | — | — | 2.2 ± 1.2 |
For most parameters, n = 10; for λz, t1/2, AUC0-∞, MRT, V, and clearance of SRB, n = 6.
Table 3.
Norbuprenorphine pharmacokinetics (mean ± 1 SD) after a single injection of LDB, HDB, or SRB in macaques
| Parameters | LDB | HDB | SRB |
| Cmax (ng/mL) | 0.9 ± 0.7 | 4.0 ± 4.0 | 1.6 ± 2.1 |
| Tmax (h) | 1.1 ± 1.7 | 0.4 ± 0.1 | 9.8 ± 10.5 |
| Tlast (h) | 15.6 ± 7.1 | 16.2 ± 5.8 | 105.6 ± 16.8 |
| λz (1/h) | — | 0.15 ± 0.00 | 0.02 ± 0.01 |
| t1/2 (h) | — | 4.5 ± 0.1 | 49.2 ± 29.4 |
| AUC0-Tlast (ng×h/mL) | 3.3 ± 1.8 | 5.1 ± 1.8 | 26.2 ± 14.6 |
| AUC0-∞ (ng×h/mL) | — | 4.9 ± 0.7 | 43.0 ± 8.1 |
| MRT (h) | — | 3.9 ± 0.2 | 44.1 ± 8.8 |
| V (L/kg) | — | 15.6 ± 27.0 | 84.2 ± 119.1 |
| Clearance (L/h/kg) | — | 2.4 ± 4.2 | 2.0 ± 2.9 |
For most parameters, n = 10; for λz, t1/2, AUC0-∞, MRT, V, and clearance, n = 2 for both HDB and SRB.
Table 4.
Buprenorphine-3-glucuronide pharmacokinetics (mean ± 1 SD) after a single injection of LDB, HDB, or SRB in macaques
| Parameters | LDB | HDB | SRB |
| Cmax (ng/mL) | 0.4 ± 0.3 | 0.7 ± 0.6 | 0.6 ± 0.7 |
| Tmax (h) | 4.5 ± 7.4 | 2.0 ± 3.8 | 26.4 ± 25.7 |
| Tlast (h) | 6.2 ± 8.7 | 17.3 ± 6.0 | 62.7 ± 27.3 |
| λz (1/h) | — | — | 0.03 ± 0.01 |
| t1/2 (h) | — | — | 24.7 ± 8.9 |
| AUC0-Tlast (ng×h/mL) | 2.5 ± 5.7 | 3.9 ± 2.0 | 28.9 ± 52.2 |
| AUC0-∞ (ng×h/mL) | — | — | 19.9 ± 7.5 |
| MRT (h) | — | — | 26.8 ± 10.6 |
| V (L/kg) | — | — | 364.0 ± 56.7 |
| Clearance (L/h/kg) | — | — | 11.2 ± 4.9 |
For most parameters, n = 10; for λz, t1/2, AUC0-∞, MRT, V, and clearance of SRB, n = 3.
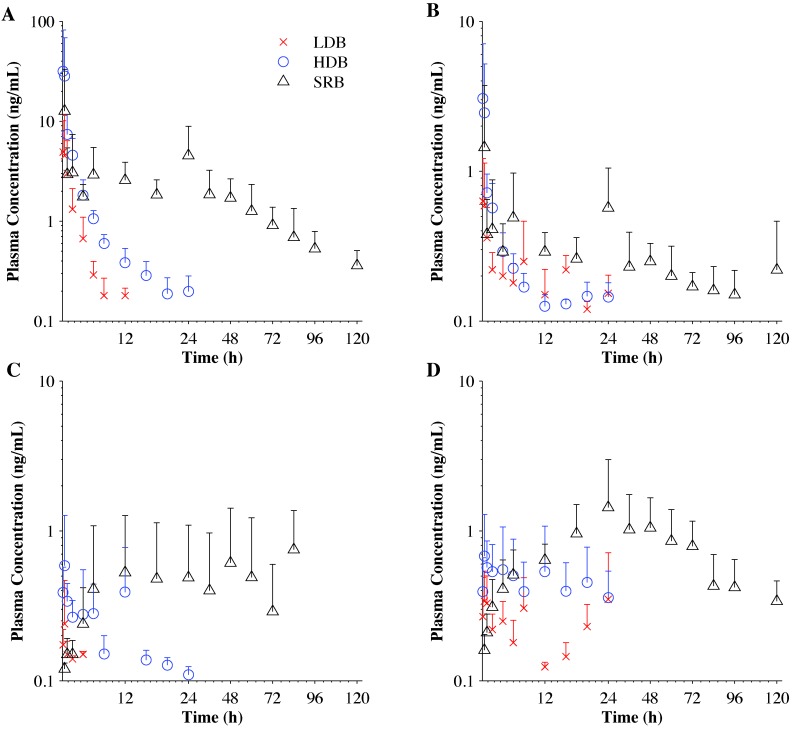
Plasma concentrations of the metabolites were variable over the sample period (Figure 2). No metabolite reached plasma concentrations similar to that of buprenorphine. Norbuprenorphine reached the highest plasma concentrations among the metabolites, and buprenorphine-3-glucuronide was present at the lowest concentrations. Norbuprenorphine-3-glucuronide had the highest relative metabolic ratio to buprenorphine for any measured metabolite in macaques (Table 6).
Figure 2.
Plasma concentration (mean ± 1 SD; n = 10) of (A) buprenorphine and its metabolites, (B) norbuprenorphine, (C) buprenorphine-3-glucuronide, and (D) norbuprenorphine-3-glucuronide, after a single intramuscular injection of LDB and HDB or subcutaneous injection of SRB.
Table 6.
Species comparison of the metabolic ratios of buprenorphine metabolites
| Metabolic ratio | Macaque | Doga | Humanb |
| AUCnorbuprenorphine: AUCbuprenorphine | 0.13 | 0.09 | 2.73 |
| AUCbuprenorphine-3-glucuronide:AUCbuprenorphine | 0.10 | 0.08 | 0.79 |
| AUCnorbuprenorphine-3-glucuronide:AUCbuprenorphine | 0.28 | 0.19 | 9.84 |
From reference 1.
From reference 19.
Discussion
To date, pain management strategies for nonhuman primates have primarily been based on compounds used in dogs. Commonly used analgesic agents include NSAIDs and opioids.2,13,14 Buprenorphine is a frequently used opioid in nonhuman primates due to its relatively long half-life.2,43 Recently, the veterinary community has sought ways to simplify opioid administration, which has led to the pharmacokinetic and clinical evaluation of transmucosal and transdermal administration as well as sustained-release formulations.1,3,7,15,38
Buprenorphine use has historically been constrained by the need for repeated injections and a lack of long-term delivery options. The transdermal fentanyl patch has been used to overcome these drawbacks, but it also has disadvantages. The patch must be worn under a jacket or bandage to prevent the patch from falling off or being removed by the monkey. In addition, to be effective, the patch must be placed at least 12 h before the anticipated onset of pain. Furthermore, no studies document clinical or experimental effectiveness of the fentanyl patch in nonhuman primates. The advent of a sustained-release formulation of buprenorphine circumvents the limitations of a transdermal opioid patch, because the drug is injected once into the animal and then slowly released from a subcutaneous depot over time to provide analgesia.
Two common approaches are used to evaluate analgesic agents: (1) behavioral response to an analgesiometric test or to postsurgical pain and (2) pharmacokinetic studies. Both approaches have their individual merit, but neither is without limitations. The behavioral response of nonhuman primates to pain is difficult to assess, and analgesiometric tests produce pain through different pathways than those of clinical significance.43 Both captive and wild nonhuman primates do not display overt signs of mild to moderate pain, making it difficult for human observers to assess behavioral responses to pain.2,29 Analgesiometric testing rarely is performed in nonhuman primates because of their behavioral tendency to hide pain, but ethograms and motor-skills testing have been used to assess lead toxicity and behavioral side effects of hydromorphone.12,26,27 Furthermore, guidelines for assessing subtle changes in response to pain in nonhuman primates have not been published as they have in other species.2 Some authors have suggested that an elevated heart rate and a decreased time spent standing in telemeterized baboons might be associated with postoperative pain due to inadequate pain management with buprenorphine dosed at 0.01 mg/kg twice daily.2 The results of the current pharmacokinetic study (Tlast = 10.8 ± 4.5 h for 0.01 mg/kg buprenorphine) support this hypothesis and warrant further evaluation, comparing pharmacokinetic data with actual analgesic management of postoperative pain in nonhuman primates.
Although pharmacokinetic studies evaluate the plasma concentrations of a given drug over time, they commonly are performed by using nonclinical routes of administration. The majority of buprenorphine pharmacokinetic studies in veterinary species involve intravenous dosing. When performed alone, intravenous dosing is of questionable clinical value for drugs like buprenorphine that are more often administered intramuscularly or subcutaneously to veterinary patients. However, when pharmacokinetic analysis of intravenous dosing is performed in combination with the recommended method of administration, additional information about bioavailability can be gained. To maximize clinical relevance, the current study was limited to intramuscular and subcutaneous injections of buprenorphine and SRB, respectively.
Comparison of buprenorphine pharmacokinetic data between studies is challenging due to the use of different assays. The method of drug analysis can affect pharmacokinetic parameters significantly, thus preventing direct comparisons.28 Mass spectrometry methods tend to be highly sensitive, measuring very low concentrations, and are highly specific for the measured metabolites.19,28,33 Although radioimmunoassay methods are also highly sensitive, their specificity is variable because of metabolite crossreactivity, which results in overestimation of plasma drug concentrations of the immunoassay target drug.28,35 Despite the increased cost, the use of mass spectrometry methods is on the rise in veterinary species and was used to elucidate buprenorphine pharmacokinetics in nonhuman primates in the current study.
Pharmacokinetic studies alone do not evaluate the physiologic effects of the drug and therefore do not correlate plasma concentration with a meaningful clinical effect. Assumptions can be made based on human and animal studies and the use of biomarkers to correlate dosages with therapeutic efficacy, but this process still requires follow-up validation in the species of interest. Interspecies scaling of drug dosages is based on body weight, body surface area, or allometry. Allometric scaling is founded on the paradigm that metabolism is higher in smaller mammals than larger mammals.34 Drugs that have low protein binding and are eliminated either by renal mechanisms or by flow-limited metabolism are more likely to be scalable across species;41,45 however, the extensively protein-bound nature of buprenorphine precludes the use of interspecies scaling.16
An alternative approach to identifying a therapeutic plasma concentration is to compare plasma concentrations and clinical efficacy directly between species. This strategy has been successfully accomplished previously with anticonvulsant drugs in dogs, rabbits, and monkeys.32 A targeted therapeutic plasma buprenorphine concentration range (0.1 to 0.5 ng/mL) has been suggested in humans, based on correlations between pharmacokinetic studies with mass spectrometry methods and clinical assessment of subjects with postoperative or chronic pain and analgesiometric tests.10,11,46 Similarly, a therapeutic buprenorphine concentration of 0.1 ng/mL has been identified in dogs, by using mass spectrometry methods, to control postoperative pain after ovariohysterectomy.24 Furthermore, a therapeutic threshold of 0.7 to 1 ng/mL has been defined in sheep and cats based on analgesiometric testing but with radioimmunoassay detection methods;35,42 this threshold is higher than previous measures due to inclusion of the metabolites in the plasma buprenorphine concentration by the low-specificity radioimmunoassay method.
The range of published therapeutic buprenorphine plasma concentrations can be used to predict clinically meaningful pain management strategies that are based on average plasma buprenorphine concentrations (Figure 2). At a hypothesized analgesic concentration threshold of 0.1 ng/mL, LDB, HDB, and SRB would provide analgesia for 10.8 ± 4.5, 20.8 ± 3.7, and more than 120 h, respectively. Moving the threshold to 0.5 ng/mL and extrapolating from the average concentration curves, the period of analgesia would decrease to approximately 4, 8, and 96 h for LDB, HDB, and SRB, respectively. Similarly, if the threshold was further moved to 1 ng/mL, the timeframe of analgesia would decrease to 2, 6, and 60 h for LDB, HDB, and SRB, respectively. These correlations bring into question the merit of using twice-daily 0.01 mg/kg buprenorphine dosing regimen in nonhuman primates and highlight the utility of a single injection of SRB. Regardless of where the threshold is set, cageside observation is crucial to assessing an individual animal's response to analgesics and identifying potential breakthrough pain.
Therapeutic opioid dosing requirements can be highly variable due to differences in individual animals’ pain sensitivity and drug pharmacokinetics.43 This inconsistency in pharmacokinetics was seen in the current study as large standard deviations in the plasma concentration curves and other pharmacokinetic parameters. The amount of variability in the data was consistent with other studies on intramuscular injection of buprenorphine in animals.9,21,30 This variability in data may be due, in part, to the lipophilic nature of buprenorphine and the inconsistent adiposity of the individual macaques. These factors could alter the absorption and metabolism of the drug due to depot formation either intracellularly or within subcutaneous fat. The variability may be compounded by the nature of an intramuscular injection and differences in liver metabolism.
In humans, buprenorphine is primarily metabolized by 2 metabolic pathways, N-dealkylation by CYP3A4 and CYP2C8 and glucuronidation.8,23,25,33 These pathways form the 3 major metabolites: norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide. The 3 metabolites have variable biologic activity that is mediated through different opioid receptor affinities and limited CNS access.6 Based solely on receptor binding, the potential activity of the compounds would be analgesia for buprenorphine; analgesia, respiratory depression, and sedation for norbuprenorphine; analgesia for buprenorphine-3-glucuronide; and respiratory depression and sedation for norbuprenorphine-3-glucuronide.6 The relative contributions of these metabolites to the overall biologic effects of buprenorphine still need to be identified, and they speak to the complexity of buprenorphine pharmacology. For LDB, HDB, and SRB in the current study, the plasma concentrations of the metabolites were approximately 10-fold lower than that of the parent drug, buprenorphine. Comparison of the plasma metabolic ratios (AUCmetabolite:AUCbuprenorphine) of macaques with dogs and humans (Table 6) suggests that dogs and macaques have similar metabolite profiles, and both species have less extensive buprenorphine metabolism than do humans.1,19 This information is consistent with other studies demonstrating decreased glucuronidation in veterinary species compared with humans.17,31,44,47
A potential factor in the metabolism, and therefore pharmacokinetic parameters of buprenorphine and SRB, was the use of ketamine sedation throughout the dosing and sampling period. Buprenorphine is metabolized primarily by CYP3A4 and CYP2C8.33 Ketamine is metabolized by CYP3A4, CYP2B6, and CYP2C9.18 In humans, ketamine is metabolized primarily by CYP3A4 due to the relative excess of the enzyme; however, ketamine is preferentially metabolized by CYP2B6 when CYP3A4 is not in overabundance.40 Although buprenorphine and ketamine can be metabolized by the same enzyme, alternative pathways minimize competition. As such, any potential effects of ketamine on buprenorphine metabolism in the current study were considered negligible.
Although buprenorphine has a desirable analgesic effect, it can cause a variety of undesirable side effects, including sedation, respiratory depression, appetite suppression, and pica.13,43 In addition, SRB has been documented to cause minor skin irritation at the injection site in rats and cats.7,15 The nonhuman primates in the current study tolerated LDB, HDB, and SRB and their associated blood collections well. No major adverse effects were noted in response to LDB, HDB, or SRB, but 4 macaques did have mild skin reactions to SRB injections that resolved with time. Macaques maintained good health over the course of the study, as assessed by body weight, physical examination, CBC, and serum chemistry analysis.
LDB, HDB, and SRB all achieved quantifiable plasma concentrations by the time the first sample was collected (15 min for LDB and HDB and 30 min for SRB). This finding indicates that both buprenorphine and SRB can be administered to rapidly address analgesic needs in a clinical situation. HDB achieved the highest Cmax, 40.7 ± 48.7 ng/mL, which was 4 times greater than either that of LDB or SRB. Cmax was hypothesized to be lower for SRB than HDB, despite the higher SRB dose, due to the controlled, slow-release from the sustained-release formulation's biodegradable DL-lactide and ϵ-caprolactone copolymer.15 SRB took longer to reach Cmax than did either LDB or HDB, but this result was expected in light of the sustained-release formation. These pharmacokinetic findings suggest that a decreased likelihood of adverse effects associated with SRB use compared with HDB, and this hypothesis is further supported by the lack of sedative or appetite-associated effects of SRB use in nonhuman primates.
The pharmacokinetic parameters (t1/2, AUC0-∞, V, MRT, and Cl) for buprenorphine (LDB and HDB) in macaque species were comparable to those for other species receiving intramuscular buprenorphine and analyzed by using mass spectrometric methods.9,21,30 These comparisons were not possible for SRB, given that no SRB pharmacokinetic studies in other species have been published. The half-life of HDB (5.3 ± 2.0 h) was greater than that of LDB (2.6 ± 0.7 h), but when the t1/2 calculation for HDB was constrained to 12 h to mimic time limits of detection for LDB, t1/2 for HDB decreased to 3.6 ± 1.6 h, and the difference was no longer significant. This finding indicates that the pharmacokinetic parameters of LDB likely were constrained due to the sensitivity of the mass spectrometry method used and the inability to detect low plasma concentrations at late time points. SRB had a 10-fold increase in half-life compared with HDB, indicating a substantially longer dosing interval for SRB. Although AUC0‑∞ was variable due to the range of doses used, this measure allows evaluation of cumulative drug exposure and determination of relative drug bioequivalence when comparing different drug formulations.28 According to AUC0-∞, there was increasing, dose proportional, buprenorphine exposure between LDB (9.3 ± 4.0 ng×h/mL) and HDB (40.4 ± 25.3 ng×h/mL), and exposure dramatically increased with SRB (180.0 ± 55.4 ng×h/mL). For buprenorphine, V is generally high due to the drug's lipophilic and protein-bound nature, but this measure was increased 20-fold for SRB due to the formation of a subcutaneous depot in a degradable copolymer matrix. Variability in MRT and clearance in the macaques themselves and between species was likely due, in part, to differences in liver metabolism both inter- and intraspecies.1,19 As expected, MRT increased for increasing doses of buprenorphine and SRB due to the increased amount of total buprenorphine present in the body and the sustained-release formulation. Clearance was similar for LDB, HDB, and SRB, indicating that the sustained-release formulation did not alter buprenorphine elimination.
Although we collected only pharmacokinetic endpoints, the current study provides insight into buprenorphine dosing and has several clinical implications. Due to the similarity in buprenorphine metabolism between dogs and macaques and given an analgesic plasma threshold of 0.1 ng/mL in dogs, we can hypothesize that the therapeutic buprenorphine plasma concentration is also 0.1 ng/mL in macaques. Based on this hypothesized therapeutic plasma concentration, the following recommendations can be made: (1) buprenorphine at 0.01 mg/kg IM should be administered every 6 to 8 h; (2) buprenorphine at 0.03 mg/kg IM should be administered every 12 h; and (3) 0.2 mg/kg SC SRB should be administered once every 5 d. However, cageside observation remains crucial to assessing the responses of individual animals to analgesics and to identifying potential breakthrough pain. Although additional studies are necessary to verify adequate pain management, SRB appears to be useful for simplifying colony management while improving animal welfare.
Acknowledgments
We thank BJ Vick, Sam Rosado, Alex Castaneda, and Jaime Murua for their assistance with sample collection and ZooPharm for their donation of sustained-release buprenorphine. The project described was supported by Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science.
References
- 1.Abbo LA, Ko JC, Maxwell LK, Galinsky RE, Moody DE, Johnson BM, Fang WB. 2008. Pharmacokinetics of buprenorphine following intravenous and oral transmucosal administration in dogs. Vet Ther 9:83–93 [PubMed] [Google Scholar]
- 2.Allison SO, Halliday LC, French JA, Novikov DD, Fortman JD. 2007. Assessment of buprenorphine, carprofen, and their combination for postoperative analgesia in olive baboons (Papio anubis). J Am Assoc Lab Anim Sci 46:24–31 [PMC free article] [PubMed] [Google Scholar]
- 3.Andaluz A, Moll X, Ventura R, Abellan R, Fresno L, Garcia F. 2009. Plasma buprenorphine concentrations after the application of a 70 µg/h transdermal patch in dogs. Preliminary report. J Vet Pharmacol Ther 32:503–505 [DOI] [PubMed] [Google Scholar]
- 4.Animal Welfare Act as Amended. 2008. 7 USC §2131–2159.
- 5.Animal Welfare Regulations. 2008. 9 CFR §2.30–2.38, 3.75–3.92.
- 6.Brown SM, Holtzman M, Kim T, Kharasch ED. 2011. Buprenorphine metabolites, buprenorphine-3-glucuronide and nobuprenorphine-3-glucuronide, are biologically active. Anesthesiology 115:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. 2011. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 72:461–466 [DOI] [PubMed] [Google Scholar]
- 8.Cone EJ, Gorodetzky CW, Yousefnejad D, Buchwald WF, Johnson RE. 1984. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos 12:577–581 [PubMed] [Google Scholar]
- 9.Davis JL, Messenger KM, Lafevers DH, Barlow BM, Posner LP. 2012. Pharmacokinetics of intravenous and intramuscular buprenorphine in the horse. J Vet Pharmacol Ther 35:52–58 [DOI] [PubMed] [Google Scholar]
- 10.Escher M, Daali Y, Chabert J, Hopfgartner G, Dayer P, Desmeules J. 2007. Pharmacokinetic and pharmacodynamic properties of buprenorphine after a single intravenous administration in healthy volunteers: a randomized, double-blind, placebo-controlled, crossover study. Clin Ther 29:1620–1631 [DOI] [PubMed] [Google Scholar]
- 11.Evans HC, Easthope SE. 2003. Transdermal buprenorphine. Drugs 63:1999–2010 [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SA, Medina RO, Bowman RE. 1993. Home-cage behavior and lead treatment in rhesus monkeys: a comparison with open-field behavior. Neurotoxicol Teratol 15:145–149 [DOI] [PubMed] [Google Scholar]
- 13.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2008. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Elsevier. [Google Scholar]
- 14.Flecknell PA. 2009. Laboratory animal anaesthesia, 3rd ed. San Diego (CA): Academic Press. [Google Scholar]
- 15.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204 [PMC free article] [PubMed] [Google Scholar]
- 16.Garrett ER, Chandran VR. 1985. Pharmacokinetics of morphine and its surrogates VI: bioanalysis, solvolysis kinetics, solubility, pKʹa values, and protein binding of buprenorphine. J Pharm Sci 74:515–524 [DOI] [PubMed] [Google Scholar]
- 17.Ghosheh O, Hawes EM. 2002. Microsomal N-glucuronidation of nicotine and cotinine: human hepatic interindividual, human intertissue, and interspecies hepatic variation. Drug Metab Dispos 30:1478–1483 [DOI] [PubMed] [Google Scholar]
- 18.Hijazi Y, Boulieu R. 2002. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 30:853–858 [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Moody DE, McCance-Katz EF. 2006. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography–electrospray ionization–tandem mass spectrometry. Ther Drug Monit 28:245–251 [DOI] [PubMed] [Google Scholar]
- 20.Hubbell JA, Muir WW. 1996. Evaluation of a survey of the diplomates of the American College of Laboratory Animal Medicine on use of analgesic agents in animals used in biomedical research. J Am Vet Med Assoc 209:918–921 [PubMed] [Google Scholar]
- 21.Ingvast-Larsson C, Svartberg K, Hydbring-Sandberg E, Bondesson U, Olsson K. 2007. Clinical pharmacology of buprenorphine in healthy, lactating goats. J Vet Pharmacol Ther 30:249–256 [DOI] [PubMed] [Google Scholar]
- 22.Institute of Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23.Iribarne C, Picart D, Dreano Y, Bail JP, Berthou F. 1997. Involvement of cytochrome P450 3A4 in N-dealkylation of buprenorphine in human liver microsomes. Life Sci 60:1953–1964 [DOI] [PubMed] [Google Scholar]
- 24.Ko JC, Freeman LJ, Barletta M, Weil AB, Payton ME, Johnson BM, Inoue T. 2011. Efficacy of oral transmucosal and intravenous administration of buprenorphine before surgery for postoperative analgesia in dogs undergoing ovariohysterectomy. J Am Vet Med Assoc 238:318–328 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi K, Yamamoto T, Chiba K, Tani M, Shimada N, Ishizaki T, Kuroiwa Y. 1998. Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Dispos 26:818–821 [PubMed] [Google Scholar]
- 26.Krugner-Higby L, KuKanich B, Schmidt B, Heath TD, Brown C. 2011. Pharmacokinetics and behavioral effects of liposomal hydromorphone suitable for perioperative use in rhesus macaques. Psychopharmacology (Berl) 216:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krugner-Higby L, KuKanich B, Schmidt B, Heath TD, Brown C, Smith LJ. 2009. Pharmacokinetics and behavioral effects of an extended-release, liposome-encapsulated preparation of oxymorphone in rhesus macaques. J Pharmacol Exp Ther 330:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KuKanich B. 2011. Clinical interpretation of pharmacokinetic and pharmacodynamic data in zoologic companion animal species. Vet Clin North Am Exot Anim Pract 14:1–20 [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre L, Carli G. 1985. Parturition in nonhuman primates: pain and auditory concealment. Pain 21:315–327 [DOI] [PubMed] [Google Scholar]
- 30.Lloyd-Jones JG, Robinson P, Henson R, Biggs SR, Taylor T. 1980. Plasma concentration and disposition of buprenorphine after intravenous and intramuscular doses to baboons. Eur J Drug Metab Pharmacokinet 5:233–239 [DOI] [PubMed] [Google Scholar]
- 31.Martin IJ, Lewis RJ, Bernstein MA, Beattie IG, Martin CA, Riley RJ, Springthorpe B. 2006. Which hydroxy? Evidence for species differences in the regioselectivity of glucuronidation in rat, dog, and human in vitro systems and dog in vivo. Drug Metab Dispos 34:1502–1507 [DOI] [PubMed] [Google Scholar]
- 32.Masuda Y, Utsui Y, Shiraishi Y, Karasawa T, Yoshida K, Shimizu M. 1979. Relationships between plasma concentrations of diphenylhydantoin, phenobarbital, carbamazepine, and 3-sulfamoylmethyl-1,2-benzisoxazole (AD810), a new anticonvulsant agent, and their anticonvulsant or neurotoxic effects in experimental animals. Epilepsia 20:623–633 [DOI] [PubMed] [Google Scholar]
- 33.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. 2002. A liquid chromatographic–electrospray ionization–tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal Biochem 306:31–39 [DOI] [PubMed] [Google Scholar]
- 34.Mordenti J. 1986. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci 75:1028–1040 [DOI] [PubMed] [Google Scholar]
- 35.Nolan A, Livingston A, Waterman AE. 1987. Investigation of the antinociceptive activity of buprenorphine in sheep. Br J Pharmacol 92:527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Office of Laboratory Animal Welfare. [Internet]. 2002. Public health service policy on humane care and use of laboratory animals. [Cited 1 January 2012]. Available at: http://grants.nih.gov/grants/olaw/references/phspol.htm.
- 37.Papp R, Popovic A, Kelly N, Tschirret-Guth R. 2010. Pharmacokinetics of cefovecin in squirrel monkeys (Saimiri sciureus), rhesus macaques (Macaca mulatta), and cynomolgus macaques (Macaca fascicularis). J Am Assoc Lab Anim Sci 49:805–808 [PMC free article] [PubMed] [Google Scholar]
- 38.Pieper K, Schuster T, Levionnois O, Matis U, Bergadano A. 2011. Antinociceptive efficacy and plasma concentrations of transdermal buprenorphine in dogs. Vet J 187:335–341 [DOI] [PubMed] [Google Scholar]
- 39.Raabe BM, Lovaglio J, Grover GS, Brown SA, Boucher JF, Yuan Y, Civil JR, Gillhouse KA, Stubbs MN, Hoggatt AF, Halliday LC, Fortman JD. 2011. Pharmacokinetics of cefovecin in cynomolgus macaques (Macaca fascicularis), olive baboons (Papio anubis), and rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 50:389–395 [PMC free article] [PubMed] [Google Scholar]
- 40.Restrepo JG, Garcia-Martin E, Martinez C, Agundez JA. 2009. Polymorphic drug metabolism in anaesthesia. Curr Drug Metab 10:236–246 [DOI] [PubMed] [Google Scholar]
- 41.Riviere JE, Martin-Jimenez T, Sundlof SF, Craigmill AL. 1997. Interspecies allometric analysis of the comparative pharmacokinetics of 44 drugs across veterinary and laboratory animal species. J Vet Pharmacol Ther 20:453–463 [DOI] [PubMed] [Google Scholar]
- 42.Robertson SA, Lascelles BD, Taylor PM, Sear JW. 2005. PK–PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 28:453–460 [DOI] [PubMed] [Google Scholar]
- 43.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 44.Sharer JE, Shipley LA, Vandenbranden MR, Binkley SN, Wrighton SA. 1995. Comparisons of phase I and phase II in vitro hepatic enzyme activities of human, dog, rhesus monkey, and cynomolgus monkey. Drug Metab Dispos 23:1231–1241 [PubMed] [Google Scholar]
- 45.Sharma V, McNeill JH. 2009. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157:907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sittl R, Griessinger N, Likar R. 2003. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther 25:150–168 [DOI] [PubMed] [Google Scholar]
- 47.Soars MG, Riley RJ, Findlay KA, Coffey MJ, Burchell B. 2001. Evidence for significant differences in microsomal drug glucuronidation by canine and human liver and kidney. Drug Metab Dispos 29:121–126 [PubMed] [Google Scholar]