Abstract
The nonsteroidal antiinflammatory drug (NSAID) ketorolac is a candidate for use as a supplemental analgesic during major surgery in anesthetized rodents. The use of ketorolac during surgery is believed to reduce the anesthetic dose required to achieve and maintain an adequate surgical plane, thus improving the physiologic condition and survival of animals during long experimental procedures. Ketorolac has reported side effects that include dizziness, ear pain, hearing loss, tinnitus, and vertigo in humans, but ketorolac has not been reported to affect the vestibular system in animals. To investigate this possibility, we evaluated the acute effects of ketorolac on vestibular compound action potentials in C57BL/6 mice. Linear vestibular sensory-evoked potentials (VsEP) were recorded during the administration of ketorolac at doses 3 to 14 times the effective analgesic dose. VsEP results for ketorolac were compared with those from a control group maintained under anesthesia for the same period. Ketorolac did not significantly affect the temporal profiles of response latencies and amplitudes or the rate of change in response measures over time between controls and ketorolac-treated mice. These findings demonstrate that ketorolac can be used as an analgesic to supplement anesthesia in mice without concerns of modifying the amplitudes and latencies of the linear VsEP.
Abbreviation: CL, plasma clearance rate; Ct, plasma concentration at time t; dB SPL, decibels, sound pressure level (reference 20μPa); Dt, dose at time t (that is, cumulative dose); ECG, Electrocardiographic; RM MANOVA, repeated-measures multivariate ANOVA; V, volume of distribution; VsEP, vestibular sensory evoked potentials
Prostaglandins are synthesized in cells throughout the body.2 The release of prostaglandins promotes inflammation, pain, and clotting function of platelets. Prostaglandins are produced by the enzyme cyclooxygenase, which is blocked by nonsteroidal antiinflammatory drugs (NSAIDs); in turn NSAIDs reduce prostaglandin release and consequently inflammation, pain, and fever. NSAIDs therefore are commonly used as systemic analgesics. Ketorolac is a NSAID that is used frequently by veterinarians to reduce “mild to moderate pain in rodents.”20 The reported side effects of ketorolac include dizziness, ear pain, hearing loss, tinnitus, and vertigo in humans.1,18,22,25 The antiinflammatory properties of ketorolac have been shown to help prevent hearing loss during experimentally induced pneumococcal meningitis,21 and its analgesic properties make it a candidate for use as a supplement to anesthesia in rodents. However, there have been no reports evaluating potential direct effects that ketorolac may have on vestibular function. Given the reported side effects in humans, it is reasonable to wonder whether acute administration of ketorolac alters vestibular function directly. The purpose of the present study was to address this question by using vestibular sensory-evoked potentials (VsEPs)7 as a direct measure of peripheral and central vestibular function. The linear VsEP has been shown to be an effective and sensitive tool for noninvasive assessment of vestibular function under a variety of conditions (for review, see reference 6). We measured VsEPs in control mice and in mice injected with increasing doses of ketorolac (5, 10, 20, 40, 80, and 160 mg/kg).
Materials and Methods
The care and use of animals in the present study was approved by the IACUC of East Carolina University and conformed to all NIH guidelines. C57BL/6 mice [n 17; weight, 23.0 g to 29.4 g (mean ± 1 SD, 27.1 ± 1.8 g); age, 1.9 to 3.8 mo old (3.1 ± 0.7 mo] obtained from The Jackson Laboratory (Bar Harbor, ME) or Charles River (Raleigh, NC) were used in this study (6 unmanipulated controls, 5 sham-treated, and 6 drug-treated). Although the C57 mouse strain exhibits age-related hearing loss, VsEP functional measures change little over their lifespan of approximately 2 y.14,15
The mice were anesthetized (ketamine [18 mg/mL]–xylazine [2 mg/mL]; 7 μL/g body weight), tracheotomized, and intubated. Mice were ventilated with oxygen-rich air (29% nitrogen in oxygen, 125 to 135 breaths per minute, 175 to 225 µL per stroke). SpO2 was measured by using a clip sensor (catalog no. TDR-43C, Med Associates, St Albans, VT) and monitored by using a pulse oximeter (model no. CANL-425SV-A, Med Associates). Blood-gas levels were maintained with SpO2 above 90%. A brain thermistor (Cole Parmer, Vernon Hills, IL) was placed in the caudal cerebrum. Brain and rectal temperatures were monitored and maintained between 36.8 and 37.5 °C by using a heat lamp and homeothermic heating pad (FHC, Bowdoin, ME), respectively. Electrocardiographic (ECG) activity was monitored on an oscilloscope (heart rate, 218 to 480 bpm [354 ± 75 bpm]). Lactated Ringers solution was administered subcutaneously as needed to prevent dehydration. Mice were included in the analysis if they were in good physiologic condition (for example, no erratic or slow heart rate), were recorded for at least 1 h after baseline recordings, and received at least 3 increasing doses of ketorolac.
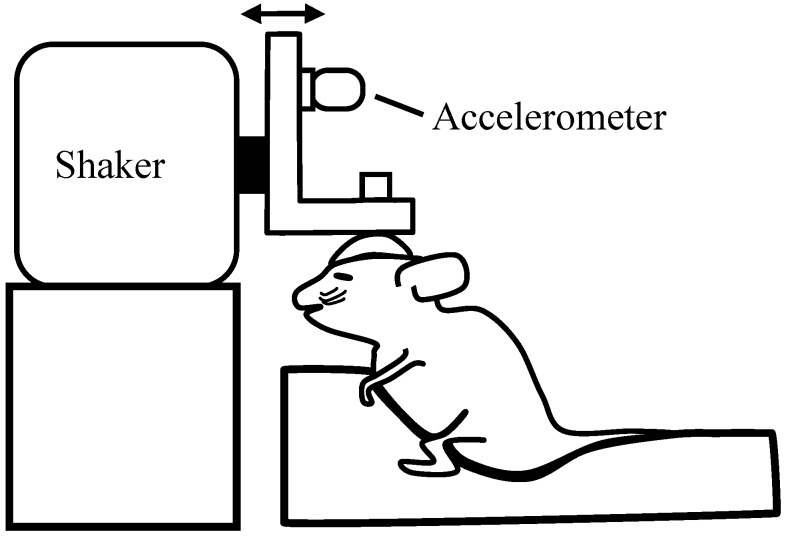
Tissue overlying the skull was shaved, incised, and retracted. Two stainless steel-anchor screws (#00 × 4.76 mm) were placed in the bone overlying the frontal sinus. To access the dura, a small hole was made parasagittally over the caudal cerebrum near the intersection of the sagittal and caudal coronal (lamdoid) sutures. A bare stainless-steel electrode was placed in the epidural space, and the hole was sealed with bone wax. Plaster was poured to encase the anchor screws, the insulated portion of the electrode, and a stainless-steel thumbnut (diameter, 1 cm; thread, #4–40) placed on the midline. The thumbnut was used to secure the skull to an aluminum plate. The aluminum plate was attached to a mechanical shaker (model no. ET-132-203, LabWorks, Costa Mesa, CA). The mechanical shaker was used to deliver linear acceleration stimuli in the nasooccipital axis (Figure 1). The motion of the shaker was monitored by an accelerometer (model no. 1018, Vibra-Metrics, Princeton Junction, NJ) and adjusted to produce the acceleration and jerk stimulus waveforms.
Figure 1.
Schematic illustration of the coupling of the skull of an anesthetized mouse to the stimulus platform. The shaker shaft produces a transient head translation in the nasooccipital axis in the direction of the arrows. An accelerometer is mounted to the L-bracket to monitor acceleration. The accelerometer output is electronically differentiated to monitor jerk levels in grams per millisecond.
VsEP stimuli were produced by applying a linear ramp voltage waveform (digital-to-analog conversion, 2 µs/point) to a power amplifier that in turn drove movements of the shaker and coupling platform. The amplitude of the applied waveform was adjusted by using an attenuator to increase or decrease stimulus level. A linear ramp acceleration of 2 ms duration was used for the stimulus. The accelerometer output was routed to a calibrated electronic differentiator, which converted acceleration to its first derivative (that is, jerk).11,12 Jerk magnitude was determined from the output of the differentiator by using an oscilloscope. The amplitude of the jerk stimulus was measured as the mean peak jerk level and expressed in units of g-force per millisecond, where g = 9.81 m/s2 and expressed in dB relative to 1.0 g/ms.8 A stimulus level of +6 dBre:1.0g/ms (that is 2.0 g/ms) was used throughout the study.
The electrode placed over the caudal cerebrum was connected to the noninverting input of a biologic amplifier. The inverting electrode was placed subcutaneously below and behind the right pinna, and the ground electrode was placed subcutaneously on the ventral neck. Electrophysiologic signals were amplified (200,000×; model no. P511, Grass, West Warwick, RI) and filtered (300 to 3000 Hz [–6 dB amplitude points]) for all VsEP recordings. Signal averaging was used to resolve responses in electrophysiologic recordings. Stimuli were presented at a rate of 17 per second. Analog-to-digital conversion was triggered at the onset of each stimulus (1024 points at 10 µs per point). Responses to normal and inverted stimulus polarities were collected and summed for a total of 256 sweeps per waveform. A binaural forward masker10 (90 dB SPL; bandwidth 50 Hz to 50 kHz) was presented during initial VsEP recordings of each study by using a free-field speaker driver (model no. FF1, Tucker Davis Technologies, Alachua, FL) to confirm the absence of auditory components in VsEPs.
Dose regimen and estimated drug levels of ketorolac.
Ketorolac is rapidly and completely absorbed—the maximal plasma concentration is achieved within approximately 15 min of administration— and its pharmacokinetics are linear, with a half-life (t1/2) of about 186 min and a reported plasma clearance rate (CL) of 0.43 mL/min/kg in mice.16 The reported effective analgesic dose range for ketorolac is 0.5 to10 mg/kg3-5,19,20,23,24 and a dose of 200 mg/kg reportedly produces a 50% mortality rate (lethal-dose 50%, LD50).20 Mice in the drug-treatment group (n = 6) received a series of 3 to 6 incremental subcutaneous doses of ketorolac (5, 10, 20, 40, 80, and 160 mg/kg; APP Pharmaceuticals, Schaumburg, IL), where each successive dose was given 20 min after the preceding dose. The treatment group included one mouse that received the first 3 doses (maximum, 20 mg/kg), one mouse that received 4 doses (maximum, 40 mg/kg), 3 mice that received 5 doses (maximum, 80 mg/kg), and one mouse that received all 6 doses (maximum, 160 mg/kg). Therefore all mice received cumulative doses well above the effective dose levels for analgesia (Table 1), and 5 of the 6 mice received at least 4 successive doses. The total volume injected was less than 400 μL. Ringers solution was administered to the sham-treatment group (n = 5) at the same volumes and times as the doses in the drug-treatment group. The unmanipulated control group (n = 6) was given Ringers solution once hourly to prevent dehydration. VsEP responses were recorded at approximately 10-min intervals for all mice throughout each study, thus including periods before and after each dose of ketorolac.
Table 1.
Dose schedules and resulting estimated cumulative doses and plasma concentrations
| Time (min) | Dose (mg/kg) | Cumulative dose (mg/kg) | Plasma concentration (mg/mL) |
| 0 | 5 | 0.000 | 0.00000 |
| 20 | 10 | 5.000 | 0.04338 |
| 40 | 20 | 14.641 | 0.12701 |
| 60 | 40 | 33.588 | 0.29138 |
| 80 | 80 | 71.174 | 0.61744 |
| 100 | — | 146.057 | 1.26706 |
| 120 | — | 135.558 | 1.17598 |
| 140 | — | 125.813 | 1.09144 |
The sequence presented represents 5 sequential doses at 20-min intervals. The following assumptions were made during the calculation of the data above: volume of distribution, 3.17 mL; weight, 0.0275 kg (the mean of the treatment group); k, 0.00373; half-life, 3.1 h.
Using the CL (0.43 mL/min/kg) and t1/2 (186 min) values reported for ketorolac in mice,16 we calculated estimates of the cumulative dose (Dt), volume of distribution (V), and plasma concentrations (Ct) resulting from our dose regimen in a mouse weighing 0.0275 kg (average weight of mice in the drug-treatment group). The estimates rely on the assumptions that the drug is completely absorbed after subcutaneous injection and that the pharmacokinetics of ketorolac remain linear over the entire dose range and behave in accordance with a simple single-compartment model. Therefore, V can be calculated from CL and t1/2 as:
where k is the elimination constant (and is given by k = 0.69315 / t1/2) and weight is that of the test animal (in kilograms). Therefore, for a 0.0275-kg mouse, V is:
The maximal concentration (milligrams per milliliter) for each dose is:
After each dose (in milligrams) is administered, the maximal plasma concentration is achieved within 15 min, and thereafter, plasma levels decrease at a rate set by the elimination constant (k). Estimated plasma concentration over time (Ct; milligrams per milliliter) is calculated as:
The dose (mg/kg) as a function of time (Dt; that is, cumulative dose) is determined as:
where k is the elimination constant; weight is 0.027 kg (the mean weight of mice receiving drug, and dose is in milligrams.
Using these equations, we estimated the ketorolac levels remaining after each initial dose over time at each 20-min time interval after injection. We then summed the levels remaining from each previous injection at each 20-min interval to produce an estimate of the total remaining dose at time t. Table 1 summarizes the dose estimates by weight and plasma concentration levels at each 20-min interval throughout the study.
Scoring response latencies and amplitudes.
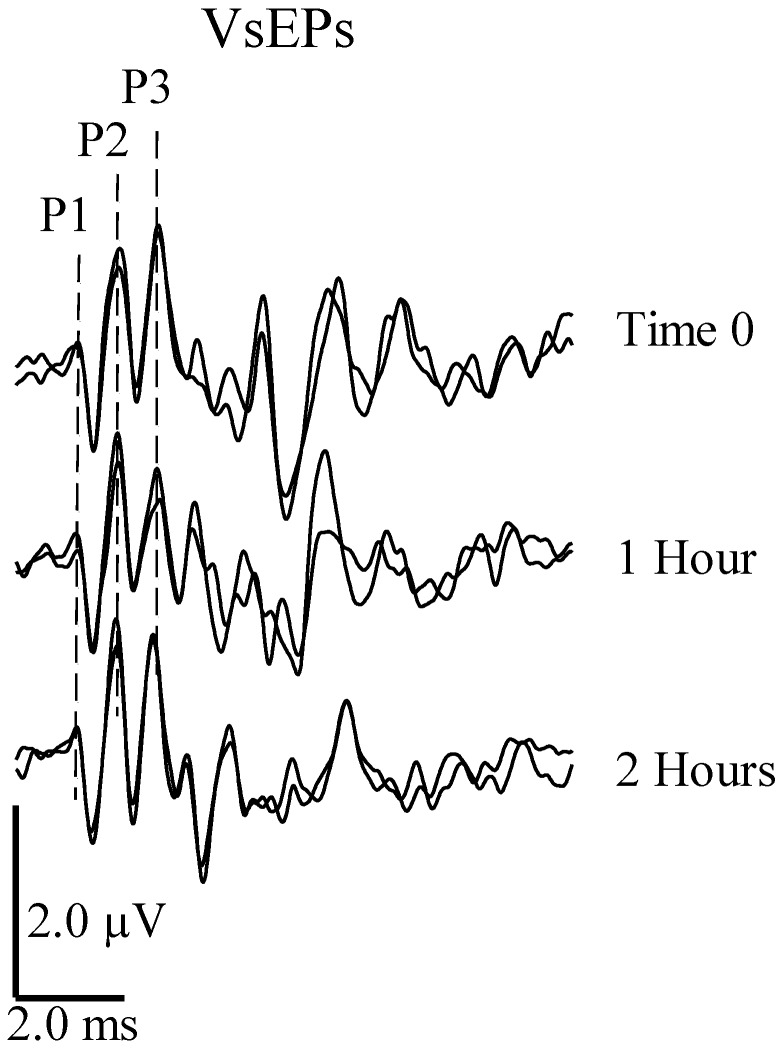
Three positive response peaks (that is, P1, P2, and P3) and 2 negative response peaks (that is, N1 and N2) were consistently present in VsEPs (Figure 2); we therefore scored their amplitudes and latencies and used this information to measure drug effects. P1 and N1 of the VsEP reflect peripheral vestibular nerve activity, whereas peaks beyond N1 reflect the activation of central vestibular pathways.17 Peaks beyond P3 are highly variable and are thought to be myogenic and labile to anesthesia.9
Figure 2.
VsEPs: stability over time. VsEPs were recorded over 2 h in a C57 BL/6 mouse with brain and rectal temperatures stabilized at 37.0 °C by using a stimulus level of +6 dBre: 1.0 g/ms. SpO2 was maintained between 95% to 100%, and heart rate remained above 300 beats per minute. Positive peaks are labeled as P1, P2 and P3. Negative peaks (N1 and N2) are not marked here but represent the next negative minimal or peak voltage amplitude after the corresponding positive peak. Negative peaks were used to define response peak-to-peak amplitudes P1N1, P2N2, and P3N2.
Response peak latencies were defined as the time (in microseconds) from the onset of the stimulus to the onset of the response peak. Peak-to-peak amplitudes were measured in microvoltsand represent the difference in amplitude between a positive peak and a negative peak (for example, P1N1). Response amplitudes and latencies were documented before and after each dose of ketorolac. Normalized response values were calculated as the ratio of the value measured after drug administration to the mean baseline value obtained before drug administration.
Because we estimated that ketorolac levels would increase monotonically with time (Table 1), we expected that dose-dependent changes in response amplitude and latency would manifest as systematic changes with time. We therefore evaluated the change in response latencies and amplitude as a function of time. Our hypothesis was that ketorolac administration has no effect on vestibular responses. We tested this hypothesis by using repeated-measures multivariate ANOVA (RM MANOVA; version 19.0, SPSS software, IBM, New York, NY).13 This analysis limited the number of mice that could be included from our sample (5 sham-treated mice, 6 unmanipulated controls, and 5 drug-treated mice) and thus restricted the number of treatment doses analyzed to 4 (estimated cumulative dose, 33.6 mg/kg; estimated plasma level, 0.29 mg/mL; number of mice, 5). Therefore for RM MANOVA, response measures were evaluated at 4 dose–time levels for within-subjects factors and compared across control and drug treatment groups for between-subjects factors.
To include all mice and doses, we performed a second, quantitative test. Here we used the linear regression slope as a single metric for characterizing the drug effects over time on each response peak tracked (latency and amplitude compared with time). Regression slopes were compared between control, sham, and drug groups by using MANOVA to determine the presence of drug- or time-associated effects. For MANOVA, response measures were evaluated across control and drug-treatment groups as fixed factors.
Results
All 17 mice had robust VsEP waveforms (Figures 2 and 3), with no significant differences in VsEP response profiles between sham-treatment and control mice over time (RM MANOVA; VsEP amplitudes: F = 1.502, df = 2, P = 0.287; VsEP latencies: F = 1.828, df = 4, P = 0.261). In addition, linear regression slopes of sham-treatment and control groups did not differ significantly for any VsEP response component (MANOVA; VsEP amplitudes: F = 1.505, df = 4, P = 0.311; VsEP latencies: F = 2.241, df = 5, P = 0.198). Therefore, the sham-treatment and control groups were combined into a single control group (n = 11), and the results from this combined control group were compared with those of the drug-treatment group (n = 5 or 6).
Figure 3.
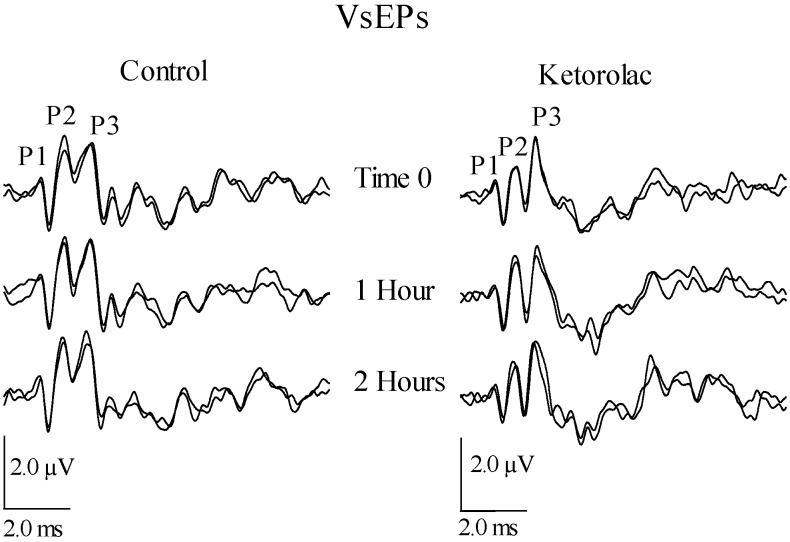
VsEP waveforms for a representative control mouse (left) and a representative mouse from the ketorolac treatment group (right). Waveforms demonstrate the stability of the VsEP over time in both the control and drug treatment groups. Estimated cumulative doses of ketorolac were 0, 33, and 135 mg/kg at times 0, 1, and 2 h respectively. Positive peaks P1 through P3 are labeled.
Response amplitude and latency profiles over time and dose level.
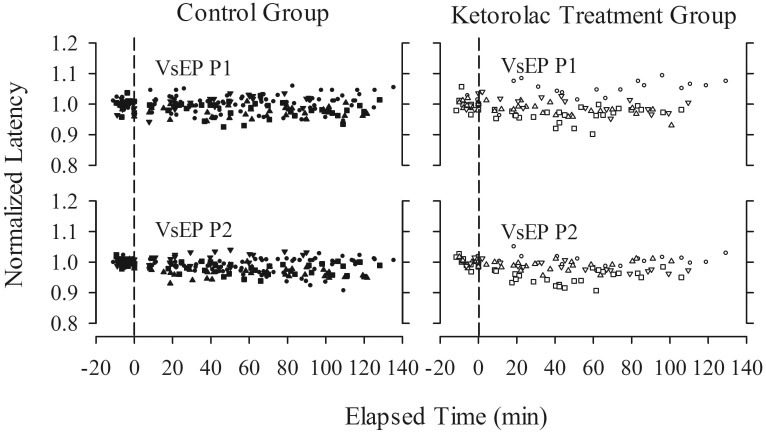
VsEP responses remained relatively stable over time, despite the administration of high doses of ketorolac. Figure 3 illustrates the stability of VsEP waveforms recorded before and after the administration of ketorolac at cumulative doses of approximately 0, 33, and 135 mg/kg (0, 1, and 2 h, respectively). In addition, response profiles over time, including normalized amplitudes and latencies for representative peripheral and central response peaks, are presented for all control and drug-treated mice (Figures 4 and 5). Baseline periods are represented to the left of the vertical dashed line, which marks time 0 (t = 0 min), when the first dose of ketorolac was administered. All response profiles were relatively flat over time, indicating no generalized trend or noteworthy systematic influence of time or drug. This impression was confirmed quantitatively: according to RM MANOVA, ketorolac administration had no significant effect on VsEP response amplitude and latency profiles. There was no effect of treatment group (drug treatment compared with control; between-subjects factor; VsEP amplitudes: F = 2.953, df = 3, P = 0.080), no effect of dose (time; within-subject factor; VsEP amplitudes: F = 0.838, df = 9, P = 0.616; VSEP latencies: F = 3.148, df = 12, P = 0.266), and no interaction between treatment groups and dose level (VsEP amplitudes: F = 2.919, df = 9, P = 0.125; VSEP latencies: F = 9.162, df = 12, P = 0.103).
Figure 4.
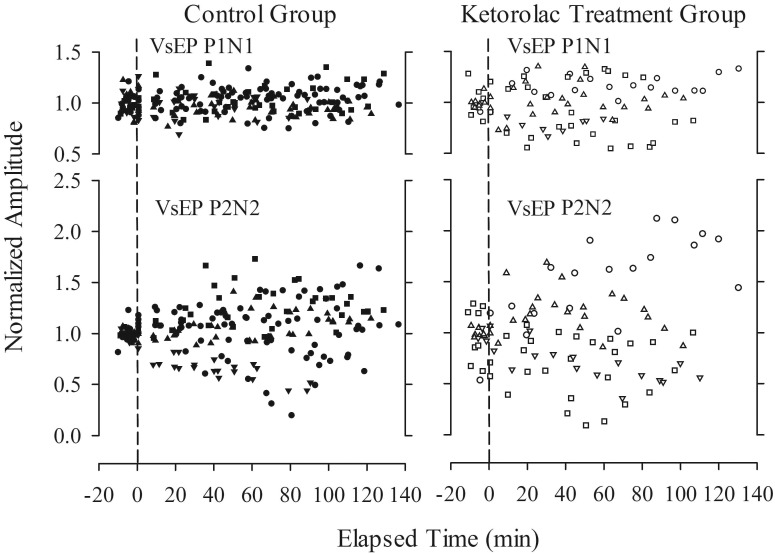
VsEP normalized response amplitudes (post drug/mean baseline) as a function of time (in min) for control (control+ sham; n = 11) and ketorolac treatment (n = 6) groups. Data reflect response-amplitude profiles over time and dose and include data from all mice. Different symbols represent individual mice. Cumulative maximal doses of ketorolac ranged from approximately 33 to more than 146 mg/kg. Time 0 is the time of the initial dose of ketorolac. Data to the left of the vertical dashed line represent baseline recordings before drug administration.
Figure 5.
VsEP normalized response latencies (post drug/mean baseline) as a function of time (in min) for control (control+ sham; n = 11) and ketorolac treatment (n = 6) groups. Data reflect response latency profiles over time and dose and include data from all mice. Cumulative maximal doses of ketorolac ranged from approximately 33 to more than 146 mg/kg. Time 0 is the time of the initial dose of ketorolac. Data to the left of the vertical dashed line represent baseline recordings before drug administration.
Linear regression and response latency and amplitude as a function of time.
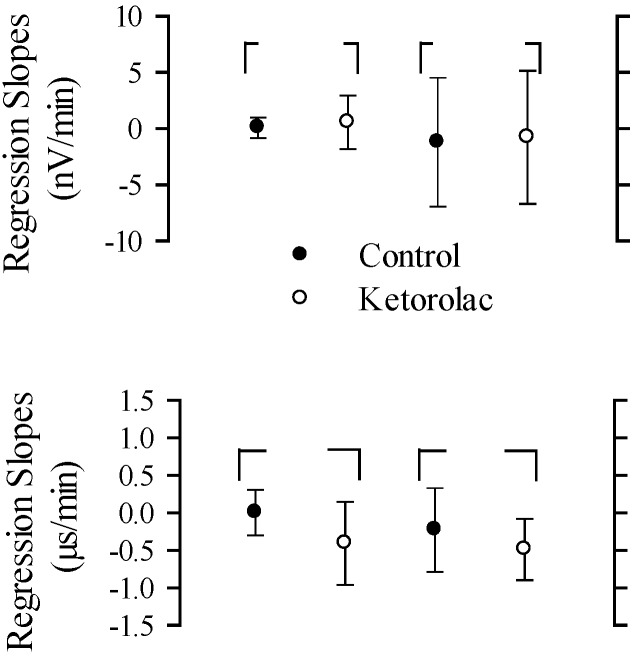
Regression slopes for amplitude and latency as functions of time were computed for all mice. The mean values of regression slopes for representative peripheral and central peaks are illustrated in Figure 6. According to MANOVA, there were no significant differences between the regression slopes of control and drug treatment groups for peak amplitudes and peak latencies of VsEPs (amplitudes: F = 0.234, df = 4, P = 0.914; latencies: F = 0.911, df = 5, P = 0.508).
Figure 6.
Linear-regression slopes (mean ± 1 SD) for nonnormalized amplitudes (top; nV/min) and latencies (bottom; µs/min) as a function of time. Two response peaks represent peripheral (P1N1) and central (P2N2) components of the VsEP amplitudes, whereas response peaks P1 and P2 represent peripheral and central components of the VsEP latencies, respectively. Solid circles, control group; open circles, ketorolac group.
Discussion
The results of the current study clearly demonstrate that VsEP response parameters were unaffected by sequential administration of increasing doses of ketorolac. The treated and control mice showed no significant differences in response profiles over time. Responses changed little over the period of drug administration despite estimated cumulative doses that approached or exceeded 146 mg/kg in most (4 of 6) mice. Given the reported effective analgesic dose range of 0.5 to 10 mg/kg, the range of doses used in the present study represents drug levels that were 3 to 14 times greater than those used for effective analgesia. These findings support the hypothesis that ketorolac has no appreciable direct action on the vestibular sensory apparatus of the inner ear or on central neural relays.
As noted in the introduction, symptoms reported by humans during ketorolac administration include dizziness, ear pain, hearing loss, tinnitus, and vertigo.1,18,22,25 Our findings in mice led us to question the likelihood of a general direct acute action of ketorolac on neurosensory function in the inner ear of the human. It is possible that other effects of ketorolac mediate the symptoms reported. The time frame for the appearance of symptoms in humans is not apparent from the literature. We evaluated the effects of ketorolac in mice over a period of about 2 h and therefore cannot rule out possible effects of ketorolac that require longer time to develop. In addition, ketorolac may have subtle actions on the inner ear that are not reflected in the VsEP.
The analgesic properties of ketorolac make it a candidate for use as a supplement to anesthesia in rodents. The present findings indicate that ketorolac may be used for that purpose acutely without producing significant changes in VsEP functional measures. However, investigators choosing to use ketorolac should consider other effects of the drug, especially those leading to prolonged clotting times.
Acknowledgments
This work was supported by NIH DC006443-04S1, The National Organization for Hearing Research Foundation, The American Academy of Audiology (AAA) and AAA Foundation (Vestibular Student Investigator Research Grant), and the Department of Communication Sciences and Disorders (East Carolina University, Greenville, NC). The work was completed in partial fulfillment of requirements for the AuD/PhD degree in the Department of Communication Sciences and Disorders (East Carolina University). We thank Drs SM Jones and A Stuart for their review of and comments on this manuscript.
References
- 1.Bauman NG. 2003. Ototoxic drugs exposed. Stewartstown (PA): GuidePost Publications. [Google Scholar]
- 2.Campbell WB, Halushka PV. 1996. Lipid-derived autocoids, eicosanoids, and platelet-activating factor, p 601–657. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG. Goodman and Gilman's pharmacological basis of therapeutics. New York (NY): McGraw–Hill. [Google Scholar]
- 3.Domer F. 1990. Characterization of the analgesic activity of ketorolac in mice. Eur J Pharmacol 177:127–135 [DOI] [PubMed] [Google Scholar]
- 4.Hillier K. 1981. BPPC. Drugs Future 6:669–670 [Google Scholar]
- 5.Jett MF, Ramesha CS, Brown CD, Chiu S, Emmett C, Voronin T, Sun T, O'Yang C, Hunter JC, Eglen RM, Johnson RM. 1999. Characterization of the analgesic and antiinflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther 288:1288–1297 [PubMed] [Google Scholar]
- 6.Jones SM. 2008. Vestibular sensory-evoked potentials, p 379–404. In: Jacobson GP, Shepard NT. Balance function assessment and management. San Diego (CA): Plural Publishing. [Google Scholar]
- 7.Jones SM, Erway LC, Bergstrom RA, Schimenti JC, Jones TA. 1999. Vestibular responses to linear acceleration are absent in otoconia-deficient C57BL/6JEi-het mice. Hear Res 135:56–60 [DOI] [PubMed] [Google Scholar]
- 8.Jones SM, Subramanian G, Avniel W, Guo Y, Burkard RF, Jones TA. 2002. Stimulus and recording variables and their effects on mammalian vestibular evoked potentials. J Neurosci Methods 118:23–31 [DOI] [PubMed] [Google Scholar]
- 9.Jones TA. 1992. Vestibular short latency responses to pulsed linear acceleration in unanesthetized animals. Electroencephalogr Clin Neurophysiol 82:377–386 [DOI] [PubMed] [Google Scholar]
- 10.Jones TA, Jones SM. 1999. Short-latency compound action potentials from mammalian gravity receptor organs. Hear Res 136:75–85 [DOI] [PubMed] [Google Scholar]
- 11.Jones TA, Jones SM, Colbert S. 1998. The adequate stimulus for avian short-latency vestibular responses to linear translation. J Vestib Res 8:253–272 [PubMed] [Google Scholar]
- 12.Jones TA, Jones SM, Vijayakumar S, Brugeaud A, Bothwell M, Chabbert C. 2011. The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs). Hear Res. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludbrook J. 1998. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol 25:1032–1037 [DOI] [PubMed] [Google Scholar]
- 14.Mock BE. 2008 Functional aging of the inner ear sensory systems in mouse models of age-related hearing loss. [Dissertation]. Greenville (NC): East Carolina University. [Google Scholar]
- 15.Mock BE, Jones TA, Jones SM. 2011. Gravity receptor aging in the CBA/CaJ strain: a comparison to auditory aging. J Assoc Res Otolaryngol 12:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mroszczak EJ, Lee FW, Combs D, Sarnquist FH, Huang B-L, Wu AT, Tokes LG, Maddox ML, Cho DK. 1987. Ketorolac tromethamine absorbtion, distribution, metabolism, excretion, and pharmacokinetics in animals and humans. Drug Metab Dispos 15:618–626 [PubMed] [Google Scholar]
- 17.Nazareth AM, Jones TA. 1998. Central and peripheral components of short-latency vestibular responses in the chicken. J Vestib Res 8:233–252 [PubMed] [Google Scholar]
- 18.Otti T, Weindel M, Bastani B. 1997. Ketorolac-induced acute reversible hearing loss in a patient maintained on CAPD. Clin Nephrol 47:208–209 [PubMed] [Google Scholar]
- 19.Pasloske K, Renaud R, Burger J, Conlon P. 1999. Pharmacokinetics of ketorolac after intravenous and oral single-dose administration in dogs. J Vet Pharmacol Ther 22:314–319 [DOI] [PubMed] [Google Scholar]
- 20.Plumb DC. 2008. Plumb's veterinary drug handbook. Ames (IA): Blackwell Publishing. [Google Scholar]
- 21.Rappaport JM, Bhatt SM, Burkard RF, Merchant SN, Nadol JB. 1999. Prevention of hearing loss in experimental pneumococcal meningitis by administration of dexamethasone and ketorolac. J Infect Dis 179:264–268 [DOI] [PubMed] [Google Scholar]
- 22.Reinhart DI. 2000. Minimizing the adverse effects of ketorolac. Drug Saf 22:487–497 [DOI] [PubMed] [Google Scholar]
- 23.Rooks WH 2nd, Maloney PJ, Shott LD, Schuler ME, Sevelius H, Strosberg AM, Tanenbaum L, Tomolonis AJ, Wallach MB, Waterbury D, Yee JP. 1985. The analgesic and antiinflamatory profile of ketorolac and its tromethane salt. Drugs Exp Clin Res 11:479–492 [PubMed] [Google Scholar]
- 24.Rooks WH 2nd, Tomolonis AJ, Maloney PJ, Wallach MB, Schuler ME. 1982. The analgesic and antiinflammatory profile of (±)-5-benzoyl-1,2-dihydro-3H-pyrrolo[1,2a]pyrrle-1-carboxylic acid (RS37619). Agents Actions 12:684–690 [DOI] [PubMed] [Google Scholar]
- 25.Schaab KC, Dickinson ET, Setzen G. 1995. Acute sensorineural hearing loss following intravenous ketorolac administration. J Emerg Med 13:509–513 [DOI] [PubMed] [Google Scholar]