Abstract
Leukapheresis is a common procedure for hematopoietic cell transplantation in adults. The main challenge in applying this procedure to human infants and small monkeys is the large extracorporeal blood volume (165 mL on average) necessary for priming the apheresis machine. This volume represents greater than 50% of the total circulating blood volume of a human neonate or small monkey. In this report, we document a safe leukapheresis protocol developed for rhesus macaques (3.9 to 8.7 kg). To avoid sensitizing donor animals undergoing leukapheresis to third-party blood products, autologous blood collected during the weeks prior to leukapheresis was used to volume-expand the same donor while priming the machine with saline on the day of leukapheresis. During the procedures, blood pressure was controlled by monitoring the inlet volume, and critical-care support was provided by the anesthesia team. Electrolytes and hemogram parameters were monitored intermittently. Overall, our research subjects underwent effective 4- to 6-h leukapheresis. A total of 9 leukapheresis procedures were performed, which yielded 1 × 109 to 6 × 109 peripheral blood mononuclear cells containing 1.1 to 5.1 × 106 CD34+ cells (assessed in 4 of 9 macaques) in a volume of 30 to 85 mL. All macaques showed decreases in Hct and platelet counts. In summary, we report a successful modified leukapheresis procedure that can be performed safely in small animals without modification of the leukapheresis machine or associated cell-collection kits.
Abbreviation: CPDA, citrate phosphate dextrose adenine solution; GCSF, granulocyte colony-stimulating factor; PBMC, peripheral blood mononuclear cell
Hematopoietic cell transplantation is a common therapy for the treatment of many neoplastic and nonneoplastic disorders.13,14 Hematopoietic stem cells are commonly collected from the peripheral blood—rather than the bone marrow—of adult humans.13,14 During this process, bone-marrow–derived stem cells are released into the circulation and collected via leukapheresis after cytokine administration, such as granulocyte colony-stimulating factor (GCSF).13,14 One of the key benefits of leukapheresis is that it requires no surgical intervention. Numerous reports have demonstrated the safety and effectiveness of leukapheresis for harvesting stem-cell–enriched peripheral blood from adults.14 However, the volume required to prime the leukapheresis machine remains one of the leading limitations of this procedure, especially in patients with low body weights.19,22 Although plasma, platelets, and RBC are returned to the donor throughout the procedure, there is inevitably some decline in blood counts due to hemodilution, harvesting into the PBMC fraction, and platelet adhesion to the leukapheresis tubing. Reports of leukapheresis in human neonates are limited, due to the insufficient blood volume and technical complications related to their small size.24 Adverse reactions in children undergoing apheresis are reported to occur during as many as 55% of all procedures and in 82% of patients (each patient generally undergoes multiple procedures).19 Hypotension, symptomatic hypocalcemia, allergic reactions, catheter-related thrombosis, and severe anemia are the most common complications,19 and 1% of the patients in the cited study19 died. In comparison, pheresis procedures in adults are much safer, with only 28% of patients having an adverse event19 associated with 4.75%18 of procedures.
Similar complications potentially can occur in small experimental animals, such as nonhuman primates. Rhesus macaques and other nonhuman primates are excellent models for hematopoietic cell transplantation.1-3 Leukapheresis of small monkeys that are similar in size and weight to human neonates and infants (3 to 10 kg) can be performed.1-3 The close phylogenetic relationship of macaques to humans has resulted in the widespread use of macaques as a preclinical model for hematopoietic cell and solid-organ transplantation.5,9-12 The safety and therapeutic efficacy of hematopoietic cell transplantation has been evaluated previously by using nonhuman primate models1,2,7 In these reports, severe anemia and thrombocytopenia and a rapid decline in Hct during leukapheresis were observed. Furthermore, modification of the mobilization kit was required to successfully leukapherese those animals; this procedure is not approved by the Food and Drug Administration and therefore is not clinically applicable.
We evaluated the safety and efficacy of leukapheresis in rhesus macaques without modification of a commercially available, clinical-grade, and approved leukapheresis kit. Macaques weighing less than 10 kg were safely used as transplant donors. Donor stem cells were mobilized to the periphery by using GCSF alone or with AMD3465. AMD3465 functions as a small-molecule antagonist of the chemokine receptor CXCR4.4 Disrupting the interaction between CXCR4 and CXCL12, a chemokine involved in the homing of hematopoietic stem cells to the bone marrow, releases stem cells into the peripheral blood. GCSF is a cytokine that mobilizes bone marrow stem cells and that has a profound effect of the maturation of cells, especially those from the granulocyte lineages.4 Cytokine-mobilized stem cells can be identified by assessing their CD34+ expression, a protein found on pluripotent hematopoietic stem cells. Engraftment of hematopoietic stem cells is important for long-lasting donor-derived immune reconstitution.
Here we report the successful harvest of peripheral blood mononuclear cells (PBMC) from rhesus macaques for transplantation purposes. The degree of hemodilution, changes in serum electrolytes, and declines in platelet and Hct levels were managed successfully in all but one case. We share our experience with this clinically relevant nonhuman primate model and demonstrate the safety of leukapheresis in the absence of modification of the leukapheresis machine or the mobilization kit.
Materials and Methods
Animals.
Nine mother–son pairs of rhesus macaques (Macaca mulatta) were used. At the time of leukapheresis, monkey weights ranged between 3.9 and 8.7 kg (Table 1). All animals were vaccinated and free of Mycobacterium tuberculosis, B virus, SIV, simian T-lymphotropic virus type 1, measles virus, and simian retrovirus types 1, 2, 3, and 5. All procedures were approved by the Massachusetts General Hospital IACUC.
Table 1.
Summary of leukapheresis procedures in rhesus macaques
| Macaque | Weight (kg) | Mobilizing agent(s) | Dose(s) | Blood volume given before leukapharesis (mL) | Duration of procedure (min) | No. of CD34+ cells recovered (× 106) | Total no. of cells (× 109) | No. of PBMC per kg of donor weight (× 108) | Total volume collected (mL) |
| S0109 | 5.2 | GCSF | 10 μg/kg | 125 | 240 | — | 6.5 | 12 | 30 |
| S0509 | 5.3 | GCSF | 10 μg/kg | 130 | 240 | 1.86 | 2.24 | 4.2 | 62 |
| S0309A | 8.1 | GCSF | 10 μg/kg | 194 | 240 | — | 1.5 | 1.9 | 60 |
| S0309B | 8.8 | GCSF | 100 μg/kg | 210 | 260 | — | 4.8 | 5.4 | 30 |
| S9010 | 4.7 | G-CSF | 100 μg/kg | 110 | 280 | 1.41 | 1.5 | 3.2 | 69 |
| S0510 | 5.3 | GCSF + AMD 3465 | 100 μg/kg, 1mg/kg | 127 | 240 | 5.16 | 2.0 | 3.7 | 62 |
| S0910 | 4.0 | GCSF + AMD 3465 | 100 μg/kg, 1 mg/kg | 96 | 275 | — | 1.7 | 4.2 | 85 |
| S8810 | 3.9 | GCSF + AMD 3465 | 100 μg/kg, 1 mg/kg | 90 | 300 | 1.19 | 2.0 | 5.1 | 48 |
| S8610 | 5.4 | GCSF + AMD 3465 | 100 μg/kg, 1 mg/kg | 129 | 300 | — | 1.5 | 2.7 | 57 |
There were no significant differences between treatment groups in the total number of cytokine-mobilized PBMC recovered.
Stem-cell mobilization.
Bone marrow derived stem cells in macaques were mobilized to the periphery by using 1 of 3 regimens designed to achieve the best stem-cell source for inducing engraftment and long-lasting chimerism in recipient monkeys (manuscript in preparation): GCSF (0.3 mg/mL; Filgrastim, Amgen, Thousand Oaks, CA) at doses of 10 μg/kg (the human clinical dose) or 100 μg/kg (high dose shown to work well in nonhuman primates)20 or GCSF (100 μg/kg) + AMD3465 (1 mg/kg; Genzyme, Cambridge, MA). Cytokines were administered subcutaneously once daily for each of the 4 d preceding the leukapheresis; AMD3465 was administered subcutaneously 4 h prior before leukapheresis.
Leukapheresis.
Donor macaques were leukapheresed under general anesthesia, and each procedure lasted 4 to 6 h. Macaques were sedated with 10 mg/kg ketamine; anesthesia was induced with isoflurane; and the animals were intubated. All fluids returned to the patients were warmed to 37 °C by using circulating blood warmers. The animals were placed on a circulating warm-water blanket to minimize hypothermia during the procedure. The leukapheresis machine (Cobe Spectra Auto PBMC, Gambro, Denver, CO) and Auto PBMC kit (Gambro) were prepared according to the manufacturer's instructions. The initial priming of the leukapheresis machine used normal saline, according to the standard procedure from the manufacturer. Before donors were connected to the leukapheresis machine, they were transfused with stored autologous blood to expand the circulating blood volume. The normal priming volume of the Auto PBMC machine ranges between 165 to 280 mL. During the leukapheresis, 2 intravenous catheters were used—a central access line and a peripheral return line. Access ports were either a central-vein or intravenous catheter (18- to 21-gauge). In our experience, the access port was most useful when a cut-down procedure was performed to isolate the femoral vein. During collection, the blood circulating through the leukapheresis machine was anticoagulated at a 10:1 ratio of blood to citrate phosphate dextrose adenine solution (CPDA). During the procedure, the WBC count, Hct, hemoglobin concentration, platelet count, and serum chemistries of donor macaques were monitored. After 4 to 6 h of leukapheresis, saline was used to rinse the collection tubing (the ‘rinseback process’); the resulting rinseback product was centrifuged at 900 × g for 12 min; and the packed RBCs were isolated and transfused back into the donor. Depending on their total protein and calcium levels, macaques were given 5% albumin boluses and 10% calcium gluconate (100 mg/kg/h; maximum, 1 g/h).
Donor blood collection.
Because we were unable to use third-party blood donors or synthetic blood products due to our experimental design, 10% of the total circulating blood volume of each leukapheresis subject was collected 4 times during the 35 d preceding the procedure. The blood volume collected at any given time was based on the assumption of 50 to 60 mL of blood per kilogram body weight for an animal weighing 5 to 9 kg; thus a maximum of 25 to 54 mL was collected during each session. Each blood collection was stored in a sterile blood-collection bag containing the anticoagulant citrate phosphate dextrose adenine at a 7:1 blood:anticoagulant ratio. All macaques were given vitamins and iron supplements, and subsequent phlebotomies were delayed when the Hct of a donor had not recovered after a previous preleukapheresis blood collection. Blood from each donor was pooled aseptically into a single large bag on the day of leukapheresis. Blood cultures were taken from the pooled product to assess for contamination.
Physiologic monitoring during leukapheresis.
During leukapheresis, all macaques were maintained under anesthesia with isoflurane. The anesthesiologists continuously monitored the vascular status and vital parameters of all macaques, including blood pressure, heart rate, respiratory rate, end-tidal CO2, and pO2, and recorded these data every 5 to 10 min. Blood samples (0.5 to 1 mL) obtained at least 3 times: immediately before the procedure, at the 2-h time point, and immediately after the procedure. More samples were taken when deemed necessary for medical reasons. Hemograms were run inhouse (HemaVet 950FS, Heska, Fort Collins, CO); we here report Hct and platelet and total WBC counts. Serum chemistries and electrolytes were monitored also (Catalyst DX, IDEXX, Westbrook, ME); we report only the calcium and total protein levels.
CD34+ phenotyping.
A sample of the leukapheresis product underwent flow cytometric assessment of CD34+ cells (FACScan, Becton Dickinson, San Jose, CA). Heparinized peripheral blood was distributed into staining tubes (100 µL per tube) and washed once by using 2 mL flow cytometry buffer (HBSS containing Ca2+ and Mg2+, 0.1% BSA, and 0.1% NaN3). Cells then were stained by incubating in 10 µL direct phycoerythrin–streptavidin-conjugated antiCD34 monoclonal antibody (clone 563, Becton Dickinson) for 30 min at room temperature. RBC were lysed and other cells fixed by using FACS lysis solution (Becton Dickinson) before acquisition of signals. Data were analyzed by using Winlist mode analysis software (Verity Software House, Topsham, ME).
Statistical analysis.
The Mann–Whitney U test (nonparametric) to assess differences in total PBMC count between treatment groups. A P value of 0.05 was considered statistically significant. The program PRISM (version 6, GraphPad Software, La Jolla,CA) was used to run all analyses.
Results
Clinical outcomes.
On physical exam, all 9 of the donor macaques were unaffected by the mobilization regimens. All maintained normal appetite throughout the mobilization period. No fevers, infections, or abnormal hematologic changes were identified secondary to the cytokines injected, with the exception of an acute increase in WBC count. All 9 macaques underwent leukapheresis, 8 of which recovered successfully, without complications. These 8 macaques all survived long-term to donate blood for immunologic assays, and each donated one of their kidneys for the assessment of tolerance in recipients (data not shown).
During leukapheresis, macaque S0509 experienced an acute drop in blood pressure, which was managed aggressively by decreasing the level of anesthesia, increasing the fluid rate, and stopping the leukapheresis machine. Abdominal ultrasonography demonstrated free fluid, and a subsequent fine-needle aspirate confirmed the fluid to be whole blood. This macaque received 4 blood transfusions during emergency laparotomy, which revealed that the bleeding originated from the liver capsule. Because the macaque was anticoagulated, we were unable to control the hemorrhage, and the macaque was euthanized in view of its poor response to treatment. No signs of trauma were observed. No abnormal preleukapheresis physical exam findings were noted; liver enzymes were always normal; and Hct values before and after stem-cell mobilization (preleukapheresis) were within normal limits. Platelet counts had decreased but remained within the normal range.
WBC counts.
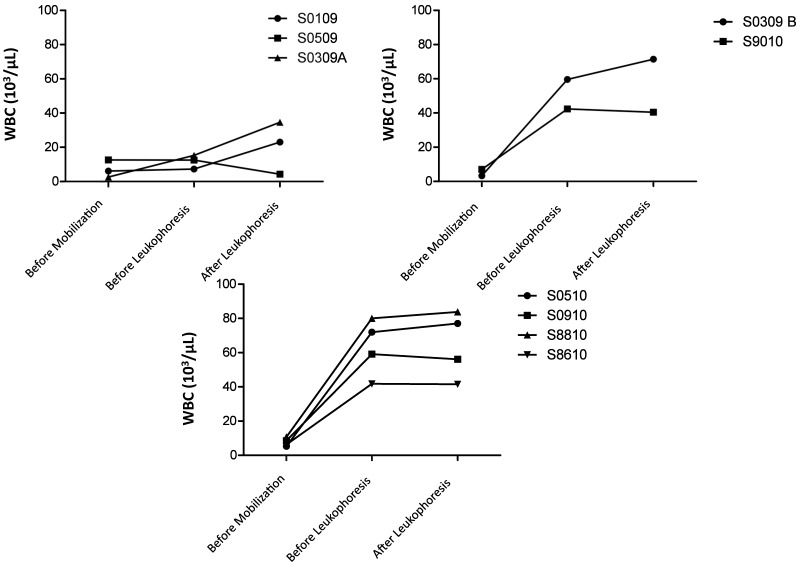
After cytokine mobilization and before leukapheresis, donors that received 100 µg/kg GCSF with (n = 4) or without (n = 2) AMD3465 showed marked increases in their WBC counts in excess of reported reference values for rhesus macaques (5000 to 19,000 cells per microliter; Table 1 and Figure 1). With the exception of animal S0309A, macaques that received 10 µg/kg GCSF showed only modest increases in WBC after mobilization; these same macaques demonstrated marked increases in WBC counts after leukapheresis. The other 2 treatment groups, which had already very high WBC counts before leukapheresis, had minimal change in their WBC counts after leukapheresis, and these levels were comparable to those preleukapheresis (and significantly [P < 0.05] higher than premobilization counts) despite temporary fluctuations in WBC counts during the procedure. None of the macaques in these 2 treatment groups 2 and 3 had a significant drop in WBC counts despite successful collection of PBMC after a 4- to 5-h leukapheresis procedure (Figure 1).
Figure 1.
WBC counts in macaques that received (top left) 10 μg/kg GCSF, (top right) 100 μg GCSF, or (bottom) 100 μg/kg GCSF + 1 mg/kg AMD3465. Macaques in all groups exhibited an increase or maintenance of WBC counts during leukapheresis. The regimens using 100-μg/kg GCSF led to markedly increased cell counts after mobilization, which were maintained throughout leukapheresis.
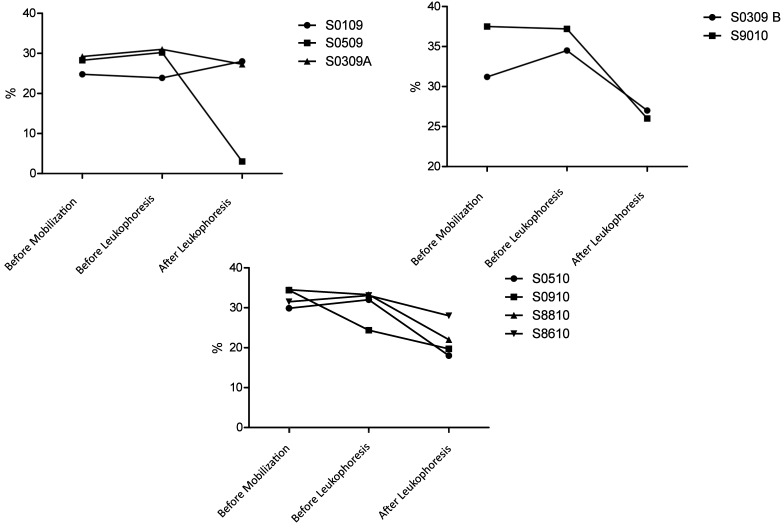
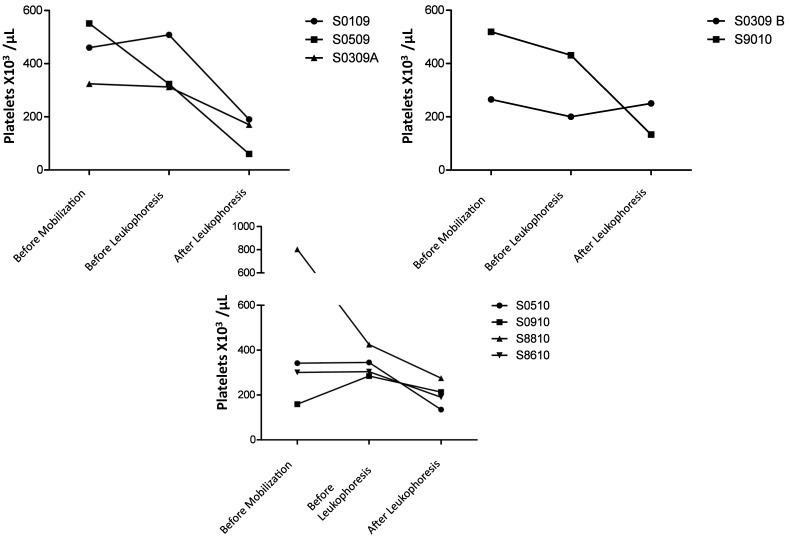
Platelet counts and Hct.
Hct (Figure 2) and platelet counts (Figure 3) were monitored closely throughout leukapheresis. With the exception of animal 0309B (100 µg/kg GCSF), which had an increase in Hct preleukapheresis, and animal S0910 (100 µg/kg GCSF + 1 mg/kg AMD3465), which had a decrease in Hct preleukapheresis, none of the macaques showed noteworthy changes in Hct after the cytokine-based stem-cell mobilization regimens. In contrast, leukapheresis led to decreases in Hct in 7 of the 9 macaques; an average Hct decline of 8% to 10% was observed (Figure 2). Except for animal S0509, which hemorrhaged secondary to the rupture of her liver capsule, macaques that received 10 µg/kg GCSF had the smallest drop in platelet count. Post leukapheresis animals recovered with HCTs of 20% to 27%. Although platelet counts had decreased in all animals by the end of leukapheresis, levels remained within a safe range (100,000 platelets per microliter), and no platelet-associated bleeding was observed. The mobilization regimen led to a decrease in platelets in 3 (10 µg/kg GCSF, n = 1; 100 µg/kg GCSF, n = 2) of the 9 macaques.
Figure 2.
Hct in macaques that received (top left) 10 μg/kg GCSF, (top right) 100 μg GCSF, or (bottom) 100 μg/kg GCSF + 1 mg/kg AMD3465. Macaques in all groups exhibited mild to moderate decreases in hematocrit. Macaque 0509 (10 μg/kg GCSF) died of hemorrhage.
Figure 3.
Platelet counts in macaques that received (top left) 10 μg/kg GCSF, (top right) 100 μg GCSF, or (bottom) 100 μg/kg GCSF + 1 mg/kg AMD3465. Macaques in all groups exhibited moderate decreases in platelet counts. With the exception of animal 0509, all macaques maintained platelet counts above 100,000 per microliter.
Chemistry and electrolyte parameters.
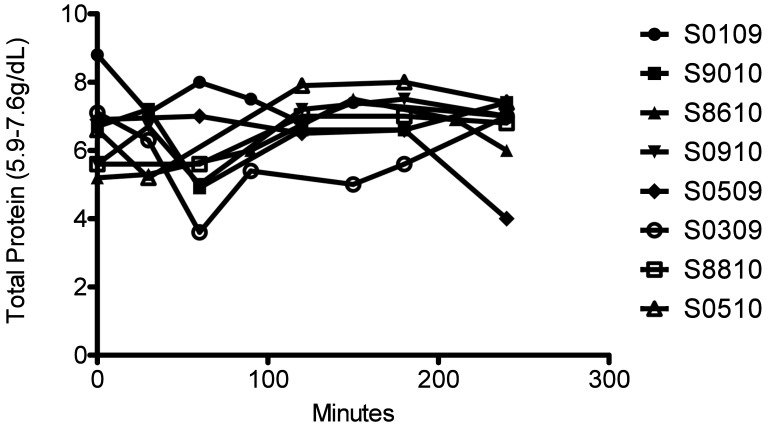
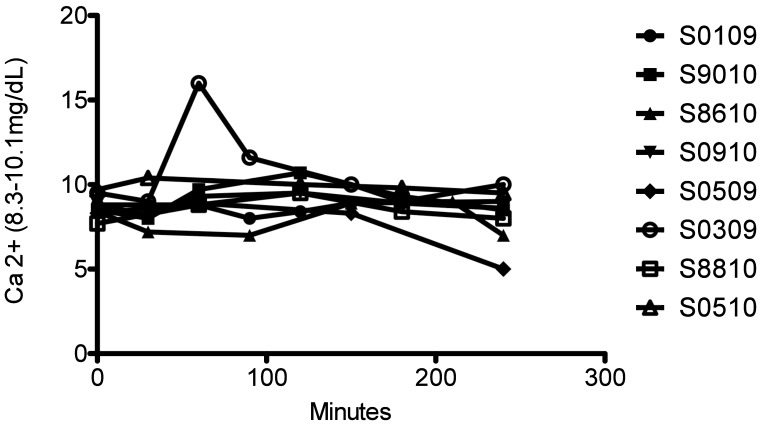
Serum chemistry values and electrolytes were monitored. Liver (AST, ALT, bilirubin) and kidney (creatinine, BUN) parameters were all within normal ranges throughout leukapheresis in all macaques (data not shown). So that we could provide appropriate oncotic and electrolyte support by using either albumin or calcium, total protein (Figure 4) and Ca2+ (Figure 5) levels were monitored closely. Overall, total protein and calcium levels remained stable throughout the procedures in all macaques except S0509, which had marked decreases in both parameters after leukapheresis (Figures 4 and 5).
Figure 4.
Total protein in leukapheresed macaques. Time 0 is preleukapheresis. Total protein was tested throughout the procedure to tailor albumin supplementation.
Figure 5.
Total serum calcium in leukapheresed. Time 0 is preleukapheresis. Calcium was tested throughout the procedure while Ca gluconate was given.
Blood pressure.
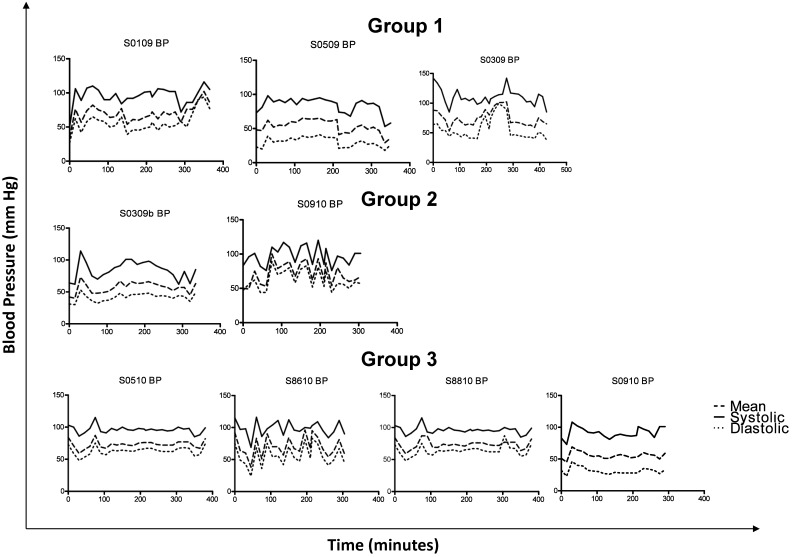
Blood pressure fluctuated during leukapheresis (Figure 6). All macaques developed minor, nonlife-threatening variations in blood pressure, which were corrected accordingly by either decreasing the depth of anesthesia, increasing the colloid–crystalloid fluid rate, or decreasing the rate at which blood was removed from the donor by the leukapheresis machine. Among the 9 macaques, 8 maintained a mean blood pressure above 50 mm Hg and systolic blood pressure above 80 mm Hg; the lone donor whose blood pressure fell below 50 mm Hg was (again) S0509. In general, systolic BP remained close to 100 mm Hg and mean blood pressure near 60 mm Hg. All macaques had an early spike in blood pressure with a subsequent nadir, during which times the anesthesia teams and transfusionists worked to achieve a stable plane of blood pressure.
Figure 6.
Blood pressure in leukapheresed macaques. The top 3 panels represent macaques given 10 μg/kg GCSF. The animals shown in the middle 2 panels received 100 μg/kg GCSF. All 4 panels on the bottom depict macaques that received 100 μg/kg GCSF + 1 mg/kg AMD3465.
Collection of stem-cell–enriched PBMC and CD34+ cell counts.
The total number of PBMC collected during leukapheresis ranged between 1.5 and 6.5 × 109 (Table 1). The average number of PBMC collected per donor was 2.63 × 109. Between 2.7 × 108 and 1.2 × 109 PBMC were collected per kilogram donor body weight. We assessed the number of CD34+ PBMC in 4 of the 9 macaques; this parameter ranged from 1.19 to 5.16 × 106 cells. The total volume collected was 30 to 85 mL (Table 1). The collection bag was never opened, nor was the product rinsed. The leukapheresed product was given directly to recipients, and a small sample was submitted for bacterial culture; these samples were negative for growth in all cases.
Discussion
Limitations of leukapheresis include, but are not limited to, adequate vascular access, the use of anticoagulants, the circulating blood volume of the patient, citrate toxicity, and maintenance of the normal physiologic parameters of the donor.
In subjects weighing more than 20 kg, vascular access sufficient to maintain adequate flow can be achieved by using peripheral (for example, saphenous) veins.17-19 However, small animals or human neonates weighing less than 10 kg require placement of central venous access catheters to achieve sufficient blood flow.19 In the macaques used in the current study, a femoral vein cut-down approach was used. It was crucial for the catheter to be advanced toward the femoral triangle to provide sufficient blood flow. The return line was always in a peripheral vessel (saphenous or cephalic) and rarely caused return pressure problems, which have often been reported in pediatric patients.19 Sporadic blood clotting of the access line (especially at the beginning of the procedure) occurred in some macaques; this complication has also been observed during pheresis of pediatric patients.8,15,16 In our case, the placement of a 3-way stop cock for occasional flushing was key to preventing or minimizing this problem, particularly at the beginning of the procedure when the least amount of anticoagulant was distributed systemically. The placement of the stopcocks close to the access points did not interfere with the procedure.
Heparin pretreatment is common during pheresis. Often, children receive heparin (20 units/kg) in addition to acid citrate dextrose during the procedure.18 We avoided heparin anticoagulation during leukapheresis of our macaques for several reasons. First, we found that a 10:1 blood:CPDA ratio during PBMC collection was sufficient to prevent clotting. Second, we never encountered any citrate-mediated toxicity (seizures, arrhythmias, nausea, vomiting) in our macaques. Third, we attempted to limit the amount of anticoagulants delivered to the recipients. Fourth, the risk of bleeding, especially in nonhuman primates, which tend to manipulate their incisions, prompted us to be very careful in our choice of anticoagulants. We therefore favored minimizing any additional medications in our donors. To avoid citrate toxicity, we provided sufficient calcium supplementation to maintain stable serum calcium levels (Figure 5). Similar to our macaques, humans undergoing leukapheresis require supplementation with calcium gluconate. Children are given oral calcium gluconate (10 mg/kg) before pheresis and every hour after starting the procedure; alternatively, a 10% solution (10 mL diluted to 50 mL) of calcium gluconate is delivered intravenously at 1 mL/kg/h.19 It is important to mention that when the CPDA:blood ratio is greater than 1:15, there is an increased risk of clotting within the components of the auto-PBMC kit.23 In pediatric patients, minimizing CPDA at the expense of heparinization has been the goal;22 we therefore suggest that macaques that receive low doses of CPDA should be anticoagulated with low-dose heparin also.
The total number of PBMC collected during approximately 4 h of leukapheresis was sufficient to achieve the target dose of 1.0 × 109 PBMC per kilogram recipient body weight. None of the recipients had any reaction to the unwashed donor inocula (data not shown). The numbers of CD34+ cells from our donors were less than those previously documented (20 × 107 CD34+ cells) for other leukapheresis protocols involving nonhuman primates.21 Part of our studies aimed to develop novel stem-cell mobilization approaches that may enhance engraftment. Therefore, strict comparisons are difficult to analyze, not only due differences regarding mobilizing agents but also because of the use of different species of nonhuman primates, such as cynomolgus macaques and baboons.
Sensitization is an immune process by which antigen-specific responses are created to a foreign antigen. One of the biggest challenges posed by our protocol was priming the leukapheresis machine without sensitizing the donor animals to blood products from other nonhuman primates. We decided to use the Auto-PBMC kit instead of the WBC Harvest kit because the Auto-PBMC kit required less volume for priming (165 mL) and collected a smaller volume (about 50 mL in 4 to 5 h), allowing us to consider using autologous blood collected prior to the procedure for priming. In comparison, the WBC kit required 280 mL blood for priming, and the approximate collection volume would have been 240 to 300 mL during the same period.23 To prime the kit, we considered the use of oxyglobin and other colloidal carriers. However, oxyglobin, which in our opinion was the best commercially available blood replacement option, would have caused the serum to become red-tinged and therefore would have interfered with the collection of the PBMC. Instead, we decided to prime the leukapheresis machine with autologous blood collected over a 1-mo period. This strategy may explain some of the decreases in Hct and platelet levels that occurred in some of our macaques before leukapheresis. In all cases, this practice provided a sufficient amount of donor blood for autotransfusion to the macaque while the leukapheresis machine was primed. Autologous blood can be used only when logistically practical (that is, the donor can be bled repeatedly over a 35-d period) and may be necessary in situations in which possible sensitization to third-party antigens is unfavorable to the desired clinical outcome.
We contemplated 2 approaches for priming the leukapheresis machine. One called for volume-expansion of the donor at the time of leukapheresis, and the second involved direct priming of the machine with the blood product. We have extensive experience in our laboratory in pheresing adult swine.6 In preliminary studies, we therefore optimized the protocol for leukapheresing 10-kg pigs to similarly sized nonhuman primates. We found that volume-expansion of the donor macaque by giving 50% of the collected blood before leukapheresis and the remaining 50% at the beginning of leukapheresis was the optimal approach to maintaining an adequate blood pressure.
The use of peripheral catheters, in addition to a central catheter, was crucial to the success of the protocol we describe here. The same practice has proven necessary in pediatric patients as well.19 Mean arterial blood pressures were maintained at or above 50 mm Hg in all 9 macaques. Only 1 of the 9 macaques (S0509) had a severe adverse event related to acute changes in blood pressure. Another animal that showed moderate blood pressure abnormalities was S0109; this macaque was the first to undergo leukapheresis and, unlike the other 8 animals, received 50% of the rinseback product rather than packed RBC. During the rinseback procedure, all RBC remaining in the Auto-PBMC kit were diluted with saline and returned to the patient. This particular macaque therefore received an additional 90 to 120 mL of the blood:saline mixture. The rapid delivery of this volume of fluid likely caused the transient increase blood pressure (Figure 5). We subsequently modified our protocol to provide only packed RBC (a volume of 30 to 40 mL)—rather than the rinseback product (120 to 200 mL)—at the end of leukapheresis. The third animal with a blood pressure abnormality (macaque S0309) showed a pressure spike at the very beginning of the procedure, during autotransfusion of whole blood. The transfusate was delivered too rapidly to this macaque, thereby accounting for the elevation in blood pressure. Decreasing the rate of the transfusion, combined with increasing the leukapheresis outlet rate and decreasing the flow of intravenous fluids into the cephalic vein (controlled by the anesthesiologists), normalized this macaque's blood pressure within minutes.
Despite our careful delivery of fluids, including supplemental albumin, colloidal support (whole-blood transfusion and packed RBC), and the use of crytalloids, some of our macaques developed edema, likely the result of volume overload. This side effect was inevitable, because the inflow into the leukapheresis machine (even at the lowest rates of 5 mL/min) often affected the blood pressure of our small nonhuman primates. However, no acute respiratory distress or emergencies related to fluid overload occurred. When lung sounds suggestive of pulmonary edema were identified or when oxygenation was compromised, macaques received furosemide (1 to 2 mg/kg) at the time of recovery. All animals recovered promptly and without complications after anesthesia. Three macaques that exhibited pronounced hemodilution and a Hct of 20 or less were autotransfused prior to recovery with packed RBC collected during rinseback of the leukapheresis machine.
We were concerned about the possibility of our macaques developing infections, which have been documented in pediatric patients that maintain intravenous catheters long-term.19 We avoided this problem by removing all catheters after the procedure. None of our blood products (blood collected for autotransfusion and leukapheresis product) were contaminated, and none of our donors required antibiotics or exhibited any signs of septicemia related to a contaminated product.
In summary, we here describe a leukapheresis procedure that can be safely and effectively applied to nonhuman primates weighing less than 10 kg. Although it has been extensively documented that young children and adults undergo leukapheresis safely,18 there is only limited literature on leukapheresis in neonates.19,22,24 Our current results suggest that it is safe to perform leukapheresis in nonhuman primates that are similar in size to human neonates. However, doing so requires sufficient planning, forethought of all potential complications, and, most importantly, the coordination of a multidisciplinary team.
Acknowledgments
We thank Amgen for providing GCSF and Genzyme for providing AMD3465. We thank Drs Thomas Spitzer and Joanne Morris for critical review of this manuscript.
Part of this work was sponsored by NCRR K01RR024466 (RDS) and NIH 5U19AI051731 (CH) and 5T32AI007529 (VP).
References
- 1.Ageyama N, Hanazono Y, Shibata H, Ohto K, Ono F, Nagashima T, Ueda Y, Donahue RE, Hasegawa M, Ozawa K, Yoshikawa Y, Terao K. 2002. Safe and efficient methods of autologous hematopoietic stem cell transplantation for biomedical research in cynomolgus monkeys. Comp Med 52:445–451 [PubMed] [Google Scholar]
- 2.Ageyama N, Hanazono Y, Shibata H, Ono F, Ogawa H, Nagashima T, Ueda Y, Yoshikawa Y, Hasegawa M, Ozawa K, Terao K. 2005. Safe and efficient collection of cytokine-mobilized peripheral blood cells from cynomolgus monkeys (Macaca fascicularis) with human-newborn–equivalent body weights. Exp Anim 54:421–428 [DOI] [PubMed] [Google Scholar]
- 3.Ageyama N, Kimikawa M, Eguchi K, Ono F, Shibata H, Yoshikawa Y, Terao K. 2003. Modification of the leukapheresis procedure for use in rhesus monkeys (Macaca mulata). J Clin Apher 18:26–31 [DOI] [PubMed] [Google Scholar]
- 4.Bodart V, Anastassov V, Darkes MC, Idzan SR, Labrecque J, Lau G, Mosi RM, Neff KS, Nelson KL, Ruzek MC, Patel K, Santucci Z, Scarborough R, Wong RS, Bridger GJ, Macfarland RT, Fricker SP.2009. Pharmacology of AMD3465: a small molecule antagonist of the chemokine receptor CXCR4. Biochem Pharmacol 78:993–1000. [DOI] [PubMed]
- 5.Buhler L, Basker M, Alwayn IPJ, Goepfert C, Kitamura H, Kawai T, Gojo S, Kozlowski T, Ierino FL, Awwad M, Sachs DH, Sackstein R, Robson SC, Cooper DKC. 2000. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation 70:1323–1331 [DOI] [PubMed] [Google Scholar]
- 6.Cina RA, Wikiel KJ, Lee PW, Cameron AM, Hettiarachy S, Rowland H, Goodrich J, Colby C, Spitzer TR, Neville DM, Jr, Huang CA. 2006. Stable multilineage chimerism without graft versus host disease following nonmyeloablative haploidentical hematopoietic cell transplantation. Transplantation 81:1677–1685 [DOI] [PubMed] [Google Scholar]
- 7.Donahue RE, Kirby MR, Metzger ME, Agricola BA, Sellers SE, Cullis HM. 1996. Peripheral blood CD34+ cells differ from bone marrow CD34+ cells in Thy1 expression and cell-cycle status in nonhuman primates mobilized or not mobilized with granulocyte colony-stimulating factor and/or stem cell factor. Blood 87:1644–1653 [PubMed] [Google Scholar]
- 8.Glaser DW, Medeiros D, Rollins N, Buchanan GR. 2001. Catheter-related thrombosis in children with cancer. J Pediatr 138:255–259 [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. 1995. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59:256–262 [PubMed] [Google Scholar]
- 10.Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, Phelan J, Boskovic S, Nadazdin O, Abrahamian G, Colvin RB, Sach DH, Madsen JC. 2002. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation 73:1757–1764 [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Wee SL, Bazin H, Latinne D, Phelan J, Boskovic S, Ko DS, Hong HZ, Mauiyyedi S, Nadazdin O, Abrahamian G, Preffer F, Colvin RB, Sachs DH, Cosimi AB. 2000. Association of natural killer cell depletion with induction of mixed chimerism and allograft tolerance in nonhuman primates. Transplantation 70:368–374 [DOI] [PubMed] [Google Scholar]
- 12.Kimikawa M, Kawai T, Sachs DH, Colvin RB, Bartholomew A, Cosimi AB. 1997. Mixed chimerism and transplantation tolerance induced by a nonlethal preparative regimen in cynomolgus monkeys. Transplant Proc 29:1218. [DOI] [PubMed] [Google Scholar]
- 13.Linenberger ML, Price TH. 2005. Use of cellular and plasma apheresis in the critically ill patient. Part 1: technical and physiological considerations. J Intensive Care Med 20:18–27 [DOI] [PubMed] [Google Scholar]
- 14.Linenberger ML, Price TH. 2005. Use of cellular and plasma apheresis in the critically ill patient. Part II: clinical indications and applications. J Intensive Care Med 20:88–103 [DOI] [PubMed] [Google Scholar]
- 15.Male C, Chait P, Andrew M, Hanna K, Julian J, Mitchell L. 2003. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood 101:4273–4278 [DOI] [PubMed] [Google Scholar]
- 16.Massicotte MP, Dix D, Monagle P, Adams M, Andrew M. 1998. Central venous catheter related thrombosis in children: analysis of the Canadian Registry of Venous Thromboembolic Complications. J Pediatr 133:770–776 [DOI] [PubMed] [Google Scholar]
- 17.McLeod BC, Price TH, Owen H, Ciavarella D, Sniecinski I, Randels MJ, Smith JW. 1998. Frequency of immediate adverse effects associated with apheresis donation. Transfusion 38:938–943 [DOI] [PubMed] [Google Scholar]
- 18.McLeod BC, Sniecinski I, Ciavarella D, Owen H, Price TH, Randels MJ, Smith JW. 1999. Frequency of immediate adverse effects associated with therapeutic apheresis. Transfusion 39:282–288 [DOI] [PubMed] [Google Scholar]
- 19.Michon B, Moghrabi A, Winikoff R, Barrette S, Bernstein ML, Champagne J, David M, Duval M, Hume HA, Robitaille N, Belisle A, Champagne MA. 2007. Complications of apheresis in children. Transfusion 47:1837–1842 [DOI] [PubMed] [Google Scholar]
- 20.Nadazdin O, Abrahamian G, Boskovic S, Smith RN, Schoenfeld DA, Madsen JC, Colvin RB, Sachs DH, Cosimi AB, Kawai T. 2011. Stem cell mobilization and collection for induction of mixed chimerism and renal allograft tolerance in cynomolgus monkeys. J Surg Res 168:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohn SK, Kim JG, Chae YS, Kim DH, Lee NY, Suh JS, Lee KB. 2003. Large-volume leukapheresis using femoral venous access for harvesting peripheral blood stem cells with the Fenwal CS 3000 Plus from normal healthy donors: predictors of CD34+ cell yield and collection efficiency. J Clin Apher 18:10–15 [DOI] [PubMed] [Google Scholar]
- 22.Stefanutti C, Lanti A, Di GS, Mareri M, De LF, Landolfo A, Isacchi G. 2004. Therapeutic apheresis in low weight patients: technical feasibility, tolerance, compliance, and risks. Transfus Apher Sci 31:3–10 [DOI] [PubMed] [Google Scholar]
- 23.Terumo Group. [Internet]. COBE Spectra Apheresis System. [Cited 18 December 2012]. Available at: http://www.terumobct.com/location/emea/products-and-services/Pages/cobe-spectra-apheresis-system.aspx#MATERIALS.
- 24.Woloskie S, Armelagos H, Meade JM, Haas D. 2001. Leukodepletion for acute lymphocytic leukemia in a 3-week-old infant. J Clin Apher 16:31–32 [DOI] [PubMed] [Google Scholar]