Abstract
Thoracic radiography has been applied extensively to cardiovascular or respiratory disorders in veterinary practice and in research using animal models. To establish baseline measures for various parameters of thoracic radiography, we used a cross-sectional method to collect the lung length (LL), maximum interior thoracic breadth (TBr), maximum breadth of the cardiac silhouette (CBr), cardiothoracic ratio (CR), and right hilar height ratio (RHHR) of random healthy captive Chinese rhesus monkeys (age, 1 to 5 y; 89 male, 64 female). Significant sex-associated differences occurred in TBr among 1-y-old subjects and RHHR in 2- and 3-y-old monkeys. In addition, LL, TBr, and CBr were significantly correlated with age in both sexes. Finally, stepwise multiple regression revealed that LL and CBr were predictors of age in female monkeys, whereas LL and TBr were age-predictive in male macaques. The current data may suggest caveats regarding the use of thoracic radiography for evaluating disease processes, including pulmonary tuberculosis, hydropericardium, and heart failure, and for assessing physical development in adolescent rhesus macaques.
Abbreviation: LL, lung length; TBr, maximum interior thoracic breadth; CBr, maximum breadth of cardiac silhouette; CR, cardiothoracic ratio; RHHR, right hilar height ratio
Rhesus macaques (Macaca mulatta) are one of the best-known species of Old World monkeys. Because of their similarities with human anatomy and physiology and relatively easy upkeep in captivity, rhesus macaques have been used extensively in medical and biologic research on human and animal health-related topics.23
Plain-film 2-dimensional radiography is a well-known, routine technique for imaging internal anatomic structures in living animals and a robust diagnostic tool in veterinary medicine. Radiography also is useful for primatologists interested in morphometric studies of primate anatomy.13 Moreover, thoracic radiography has been widely applied to animal disease model research and in veterinary practice involving nonhuman primates.2,3,16 Knowing the baseline, normal measures of thoracic radiographic parameters is essential for defining abnormalities. In this context, previous studies in long-tailed macaques (M. fascicularis) and Formosan monkeys (M. cyclopis) have focused on measurements of cardiac size or the cardiothoracic ratio.9,13-15 However, we have not found any information about the thoracic radiographic measurements of normal captive rhesus monkeys.
The current study focuses on thoracic radiographic measurements in captive rhesus macaques from China by using a cross-sectional method. We selected 153 random subjects that were 1 to 5 y old from the colony population. We then collected 5 important measurements from thoracic radiographs that yielded information regarding thoracic size, cardiac size, and hilar position and investigated the sex-associated differences in these measurements. In light of these observations, we hypothesized that at least some of the measurements obtained from thoracic radiographs would correlate with age.
Materials and Methods
Animals.
All 153 monkeys were obtained from Xishan Zhongke Laboratory Animals (Suzhou, China) in April 2011. All enrolled monkeys were born in China. The housing conditions and animal care procedures were detailed in previous report22 and were in accordance with Chinese regulatory requirements and AAALAC guidelines. After weaning, juvenile rhesus macaques were peer-reared in 30 indoor enclosures each measuring 8 m × 3 m × 3 m. Each group contained about 20 subjects. All enclosures were maintained on a 12:12-h light:dark cycle (lights on, 0600 to 1800). The macaques were given water ad libitum and were fed daily with fresh fruit, vegetables, and a commercial high-nutrition monkey food. All procedures involving nonhuman primates were approved by the Animal Care and Use Committee of Chongqing Medical University and were in compliance with Guide for the Care and Use of Laboratory Animals.8
Using simple randomization, we selected 153 (89 male, 64 female) healthy juvenile rhesus macaques (Table 1) from the colony population (n = 630) that was 1 to 5 y old. The seed value of the simple randomized sample was set as 201104. Physical examinations, CBC, and liver-, kidney-, and pancreas-specific biochemical tests were performed on all enrolled macaques prior to study initiation; all results were within normal limits.
Table 1.
Thoracic radiography measurements of rhesus macaques (age, 1 to 5 y)
| Age (y) | Sex | n | LL (mm) | TBr (mm) | CBr (mm) | CR | RHHR |
| 1 | Male | 16 | 66.04 ± 5.45a | 87.14 ± 4.66ac | 49.90 ± 2.93a | 0.57 ± 0.03a | 1.86 ± 0.24a |
| Female | 11 | 69.53 ± 8.44a | 82.96 ± 3.45ac | 48.62 ± 3.37a | 0.59 ± 0.05a | 1.74 ± 0.16a | |
| 2 | Male | 42 | 70.89 ± 8.11a | 88.18 ± 6.38a | 52.91 ± 4.40a | 0.60 ± 0.04a | 1.96 ± 0.36ad |
| Female | 15 | 70.84 ± 9.34a | 85.72 ± 6.43a | 52.30 ± 4.10a | 0.61 ± 0.03a | 1.72 ± 0.26ad | |
| 3 | Male | 15 | 80.32 ± 7.49a | 92.85 ± 4.75a | 53.30 ± 5.97a | 0.57 ± 0.05a | 1.95 ± 0.44ae |
| Female | 23 | 84.27 ± 10.20a | 94.82 ± 9.89b | 54.45 ± 4.13a | 0.58 ± 0.05a | 1.69 ± 0.22ae | |
| 4 | Male | 5 | 82.38 ± 12.98a | 100.55 ± 8.26a | 59.19 ± 3.82a | 0.59 ± 0.06a | 1.80 ± 0.53a |
| Female | 11 | 92.86 ± 7.89a | 99.32 ± 7.11b | 56.05 ± 5.27a | 0.57 ± 0.07a | 1.59 ± 0.21a | |
| 5 | Male | 11 | 104.17 ± 7.15a | 104.51 ± 13.74a | 60.40 ± 8.60a | 0.58 ± 0.05a | 1.73 ± 0.15a |
| Female | 4 | 103.58 ± 4.15a | 104.57 ± 9.24a | 62.35 ± 3.91a | 0.60 ± 0.05a | 1.69 ± 0.20b |
Data are shown as mean ± 1 SD.
Data conformed to normal distribution
Data conformed to nonnormal distribution.
P = 0.018 between TBr values of 1-y-old male and female macaques.
P = 0.018 between RHHR values of 2-y-old male and female macaques.
P = 0.049 between RHHR values of 3-y-old male and female macaques.
Radiographic examination.
The protocol for radiography has been described in detail elsewhere.13,14,18 In the current study, a digital radiography unit with a flat-panel digital detector (PLX8200, Perlove, Nanjing, China) was used for obtaining thoracic radiographs. The digital detector were exposed to X-ray at 60 kVp, with an approximate detector-to-tube distance of 1 m. Exposure times were no greater than 0.1 s, resulting in 4.0 milliamp–seconds of exposure. The macaques were anesthetized by using ketamine (10 mg/kg IM) before each procedure. Anesthetized macaques were positioned and immobilized on a platform under the detector, with their arms extended laterally by 2 assistants, who wore appropriate shielding to avoid radiation exposure. Images were obtained during the inspiratory phase of respiration. Right-to-left lateral and posteroanterior thoracic radiographs were obtained on all 153 macaques. All digital films were labeled by using the identification numbers of the subjects.
Radiographic measurement.
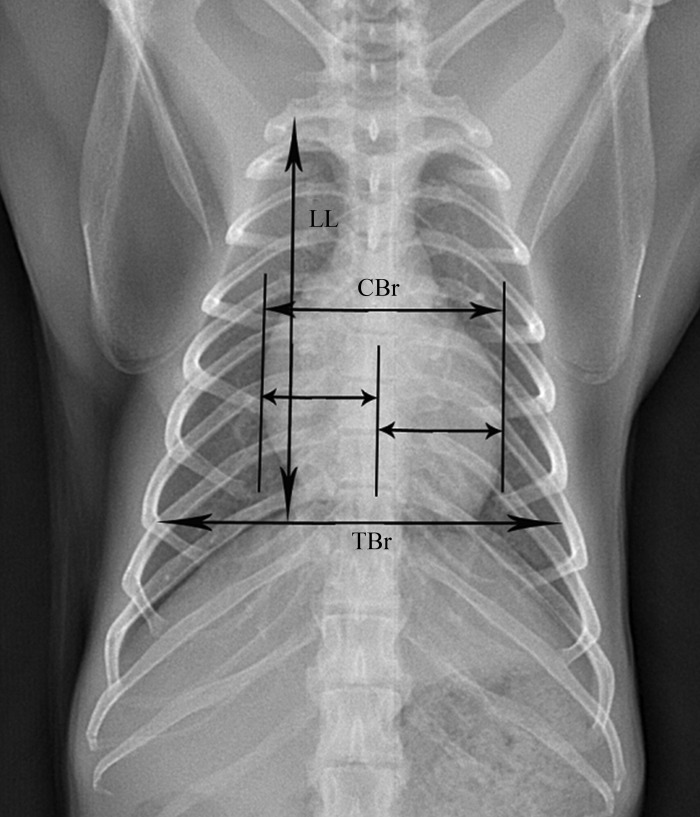
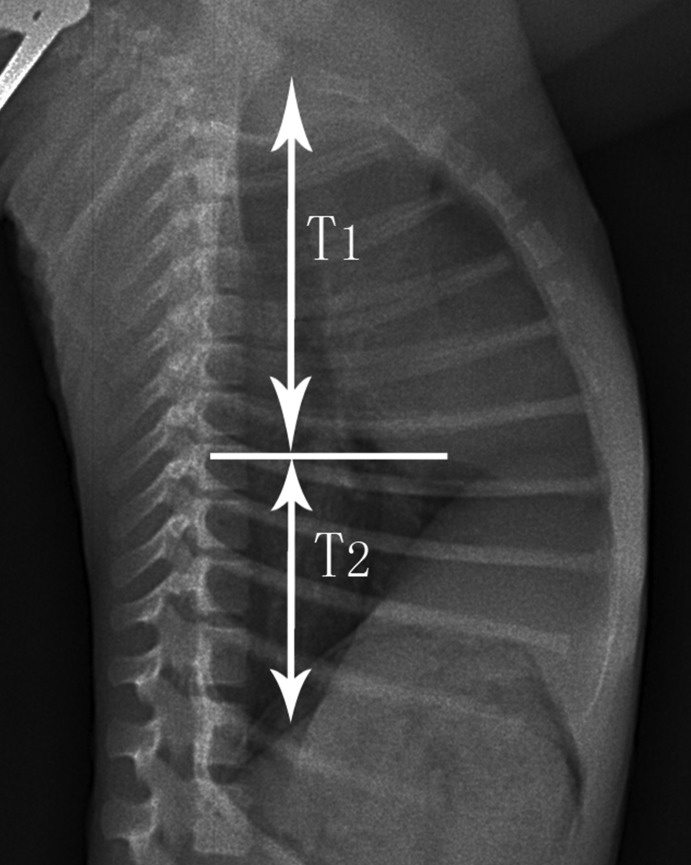
The digital radiographs were interpreted and measured by a licensed veterinarian, who was blinded to the study design. Five different radiographic measurements (Figures 1 and 2) were obtained from each film by using appropriate software (Centricity DICOM Viewer 3.0, GE Healthcare IT, Piscataway, NJ): lung length (LL), measured as distance from the tubercle of the first rib to the top of the dome of the right diaphragm;12 maximal interior thoracic breadth (TBr), measured as the linear distance between the internal margins of the ribs near the superior margin of the hemidiaphragm;17 maximal breadth of cardiac silhouette (CBr), measured as the sum of the distance from midline to the right border of the cardiac silhouette plus the distance from the midline to the left border of the cardiac silhouette;17 cardiothoracic ratio (CR), calculated as CBr divided by TBr;17 and right hilar height ratio (RHHR). This measure was obtained from right-to-left lateral radiographs by drawing a line parallel to the thoracic spine from the highest point of the pulmonary apex to the diaphragm; this line did not always end at the highest point on the diaphragm. An intersecting line then was drawn from the midpoint of the hilus pulmonis perpendicular to the first line. The ratio of the distances from the pulmonary apex to the hilus pulmonis (T1) and the hilus pulmonis to the diaphragm (T2) was calculated as the value of RHHR.6
Figure 1.
Posteroanterior thoracic radiograph of a rhesus macaque, showing lung length (LL), maximal interior thoracic breadth (TBr), and maximal breadth of cardiac silhouette (CBr).
Figure 2.
Right-to-left lateral thoracic radiograph of a rhesus macaque, showing the distance from the pulmonary apex to the hilus pulmonis (T1) and the distance from the hilus pulmonis to the diaphragm (T2).
Statistical analysis.
Data were grouped by age and sex; a Shapiro–Wilk test was performed to test for normality of distribution. For variables that showed normal distribution in each age group, differences between sexes were analyzed by using independent-sample t tests; otherwise the Mann–Whitney test was performed. Furthermore, the Spearman rank-correlation test was used to determine whether measurements correlated significantly with age. In addition, stepwise multiple-regression analysis was performed to select predictors for age from among the 5 measurements and to establish a prediction model. Finally 2-way ANOVA was performed to investigate the interaction effect of age and sex in the thoracic radiographic measurements. Statistical significance was defined as a P value of less than 0.05, and all reported P values are from 2-sided tests. All analyses were performed by using IBM SPSS Statistics 19 (SPSS, Chicago, IL).
Results
All macaques were allocated into groups by age and sex; descriptive data (mean ± 1 SD) are presented in Table 1. TBr showed significant sex-associated differences in 1-y-old macaques (male, 87.14 ± 4.66 mm; female, 82.96 ± 3.45 mm; P = 0.018). RHHR was significantly different between male and female 2-y-old (male, 1.96 ± 0.36; female, 1.72 ± 0.26; P = 0.018) and 3-y-old (male, 1.95 ± 0.44; female, 1.69 ± 0.22; P = 0.049) macaques.
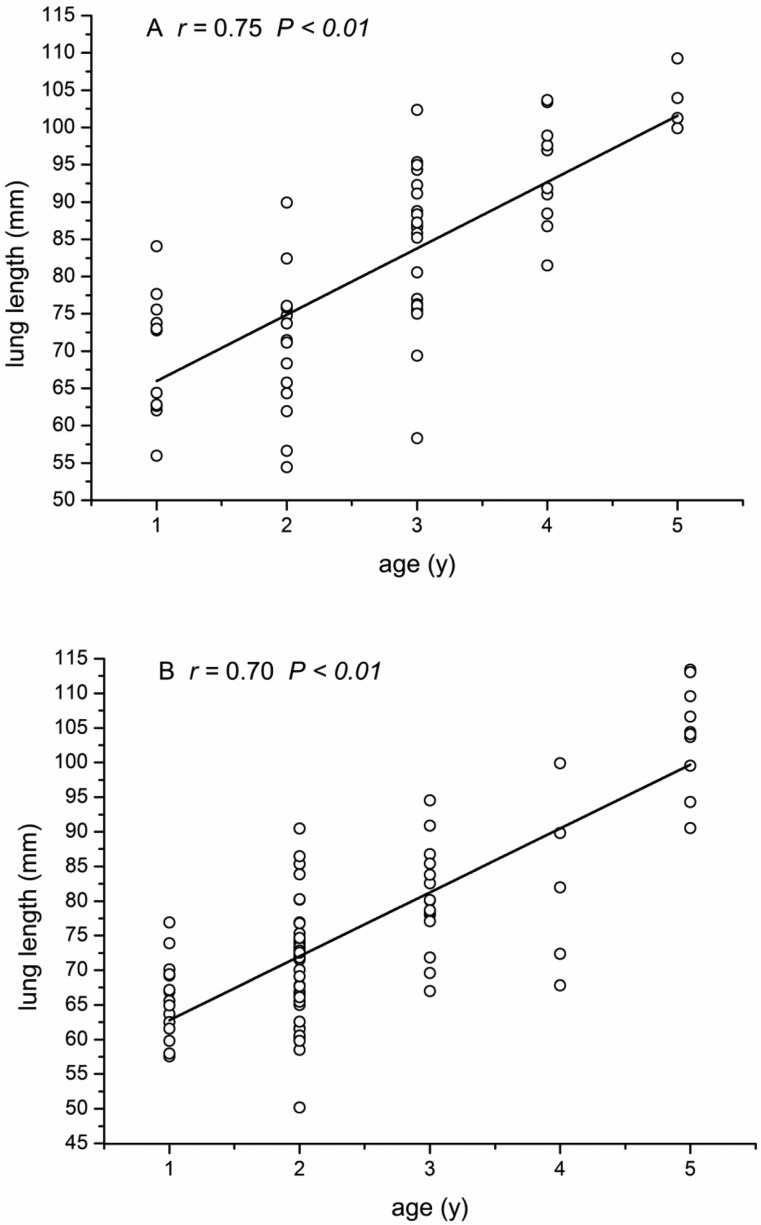
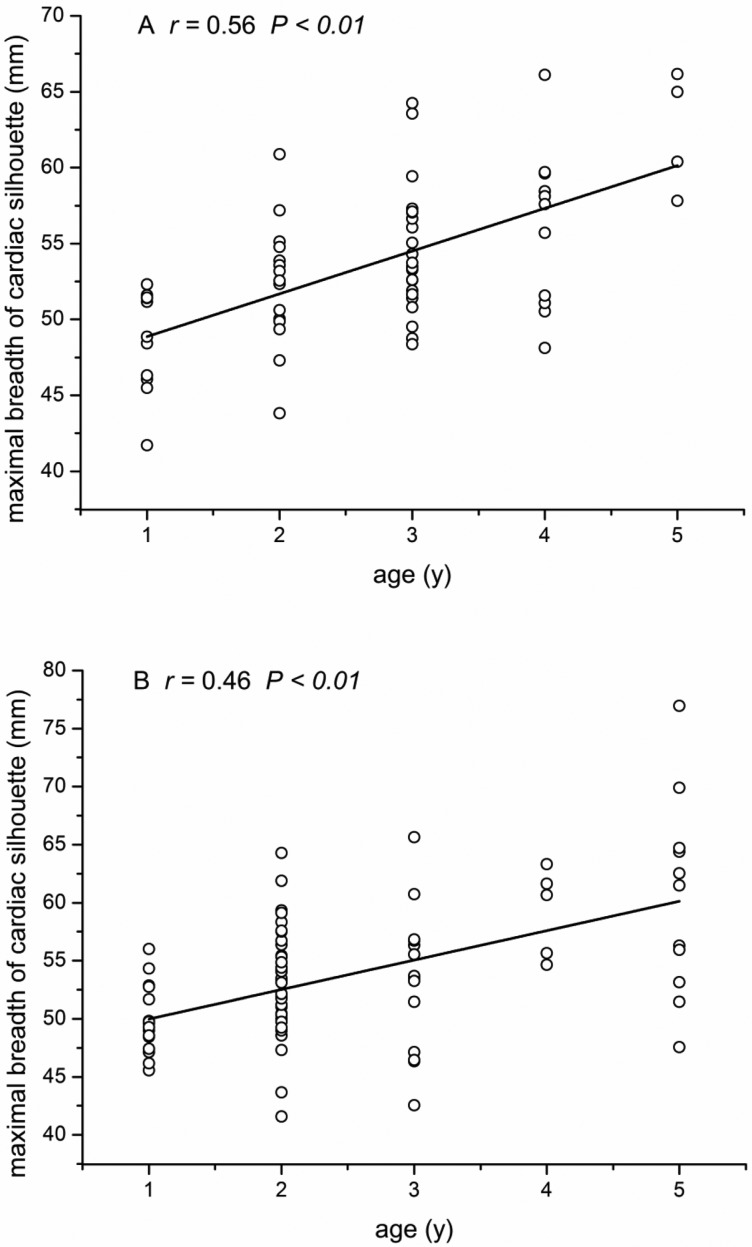
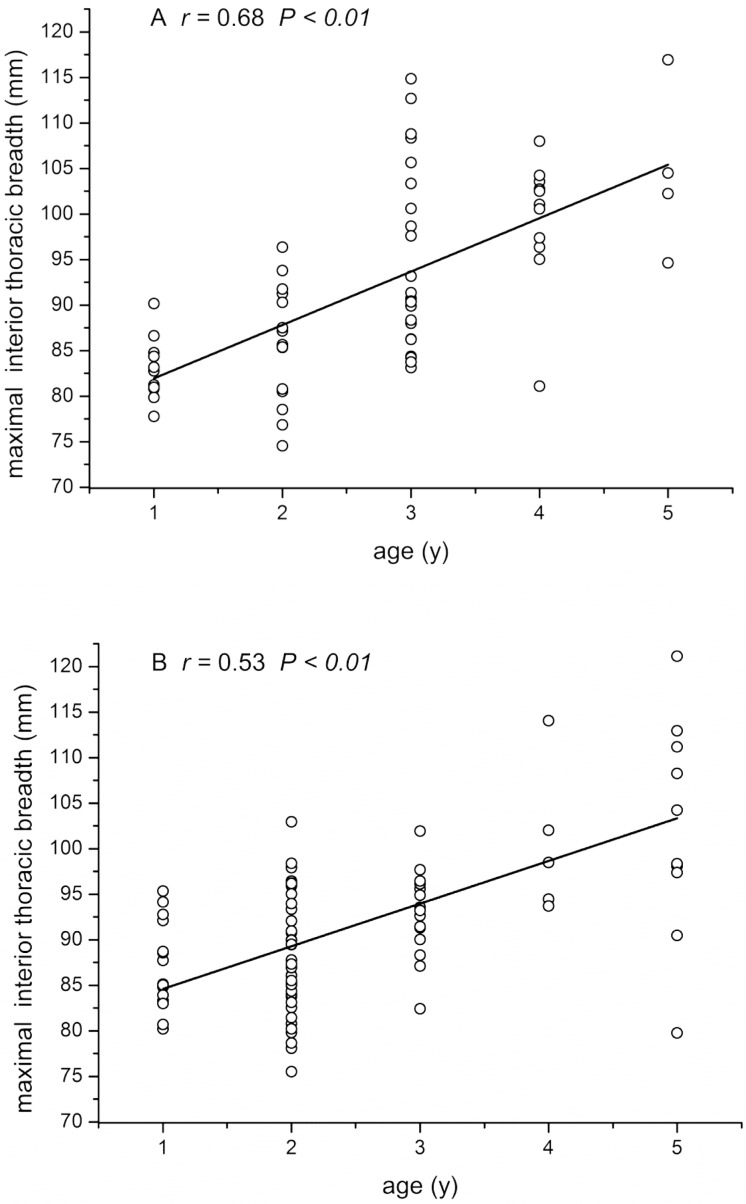
LL, TBr, and CBr showed significant (P < 0.01 for all parameters) positive correlation with age in both male and female macaques (Figures 3 through 5), whereas CR (male: r = 0.01 P = 0.90, female: r = −0.18 P = 0.15) and RHHR (male: r = −0.11, P = 0.31; female: r = −0.18, P = 0.16) showed no such age-associated correlation. Stepwise multiple-regression analysis indicated that LL and CBr were predictive of age in female macaques (r2 = 0.591; Table 2). In male macaques (Table 3), LL and TBr were selected as factors predictive of age (r2 = 0.679). The 2-way ANOVA revealed no significant interaction effect of age or sex in any of the 5 measurements.
Figure 3.
Correlation between lung length (LL) and macaque age. (A) Female macaques. (B) Male macaques.
Figure 5.
Correlation between maximal breadth of cardiac silhouette (CBr) and the age of macaques. (A) Female macaques. (B) Male macaques.
Table 2.
Stepwise multiple-regression analysis in female rhesus monkeys
| Estimate | SE | t | P | |
| Intercept | −4.239 | 0.955 | −4.439 | 0.000 |
| LL | 0.049 | 0.008 | 5.729 | 0.000 |
| CBr | 0.056 | 0.022 | 2.537 | 0.014 |
y (age) = 0.049*x1(LL) + 0.056*x2 (CBr) – 4.239 (r2 = 0.591)
Table 3.
Stepwise multiple-regression analysis in male rhesus monkeys
| Estimate | SE | t | P | |
| Intercept | −4.531 | 0.740 | −6.121 | 0.000 |
| LL | 0.060 | 0.007 | 8.950 | 0.000 |
| TBr | 0.026 | 0.010 | 2.613 | 0.011 |
y (age) = 0.06*x1(LL) + 0.026*x2 (TBr) – 4.531 (r2 = 0.679)
Figure 4.
Correlation between maximal interior thoracic breadth (TBr) and macaque age. (A) Female macaques. (B) Male macaques.
Discussion
Thoracic radiography is a common noninvasive diagnostic tool in human and veterinary clinical medicine practice, where it is used to evaluate both cardiac and noncardiac thoracic structures.11 Particularly in research involving animal models of respiratory or circulatory disorders, thoracic radiologic evaluation remains a simple and cost-effective tool for further characterizing disease processes.2,3 Specialized chest radiographic research has been conducted in various other nonhuman primates,13,14 but the thoracic radiograph measurements of healthy captive rhesus macaques, one of the most important species used in medical research, have not been reported previously.
The results of the current study demonstrated that some thoracic parameters showed sex-associated differences, depending on the age group. In particular, TBr displayed significant sex-associated differences in 1-y-old rhesus macaques, and RHHR showed similar bias in the 2- and 3-y-old cohorts. However, growth-associated changes in thoracic radiograph measurements did not differ between adolescent male and female macaques in our study. In terms of physiology and morphologic aspects of macaques, some trunk-related parameters, including chest breadth and chest depth, show greater acceleration of growth in male macaques during puberty, whereas caged group-housed female macaques show no such trends.19 Puberty is a process typically defined as the attainment of adult reproductive function.24 Some 3.5-y-old male rhesus macaques have systemic testosterone concentrations comparable to those of adult males during the mating season, whereas others do not achieve this concentration until 5.5 y of age.1 In laboratory-housed female rhesus monkeys, first ovulation occurs during the fourth year.21 The testes in male macaques are fully mature at about 10 y of age, and the mammary glands mature at about 7 y of age in female macaques.4 Therefore, a future study that enrolls more subjects over an older age range (3 to 7 y old) may demonstrate sex-associated differences in thoracic radiograph measurements, similar to differences seen in previous morphologic studies.
The process of sexual maturation in rhesus monkeys is concomitant with significant physiologic changes.21 Corresponding to the initiation of maturation, adolescent macaques manifest a pronounced physical change, termed the ‘adolescent spurt.’20 We therefore hypothesized that at least some measurements of thoracic radiography would correlate with age to some degree. Consequently we did find that LL, TBr, and CBr were significantly positive correlative with age in both sexes, in agreement with previous morphologic reports revealing that the Japanese macaques had a sustained rise in chest breadth and depth from 1 to 5 y old.19
We screened for valid age-predictive factors from among the 5 thoracic measurements and established predictive models for female (Table 2) and male (Table 3) rhesus macaques. The models may be useful in veterinary practice to facilitate monitoring of subjects with abnormal physical development. The models we developed need to be verified and modified in further studies with larger sample sizes and subjects spanning a larger age range.
In human studies, CR is an accepted measurement of quantifying cardiac size and carries prognostic information in acquired heart disease.5,7 The CR of the macaques in our current study was relatively constant, ranging from 0.57 to 0.61 across 1- to 5-y-old subjects. However, CR did not display any sex-associated difference or correlation with age. Similarly, the distribution of CR values a feral population of long-tailed macaques did not deviate significantly from normality; CR was not correlated with age or body weight; and no significant sex-associated difference was found.14
The hilar height ratio is a value that expresses the normal position of a hilus in its hemithorax;6 this parameter is rarely applied in nonhuman primate research. In human medical practice and research, the hilar height ratio is used an important indicator of loss of pulmonary volume.10 Here we present the mean RHHR of adolescent (age, 1 to 5 y) captive rhesus monkeys. This information might be helpful in evaluating the prognosis of respiratory system lesions in adolescent rhesus macaques.
Collectively, our current study characterized 5 important thoracic radiographic measurements (LL, TBr, CBr, CR, RHHR) in healthy captive adolescent rhesus monkeys. Moreover we showed that, in adolescent rhesus monkeys, the measurements of LL, TBr, and CBr are positively correlative with age in both male and female adolescent macaques, and these measurements are valid predictors for age. These current results may provide a basis for future research on thoracic radiography of rhesus monkeys. In addition, the parameters presented here may be useful in veterinary practice, research involving nonhuman primate models of respiratory or circulatory disorders, and morphologic studies of rhesus macaques.
Acknowledgments
We thank the Xishan Zhongke Laboratory Animal for providing the facilities to conduct the study. This work was supported by the National Basic Research Program of China (the 973 Program; grant no. 2009CB918300), the National Natural Science Foundation of China (grant no. 31271189).
References
- 1.Bernstein IS, Ruehlmann TE, Judge PG, Lindquist T, Weed JL. 1991. Testosterone changes during the period of adolescence in male rhesus monkeys (Macaca mulatta). Am J Primatol 24:29–38 [DOI] [PubMed] [Google Scholar]
- 2.Brandt DJ, Canfield DR, Peterson PE, Hendrickx AG. 2002. Persistent truncus arteriosus in a rhesus monkey (Macaca mulatta). Comp Med 52:269–272 [PubMed] [Google Scholar]
- 3.Brining DL, Mattoon JS, Kercher L, LaCasse RA, Safronetz D, Feldmann H, Parnell MJ. 2010. Thoracic radiography as a refinement methodology for the study of H1N1 influenza in cynomologus macaques (Macaca fascicularis). Comp Med 60:389–395 [PMC free article] [PubMed] [Google Scholar]
- 4.Hamada Y, Suzuki J, Ohkura S, Hayakawa S.2005. Changes in testicular and nipple volume related to age and seasonality in Japanese macaques (Macaca fuscata), especially in the pre- and postpubertal periods. Primates 46: 33–45. [DOI] [PubMed]
- 5.Hammermeister KE, Chikos PM, Fisher L, Dodge HT. 1979. Relationship of cardiothoracic ratio and plain-film heart volume to late survival. Circulation 59:89–95 [DOI] [PubMed] [Google Scholar]
- 6.Homer MJ. 1978. The hilar height ratio. Radiology 129:11–16 [DOI] [PubMed] [Google Scholar]
- 7.Hubbell FA, Greenfield S, Tyler JL, Chetty K, Wyle FA. 1985. The impact of routine admission chest x-ray films on patient care. N Engl J Med 312:209–213 [DOI] [PubMed] [Google Scholar]
- 8.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press.
- 9.Liang S, Chin S, Yang H, Yeh L. 2005. Radiographic measurements of cardiac size in the Formosan monkey (Macaca cyclopis). Taiwan Vet J 31:85–91 [Google Scholar]
- 10.Lim MK, Im JG, Ahn JM, Kim JH, Lee SK, Yeon KM, Han MC. 1997. Idiopathic pulmonary fibrosis versus pulmonary involvement of collagen vascular disease: HRCT findings. J Korean Med Sci 12:492–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens JM, Biery DN. 1998. Radiographic interpretation for the small-animal clinician. Baltimore (MD): Williams and Wilkins. [Google Scholar]
- 12.Reich SB, Weinshelbaum A, Yee J. 1985. Correlation of radiographic measurements and pulmonary function tests in chronic obstructive pulmonary disease. AJR Am J Roentgenol 144:695–699 [DOI] [PubMed] [Google Scholar]
- 13.Schillaci MA, Jones-Engel L, Heidrich JE, Benamore R, Pereira A, Paul N. 2008. Thoracic radiography of pet macaques in Sulawesi, Indonesia. J Med Primatol 37:141–145 [DOI] [PubMed] [Google Scholar]
- 14.Schillaci MA, Lischka AR, Karamitsos AA, Engel GA, Paul N, Ramoul R, Rompis A, Putra A, Wandia IN, Jones-Engel L. 2010. Radiographic measurement of the cardiothoracic ratio in a feral population of long-tailed macaques (Macaca fascicularis). Radiography 16:163–166 [Google Scholar]
- 15.Schillaci MA, Parish S, Jones-Engel L. 2009. Radiographic measurement of the cardiothoracic ratio in pet macaques from Sulawesi, Indonesia. Radiography 15:e29–e33 [Google Scholar]
- 16.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. 2010. Establishment of an aerosol-challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol 17:1170–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shivkumar K, Ravi K, Henry JW, Eichenhorn MS, Stein PD. 1994. Chest radiographs fail to detect right ventricular enlargement and right atrial enlargement in patients with a pure restrictive ventilatory impairment. Chest 106:381–384 [DOI] [PubMed] [Google Scholar]
- 18.Silverman S, Morgan J. 1980. Thoracic radiography of the normal rhesus macaque (Macaca mulatta). Am J Vet Res 41:1704–1719 [PubMed] [Google Scholar]
- 19.Suzuki J, Miwa N, Kumazaki K, Abe M, Kamanaka Y, Matsubayashi N, Gotoh S, Matsubayashi K. 2001. The influence of rearing conditions on the physical growth of captive Japanese macaques (Macaca fuscata). J Vet Med Sci Japan Soc Vet Sci 63: 361–366 [DOI] [PubMed] [Google Scholar]
- 20.van Wagenen G, Catchpole HR. 1956. Physical growth of the rhesus monkey (Macaca mulatta). Am J Phys Anthropol 14:245–273 [DOI] [PubMed] [Google Scholar]
- 21.Wilson ME, Gordon TP, Blank MS, Collins DC. 1984. Timing of sexual maturity in female rhesus monkeys (Macaca mulatta) housed outdoors. J Reprod Fertil 70:625–633 [DOI] [PubMed] [Google Scholar]
- 22.Xu F, Xie L, Li X, Li Q, Wang T, Ji Y, Kong F, Zhan Q, Cheng K, Fang L, Xie P. 2012. Construction and validation of a systematic ethogram of Macaca fascicularis in a free enclosure. PLoS ONE 7:e37486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan G, Zhang G, Fang X, Zhang Y, Li C, Ling F, Cooper DN, Li Q, Li Y, van Gool AJ, Du H, Chen J, Chen R, Zhang P, Huang Z, Thompson JR, Meng Y, Bai Y, Wang J, Zhuo M, Wang T, Huang Y, Wei L, Li J, Wang Z, Hu H, Yang P, Le L, Stenson PD, Li B, Liu X, Ball EV, An N, Huang Q, Fan W, Zhang X, Wang W, Katze MG, Su B, Nielsen R, Yang H, Wang X. 2011. Genome sequencing and comparison of 2 nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol 29:1019–1023 [DOI] [PubMed] [Google Scholar]
- 24.Zehr JL, Van Meter PE, Wallen K. 2005. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod 72:1087–1094 [DOI] [PubMed] [Google Scholar]