Abstract
In early 2009, we experienced a widespread outbreak of mouse parvoviruses 1 and 2 (MPV) at our institution, which encompasses approximately 50,000 cages located in 7 campus vivaria. MPV had not been detected for several years; however, during a single 4-mo sentinel-testing rotation comprising all racks at the institution, 72 of 927 rack sentinels tested serologically positive for MPV1, MPV2, or both. PCR of fecal samples from several index cases confirmed MPV. Each sentinel-positive rack contained between 0 and 10 infected colony cages. Positive racks appeared to be randomly distributed, although several small facilities escaped infection. We investigated how this infection may have entered the facilities, in which mice were maintained in barrier caging with sterilized feed, bedding, and equipment, and procedures were in place to prevent incoming infection and cross-contamination. The only widespread change that occurred during the 3 mo preceding the first positive test was that every cage had been treated for 12 wk with an unsterilized fenbendazole-medicated diet. At the completion of fenbendazole treatment, sterilized feed was reinstituted. Evidence of MPV infection was eliminated within 7 mo via an intensive test-and-remove policy in combination with movement controls, and we have had no further positive tests in the 3.5 y since the outbreak. Although the possibility remains that MPV infection resulted from fomites or undetected infections in incoming mice, the timing and extent of this outbreak together with the complete absence of new cases after sterilized feed was reinstituted strongly implicate unsterilized feed as the source of this MPV outbreak.
Abbreviation: HRR, high-risk return; MPV, mouse parvovirus
Mouse parvoviruses (MPV) are routinely excluded from laboratory mouse colonies because of their predilection for lymphoid tissues and potential to affect research involving host immunologic responses, such as by rejection of tumors or skin grafts.16,17 Nevertheless, mouse parvoviruses are among the pathogens most commonly identified in research and pet shop mice.4,6,12,15,18,20
Despite the use of barrier caging and procedures designed to exclude rodent pathogens, sporadic MPV outbreaks are common, as evidenced by contributors to the NAALAS 2009 panel discussion on parvoviruses.7 A source for such infections is rarely identified, although several possibilities exist. First, wild house mice are commonly infected with parvoviruses,1 and viruses shed by wild rodents could be transferred as fomites into a facility and therefore into cages during lapses in barrier procedure. Indeed, parvoviruses are among the most environmentally persistent viruses and are resistant to many common disinfectants.5 Second, there may be undetected infections in incoming laboratory mice. However quarantine testing of mice from other institutions and restricting purchases to vendors with comprehensive health monitoring should mitigate this risk. Third, MPV may persist at low prevalence in a facility and only be detected intermittently. Transmission to sentinels from colony mice is efficient only during the first 2 wk of shedding,14 and susceptibility of sentinels to infection decreases with increasing age.2 Even evaluation of colony animals can be problematic: mice on C57BL/6 backgrounds are common in research facilities yet are relatively resistant to infection,2,3 so detecting MPV in colonies that contain many C57BL/6 mice may be confounded by extremely low infection prevalence.
Until recently, feed had not been suspected as a source of MPV infections, however unsterilized feed was discussed as a potential source of parvovirus infections at a 2009 NAALAS panel discussion on parvoviruses,7 and a subsequent report described a reduction in new parvovirus detections after feed was switched from unsterilized to irradiated feed and disinfectants were upgraded.19 Unsterilized feed is not commonly viewed as a source of MPV infection because the sporadic incidence and low prevalence of MPV infections argue against a source such as feed that is routinely applied to every cage. Further, pelleted feed is subjected to 65 to 85 °C, pressure, and steam,22 which likely kills many organisms. Nevertheless we here present compelling evidence that a temporary change from sterilized to unsterilized feed resulted in a widespread mouse parvovirus infection at our institution, where parvovirus had not been detected in recent history. The infection was distributed randomly throughout approximately 42,000 (of a total of approximately 50,000) barrier-maintained mouse cages in 4 different buildings. After positive cages were removed and sterilized feed was reinstated, no additional unrelated cases were detected. The barrier cage system prevented spread to adjacent cages, and an intensive test-and-remove policy in combination with movement controls succeeded in eliminating detectable infection from the institution within 6 mo.
Case Report
Routine husbandry and infection monitoring.
Facilities at the Johns Hopkins Medical Institutions are AAALAC-accredited, and all procedures were IACUC-approved and in compliance with the Guide for the Care and Use of Laboratory Animals.9 Vivaria contained approximately 50,000 mouse cages, 86% in the 3 largest facilities and the remainder in several separate, smaller facilities. The majority of mice were housed in individually ventilated cages (Allentown Caging Equipment, Allentown, NJ and Thoren Caging Systems, Hazelton, PA) on autoclaved corncob or TEK-Fresh bedding (Harlan Teklad, Indianapolis, IN). Autoclave performance (4 min of sterilization at 132 °C) was recorded on autoclave printouts and validated by steam-sterilization strips (OK Strips, Propper Manufacturing, Long Island City, NY) applied to every load, by steam chemical integrators (ComplySterigage, 3M, St Paul, MN) in one load daily, and by biologic indicators (B/T Sure, Thomas Scientific, Swedesboro, NJ) monthly. Water was reverse-osmosis–treated, either acidified or hyperchlorinated, and delivered by means of incage automated watering systems (Edstrom Industries, Waterford, WI, and Systems Engineering, Napa, CA). A smaller number of mice consisting of several hundred cages in satellite facilities were housed in shoebox-style cages with filter tops and provided with filtered municipal water via automated watering systems external to the cage or via water bottles. Mice were fed either autoclaved or irradiated diet (2018SX or 2918 Teklad Global, Harlan Laboratories). Ventilated cages were changed on a 2-wk cycle by using chlorine-dioxide–based disinfectant (MB10 Tabs, 100-ppm solution, Quip Laboratories, Wilmington, DE) in filtered-air change stations (Lab Products, Seaford, DE, and Allentown Caging) to minimize cross contamination between cages. A few shoebox cages were changed weekly by using aseptic procedures either in change stations or on the tabletop. Vermin control (Regional Pest Management, Baltimore, MD) by using live mouse traps and sticky insect traps indicated minimal vermin problems. Loose mice caught in the live traps were evaluated as for sentinels, but no pathogens had been detected in mice caught in traps during the previous 3 y.
Mouse housing areas were assigned to one of several risk levels based on infection status, role, and risk of infection. Both mouse transfers and personnel movement were controlled to minimize transfer of infection between facilities. Requests to move mice between facilities were reviewed and approved by veterinary staff, and personnel were required to move between facilities in a ‘clean’ (low risk) to ‘dirty’ (high risk) progression during the course of a single day. Assigned risk levels (from low to high) were: (1) transgenic core; (2) Helicobacter-negative; (3) Helicobacter-positive; (4) low-risk quarantine and high-risk return (HRR), and (5) areas quarantined for known or suspected infections. HRR housing was reserved for mice that had been used outside the biosafety cabinet (for example, for imaging and behavioral tests or used in laboratories). Mice in HRR housing were considered to be at increased risk for infection, but HRR facilities were maintained at the same infection-exclusion status as were regular colonies. Mice were not permitted to return from HRR to regular colonies without returning to quarantine for additional pathogen testing.
Mouse colonies were evaluated for infection by using sentinels: 2 outbred female sentinels (Hsd:ICR[CD1] or Crl:CD1[ICR]; age, 4 to 6 wk) were placed per single-sided rack and exposed to approximately 15 mL soiled bedding from each cage on the rack at every cage change for 3 to 4 mo. Sentinels were submitted for gross necropsy and evaluation according to a rotating schedule such that 25% of the racks in each room was sampled every month, with all racks being sampled over a 4-mo period. Sentinel serum samples were evaluated by Charles River Laboratories (Wilmington, MA) by using multiplexed fluorometric immunoassays for multiple pathogens, and fur, anal tape, and fecal samples were evaluated inhouse for fur mites and pinworms. Initial tests were conducted on the first sentinel; the serum plus mesenteric lymph nodes or spleen from the second sentinel were frozen and tested in the event of a positive test result from the first sentinel. In addition, Helicobacter-negative facilities were monitored inhouse for Helicobacter spp. by PCR of fecal samples.
Mice from the transgenic core facility and quarantined animals from outside institutions were monitored intensively before being released into the facilities. Recipient female mice from every new litter leaving the transgenic core were each tested as for sentinels. For incoming mice from other institutions, health reports from the source institution were screened by a veterinarian and assigned either to low-risk (no excluded pathogens detected) or high-risk (excluded pathogens detected or not evaluated) quarantine. Low-risk mice were assessed by serology and parasitology after 6 wk of exposure to both contact and soiled-bedding sentinels. High-risk mice were screened by parasitology and PCR of pooled fecal samples (PRIA, Charles River Laboratories) from all incoming mice in the shipment. All quarantined mice were treated for pinworms (with fenbendazole-impregnated diet) and for mites (selamectin 10 mg/kg applied to the skin between the shoulder blades; Revolution, Pfizer, New York, NY). Access to quarantine was limited to veterinary and dedicated husbandry staff.
Events leading to the outbreak.
Prior to this report, sentinel testing indicated that all colonies were free of Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mouse minute virus, mouse parvovirus, mouse encephalomyelitis virus, reovirus, epizootic diarrhea of infant mice, lymphocytic choriomeningitis virus, ectromelia virus, murine adenovirus, murine cytomegalovirus, Mycoplasma pulmonis, and fur mites. However, pinworms (Aspiculuris tetraptera) had been detected sporadically in the larger facilities for several years, and despite repeated local treatments, eradication had not been successful. Therefore, it was determined to treat all mice at the institution with fenbendazole-medicated diet and simultaneously perform a thorough environmental cleaning in an attempt to eradicate pinworms from the institution. Although stock sterilizable fenbendazole-medicated pelleted diet was available, it could not be used because it required additional labor for processing to break up the clumps that form when pelleted diet is autoclaved. Conversely, the stock diet could not be used without autoclaving because it contained additional vitamins to compensate for autoclave loss, and our previous report had shown that tumor growth was inhibited when fenbendazole was fed in combination with supplementary vitamins.8 Therefore, our usual feed (Harlan Teklad Global 2018) was compounded with 150 mg/kg fenbendazole into pellets without the additional vitamins needed for autoclaving, so that it could be fed without sterilization. Irradiation of this compounded diet was considered; however, in light of the absence of reported infections due to unsterilized feed, the substantial additional cost did not seem justified. Therefore, between October and December 2008, immediately prior to the parvovirus outbreak, all mice throughout the institution were treated continuously for 12 wk with medicated unsterilized feed. After treatment was completed, sterilized diet was reinstituted.
In the 3 y prior to the outbreak, more than 8000 sentinel tests had revealed only 5 tests positive for MPV. Of those, only one was confirmed by subsequent testing of contributing colony cages. In every case, affected racks were quarantined, and colony cages tested negative by PCR or serology before being released from quarantine. The source of these isolated infections was never identified, but in retrospect the labs may have been using unsterilized diets during experiments.
Outbreak: testing and eradication procedures.
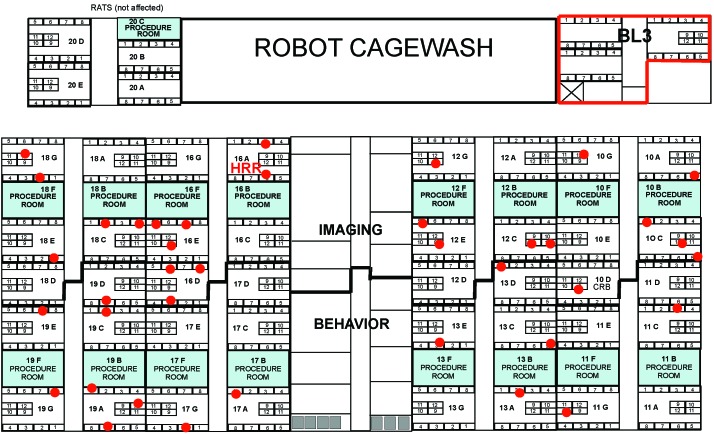
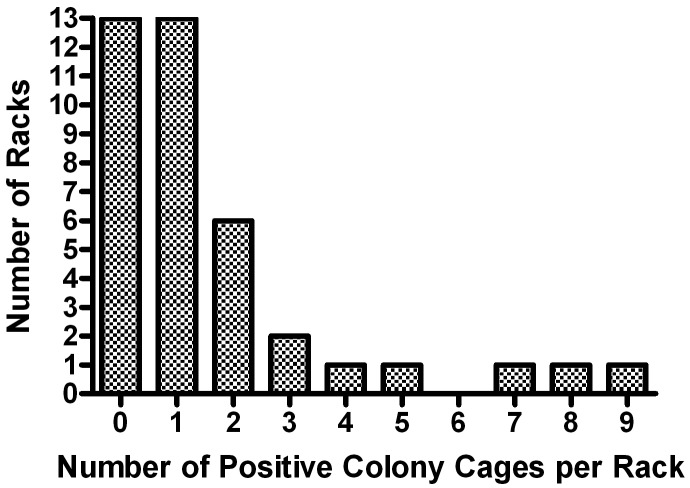
The first MPV-positive sentinel test in this outbreak occurred in January 2009, less than 3 mo after the start of treatment with unsterilized diet. Initial positives were confirmed by a second serologic test on the same sample, by repeating the serologic tests on the frozen serum from backup sentinels, and by positive PCR tests of spleen or fecal samples. Subsequently only serologic testing was used to detect infections. During the 4 mo after the first positive tests (one complete testing cycle that encompassed all racks at the institution), 72 sentinels in 4 buildings (from a total of 927 sentinels) tested serologically positive for MPV. Serum tested positive for MPV1 (29%), MPV2 (11%), or both (60%) by multiplexed fluorometric immunoassay. Tests for MPV1 were confirmed with immunofluorescent assays and for MPV2 with ELISA. Only 28% of MPV1- or MPV2-positive sera detected by using the rVP2 viral capsid protein were also positive by the conserved nonstructural protein NS1, which is consistent with other reports that NS1 antibody response can be reduced or delayed compared with response to VP antigens.2 The 3 largest housing areas (encompassing approximately 42,000 cages) and one smaller facility (containing approximately 4000 cages) were affected. The distribution of positives appeared to be random: Figure 1 shows the distribution of sentinel-positive racks in the largest facility (BR), which contained approximately 28,000 mouse cages. The percentage of affected racks in each facility varied (Table 1), and even on sentinel-positive racks, there were commonly either no or few infected colony cages (Figure 2).
Figure 1.
Distribution of racks with positive sentinels (red dots) in facility BR, which contained approximately 28,000 mouse cages.
Table 1.
Number and percentage of rack sentinels positive for MPV in each facility.
| Facility | Total no. of racks | No. of positive racks | % of positive racks |
| BR | 518 | 38 | 7.3 |
| CR1 | 108 | 16 | 14.8 |
| CR2 | 85 | 15 | 17.7 |
| SH | 57 | 3 | 5.3 |
| RO5 | 30 | 0 | 0 |
| RO3 | 24 | 0 | 0 |
| WW | 21 | 0 | 0 |
| HW | 36 | 0 | 0 |
| AA | 48 | 0 | 0 |
| Total | 927 | 72 | 7.8% |
Figure 2.
Frequency distribution of MPV-positive colony cages on racks with MPV-positive sentinels.
Once we recognized that a widespread MPV outbreak was occurring, a plan was developed to contain the outbreak and eradicate the virus through a test-and-remove process. Preventing further spread required the quarantining of racks in place: the sheer scale of the outbreak prevented implementation of the usual procedure of moving infected racks to high-risk quarantine. Instead an intensive communication campaign was mounted to inform both affected and unaffected investigators about the quarantine and testing plan. Emphasis was placed on informing investigators that the majority of cages were unaffected and would likely remain so if personnel were careful to follow existing procedures to prevent spread of infection. Mice on affected racks were not permitted to be moved from their current positions, and mice on unaffected racks could be relocated only after testing negative. Personnel were required to attend to mice on positive racks after those on unaffected racks during the course of each day. Mice in positive cages were euthanized or moved to high-risk quarantine as soon as they were identified. In addition, current procedures for preventing spread of infection were emphasized: personnel were reminded to clean and disinfect biosafety cabinets before use, to spray MB10 (Quip Laboratories) on all surfaces allowing 10-min contact time, to spray gloved hands and the outside of cages before opening each cage, to change gloves between racks and risk groups, and to thoroughly clean and disinfect hoods after use. Furthermore, we emphasized that investigators should not share mice with others unless veterinary staff had tested the cages.
Several routes of communication were used, including the university rodent advisory committee and email notifications and by posting highly visible facility, room, and rack signage. In addition, cage cards on infected racks were marked with rack, row, and column position to facilitate identification and detect unauthorized movement. However, the success of the operation relied heavily on the cooperation of investigators and their staff. To encourage compliance, we emphasized that continued research would be supported, providing that veterinary staff was notified so that procedures could be instituted to minimize the risk of infection spread. For example, mice on affected racks could be removed for euthanasia or acute procedures, but a dedicated staff person monitored or assisted with site decontamination afterward. Continued breeding was permitted provided that cages were labeled to allow tracking back to parent cages, and relocation of cages for research purposes was permitted after individual cage tests.
To eliminate positive cages from affected racks, all colony cages on affected racks were evaluated by serologically testing one mouse per cage for antibodies to MPV1, MPV2, and NS1 antigens. Mice in positive colony cages were either euthanized or moved to quarantine pending completion of the experiment. All colony cages on positive racks were retested monthly, and new positives were removed until all cages on the rack tested negative. At the beginning of the outbreak, a subset of 38 sentinel-positive racks were tested by colony cage bleeds 5 times at monthly intervals to determine whether 3 colony cage bleeds would be sufficient to ensure that racks were negative. At the first colony test, 56 positive colony cages were removed from 26 of 38 racks, and 12 of 38 racks had no colony positives. At the second colony test, 4 of the 26 racks with prior positives had 6 additional positive cages, which were removed, and 2 of the 12 racks that previously had no positives had 8 positive colony cages, which were removed. After removal of these cages, no further cages tested positive on the 3 subsequent colony tests; therefore, it was concluded that 3 colony tests were sufficient to ensure that all cages would continue to test negative. Once all colony cages tested negative, a new sentinel cage was exposed to all cages on the rack for 6 wk and then was tested; and this process was repeated once. No racks tested positive on these sentinel tests, and racks were released from quarantine after 2 negative sentinel tests.
Racks that had not initially tested positive for MPV by sentinel were reevaluated on an accelerated schedule: sentinels were evaluated and replaced every 6 wk for 2 tests. However, only 2 positive racks (housing mice from the same laboratory as previously positive racks) were identified, and by July 2009, all racks in the institution were testing negative. The outbreak was concluded in October 2009, and there have been no further MPV-positive sentinel tests in the 3.5 y since the outbreak.
Discussion
A number of possible explanations for this MPV outbreak other than unsterilized feed were considered, included false positives, infected sentinels, water, feed bag or biologic contamination, dissemination via shared equipment or resources, and introductions from a vendor or from the transgenic core facility. False positives were discounted because the initial cases were all confirmed by PCR evaluation of spleen or fecal samples, and all positive tests by multiplexed fluorometric immunoassay were confirmed by a second test. Sentinel contamination was ruled out because 2 different sentinel vendors (Harlan and Charles River) were used for the affected facilities, there were no vendor reports of infection, and colony cages in addition to sentinel cages tested positive. Water as a source was discounted because 4 buildings were affected, each of which had its own water treatment system. Feedbag contamination could not be discounted; however, it is highly unlikely that an outbreak this widespread (4 separate buildings, 72 racks) could have resulted via fomite transfer from contaminated bags, particularly given that the feed was delivered to us immediately after manufacture. Feed bags were delivered to our facility in batches on shrink-wrapped pallets and the plastic removed before bags were taken into facilities. Bags were not sprayed with disinfectant before opening: indeed disinfectants are relatively ineffective at reducing viral titers on paper products,11 so the value of this practice is unproven. Investigators were required to test biologics passaged through, or derived from, outside rodent sources by PCR assays for rodent pathogens. In practice, few samples were submitted for testing, and the only organism commonly detected was Mycoplasma spp. The sudden appearance of this outbreak argued against dissemination via shared resources or collaborations, and we were unable to detect a common relationship between the numerous affected investigators. Shared resources were ruled out rapidly, because all mice used in those areas were subsequently housed in high risk areas, and this MPV outbreak affected all areas. Mice from vendors and the transgenic core unit were placed directly into facilities; however, no vendors reported MPV during this period, and the transgenic core facility, in which every outgoing cage is tested routinely, continued to test negative for parvoviruses throughout.
In short, the systems at our institution have maintained, and continue to maintain, our colonies free of detectable parvoviral infections for many years. Given the sudden, widespread nature of this outbreak followed by its rapid resolution, the cause had to have been applied and then withdrawn from a majority of cages over a short period. The only change in husbandry that met that criterion was the change from sterilized to unsterilized feed for 12 wk of pinworm treatment immediately prior to the MPV outbreak. Unsterilized feed was used from October through December, and sterilized feed was reinstituted in January. The outbreak was detected during the first full cycle of sentinel testing after the use of the unsterilized feed, that is, from January through April. Only 2 additional positive racks were detected during the subsequent testing cycle, and no positive tests have occurred in the 3.5 y since. Feed can be contaminated via wild house mice, which are commonly infected with parvoviruses1 and are present in crop fields.10 Susceptible young mice could shed quantities of virus on grain, particularly during breeding periods21. Once grain is contaminated, the resistant nature of parvoviruses may allow small quantities to remain viable through the pelleting process. Wheat and corn are harvested in the United States during late summer and fall. Grain then is stored prior to being delivered to manufacturers for processing, potentially providing an opportunity for contamination during storage. Indeed, a previous report19 suggested that MPV infection in one institution spiked during the quarterly June sentinel evaluation, implying infection during the preceding 3 mo, perhaps from stored grain contamination by wild mice during an early-spring breeding period. In contrast, the outbreak at our institution resulted from feed manufactured during late fall, suggesting that contamination may have occurred prior to harvest the same year.
A remaining question is why none of the 5 smallest facilities had even a single positive sentinel test when all cages received medicated diet. Extrapolating from the 10.5% sentinel incidence in the 4 facilities that did test positive, an additional 17 positive racks would have been expected. Possible explanations include fewer susceptible mice in those facilities (for example, fewer breeding cages), or they simply may have received different batches of diet that were not infected. Unfortunately we did not record those data. Another factor could have been the increased proportion of shoebox-style caging in the smaller facilities—infection transmission to sentinels in shoebox caging is less efficient than is transmission in ventilated cages,21 although whether MPV survival differs between the 2 caging types is unknown. In an attempt to confirm that diet was the cause, we did submit several samples of medicated diet to the manufacturer for PCR testing for parvoviruses; however, none tested positive. However, we received and used multiple shipments of diet over the course of the treatment, and none of the original batches remained for testing at the time we detected the outbreak. Furthermore, we tested a few hundred grams only, whereas the total quantity of medicated diet used was in excess of 165,000 kg. Assuming that diet was the source of the outbreak, more than 4.2 million cage-days of exposure resulted in a relatively small percentage (0.06%) of infected cages, an exceedingly low rate of infection. The only direct evidence we have that the diet contained infectious parvoviruses came from one cage in a small pilot study that we conducted in the early stages of the outbreak when we suspected that feedbag contamination was the cause. We exposed 4 cages (2 mice each) of newly arrived sentinel mice to parts of feed bags in their cages; an additional cage without feed bags acted as control. Mice in all 5 cages received medicated diet. The only mice to seroconvert to MPV were the 2 mice in the control cage. Unfortunately time constraints during the height of the outbreak prevented us from pursuing this line of enquiry.
Although not the most sensitive detection method, we chose to test colony cages by evaluating the serology of only one mouse per cage, to minimize cost. Cages that had only recently been exposed and cages in which not all mice in the cage seroconverted likely would have been missed. More recently, PCR screening of environmental samples has become available as a potential alternative to live animal samples. Nevertheless, our strategy of repeatedly testing colony cages and removing positive cages, similar to that since reported,1 worked well: 82% of affected racks were negative on the second colony cage test, and all tested negative on the third colony cage test. A subset of 38 racks were tested on 2 subsequent colony tests (a total of 5 colony cage tests) at monthly intervals, but no further positives were identified, and no racks subsequently tested positive by using sentinels. Positive colony cages were not identified on 33% of sentinel-positive racks. The most likely explanation for this result is that outbred sentinels succumbed to a lower infectious dose of MPV in feed than did the more resistant mice (such as C57BL/6) in colony cages. In addition, several investigators chose to eliminate mice from infected racks rather than to wait for testing, thus removing potentially positive colony cages.
This parvovirus outbreak did not continue to spread: instead it was contained and eliminated via test-and-remove policies in combination with strict movement controls and barrier cage procedures. The efficacy of these measures lends weight to the argument that the initial MPV source was no longer present. In a prior case report,19 detection of parvoviruses in a small colony spontaneously decreased once sterilized feed was instituted; however, whether simple attrition played a role in our eradication process is unknown.
In summary, the timing and extent of this event and its subsequent resolution provide strong circumstantial evidence that unsterilized feed was the source of this MPV outbreak. In addition, our successful eradication strategy provides evidence that barrier cage procedures are effective in preventing spread of MPV between cages and that test-and-remove procedures are a viable option for MPV eradication under cage–barrier conditions, providing that the source of the infection is no longer present.
Acknowledgments
I thank Bob Adams and Sarah Poynton for helpful suggestions for the manuscript and members of the rodent technical support team—Eric Syversen, Mihoko Anderson, Dee Westerfeld-Vaughn, Craig Zikan, and Steve Simpson—whose diligence in obtaining several thousand individual blood samples in addition to performing their routine duties enabled the successful resolution of this outbreak.
References
- 1.Becker SD, Bennett M, Stewart JP, Hurst JL. 2007. Serological survey of virus infection among wild house mice (Mus domesticus) in the UK. Lab Anim 41:229–238 [DOI] [PubMed] [Google Scholar]
- 2.Besselsen DG, Wagner AM, Loganbill JK. 2000. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp Med 50:498–502 [PubMed] [Google Scholar]
- 3.Compton SR, Paturzo FX, Macy JD. 2010. Effect of murine norovirus infection on mouse parvovirus infection. J Am Assoc Lab Anim Sci 49:11–21 [PMC free article] [PubMed] [Google Scholar]
- 4.Dammann P, Hilken G, Hueber B, Kohl W, Bappert MT, Mahler M. 2011. Infectious microorganisms in mice (Mus musculus) purchased from commercial pet shops in Germany. Lab Anim 45:271–275 [DOI] [PubMed] [Google Scholar]
- 5.Eterpi M, McDonnell G, Thomas V. 2009. Disinfection efficacy against parvoviruses compared with reference viruses. J Hosp Infect 73:64–70 [DOI] [PubMed] [Google Scholar]
- 6.Filipovska-Naumovska E, Abubakar SM, Thompson MJ, Hopwood D, Pass DA, Wilcox GE. 2010. Serologic prevalence of MPV1 in mouse strains in a commercial laboratory mouse colony determined by using VP1 antigen. J Am Assoc Lab Anim Sci 49:437–442 [PMC free article] [PubMed] [Google Scholar]
- 7.Gallaugher LD, Hickman-Davis JM. 2009. Institutional approaches to the control and eradication of MPV, p 66. National Meeting Program. Memphis (TN): American Association for Laboratory Animal Science. [Google Scholar]
- 8.Gao P, Dang CV, Watson J. 2008. Unexpected antitumorigenic effect of fenbendazole when combined with supplementary vitamins. J Am Assoc Lab Anim Sci 47:37–40 [PMC free article] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 10.Kaufman DWKG. 1990. House mice (Mus musculus) in natural and disturbed habitats in Kansas. J Mammal 71:428–432 [Google Scholar]
- 11.Lee H, Purdy GA, Riley LK, Livingston RS. 2007. Efficacy of disinfectants against MVM- and MNV-contaminated surfaces. J Am Assoc Lab Anim Sci 46:94–95 [Google Scholar]
- 12.Liang CT, Shih A, Chang YH, Liu CW, Lee YT, Hsieh WC, Huang YL, Huang WT, Kuang CH, Lee KH, Zhuo YX, Ho SY, Liao SL, Chiu YY, Hsu CN, Liang SC, Yu CK. 2009. Microbial contaminations of laboratory mice and rats in Taiwan from 2004 to 2007. J Am Assoc Lab Anim Sci 48:381–386 [PMC free article] [PubMed] [Google Scholar]
- 13.Macy JD, Cameron GA, Smith PC, Ferguson TA, Compton SR. 2011. Detection and control of mouse parvovirus. J Am Assoc Lab Anim Sci 50:516–522 [PMC free article] [PubMed] [Google Scholar]
- 14.Macy JD, Paturzo FX, Ball-Goodrich LJ, Compton SR. 2009. A PCR-based strategy for detection of mouse parvovirus. J Am Assoc Lab Anim Sci 48:263–267 [PMC free article] [PubMed] [Google Scholar]
- 15.Mahler M, Kohl W. 2009. A serological survey to evaluate contemporary prevalence of viral agents and Mycoplasma pulmonis in laboratory mice and rats in Western Europe. Lab Anim (NY) 38:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKisic MD, Macy JD, Jr, Delano ML, Jacoby RO, Paturzo FX, Smith AL. 1998. Mouse parvovirus infection potentiates allogeneic skin graft rejection and induces syngeneic graft rejection. Transplantation 65:1436–1446 [DOI] [PubMed] [Google Scholar]
- 17.McKisic MD, Paturzo FX, Smith AL. 1996. Mouse parvovirus infection potentiates rejection of tumor allografts and modulates T-cell effector functions. Transplantation 61:292–299 [DOI] [PubMed] [Google Scholar]
- 18.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 19.Reuter JD, Livingston R, Leblanc M. 2011. Management strategies for controlling endemic and seasonal mouse parvovirus infection in a barrier facility. Lab Anim (NY) 40:145–152 [DOI] [PubMed] [Google Scholar]
- 20.Schoondermark-van de Ven EM, Philipse-Bergmann IM, van der Logt JT. 2006. Prevalence of naturally occurring viral infections, Mycoplasma pulmonis and Clostridium piliforme, in laboratory rodents in Western Europe screened from 2000 to 2003. Lab Anim 40:137–143 [DOI] [PubMed] [Google Scholar]
- 21.Smith PC, Nucifora M, Reuter JD, Compton SR. 2007. Reliability of soiled bedding transfer for detection of mouse parvovirus and mouse hepatitis virus. Comp Med 57:90–96 [PubMed] [Google Scholar]
- 22.Tobin G, Stevens KA, Russell RJ. 2007. Nutrition, p 321–384. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]