Summary
Angiogenesis characterizes embryonic development, but also occurs in adulthood in physiological situations such as adaptation to muscle exercise, and in pathological conditions like cancer. Major advances have been made in understanding the molecular mechanisms responsible for vasculogenesis and angiogenesis, largely due to the use of “knock-out mice”, i.e. mice in which the gene coding for the protein under investigation has been inactivated. Interestingly, the same growth factors and their receptors are equally involved in the different aspects of vasculogenesis and angiogenesis during development and in adulthood. This review aims to describe in detail their respective roles and how interactions between them lead to a newly formed vessel.
Key words: vasculogenesis; angiogenesis; growth factors
Definitions
During embryonic development, the cardiovascular system is formed early on in response to growing metabolic demand of the embryo’s organs due to both the increase in organ size and the increased distance between capillaries and cells and hence reduced oxygen diffusion in these organs.
The vascular tree develops by differentiation of one or more vascular precursors into angioblasts, the migratory precursors of endothelial cells (EC) and smooth muscle cells (SMC). Assembly of the endothelial cells leads to the formation of primitive endothelial tubes, followed by the recruitment of SMC to form the primitive vascular plexus. Lastly, the elongation, division and maturation of these tubes give rise to a three-dimensional structure which forms the arterial and venous axes. Schematically, vasculogenesis, defined as the differentiation of an embryonic stem cell, precursor of the endothelial cell or angioblast, can be distinguished from the aggregation of such cells to form the primitive blood vessels or endothelial tubes. Vasculogenesis can be divided into type I when association of angioblasts starting from the differentiation of the stem cell occurs in situ, and vasculogenesis type II when the association of angioblasts occurs after their migration34. Angiogenesis is defined as the extension of the vascular tree starting from pre-existing vessels. When these two steps occur during embryonic life, the whole extension of the vascular system after birth basically concerns the processes started during angiogenesis. Nevertheless, progenitors shared by the EC and SMC able to differentiate in sites of new vascularization were recently disclosed in the adult8.
The circumstances in which angiogenesis is resumed after birth involve “compulsory” physiological events such as changes in the endometrium during the menstrual cycle, “adaptive” physiological events under certain conditions like muscular exercise or scarring, but also a variety of pathological conditions: cancer, diabetic retinopathy, psoriasis, rheumatoid polyarthritis and the development of atherosclerotic plaque... Likewise, arterial rarefaction reflecting vascular regression or non appearance of new vessels is encountered during development (non circulating vessels subside and disappear) or in pathological conditions like digestive atresia or unilateral facial atrophy in which the absence of normal vascular tree development or its regression give rise to the absent development of the poorly vascularized organ. Lastly, the microcirculation is rarefied in the course of arterial hypertension in most organs affected by hypertension complications.
The major growth factors involved in the formation of a new vessel, either de novo (vasculogenesis) or starting from a pre-existing vessel (angiogenesis) have been identified in the last ten years. Knowledge of the sequence of events implicated in the formation of a new vessels is crucial since, starting during embryonic development, the process continues physiologically during adulthood, e.g. during the menstrual cycle or in physical exercise or scarring, and in pathological conditions such as cancer or cerebral arteriovenous malformations. Likewise, this knowledge serves in gene therapy by injection of growth factors, like Fibroblast Growth Factor or FGF, in critical ischaemia of the lower limb characterized by insufficient regional vascularization9, flanked by numerous experimental studies in cancer using anti-angiogenic factors36.
We review the growth factors implicated in vasculogenesis and angiogenesis, and their role in the temporal sequences leading up to the formation of new vessels. Furnishing some examples, we also show how knowledge of these mechanisms has revolutionised our understanding of certain diseases. For the sake of simplicity, the factors which have a major impact on angiogenesis such as hypoxia, flow or the hormonal system will not be tackled herein.
Introduction
A vessel is made up of an internal layer of endothelial cells and an outer layer of pericytes or smooth muscle cells forming the media “bound” to the extracellular matrix. The endothelium is made up of an aggregation of cells whose cohesion is ensured by homophilic intercellular adhesion molecules such as vascular endothelial cadherin (VE-Cadherin). Vessel cohesion to its environment is ensured by heterophilic adhesion molecules like integrins. These elements are essential to vessel stability.
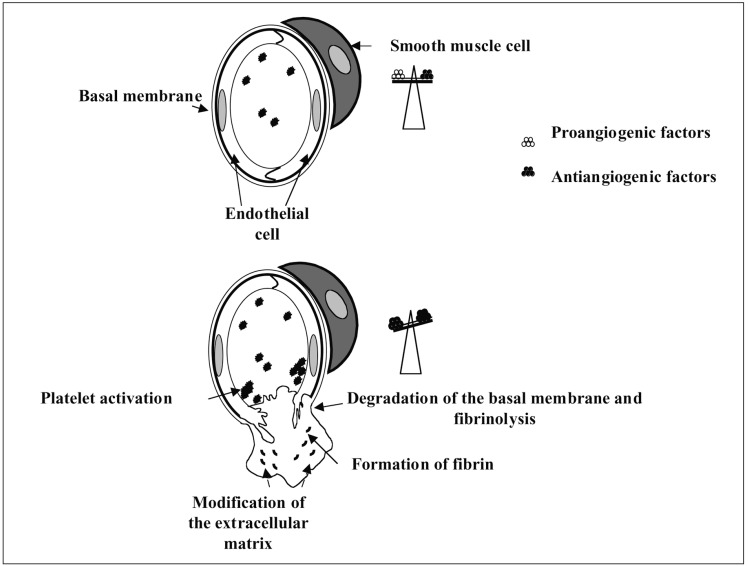
The formation of a new vessel from a pre-existing vessel, or angiogenesis, involves compulsory remodelling (figure 1). Firstly, the extension of a new vessel is characterised by detachment of the vessel from its environment (the other endothelial cells, pericytes or SMC and the extracellular matrix), a proliferation phase, a migration phase and a reassembly phase serving to stabilize the newly formed vessel.
Figure 1.
Description of remodelling induced by angiogenesis (redrawn from Suh, 2000).
The Activation Phase Includes:
— triggering the angiogenesis process following a stimulus by so-called angiogenic molecules or “growth factors”
— increased cell permeability and the formation of extracellular fibrin deposits
— deassembly of the vessel wall with enzymatic degradation and dissolution of the ECM architecture
— degradation of the basal lamina
— migration of the EC into the perivascular space under angiogenic stimulation known as chemotactism, and invasion of the ECM
— proliferation of EC following the loss of cell-to-cell contact inhibition
— formation of the capillary lumen by coalescence of EC.
The Resolution Phase Involves:
— inhibition of EC proliferation
— a stop to cell migration
— reconstitution of the basal membrane
— maturation of junctional complexes
— assembly of vessel walls
— recruitment and differentiation of SMC and pericytes as the endothelial tube elongates
— organisation of the three-dimensional architecture of the vascular tree.
Two separate mechanisms of angiogenesis have been described: sprouting or budding which includes the growth phases of the new vessel and its stabilization as described above and intussusception33. Intussusception is characterized by the insertion of interstitial cell columns into the lumen of the pre-existing vessel, to partition the vessel and remodel the local vascular network.
Neovascularization entails the cooperation of a series of growth factors whose concomitant or successive intervention must be coordinated. These factors may have a proliferative or chemotactic role or stabilize the newly formed structure. An isolated factor may be pro-angiogenic without being responsible for the multiplication of endothelial cells and hence only have a chemotactic role.
This concept is important because it explains why certain factors were not deemed pro-angiogenic factors as they were not involved in the vascular proliferation process when they served to stabilize the newly formed vessel. The exact role of numerous molecules during angiogenesis was only confirmed by the vascular phenotype of knock-out mice for this factor (e.g. TGFß) and the processing of transgenic mice has revolutionised our understanding of these events.
Growth Factors and their Receptors
The growth factor receptors involved in vasculogenesis and angiogenesis are transmembrane receptors with tyrosine kinase activity, except for the TGFß receptor which has serine/threonine kinase activity (classes I, II and III).
— Tyrosine kinase receptors have a single transmembrane domain, whereas their cytoplasmic domain contains one to two domains with tyrosine kinase activity. This enzymatic activity involves phosphorylation of the cytoplasmic protein by addition of a phosphorus group at the level of a tyrosine residue. The binding of the ligand with its receptor leads to the formation of receptor dimers involving the autophosphorylation of the receptor and its activation. The activated receptor is responsible for the phosphorylation of cytoplasmic proteins conveying the intracellular signal.
— Serine/threonine kinase receptors form a family of three classes of single transmembrane domain proteins (I, II, III). These proteins contain a serine/threonine kinase domain in the cytoplasmic part. Classes I and II receptors transmit a cell signal when they bind to the ligand. On the contrary, class III receptors (betaglycan and endoglin) modulate classes I and II receptors and do not transmit a cell signal directly. Class I receptors differ from those of class II as they have a cytoplasmic GS pattern preceding the kinase pattern. The enzymatic activity of class I and II receptors involves the phosphorylation of cytoplasmic proteins at the level of serine or threonine residues.
Transduction of the signal leads to phosphorylation of the proteins implicated in cell proliferation.
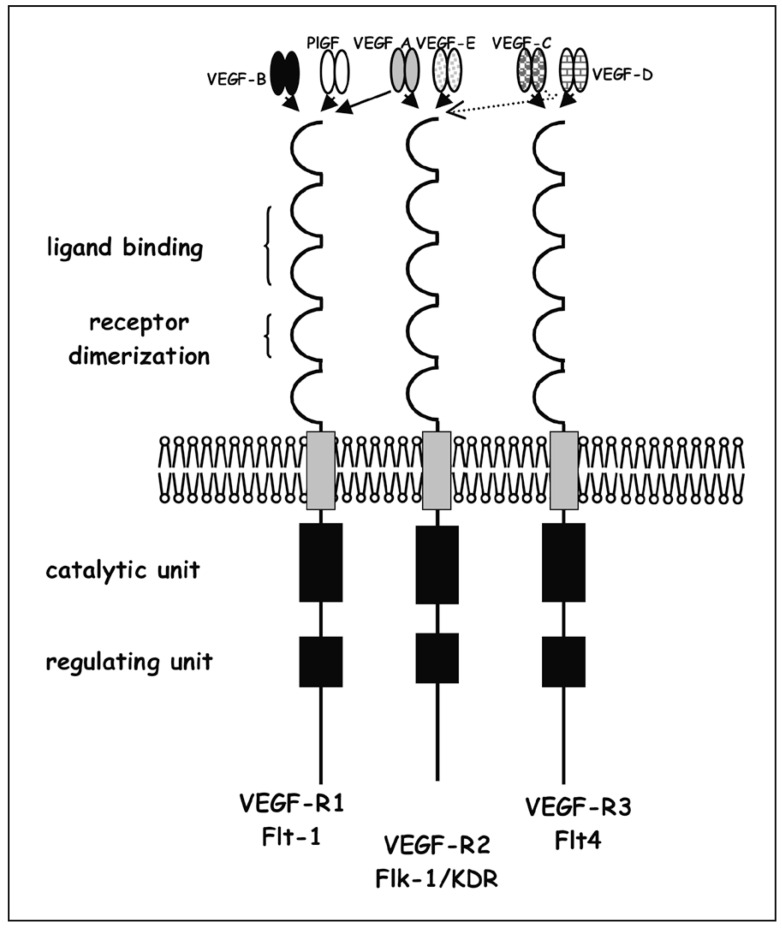
Families of the Vascular Endothelial Growth Factor (VEGF) (A,B,C,D,E), and Placenta Growth Factor (PlGF) and their Receptors
VEGF receptors are: VEGF-R1 (flt-1); VEGF-R2 (flk-1/KDR); VEGF-R3 (flt-4); names in brackets correspond to the names given to man/mouse receptors. These receptors are activated by ligands in dimeric form according to the following schemes: VEGF A-PlGF / VEGF A-VEGF B / VEGF A-VEGF A / PlGF-PlGF (figure 2).
Figure 2.
Schematic diagram of different VEGF and their respective receptors, tyrosine kinase receptor models.
The ligands have preferential binding for one receptor: PlGF binds to VEGF-R1; VEGF A binds to receptors VEGF-R1 and VEGF-R2; VEGF C and D bind to receptors VEGF-R2 and VEGF-R3; VEGF B binds to VEGF-R1 (Ferrara, 2000).
Little is known about the specific role of the different forms of the VEGF ligand, except for VEGF C which is implicated in the formation of lymphatic vessels24. VEGF A, the most widely studied VEGF ligand, is a homodimeric glycoprotein which binds to heparin and has a signal sequence which allows its secretion. The alternative splicing of its RNA messenger gives rise to four isoforms: VEGF-121 secreted in free form, VEGF-165 the main form secreted bound to heparan sulphate, VEGF-189 and VEGF-206 bound to heparin and sequestered in the ECM.
These forms bound to the ECM are released by heparin or heparinases suggesting that their binding site is made up of proteoglycans containing heparin-like residues; the long form is also released by plasmin. Likewise, the ligands are made available both by free secretion and after degradation of the ECM. The gene coding the VEGF ligand is widely expressed throughout the body, especially by smooth muscle cells, but not by endothelial cells, when VEGF receptors are expressed by endothelial cells. This differential expression suggests a paracrine mechanism of receptor activation. The -165 form of VEGF A can bind not only to its receptor VEGF-R2, but also to neuropilins -1 and -2 able to heterodimerize to VEGF-R2 and to the heparan sulphate proteoglycans.
The gene coding for VEGF A is regulated by hypoxia, hormones and cytokines, but also by cell differentiation and transformation.
Fibroblast Growth Factor (FGF) (1) (a) Acid, and (2) (b) Basic
The FGF belong to a family of polypeptides with a strong affinity for heparin, coded by 19 genes.
The special features of these growth factors is that they do not have a signal sequence and hence cannot be secreted a priori from the cellvia the complex endoplasmic reticulum-Golgi apparatus.
This indicates that the cell producing the FGF must be broken down in order to exercise the FGF function. However, recent evidence has shown that bFGF or FGF2 can be released into the extracellular environment4. The binding of FGF-1 and FGF-2 to heparan sulphate enhances their autocrine and paracrine activity, allowing tissue storage of FGF as a protection against proteolysis and an improved presentation of FGF to its receptors.
There are four types of cytoplasmic tyrosine kinase FGF receptors: (R1 to R4) giving rise to numerous variants by alternative splicing and identified in endothelial and smooth muscle cells.
FGF ligands have a high affinity for ECM proteins containing heparin (like heparan sulphate proteoglycans) located at the cell surface or within the ECM. ECM proteins can sequester the ligand or present it to its high affinity receptor (Baird, 2000). FGF can bind to their receptor in the form of monomers and the receptor binding site differs from the heparin binding site. Binding of an FGF molecule bound to heparin weakly activates the receptor where as if a second FGF molecule binds to the complex, receptor activation is much stronger.
Transforming Growth Factor Beta (TGFß) Family
The TGFß family has more than 30 members. TGFß has been isolated in three isoformsin mammals: TGFßl, 2 and 3. This growth factor is a protein secreted in a latent form (C-terminal tip containing the mature peptide) bound to a binding protein which is called LAP and activated by an acid or plasmin. The proteins act in the form of homo or heterodimers joining two peptides of this family.
Members of the TGFß family bind to a heteromeric complex comprising two types of serine-threonine kinase receptors known as type I and type II. Activated by ligand binding, the type II receptor recruits and transphosphorylizes the type I receptor, triggering the activation of specific intracellular transcriptional factors, the Smad. TßRI or ALK-5 (Activin receptorlike kinase 5) is a more widely expressed type I TGF receptor than ALK-1. TGFß can bind to another accessory receptor, endoglin, which is not a serine-threonine kinase receptor26.
Regulation is basically extracellular: the proteins binding to the LAP are bound together by disulfide bridges and seem to target TGFß1 towards its potential action sites. Plasmin and other proteases allow cleavage of the LAP. Thrombospondin-1 (TSP-1) binds to the LAP and changes the conformation of TGFß1 to stimulate its activation. TSP-1-/-mice have a phenotype similar to that of TGFßl-/-mice. Likewise, interactions between avb6 integrins and the LAP activate TGFß1 in settings of specific cell presentation10,30.
Platelet Derived Growth Factor (PDGF) Family
PDGF does only acts in a dimeric form which is joined by disulphide bridges. Two genes each code for one protein, form A and form B, and the different associations give rise to three different molecules (AA, AB, BB). A rise in shear stress and hypoxia are potent stimuli of PDGF-BB expression in endothelial cells and macrophages. Shear stresses are longitudinal frictional forces along the endothelium surface and proportional to blood flow velocity and viscosity and three times inversely proportional to vessel radius. PDGFαR and PDGFßR are the receptors for these ligands. The PDGFßR form is expressed by endothelial cells, smooth muscle cells and pericytes and is stimulated by increased shear stress.
Likewise, when intravascular blood flow increases, shear stress increases thereby favouring the expression of PDGF-BB and its receptor.
The Angiopoietins Family
Angiopoietins -1 and -2 are growth factors intervening early in angiogenesis. Angiopoietins -3 and -4 were recently cloned by sequence homology41. Their structure can be divided into three domains: an N-terminal region, a segment rich in patterns implicated in multimerization, and a fibrinogen-like domain, the most preserved region of the angiopoietins which determines the agonist or antagonist features of the molecule.
Ang-1 is an agonist of the tyrosine kinase receptor TIE-2 (TEK) whereas ang-2 is its antagonist. Ang-3 in mice is thought to act as an antagonist of TIE-2 and ang-4 in man as an agonist of TIE-2. The TIE family has another tyrosine kinase receptor, TIE-1, whose ligand is not known.
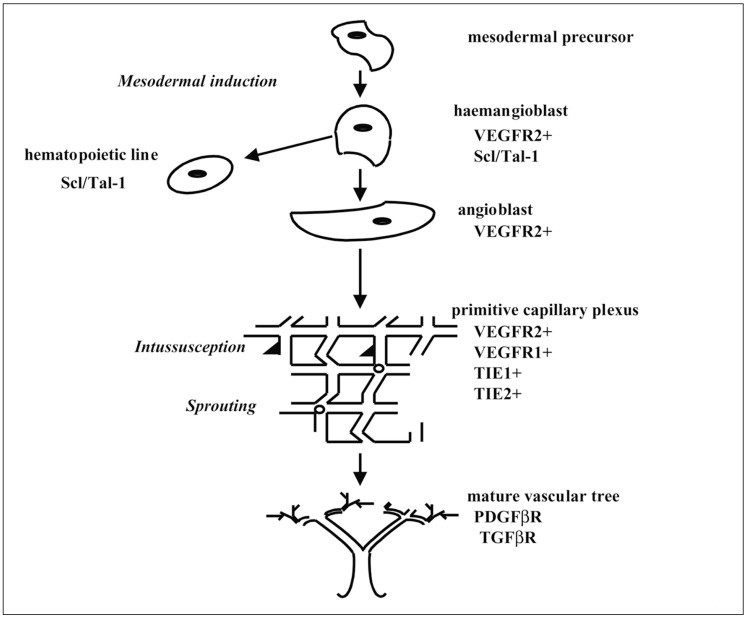
Role of the Growth Factors Involved in Vasculogenesis and Angiogenesis (figure 3)
Figure 3.
Schematic summary of the different growth factors controlling vasculogenesis and angiogenesis (redrawn from Risau, 1997).
Roles of FGF, VEGF and their Receptors in Vasculogenesis
In mice, the vascular tree starts to develop at E7.5, i.e. on day 7.5 of embryonic development. Certain regions of the epiblast, a germinal layer giving rise to the whole embryo, undergo mesodermal induction under the influence of FGF.
This cell sub-population differentiates into haemangioblasts, the precursors of the haematopoietic and endothelial cell lines (angioblasts). Haemangioblasts express VEGF-R2 (Flk-1) and SCL/TAL-1, a transcriptional factor with a helix-loop-helix domain. Mice knocked out for VEGF-R2 (Flk-1) have a deficit of the two haematopoietic and endothelial cell lines38.
In embryos not expressing the transcriptional factor SCL/TAL-1, only haematopoietic cells are affected. After differentiation, VEGF-R2 (Flk-1) is negatively regulated in the cells of the haematopoietic line but not in the endothelial cells. VEGF-R2 (Flk-1) therefore plays a crucial early role in angioblast formation.
VEGF-R1 (Flt-1) is involved later in angiogenesis: Flt-1-/-mice have angioblasts but they are unable to aggregate. These two receptors differ in their affinity for VEGF (higher for Flt-1) and tyrosine kinase activity (higher for Flk-1). VEGF-R1 has an unexpected mode of action: in a construction in which only the cytoplasmic ty-rosine kinase domain is lacking, the vascular system in mice develops normally21. In this way, the role of this receptor in angiogenesis is ensured by its binding to the ligand and not by the tyrosine kinase site.
The VEGF ligand is implicated very early in vascular development and acts by binding to its tyrosine kinase receptors31. The roles of the ligand and its receptors were recently disclosed using transgenic mice whose genes coding for VEGF or its receptors had been inactivated. VEGFA-/-mice present relative but abnormal endothelial differentiation leading to death of the homozygote mouse. Heterozygote VEGFA-/+ mice die at E10.57 or E11.517 indicating the need for a critical amount of VEGF for embryonic development.
The VEGF ligand is produced in situ by the endoderm (paracrine) whenever the receptors are expressed by cells produced by the mesoderm. Angioblasts differentiate in situ or migrate to some distance in adaptation to the environment. Cells proliferate in situ then extend and interconnect in a network forming a cranial-to-caudal and dorsal-to-ventral gradient14.
This differentiation of an undifferentiated cell into an endothelial cell was first reported only in prenatal vasculogenesis. Nevertheless2, found putative progenitor endothelial cells expressing VEGF-R2 (Flk-1) in the peripheral blood circulation of different animal models and able to differentiate into endothelial cells in vitro and be incorporated into active sites of angiogenesis. More recently still, putative progenitors of both endothelial and smooth muscle cells were identified. Differentiation into EC is determined by VEGF and SMC by PDGF BB44.
Role of TIE-1 and TIE-2 Receptors in Vasculogenesis
Dumont 15 highlighted another family of tyrosine kinase receptors in the vascular endothelium: TIE-1 and TIE-2. Using in situ hybridization, Davis 12 showed that these receptors appear later than the VEGF-R2. Indeed, TIE-2-/-mice present later vascular abnormalities implicating the VEGF ligand and its receptor. TIE-2-/-mice die within E9.5 and E10.5 of the vascular system malformation, the vessels appearing dilated, of uniform size and without ramifications16, whereas TIE-1-/-mice die later within E13.5 and P0 from respiratorydistress and oedema ascribed to the increased permeability due to the vascular defect37. This type of abnormality is related to human disease in families with a TIE-2 mutation leading to venous malformations such as dilated vessels without increase in the ratio of smooth muscle cell layers 42.
Role of TIE-2 ligands: Angiopoietin 1
Vascular expression of angiopoietin 1 is later than that of VEGF-R2 (Flk-1)12. Angiopoietin 1 phosphorylates TIE-2. On human umbilical vein endothelial cell (HUVEC) culture or on fibroblasts culture, the proliferative effect of TIE-2 and Ang-1 on these cells is not seen12. Ang-1-/- mice present a simplified appearance of heart and vessels with a “syncytium” aspect, i.e. dilated without branching, but with a slightly less severe phenotype than that of TIE-2-/-mice 40.
Endothelial cells do not appear correctly joined to the underlying matrix and no recruitment of other peri-endothelial cells is observed.
Role of TIE-2 ligands; Angiopoietin 2
Angiopoietin 2 was revealed by low stringency screening of a bank of DNAc with an angiopoietin 1 probe. Unlike ang-1, ang-2 dephosphorylates TIE-2. Coexpression of the ang-1 and ang-2 transcripts is observed and ang-2 is expressed in the adult in major angiogenesis sites like the uterus. Ang-2 expression, just like that of VEGF, precedes then accompanies that of ang-1 and vessel formation. Ang-2 expression forced in endothelial cells by transgenesis with the TIE-2 promoter leads to vessel defects of the same type as those induced by a lack of TIE-2 or ang-1. As a whole, these results suggest that ang-2 is a natural antagonist of ang-1.
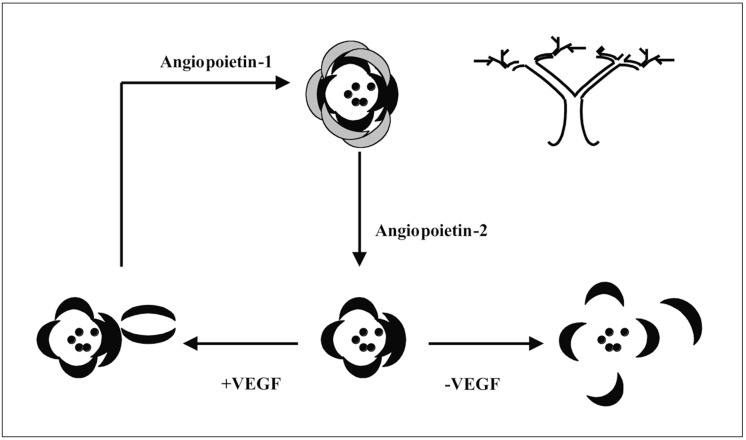
Hanahan 20 proposed the following activation pattern: endothelial cells differentiate, aggregate and form the vascular lumen and endothelial tube. Ang-1, an agonist, serves to stabilize this new formation, the presence of VEGF maintaining the activation sequence, the absence of VEGF leading to vascular regression. Ang-2, an antagonist, is thought to inhibit the maturation and stabilization of the endothelial tube or favour detachment of the EC thereby allowing their migration (figure 4).
Figure 4.
The role of angiopoietins -1 and -2 during angiogenesis.
Role of PDGF in Vasculogenesis
In culture, exogenous PDGF:
— induces the proliferation of endothelial cells;
— is mitogenic and chemotactic for SMC and pericytes;
— antibodies neutralising PDGF-BB block the migration of SMC22.
PDGF is positively regulated by the proliferation of endothelial cells and increased shear stress; the ligand activates its receptor expressed on the perivascular mesenchymal cells.
Knockout mice for the PDGF ligand or receptor die in utero from haemorrhage and impaired attachment of pericytes to the endothelial cells 5,27. The PDGF-BB ligand and its receptor PDGFßR are coexpressed at an early stage in endothelial cells 23. In culture, these two molecules induce a proliferation of endothelial cells suggesting they function by an autocrine route. Later on, ligand expression is maintained in the endothelial cell when the RNAm of the receptor becomes undetectable there, but is nonetheless present in fibroblasts which can differentiate into pericytes and SMC22, and the surrounding SMC. Hence the observed cell proliferation indicates they function by a paracrine route.
Role of TGF in Vasculogenesis
TGFß1 in culture inhibits the proliferation of endothelial cells and pericytes 11. Cocultures of pericytes and endothelial cells disclosed TGFß1 secretion by two cell types. TGFß1 inhibits the proliferation and migration of endothelial cell when the EC and pericytes enter into contact and this contact is favoured by TGFß1 activation 1. Lastly, TGFß1 knock out mice present late onset vascular abnormalities leading to impaired cell contact and endothelial differentiation 13, i.e., a phenotype identical to that observed for TGFßR type II knock out mice32.
The TGFß gene is positively regulated by increased shear stress and endothelial cells.
Endoglin and ALK-1 mutations are responsible for certain forms of the autosomal dominant human disease hereditary haemorrhagic telangiectasia. On the one hand, endoglin is a class III TGFß homodimeric receptor which binds to receptors of classes I and II thought to initiate the growth factor response. On the other, ALK-1 is a class I receptor which binds to the class II receptor. During the disease, epitaxis and gastrointestinal bleeding occur, sometimes from childhood, caused by multiple telangiectasias characterized by dilation of post-capillary venules joined to dilated arterial segments with disappearance of capillary segments 25,28.
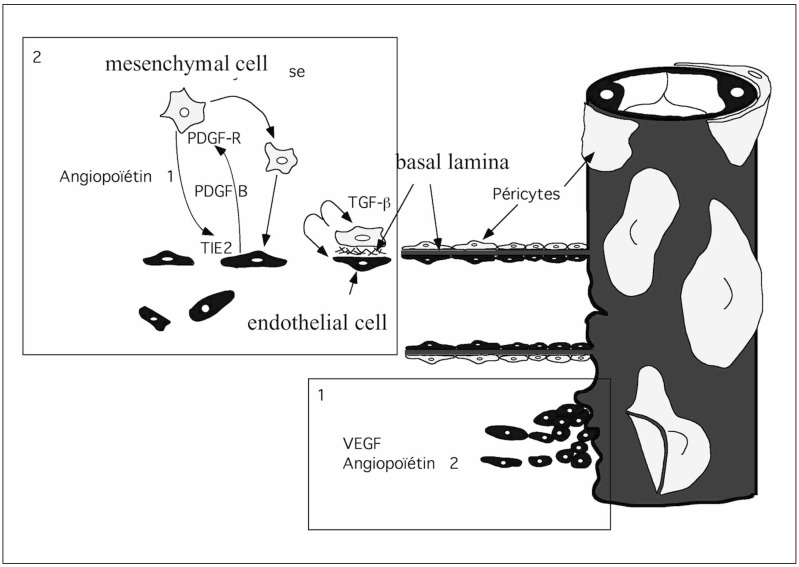
Joint Roles of VEGF, ang-1, ang-2, PDGF and TGFb and their Receptors in Vasculogenesis
In an overview of these findings, Folkman and d’Amore19 and Hanahan20 proposed that initially angiopoietin-2, by attenuating the interactions among endothelial cells, extracellular matrix and pericytes, is thought to make the cells sensitive to VEGF. Like PDGF-BB, angiopoietin-2 is thought to participate in the recruitment of nearby mesenchymal cells migrating towards the capillary. When endothelial and mesenchymal cells come into contact, the TGFb secreted by these cells induces the differentiation of mesenchymal cells into pericytes, inhibiting the proliferation of endothelial cells and stimulating extracellular matrix fixation. Angiopoietin-1 then stabilizes the interactions between the different cell types and the extracellular matrix (figure 5).
Figure 5.
Schematic diagram of the different interactions between growth factors during angiogenesis (redrawn from Mattot et Al, 1998).
Differentiation into Arteries or Veins
The differentiation of vessels into arteries or veins seems to be determined not only by the direction and rate of blood flow, but also by the presence or absence of molecules known as ephrins. These molecules, already known for their role in guiding axons, play a key role in the differentiation into arteries and veins. Ephrins are transmembrane molecules expressed by cells adjacent to cells expressing the corresponding receptor. Ephrine receptors or Eph are also tyrosine kinase activity receptors. Wang43 showed that ephrin B2 is expressed in the EC of arteries but not in veins, and that inversely its receptor Eph B4 is expressed in the EC of veins but not arteries. In addition, inactivation of ephrin B2 leads to an absence of artery-vein differentiation at E9 in the yolksac, head and heart. Likewise, the pair act reciprocally in endothelial cells destined to arteries and veins.
Interactions between Nerve and Artery Growth
Recent evidence suggests that during development the peripheral nervous and peripheral arterial systems are related in a way over and above anatomical and functional. Arteries run the length of nerves, feeding them and supplying oxygen, and nerves control arterial tonus. Moukouyama 29 described an impairment of arterial arborization in the foot skin of mutant mice in which genes coding for peripheral sensory nerve formation or Schwann cells had been inactivated (neurogenin1/neurogenin 2 homozygote double mutants and semaphorin 3A homozygotes).
They showed that sensory nerves express VEGF and that coculture of sensory nerves and Schwann cells was responsible for expression of the marker of arterial differentiation of embryonic endothelial cells.
In addition, Cantarella 6 highlighted the angiogenic role of the nerve growth factor on HUVEC cells and in the chick chorio-allantoic membrane model. These important findings suggested that the neurons in these models have a growth guide for arterial arborization in the same territory via a vascular growth factor VEGF and a neuronal growth factor NGF.
Conclusions
Understanding these mechanisms will pave the way for novel therapeutic strategies for the many diseases resulting from the excess formation of new abnormal vessels as in cancer, or in ischaemic disease in which angiogenesis is no longer repressed, but stimulated. Plainly, these targets are the growth factors described herein, but they will also involve other molecules playing a major role and allowing the destruction of the surrounding extracellular matrix and the progression of the endothelial cell and its daughter cells to extend the new vessels. These cellular interactions are described in the second part of this review.
References
- 1.Antonelli-Orlidge A, Saunders KB, et al. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Baird A. Angiogenesis in health and disease, Richmond, Berlex Biosciences. 2000:75–88. [Google Scholar]
- 4.Bastagli L, Lazzarotto T, et al. Presence of basic fibroblast growth factor in cultured rat cardiomyocytes and its release in culture medium. Ann N Y Acad Sci. 1995;752:417–421. doi: 10.1111/j.1749-6632.1995.tb17449.x. [DOI] [PubMed] [Google Scholar]
- 5.Beck L, Jr, D’Amore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. [PubMed] [Google Scholar]
- 6.Cantarella G, Lempereur L, et al. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Ferreira V, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Developmental biology. One cell, two fates. Nature. 2000;408:43–45. doi: 10.1038/35040684. [DOI] [PubMed] [Google Scholar]
- 9.Comerota AJ, Throm RC, et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J Vasc Surg. 2002;35:930–936. doi: 10.1067/mva.2002.123677. [DOI] [PubMed] [Google Scholar]
- 10.Crawford SE, Stellmach V, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 11.D’Amore PA, Smith SR. Growth factor effects on cells of the vascular wall: a survey. Growth Factors. 1993;8:61–75. doi: 10.3109/08977199309029135. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Aldrich TH, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 13.Dickson MC, Martin JS, et al. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 14.Drake CJ, Brandt SJ, et al. TAL1/SCL is expressed in endothelial progenitor cells/angioblasts and defines a dorsal-to-ventral gradient of vasculogenesis. Dev Biol. 1997;192:17–30. doi: 10.1006/dbio.1997.8751. [DOI] [PubMed] [Google Scholar]
- 15.Dumont DJ, Yamaguchi TP, et al. Tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- 16.Dumont DJ, Gradwohl G, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Carver-Moore K, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. Angiogenesis in health disease, Richmond, Berlex Biosciences. 2000:47–73. [Google Scholar]
- 19.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 21.Hiratsuka S, Minowa, et al. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirshi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc. Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 23.Holmgren L, Glaser A, et al. Angiogenesis during human extraembryonic development involves the spatiotemporal control of PDGF ligand and receptor gene expression. Development. 1991;113:749–754. doi: 10.1242/dev.113.3.749. [DOI] [PubMed] [Google Scholar]
- 24.Jeltsch M, Kaipainen A, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 25.Johnson DW, Berg JN, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Na. Genet. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 26.Larsson J, Goumans MJ, et al. Abnormal angiogenesis but intact haematopoietic potential in TGF-beta type I receptor-deficient mice. Embo J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattot V, Pourtier A, et al. La morphogénèse de l’arbre vasculaire. de la compréhension des mécanismes moléculaires aux perspectives therapeutiques. Med Sci. 1998;14:437–447. [Google Scholar]
- 28.McAallister KA, Grogg KM, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 29.Mukouyama YS, Shin D, et al. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 30.Munger JS, Huang X, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 31.Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima M, Oshima H, et al. TGF-beta receptor type II deficiency results in defects of yolk sac haematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 33.Patan S, Alvarez MJ, et al. Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch Histo l Cytol. 1992;55:65–75. doi: 10.1679/aohc.55.suppl_65. [DOI] [PubMed] [Google Scholar]
- 34.Poole TJ, Finkelstein EB, Cox CM. The role of FGF and VEGF in angioblast induction and migration during vascular development. Developmental Dynamics. 2001;220:1–17. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 36.Rubenstein JL, Kim J, et al. Anti-VEGF antibody treatment of glioblastoma prologs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato TN, Tosawa Y, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 38.Shalaby F, Rossant J, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 39.Suh DY. Understanding angiogenesis and its clinical applications. Ann Clin Lab Sci. 2000;30:227–238. [PubMed] [Google Scholar]
- 40.Suri C, Jones PF, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 41.Valenzuela DM, Griffiths JA, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci. 1999;96:1904–1909. doi: 10.1073/pnas.96.5.1904. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vikkula M, Boon LM, et al. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- 43.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita J, Itoh H, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]