Summary
Stent assisted coil embolization is a useful therapeutic modality for wide-necked and geometrically difficult aneurysms, as the stent provide a buttress that allows for coil deposition while preventing coil haerniation into the parent vessel lumen, and placement of an endovascular stent within the parent artery across the aneurysm neck may divert the blood from the aneurysm inflow tract and promote intra-aneurysm stasis and thrombosis. We report herein a 3 patients treated with endovascular stent-assisted coil embolization for symptomatic or enlarging wide-necked, dissecting, and fusiform aneurysms of the carotid and vertebrobasilar arteries.
One patient had intracranial mass effect, the second had subarachnoid haemorrhage, and the third had angiographic evidence of enlarging aneurysm. The aneurysm was located in the petrous segment of internal carotid artery in one patient and in the intracranial vertebral artery in the other two patients. For all patients, we used balloon expandable stent (such as GFX, S-670) in this technique. Complete obliteration of the aneurysms could be achieved in all cases, with preservation of distal circulation.
Key words: Intracranial aneurysm, wide necked, dissection, fusiform, stent, Guglielmi detachable coil
Introduction
Coil embolization with Guglielmi detachable coils (GDCs) for wide-necked, dissecting, and fusiform aneurysms is difficult because of the complex wide necks or irregular shapes of these aneurysms. Aneurysm coiling can result in parent vessel occlusion secondary to coil haerniation.
With the recent availability of the flexible intravascular stents, a new therapeutic approach has become possible, heralding a new era in the endovascular treatment of intracranial aneurysms3,4,8-11. Placement of an endovascular stent within the parent artery across the aneurysm neck may divert the blood from the aneurysm inflow tract and promote intra-aneurysm stasis and thrombosis1,6,12,13. Additionally, stents provide a rigid scaffold that allows improved coil deposition with reduced risk of coil haerniation into the parent vessel lumen.
We report herein a series of three consecutive patients treated with endovascular stent-assisted coil embolization for symptomatic or enlarging wide-necked, dissecting aneurysms and fusiform aneurysms of the carotid and vertebrobasilar arteries
Case Reports
Case 1
A 48-year-old woman was admitted our hospital following sudden onset of occipitalgia and vomiting.
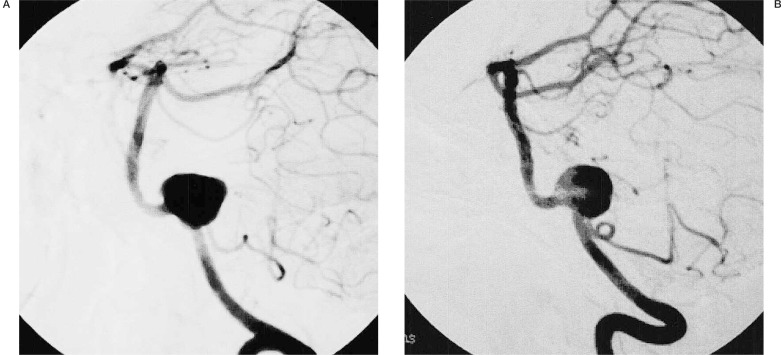
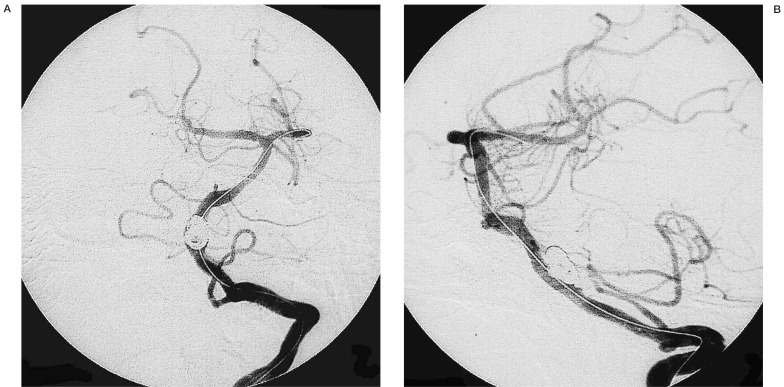
Computed tomography (CT) of the head demonstrated subarachnoid haemorrhage (SAH). Magnetic resonance angiography (MRA) demonstrated an aneurysm of the left vertebral artery. Cerebral angiography showed left vertebral artery aneurysm with a wide neck. The left posterior inferior cerebellar artery (PICA) arose from the neck of the aneurysm (figure 1A,B).
Figure 1.
Initial diagnostic cerebral angiograms of the left vertebral artery (A: oblique view, B: lateral view) demonstrating a saccular wide-necked vertebral aneurysm (Case 1).
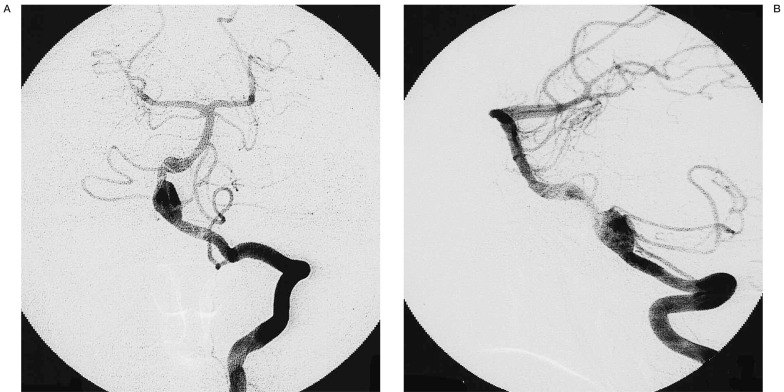
The aneurysm was judged to be unsuitable for surgical clipping or endovascular coil placement because of its location and configuration. A 3.6 mm in diameter × 24 mm in length GFX stent (AVE Medtronics) was placed across the aneurysm neck (figure 2), and then 19 GDCs were placed in the aneurysm via a microcatheter (figure 3). Satisfactory aneurysm occlusion was achieved without any procedural complications (figure 4A,B). The patient was discharged after 14 days. A follow-up angiographic study performed 24 months later revealed persistent aneurysm obliteration and no evidence of in-stent stenosis with good flow distal to the stented segment. She is currently neurologically intact.
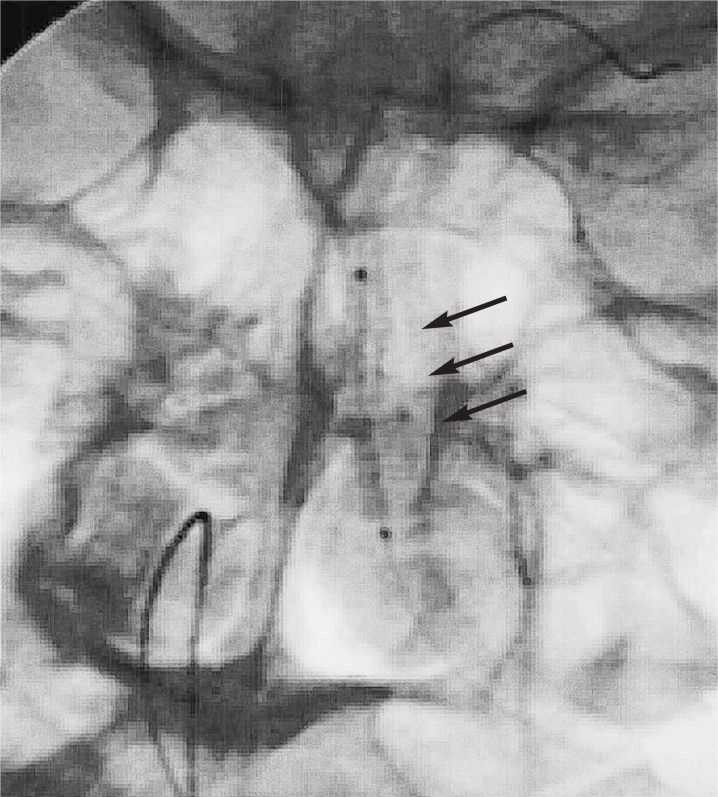
Figure 2.
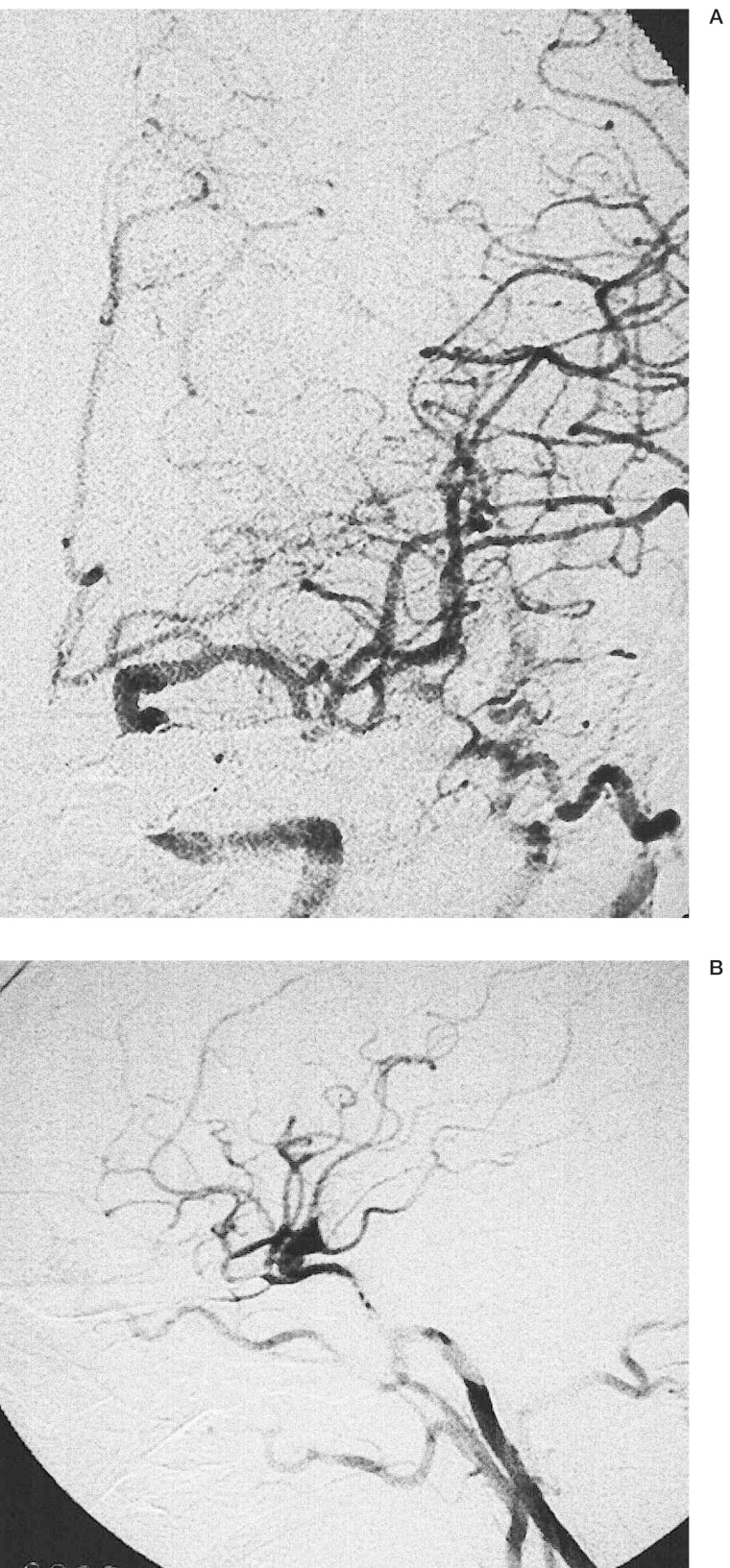
Plain X-ray showing the intravascular stent positioned from the inflow to the outflow of the vertebral artery aneurysm, allowing bridging and creation of an aneurysm neck (arrows).
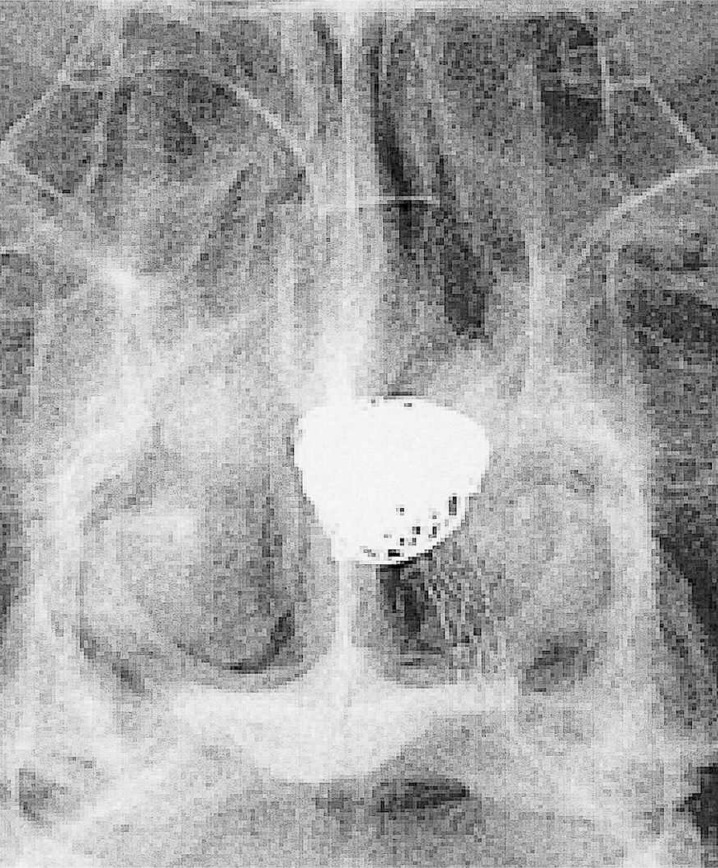
Figure 3.
Unsubtracted image demonstrating the relationship of the stent and coils.
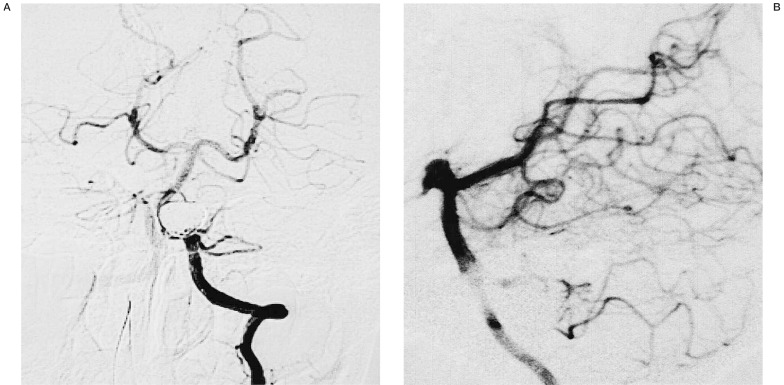
Figure 4.
Immediately post-procedure left vertebral artery angiograms (A: antero-posterior view, B: lateral view) after stenting and placement of GDCs showing total occlusion of the aneurysm, while the posterior inferior cerebellar artery is clearly seen.
Case 2
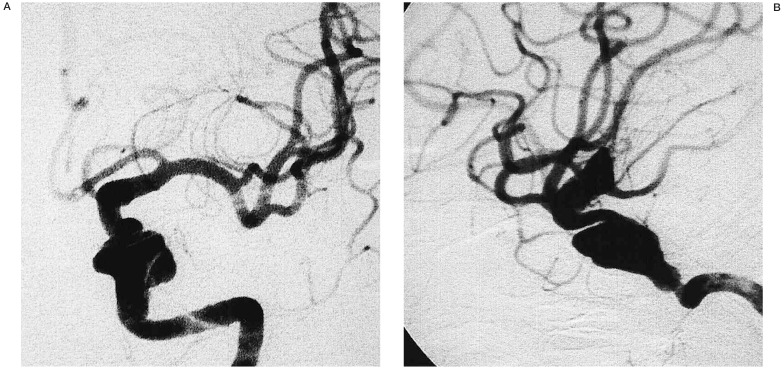
A 51-year-old woman suffered from occipitalgia of sudden onset not associated with any dizziness or loss of consciousness. A dull headache persisted till admission into a neighboring hospital three days later. At that time, she had no focal neurological signs, and MRA demonstrated no evidence of aneurysm, but showed stenosis of the petrous portion of the left internal carotid artery (ICA) (figure 5). Following conservative treatment for 2 weeks, the patient developed left oculomotor and abducent nerve palsies. Repeated MRA and four-vessel angiography demonstrated dissection of the left ICA in the horizontal petrous segment with large pseudoaneurysm formation and persistent left ICA stenosis (figure 6A,B).
Figure 5.
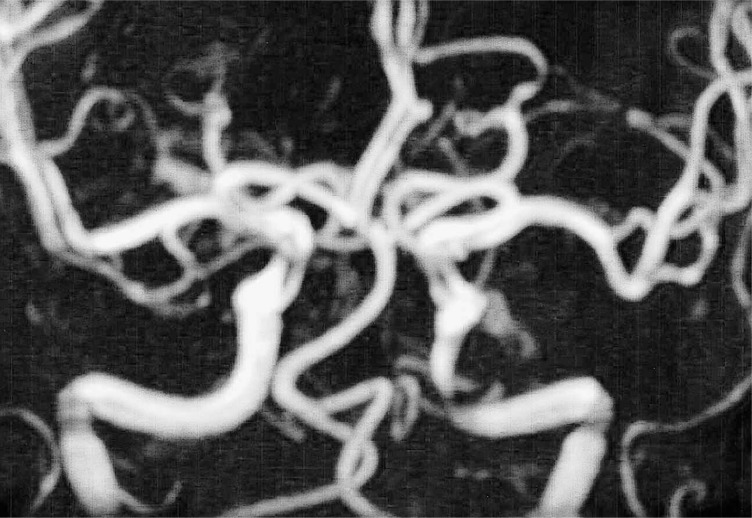
Magnetic resonance angiography (MRA) demonstrated no aneurysm but only stenosis of petrous segment of the left internal carotid artery (Case 2).
Figure 6.
Angiograms of the left internal carotid artery (A: antero-posterior view, B: lateral view), showing an intracranial ICA pseudoaneurysm of the horizontal portion of the petrous segment of the intracranial left internal carotid artery (ICA). The aneurysm is associated with parent artery stenosis.
A 3.5 mm in diameter × 25 mm in length SR670 (AVE Medtronic) stent was deployed across the neck of the pseudoaneurysm, and 11 GDCs of various sizes were then progressively positioned within the aneurysm. Post-treatment angiography confirmed complete angiographic occlusion of the aneurysm (figure 7A,B).
Figure 7.
Angiograms of the left internal carotid arteries (A: antero-posterior view, B: lateral view) showing the final angiographic results after stent placement and coil deployment.
The patient showed neither neurological changes nor adverse events throughout the procedure. Six months after treatment, the left oculomotor and abducens palsies completely disappeared.
Case 3
A 46-year-old man presented with dizziness, and numbness of right upper limb. Three-dimensional (3D) CT angiography revealed a fusiform aneurysm in the right vertebral artery. Althrough he was managed conservatively for six months, repeated 3D CT angiography demonstrated enlargement of this aneurysm.
Cerebral angiography confirmed the presence of a fusiform aneurysm originating from the right vertebral artery (figure 8A,7B). A 4 mm in diameter × 2 cm in length S670 (AVE Medtronic) stent was deployed across the aneurysm neck, and a total of 5 GDCs were delivered into the aneurysm sac. Post-treatment angiography confirmed complete angiographic occlusion of the aneurysm (figure 9A,B). The patient remained symptom-free and his neurological baseline was maintained during a 2-month follow-up. All patients received heparin infusion to obtain an activated clotting time of 2 to 2.5 × baseline during the procedure. After treatment, heparin was discontinued, and the patients were given oral aspirin (325 mg/day for 6 months).
Figure 8.
Initial diagnostic cerebral angiogram of the left vertebral artery (A: antero-posterior view, B: lateral view) showing fusiform aneurysm of the left vertebral artery (Case 3).
Figure 9.
Angiogram of the left vertebral artery (A: antero-posterior view, B: lateral view) demonstrating final angiographic results after stent placement and coil deployment. There is no change in the distal filling of the vertebrobasilar circulation.
Discussion
With the development and refinement of the intravascular stents, the potential for treatment of both extracranial and intracranial aneurysms has been realized. In the experimental models of intracranial aneurysms, the blood fiow within the aneurysm decreased after stent placement7, resulting in stasis and thrombosis 1,13. The stent serves as a mechanical scaffold for placement of intra-aneurysmal coils, preventing their haerniation into the parent artery and allowing a denser coil packing5,6,9,12.
This advantageous alteration of the flow dynamics, combined with the ability to achieve a denser coil packing in the aneurysm, may reduce the likelihood of future coil compaction, a phenomenon particulary observed in the wide-necked aneurysms.
The advent of new-generation of flexible stents that permit safe and reliable percutaneous access of the intracranial and extracranial vasculature, has transformed this technique into a viable therapeutic option. Higashida 2 first reported the use of this technique for an intracranial aneurysm. Previously, Lanzino 6 described a series of 10 patients treated with stent-assisted coil embolization. One of these 10 patients had an intracranial vertebral artery aneurysm, while the other patients had intracranial ICA aneurysms. The short-term clinical follow-up was favorable for all patients, and angiographic follow-up demonstrated that only one patient (with a vertebral artery aneurysm) had mild-to-moderate in-stent stenosis.
However, the stent-assisted coil embolization for the intracranial aneurysms has several limitations. Covering of the ostia of the side wall branches by the stent metal work commonly occurs in the intracranial aneurysms, in which the small but important perforating vessels relative to their diameter and angle of origin, remain patent if less than 50% of the ostial diameter is covered by the stent struts 6,13. There was no clinical or angiographic evidence of brainstem perforator occlusion after placement of the vertebrobasilar stent in our patient.
Another potential problem with the use of the stent-assisted coil embolization technique is accurate intraprocedural fluoroscopic visualization of the coils in relation to the parent arterial lumen 12.
Coil loop protrusion through the stent interstices back into the parent artery may be difficult to detect because of superimposition of the coils and stent meshwork. The use of an inflated balloon in the stent during coil embolization has been suggested as a possible solution.
Our cases illustrate the successful use of stent-assisted coil embolization to treat wide-necked, dissecting, and fusiform aneurysms. Although this and similar reports demonstrated good short-term results a long-term follow-up of these patients is of utmost importance.
Conclusions
Stent-assisted coil embolization appears to be a viable and durable treatment for wide-neck, fusiform, and dissecting aneurysms. However, certain technical problems remain, such as adequately distinguishing the parent artery from the coiled aneurysm fluoroscopically. Rapid continued improvement in the endovascular technology will undoubtedly further facilitate its use and extend its applications.
References
- 1.Geremia G, Haklin M, et al. Embolization of experimentally created aneurysms with intravascular stent devices. Am J Neuroradiol. 2000;15:1223–1231. [PMC free article] [PubMed] [Google Scholar]
- 2.Higashida RT, Smith W, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg. 1997;87:944–949. doi: 10.3171/jns.1997.87.6.0944. [DOI] [PubMed] [Google Scholar]
- 3.Holowitz MB, Levy EI, et al. Transluminal stent-assisted coil embolization of a vertebral confluence aneurysm: Technique report. Surg Neurol. 2001;55:291–296. doi: 10.1016/s0090-3019(01)00421-9. [DOI] [PubMed] [Google Scholar]
- 4.Irie K, Kawanishi M, et al. Combined endovascular stent implantation and endosaccular coil placement for treatment of a wide-necked vertebral artery aneurysm: A case report. Jpn J Neurosurg. 2000;9:696–701. doi: 10.1097/00006123-199808000-00127. Tokyo. [DOI] [PubMed] [Google Scholar]
- 5.Lanzino G, Guterman LR, et al. The case for stenting (Review) Clin Neurosurg. 1999;45:249–150. [PubMed] [Google Scholar]
- 6.Lanzino G, Wakhloo AK, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538–546. doi: 10.3171/jns.1999.91.4.0538. [DOI] [PubMed] [Google Scholar]
- 7.Lieber BB, Stancampiano AP, et al. Alteration of haemodynamics in aneurysms in aneurysms model following by stenting. J Neurosurg. 2000;91:538–546. [Google Scholar]
- 8.Lylyk P, Cohen JE, et al. Combined endovascular treatment of dissecting vertebral artery aneurysm by using stents and coils. J Neurosurg. 2001;94:427–432. doi: 10.3171/jns.2001.94.3.0427. [DOI] [PubMed] [Google Scholar]
- 9.Mericle RA, Lanzino G, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery. 1998;43:1229–1234. doi: 10.1097/00006123-199811000-00130. [DOI] [PubMed] [Google Scholar]
- 10.Phatouros CC, Sasaki TYJ, et al. Stent-assisted coil embolization: The treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000;47:107–115. doi: 10.1097/00006123-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Sekhon LH, Morgan MK, et al. Combined endovascular stent implantation and endosaccular coil placement for the of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 1998;43:380–383. doi: 10.1097/00006123-199808000-00127. [DOI] [PubMed] [Google Scholar]
- 12.Wakhloo AK, Lanzino G, et al. Stent for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery. 1998;43:377–379. doi: 10.1097/00006123-199808000-00126. [DOI] [PubMed] [Google Scholar]
- 13.Wakhloo AK, Tio FO, et al. Self-expanding nitinol stents in canaine vertebral arteries: haemodynamics and tissue response. Am J Neuroradiol. 1995;16:1043–1051. [PMC free article] [PubMed] [Google Scholar]