Abstract
The Lophotrochozoa includes disparate tentacle-bearing sessile protostome animals, which apparently appeared in the Cambrian explosion, but lack an uncontested fossil record. Here we describe abundant well preserved material of Cotyledion tylodes Luo et Hu, 1999, from the Cambrian (Series 2) Chengjiang deposits, reinterpreted here as a stem-group entoproct. The entoproct affinity is supported by the sessile body plan and interior soft anatomy. The body consists of an upper calyx and a lower elongate stalk with a distal holdfast. The soft anatomy includes a U-shaped gut with a mouth and aboral anus ringed by retractable marginal tentacles. Cotyledion differs from extant entoprocts in being larger, and having the calyx and the stalk covered by numerous loosely-spaced external sclerites. The description of entoprocts from the Chengjiang biota traces the ancestry of yet another lophotrochozoan phylum back to the Cambrian radiation, and has important implications for the earliest evolution of lophotrochozoans.
The extant members of the Phylum Entoprocta are all small sessile suspension-feeders that consist of a calyx, stalk and basal attachment, but lack a skeleton1. The only uncontested fossil entoproct comes from the late Jurassic2. The Cambrian Dinomischus from the Burgess Shale and Chengjiang biotas was originally suggested to belong within the Entoprocta3,4; however, the supposed filter-feeding apparatus of Dinomischus is composed of stiff bracts and lacks evidence of the flexible tentacles that are characteristic of extant entoprocts2,5. Here we report for the first time a probable stem entoproct, originally described as Cotyledion tylodes Luo et Hu, 1999 in Problematica6, from the Chengjiang Konservat-Lagerstätte [about 520 Ma], Kunming, Yunnan, China. The entoproct affinity is supported by the presence of a lower fleshy stalk and an upper cup-shaped calyx containing a U-shaped gut with mouth and projected anal opening, both of which are surrounded by a marginal crown of flexible and foldable tentacles. This combination of characters is closely comparable with that of extant Entoprocta. However, Cotyledion differs from all crown entoprocts in having a relatively large size (8–56 mm, compared to a typical extant entoproct of 0.1–7.0 mm). The most notable difference, however, is that the entire outer surface of the body of Cotyledion is covered by numerous closely-spaced, pyrite-coated sclerites that originally might have been mineralized. In conjunction with studies of articulated tommotiids more recently interpreted as members of the brachiopod/phoronid stem group7,8,9, this sclerite-bearing tentaculate animal demonstrates that external sclerites may be more widely distributed within stem lophotrochozoans than previously suspected10, raising the possibility that sessile extant lophotrochozoan phyla probably were rooted in the earliest Cambrian Small Skeletal Fauna (SSF).
Results
Systematic Palaeontology
Total group Lophotrochozoa
Stem group Entoprocta
Cotyledion tylodes Luo et Hu, 1999
Referred Material
ELI-C 0001-0418, all the specimens are housed in the Early Life Institute and Key Laboratory of Continental Dynamics, Northwest University, Xi′an, China (Prefix: ELI).
Locality and Stratigraphy
Heilinpu (Chiungchussu or Qiongzhusi) Formation, Yu'anshan Member (Eoredlichia-Wutingaspis Trilobite Biozone, ordinarily correlated with the late Atdabanian or Botoman Stage in Siberia)6, Cambrian Stage 3. A total of 162 specimens come from the Jianshan Section at Haikou town, about 48 km south of Kunming, and the remaining 256 are mostly from the Erjie section, about 24 km southwest of Haikou.
Revised diagnosis
Sessile bipartite body, comprising an upper cup-shaped calyx and lower stalk terminated by an attachment disc, completely covered by mineralized or possibly sclerotized, oval sclerites; internal anatomy with U-shaped alimentary canal: comprising a narrow anterior esophagus, extending from a recessed mouth cavity and followed by an enlarged stomach and a recurved narrow intestine, terminating in an anal opening. Both mouth and anal opening are enclosed within the same tentacle ring. Tentacles, slender, protruding from the upper margin of calyx. Stalk variable in length and terminated by knob-shaped holdfast for attachment to hard substrates.
Description of new material
Continued collecting has produced important new data on Cotyledion tylodes11 since the original report and brief description of Cotyledion6,12. All available material from two localities can unequivocally be referred to the same species. The majority of C. tylodes specimens occur as compressed moulds that replicated the internal or external surface of this animal; some individuals were strongly flattened and preserved as reddish-brown flattened films with exceptionally preserved details of internal anatomy (n = 26).
The body of C. tylodes is shaped like a goblet, composed of an upper cup-like calyx and a lower cylindrical stalk (Fig. 1a–h). The calyx is usually compressed in the fossil state, with an elliptical transverse section (Fig. 2a–d, Fig. S2a, S4a). Calyx length ranges between 4.3 mm and 42 mm (mean 13.3 mm; n = 292), and the maximum width ranges from 3 mm– 23 mm with mean of 8.82 mm (n = 292) (Fig. S1). The ratio of maximum height/width of the calyx ranges from 1.05 to 2.25 (mean 1.61) (Table S1). The interior of the calyx, in a few cases, contains substantial sediment infill, apparently more pronounced in the upper part (Fig. S2a). This sediment infill suggests the interior originally contained a body cavity, probably accommodating the visceral organs, such as the digestive tract.
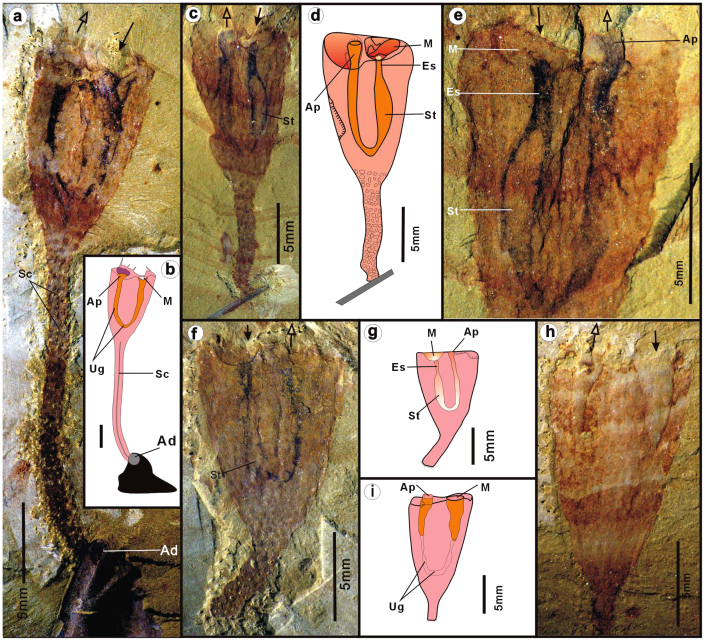
Figure 1. Cotyledion tylodes Luo & Hu 1999 from the Cambrian Stage 3 Chengjiang fauna (Yunnan, China).
The hemispheric buccal cavity with basal mouth (anterior) and elongate anal papillae (posterior) marked with solid arrows and hollow arrows, respectively. (a), ELI-C-0369, a complete flattened specimen attached by long stalk to exoskeleton of trilobite; note the circular attachment disc (Ad); (b), interpretative drawing; (c), ELI-C-0359A, a laterally compressed specimen attached to gena of a trilobite, showing U-shaped gut with 3-D buccal cavity and enlongate anal tube enclosed inside apertural cavity; (d), interpretative drawing; (e), ELI-C-0359A, counterpart of C, close-up view of the calyx showing the U-shaped gut with buccal cavity and anal papillae; (f), ELI-C-0334A, a flattened specimen showing U-shaped digestive tract; (g), compare to f; (h), ELI-C-0338A, showing mud-filled buccal cavity and anal tube; (i), Compare to h; Scale bars are indicated. Abbreviations: Ad, attachment disc; Ap, anal papilla; Bc, buccal cavity; Cc, the interior cavity of calyx; Es, esophagus; In, intestine; M, mouth; Sc, the central cavity of stalk; St, the distended stomach; Ug, the U-shaped gut.
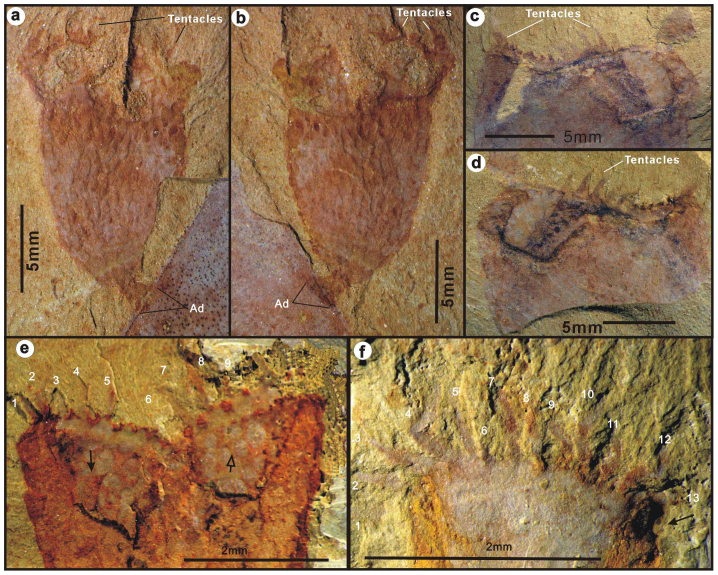
Figure 2. Cotyledion tylodes Luo & Hu 1999 from the Cambrian Stage 3 Chengjiang fauna (Yunnan, China).
(a–b), ELI-C-0340AB, part and counterpart, a small individual attached by a distal attachment disc (Ad) onto a big exoskeleton of unknown affinity; note the oval tentacle crown. (c–d), ELI-C-0358AB, part and couterpart, top view of a compressed and distorted tentacle crown ringed by tentacle stubs. (e), ELI-C-0107A, Internal mould of the banded tentacle crown with dotted impression of tentacles marked with numbers; note the putative buccal cavity (solid arrow) and anal vestibule (hollow arrow); (f), ELI-C-0361A, External mould of a crown of tentacles marked with numbers.
As seen in specimens ELI-C 0359B (Fig. 1c, d), a recurved tube, delineated by two undulating parallel curved black lineations, is interpreted as the U-shaped digestive tract. The U-shaped gut occurs either as a flattened tubular impression with blackish pigment (Fig. 1a–g), or as a partly (Fig. 1h–i) or completely mud-filled tube with some relief (Fig. S2b–d). A distended kidney-shaped region is interpreted to represent the stomach, which makes up the lower 2/3 of the U-shaped gut (Fig. 1c–e). Anterior to the stomach is a dark-stained tube, interpreted as an esophagus, which leads anteriorly into a hemi-spherical chamber filled with sediment (Fig. 1c–d; Fig. S2b,f,g). This is also readily observable in the counterpart specimen ELI-C 0359A (Fig. 1e), where the chamber is interpreted as a funnel-like buccal cavity (Fig. 1c–e). Beyond the bulbous stomach, the digestive canal shrinks to become a short tapering tube; it then recurves, forming a tubular intestine that extends upward reaching the main aperture of the calyx. The digestive canal terminates at a slightly projected anal opening, evidently located in the apertural vestibule (Fig. 1d). The buccal cavity and anal opening are often obscured by in-filled sediment within the apertural vestibule (Fig. 1a–b). In several flattened specimens (Fig. 1f, h), the funnel-like buccal cavity and anal opening form separate units, each of which possesses relief and are in-filled by sediment. The cavity is also encircled by the upper margin of the calyx. The mouth and anus are at opposite sides of the long axis of the suboval vestibule, here regarded as marking the anterior and posterior respectively (Fig. 1a–i).
A circle of tentacles originate from the margin of the calyx, preserved as reddish, flat banded impressions within the fine-grained bedding planes (Fig. 2; Fig. S2e–g). The bases of the tentacles are connected to the calyx margin by an undulating constriction of reddish, indented, membranous band (Fig. 2c–d). Each tentacle originates from an indentation in the membrane and protrudes outwards up to 1.6–3.2 mm in length (Fig. 2e, Fig. S3). We interpret each of these reddish indentations to represent the insertion point of an original tentacle, although only 9 well-preserved tentacles can be recognized with any certainty in Fig. 2e. In one, possibly anterior-posteriorly compressed specimen, with some relief, 13 separate tentacles stretch outward to form a semicircle (Fig. 2f). The tentacles are evenly spaced, with approximately six tentacles per millimeter. The maximum estimated number of tentacles based on available marginal space is 3412 (Fig. S3). The tentacles appear to be parallel-sided, and project straight up from the calyx in most cases (Fig. 2). In a few specimens (Fig. S4a), the tentacles evidently radiate outward away from the calyx aperture. In contrast, in some specimens curved tentacles appear to be retracted, and are converged toward the center of the calyx (Fig. S3c, S4b–c, I; also see Clausen et al. 2010: Fig. 2.3–411). In these specimens, the tentacle membrane bands were apparently tightened resulting in the maximum diameter of the calyx occurring slightly below the top of the apertural margin (Fig. S4b–c).
Figure 4. Reconstruction of Cotyledion tylodes Luo & Hu 1999 in life position.
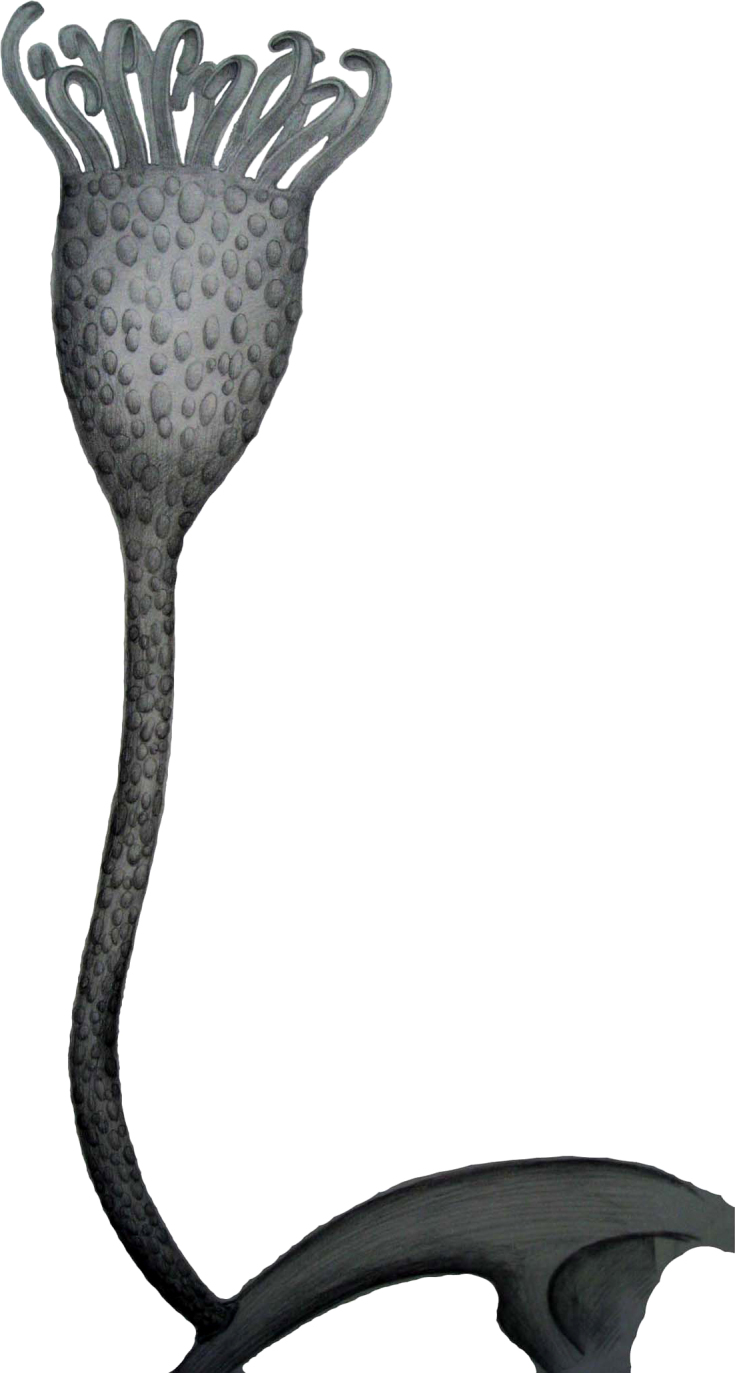
The high variability of calyx height to width ratio is a direct result of differing compaction of this cup-like structure. The ovoid and conical shape is demonstrated clearly by specimens compacted along the direction of the stalk, showing an ovoid tentacle crown (Fig. S4a, c). As seen in specimen ELI-C 0370, the calyx was compressed along the mouth-anus direction, and thus exhibits a parallel-sided tongue-shaped contour (Fig. S4d).
The central base of the calyx connects directly to an extended stalk, the distal end of which is normally attached to exoskeletal debris of other organisms (Fig. 1a, c; Fig. S4d–h). The animals were singly or multiply attached to a common hard substrate provided by other bioclasts (Figs. 1a–d, 2a,b; Fig. S4 d–h). In several specimens, the terminal end is free of bioclasts, revealing a knob-shaped attachment disc (Fig. S4j–k). The length of the stalk varies from 2.7– 38.1 mm, with a diameter between 0.8–3 mm (see: Supplementary Table. S1). The central section of some stalks contains a darkish sinuous lineation (Fig. 1a–b; Fig. S4g, l), here interpreted as a lumen extending from the main calycal cavity.
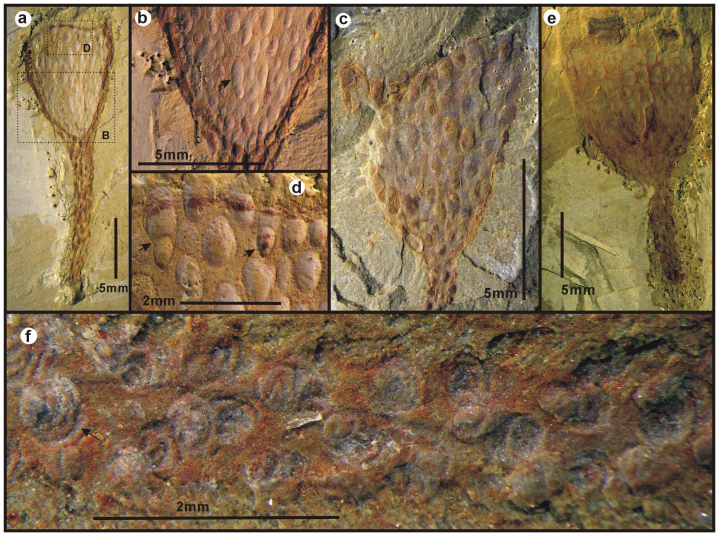
The outer surface of the calyx and stalk is completely covered by loosely-spaced, rounded to elliptical external sclerites that have a quite pronounced relief (Fig. 3a–e). The sclerites are almost invariably composed entirely of pyrite pseudomorphs (Table S2), most commonly framboids (Fig. S5c–f). The length of sclerites is estimated to range from 0.2 mm to 2.8 mm, with a mean of 0.7 mm (n = 769), although most sclerites are strongly deformed and sometimes compressed (Fig. 1a,c,f,h). In general, they are seemingly arranged in longitudinal rows. The number of sclerites in the calyx varies from 39 to 203 in the available specimens. The sclerites in calyx and stalk are comparable in size and width. Arrays of sclerites radiated from the base of calyx, are seemingly in alignment with those on the stalk, where 3 rows of sclerites are usually recognizable (Fig. 3a–c). Sclerite rows evidently increased in number as the conical calyx grew. At least 3 pairs of sclerite rows radiate from the base of the calyx, and 2 mm away from the base new rows of sclerites arise and intervene between the earlier ones (Fig. 3a,b). The largest sclerite (Fig. 3b), probably representing two sclerites merging in the same row, is found in the uppermost part of the calyx (Fig. 3d). In the internal moulds of calices, the sclerites are expressed as loosely spaced shallow imprints (Fig. 3e, Fig. S2e–f, S5a–b). As seen in Fig. 3f, the outer surface of individual sclerites is marked by a series of tightly spaced lineations that we consider to represent growth lines and evidence for accretionary growth. The semicircular growth lines suggest that the sclerites may have grown unidirectionally towards the aperture of the calyx, although this interpretation may require further evidence to be conclusive.
Figure 3. Sclerites on the calyx and stem of the stem entoproct Cotyledion tylodes Luo & Hu 1999 from the Cambrian Chengjiang fauna (Stage 3) of Yunnan, China.
(a), ELI-C-0211A, dashed boxes indicate positions of b and d, note the seemingly increased arrays of sclerites; (b), details of a indicated; note the elongate sclerites marked by an arrow; (c), ELI-C-0344B, an external mould; showing the sclerites in some distinct relief; (d), Enlargement of a indicated, showing the merged two sclerites indicated by two arrows; (e), ELI-C-0186A, an interior view of calyx showing the impressions of sclerites; (f), ELI-C-0069A, close-up view of sclerites on the stalk to show the concentric lamellae.
Discussion
The first description of the enigmatic Cotyledion was based on only two incomplete specimens, and it was accordingly referred to Problematica6 on account of the paucity of characters. Later, an additional well preserved specimen distinctly revealed a circle of tentacles around the upper margin of the calyx12, suggesting an uncertain tentaculate affinity of Cotyledion. Clausen et al.11 re-examined 27 specimens of Cotyledion and concluded that it belonged in the stem group of cnidarians. Our interpretations are based on observations of approximately 400 new specimens collected by the work-team of the Early Life Institute (Prefix: ELI). Well-preserved specimens provide considerable new morphological and anatomical data that form the basis for a major reconsideration of the structure, function and affinities of this peculiar tentaculate animal. The new material shows that Cotyledion was a solitary, sessile and benthic, tentaculate presumed suspension feeder (Fig. 4). Its body consisted of a lower stalk that normally attached (often gregariously) to hard bioclasts, and an upper cup-shaped calyx that contained a U-shaped gut and a tentacle crown that surrounded the mouth and anus (Figs. 1, 2). The presence of a U-shaped digestive tract unequivocally rule out an affinity of Cotyledion with the cnidarians11, but rather points to its inclusion in the sessile lophotrochozoans13,14. In contrast filter-feeding deuterostomes usually have bifurcating tentacles15.
The Lophotrochozoa13,16, represents a clade of remarkably diverse and anatomically disparate spiralian animals, comprising a number of animal phyla, specially Annelida, Mollusca and several sessile tentaculate groups (Bryozoa, Cycliophora, Brachiopoda, Entoprocta, Phoronida)10. Anatomically, the Entoprocta differs markedly from other sessile lophophorates in that both the mouth and anus lie inside the “crown” of tentacles. Not least because of differences in cleavage patterns and body cavities17, the Entoprocta is usually placed outside the Superphylum Lophophorata18. Recently, molecular phylogenies confirm that the entoprocts are monophyletic19 and invariably nest within the Lophotrochozoa, with recent results suggesting they are sister group to the cycliophorans and with weak support for forming the Polyzoa together with the bryozoans14,16,20.
Despite the relatively stable composition of the Lophotrochozoa, the precise internal relationships of the clade, and its morphological origins remains obscure, largely owing to the widely disparate nature of the phyla contained. As a result, reconstruction of the evolution of fossil stem-groups to extant crown groups is a necessary step to understanding this large group's early history. Such information has largely relied on exceptionally preserved fossils, such as from the Burgess Shale and Chengjiang15,21. Exceptionally preserved tentaculate fossils including eldoniids22, Phlogites6 and the recently reported Herpetogaster15, commonly characterized by a coiled intestine and paired bifurcated tentacles that encircle the mouth but not anus, are best referred to primitive deuterostomes rather than any lophotrochozoans15, let alone entoprocts typified by unbranched tentacles surrounding a mouth and anus in a whole5. Although originally compared to entoprocts3,4, the affinities of Dinomischus were later questioned owing to its lack of pliable and foldable tentacles2 and any further information on its interior anatomy5,15,23.
Although the exact phylogenetic placement of Cotyledion is still open to some speculation, the discovery of a U-shaped gut represents a significant progress in the understanding of these enigmatic Cambrian fossils, and demonstrates a bilaterian, lophophorate affinity of Cotyledion with certainty. The morphology (a U-shaped gut with a mouth and anus enclosed within a circle of tentacles) and sessile lifestyle of Cotyledion is compatible with an entoproct affinity1, and accords well with the recent suggestion based on molecular analyses that the entoproct ancestor had a “marine, probably solitary, epizoic adult stage”19. In addition, there is some evidence that the tentacles may have been contractible and could have been retracted into the membranous band where they originate from (Fig. S4a–c), suggesting some degree of retractability of the tentacles that corresponds functionally and structurally to that seen in those of the extant entoprocts1. Other entoproct characters include presence of a slit-like mouth and funnel-like buccal cavity (Fig. 1). By contrast, all extant solitary entoprocts are relatively diminutive, with height of individual zooids ranging from 0.3 to 30 mm and that of calyxes between 0.2 and 1.2 mm1, markedly less than the average calyx (4.3–42 mm) and individual height (8–56 mm) of Cotyledion (Fig. S1, Table S1). In addition, recent entoprocts are pseudocoelomate, with the cavity surrounding the calycal organs and extending into the stalk in-filled by a hydrostatic skeleton of loose mesenchyme cell or narrow primary body cavity1.
It is notable that a significant number of animals that arose in Cambrian explosion have combinations of very unfamiliar morphologies and anatomies21,24, not encountered in extant phyla. Nevertheless, the key utility of fossils in systematics is not supposedly insuperable difference, but shared similarities or homologous characters. Even when weak, such characters provide provisional support for particular stem-group placement of enigmatic taxa such as Cotyledion and assist in reconstruction the sequence of character acquisition in the Cambrian, and indeed can polarize hypothesis of character loss or gain in related derived extant crown groups. Thus, although Cotyledion i) is larger; ii) apparently possesses a more extensive body cavity; iii) has an external skeletal organization, it nevertheless shares features with entoprocts such as the holdfast + stalk + calyx bearing tentacles surrounding both mouth and anus, both linked by a U-shaped gut. Together, these are features possessed only by the extant entoprocts, and - despite the notable differences – suggest a placement of Cotyledion within the entoproct stem-group.
If correctly interpreted, this placement of Cotyledion has several novel implications for lophotrochozoans. Although living entoprocts are markedly smaller in adult size than Cotyledion, the size decrease could be taken as a result of miniaturization that is a widespread phenomenon in animals25. It is well known that miniaturization involves not only small body size, but also consequent and often dramatic effects of extreme size reduction on anatomy, ecology and life history25. Such consequences were well exemplified in Cotyledion. Along the stalk of Cotyledion (Fig. 1a, b; Fig. S4g), there is a central lineation of darkish color suggesting a central canal, interpreted here as an extension of the calycal cavity. Given that recent molecular results place the entoprocts within a clade of coelomates14,16, it is possible that the body cavity of Cotyledion was indeed a coelom. If so, the loss of a coelom may represent an apomorphic character in living entoprocts, since reduction and structural simplification is a common effect of miniaturization25, and an adaptation that may be associated with a change to colonial life. Moreover, the body wall of living entoprocts is very thin, even transparent on the calyx, solely covered by a glycoprotein cuticle with a trace of chitin25. By contrast, the outer body surface of Cotyledion hosts numerous pronounced sclerites that presumably stiffened the calyx or stalk (Fig. 3,4). Despite providing protection from durophagy26, the strongly armoured scleritome of Cotyledion might thus have precluded the organism from performing the flexible “nodding” motions as seen in recent entoprocts. The single-layered epithelial body wall in extant entoprocts likely represents another consequence of size decrease. It is apparent that Cotyledion had a solitary lifestyle and was attached to the exoskeletons of other organisms (Fig. 1a, c; Fig. S4c, e-g). Taking into account the evolutionary changes of the characters discussed above, solitary life could be a plesiomorphic state in contrast to the colonial one of modern entoprocts. The assumption is supported by the younger colonial entoprocts, so far known from the upper Jurassic rocks in Great Britain2. If so, the interpretation of Cotyledion among stem entoprocts demonstrates that a stolon as attachment structure is an apomorphy for the Order Coloniales within the Entoprocta19. The Cambrian (Stage 3) fossils discussed here are, therefore, a very significant extension of the geological range of total group entoprocts, set a minimum time frame for the divergence of entoprocts from other lophotrochozoans, and first shed light on their scleritomous body plan (Fig. 4).
The cuticular sclerites represent the most unique feature of Cotyledion. The discovery that Cotyledion is allied to entoprocts demonstrates that entoprocts developed distinctive armoured bodies early in their history. Comparable to Cotyledion, armoured scleritome-bearing bodyplans were also known from stem groups of annelids27, molluscs21,28,29, brachiopods8 and phoronids9, presumably thought as evidence of a close affinity between lophotrochozoans. Abundant varieties of sclerites commonly found in Cambrian SSF assemblages such as siphogonuchitids, sachitids and halkieriids have been associated with the stem groups of various branches of the lophotrochozoan tree (i.e. Mollusca and Annelida)21,27,28,29, and the phosphatic tommotiids have been placed in the stem groups of the Phoronida9 and Brachiopoda7,8. In terms of morphology, the scleritome of Cotyledion (particulary in the stalk) is most comparable to the tommotiid Eccentrotheca9,30, which has a tubular articulated scleritome composed of highly variable phosphatic sclerites. However, the original mineralogy of the sclerites in Cotyledion cannot be ascertained, and although there is some evidence for accretionary growth, the detailed shape and loose configuration of the scleritome of Cotyledion clearly differs from that of the tightly interlocking and organized configuration of Eccentrotheca. In Eccentrotheca, the sclerites are arranged in transverse, ring-shaped units that are eventually fused during ontogeny9,30. This contrasts sharply with the roughly longitudinal arrangement and universal separation of the sclerites in Cotyledion. The sclerites of Cotyledion (Fig. 3) also differ from those of Eccentrotheca and other tommotiids by the existence of a unidirectional mode of growth (Fig. 3f). Based on these differences, Cotyledion cannot be included in the tommotiids and the structural similarities of the stalk in Cotyledion with the scleritome of Eccentrotheca are superficial. The presence of external sclerites in the stem entoproct Cotyledion as well as in the proposed stem groups of phoronids9, brachiopods7,8, annelids27 and molluscs21,28,29 suggests that sclerites themselves are distributed more widely than thought before, and may even be plesiomorphic for the Lophotrochozoa. Despite the differences in organization, growth and composition, the discovery of Cotyledion as a sclerite-bearing entoproct strengthens the view that the stems of many of lophotrochozoan phyla may be traced within the early Cambrian Small Skeletal Fauna (SSF).
Methods
All the material of Cotyledion was collected from the Yu'anshan Member (Eoredlichia-Wutingaspis Zone) of the upper part of the Lower Cambrian Heilinpu Formation by the team from the Early Life Institute (ELI) of Northwest University, Xi'an, China. The fossils were examined by Z. Zhang with a binocular Olympus Zoom Steromicroscope and photographed with a Nikon camera mounted on a photomicrographic system, with different illuminations for particular views when high contrast images are required. Measurements were directly made by means of a millimeter ruler. The SEM microphotographs of uncoated fossils were taken at Stage Key Laboratory of Continental Dynamics, Northwest University, Xi'an and some assisted by Leonid Popov at National Museum of Wales, Cardiff, UK.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the International Code of Zoological Nomenclature (ICZN). The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix ‘http://zoobank.org/'. The LSID for this publication is: urn:lsid:zoobank.org:pub:4810D25C-02BB-4674-B508-5D5D67DEF9EE, and urn:lsid:zoobank.org:act:51669C19-D7EC-469D-A537-DDA51655E4FA.
Author Contributions
All authors participated in the discussion and analysis of these fossils. Collection of material was made by Chinese members (ELI). Z.F. Zhang designed the study and made statistics of these fossils. Z.F. Zhang, L.E. Holmer, G.A. Brock and C.B. Skovsted prepared the earlier manuscript, and G. Budd corrected the English and improved the final version. Z.F. Zhang, D.J. Fu and J. Han made the artistic reconstruction of Cotyledion.
Supplementary Material
Supplementary information
Acknowledgments
This work is financially supported by National Natural Science Foundation of China (Grant 41072017 and 40830208), the 973 Project (2013CB835002), the Programme of Introducing Talents of Discipline to Universities (P201102007), and the Swedish Research Council (VR to LEH, 2009–4395, 2012–1658), and Australian Research Council Discovery Project (#120104251 to GAB), and the State Key Laboratory of Palaeobiology and Stratigraphy in Nanjing Institute of Geology and Palaeontology, CAS (Grant: 123117, KZCX2-EW-115). Z.F. Zhang also acknowledges financial supports from Ministry of Education of China (FANEDD200936, NCET-11-1046), Postdoctoral Science Foundation (2011M501273), Shaanxi Bureau of Science and Techonology (2011kjxx37, 2011JZ006), and the Fok Ying Tung Education Foundation (G121016). Thanks are also due to J.P. Zhai and M.R. Cheng (Xi'an) for fossil preparation and technical assistance in laboratory, NWU, Xi'an.
References
- Wasson K. A review of the invertebrate phylum Kamptozoa (entoprocta) and synopsis of Kamptozoan diversity in Australia and New Zealand. Transactions of the Royal Society of S. Aust. 126, 20 (2002). [Google Scholar]
- Todd J. A. & Taylor P. D. The first fossil Entoproct. Naturwissenschaften 79, 311–314 (1992). [Google Scholar]
- Chen J. Y. & Zhou G. Q. Biology of the Chengjiang fauna. Bulletin of the National Museum of Natural science 10, 11–106 (1997). [Google Scholar]
- Conway Morris S. A new entoproct-like organism from the Burgess Shale of British Columbia. Paleontology 20, 833–845 (1977). [Google Scholar]
- Nielsen C. Animal evolution-interrelationships of the Living phyla (2nd edition). (Oxford University press, 2001). [Google Scholar]
- Luo H. L., Hu S. X., Chen L. Z., Zhang S. S. & Tao Y. H. Early Cambrian Chengjiang Fauna from Kunming Region, China 129 (Yunnan Science and Technology Press, 1999). [Google Scholar]
- Skovsted C. B. et al. The scleritome of Paterimitra: an Early Cambrian stem group brachiopod from South Australia. P R Soc B 276, 1651–1656 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer L. E., Skovsted C. B., Brock G. A., Valentine J. L. & Paterson J. R. The Early Cambrian tommotiid Micrina, a sessile bivalved stem group brachiopod. Biol Letters 4, 724–728 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovsted C. B., Brock G. A., Paterson J. R., Holmer L. E. & Budd G. E. The scleritome of Eccentrotheca from the Lower Cambrian of South Australia: Lophophorate affinities and implications for tommotiid phylogeny. Geology 36, 171–174 (2008). [Google Scholar]
- Erwin D. H. et al. The Cambrian Conundrum: Early Divergence and Later Ecological Success in the Early History of Animals. Science 334, 1091–1097 (2011). [DOI] [PubMed] [Google Scholar]
- Clausen S., Hou X.-G., Bergström J. & Franzén C. The absence of echinoderms from the Lower Cambrian Chengjiang fauna of China: Palaeoecological and palaeogeographical implications. Palaeogeography, Palaeoclimatology, Palaeoecology 294, 133–141 (2010). [Google Scholar]
- Zhang X. L., Shu D. G., Li Y. & Han J. New sites of Chengjiang fossils: crucial windows on the Cambrian explosion. J. geol. Soc., Lond 158, 211–218 (2001). [Google Scholar]
- Paps J., Baguna J. & Riutort M. Lophotrochozoa internal phylogeny: new insights from an up-to-date analysis of nuclear ribosomal genes. P R Soc B 276, 1245–1254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribet G. Assembling the lophotrochozoan ( = spiralian) tree of life. Philos T R Soc B 363, 1513–1522 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. B., Morris S. C. & Shu D. G. Tentaculate Fossils from the Cambrian of Canada (British Columbia) and China (Yunnan) Interpreted as Primitive Deuterostomes. Plos One 5, A189–A201 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. W. et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–U745 (2008). [DOI] [PubMed] [Google Scholar]
- Wanninger A. Shaping the Things to Come: Ontogeny of Lophotrochozoan Neuromuscular Systems and the Tetraneuralia Concept. Biol Bull-Us 216, 293–306 (2009). [DOI] [PubMed] [Google Scholar]
- Nielsen C. The phylogenetic position of Entoprocta, Ectoprocta, Phoronida, and Brachiopoda. Integr Comp Biol 42, 685–691 (2002). [DOI] [PubMed] [Google Scholar]
- Fuchs J., Iseto T., Hirose M., Sundberg P. & Obst M. The first internal molecular phylogeny of the animal phylum Entoprocta (Kamptozoa). Mol Phylogenet Evol 56, 370–379 (2010). [DOI] [PubMed] [Google Scholar]
- Edgecombe G. D. et al. Higher-level metazoan relationships: recent progress and remaining questions. Org Divers Evol 11, 151–172 (2011). [Google Scholar]
- Conway Morris S. & Caron J. B. Halwaxiids and the early evolution of the lophotrochozoans. Science 315, 1255–1258 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu M. Y., Zhao Y. L. & Chen J. Y. Revision of the Cambrian discoidal animals Stellostomites eumorphus and Pararotadiscus guizhouensis from South China. Geobios-Lyon 35, 165–185 (2002). [Google Scholar]
- Peng J., Zhao Y. L. & Lin J. P. Dinomischus from the Middle Cambrian Kaili Biota, Guizhou, China. Acta Geol Sin-Engl 80, 498–501 (2006). [Google Scholar]
- Shu D. G., Morris S. C., Zhang Z. F. & Han J. The earliest history of the deuterostomes: the importance of the Chengjiang Fossil-Lagerstatte. P R Soc B 277, 165–174 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken J. & Wake D. B. Miniaturization of Body-Size - Organismal Consequences and Evolutionary Significance. Annu Rev Ecol Syst 24, 501–519 (1993). [Google Scholar]
- Zhang Z. F. et al. First record of repaired durophagous shell damages in Early Cambrian lingulate brachiopods with preserved pedicles. Palaeogeography, Palaeoclimatology, Palaeoecology 302, 206–212 (2011). [Google Scholar]
- Vinther J., Van Roy P. & Briggs D. E. G. Machaeridians are Palaeozoic armoured annelids. Nature 451, 185–188 (2008). [DOI] [PubMed] [Google Scholar]
- Conway Morris S. & Peel J. S. Articulated Halkieriids from the Lower Cambrian of North Greenland and Their Role in Early Protostome Evolution. Philos T Roy Soc B 347, 305–358 (1995). [Google Scholar]
- Vinther J. & Nielsen C. The Early Cambrian Halkieria is a mollusc. Zool Scr 34, 81–89 (2005). [Google Scholar]
- Skovsted C. B., Brock G. A., Topper T. P., Paterson J. R. & Holmer L. E. Scleritome Construction, Biofacies, Biostratigraphy and Systematics of the Tommotiid Eccentrotheca Helenia sp. nov from the Early Cambrian of South Australia. Palaeontology 54, 253–286 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information