Abstract
There is a paucity of information on the utilization patterns of liver transplantation (LT) for HIV-positive individuals. The aim of this study is to examine the trends in LT of HIV patients in the US. This study was a retrospective analysis using the UNOS database (1999–2008). There were 135 HIV-positive patients. There was a steady increase in the number of LT recipients over time as well as regional variation. Ethnic minorities accounted for 33.3% and there was no ethnic difference in survival. Though LT for HIV-positive patients is on the rise, significant variations exist in patient demographics, geographic location, and insurance payer.
Keywords: HIV, Liver transplantation, Disparity, Survival
Introduction
Since the introduction of highly active antiretroviral therapy (HAART) in 1996, the life expectancy of individuals infected with the human immunodeficiency virus (HIV) has dramatically improved. Consequently, deaths due to AIDS-related opportunistic infections have decreased, while morbidity and mortality due to end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) has increased [1]. The most common etiology of ESLD in HIV-positive patients is HBV or HCV co-infection, and compared to the mono-infected individual (HCV or HBV alone), reports from natural history studies suggest a significantly shorter duration of survival following hepatic decompensation [2].
Liver transplantation (LT) has been widely accepted as the therapeutic option of choice for individuals with ESLD and HCC. In the HAART era, there is a growing experience with liver transplantation in the HIV-positive patient. Many no longer consider this life-saving procedure a contraindication. Furthermore, recent reports from several studies in Europe and North America have consistently shown a reasonable patient and graft survival of LT recipients with HIV infection [3–6]. The aim of this study is to examine the recent trends in liver transplantation of HIV-infected patients in the US based upon an analysis of the UNOS registry. We focused our analysis on evaluation of the breadth of transplantation and the demographics of HIV transplanted patients compared to all transplanted individuals including characteristics that might influence transplant availability and success.
Methods
Using the UNOS database, we first identified all adults (≥18 years) who received deceased donor liver transplant between 1999 and 2008. Patients who received multi-organ transplantation or re-transplantation were excluded. The data collected included demographics, insurance payer, liver diagnosis, survival information, and UNOS regions. Insurance payer was categorized as private and non-private (i.e., government) insurance. The non-private group was further divided into Medicaid and non-Medicaid Insurance. The race and ethnicity of the study population was categorized based on the classification used in the UNOS database. Race and ethnic group are captured in the UNOS database as ‘‘ethnicity’’ and categorized into the following: white, black, Hispanic, Asian, Pacific Islander and American Indian/Alaska Native. The underlying liver disease was categorized into (1) hepatitis B, (2) hepatitis C, (3) hepatocellular carcinoma (HCC) and (4) others. No data was available on recipients with dual HCV-HCC or HBV-HCC.
The results are reported as medians and ranges for continuous variables and as proportions (%) for categorical variables. Univariate t test was used to identify variables that differed significantly between the HIV and non-HIV groups. The Cochran-Armitage tests and χ2 test was used to evaluate temporal trends in transplantation over the years. The Kaplan–Meier method was used to calculate overall survival and logrank test to compare survival. All statistical analysis was performed using SAS version 9.1 (Statistical Analysis Software; SAS Institute, Cary, NC). P values <0.05 were considered statistically significant in all cases.
Results
Study Population
A total of 48,334 patients received liver transplantation during the study period. Of these, 135 were documented as HIV-positive in the UNOS registry. The demographics and clinical characteristics of the study population are shown in Table 1. There were significant differences in age, gender, ethnicity, and underlying liver disease between both groups (HIV and non-HIV). The HIV-positive group was younger and had a higher proportion of male recipients. In addition, there was a significant ethnic variation with a notable higher proportion of African Americans in the HIV group. Though a significant difference in underlying liver disease was observed in both groups, the lack of data on LT recipients with dual diagnosis (HBV-HCC or HCV-HCC) limits our interpretation of this finding.
Table 1.
Demographics and clinical characteristics of the study population
| Characteristic | Variable | HIV | Non-HIV | Total | p value |
|---|---|---|---|---|---|
| Age | n | 135 | 48,199 | 48,334 | <0.0001 |
| Mean ± SD | 47.6 ± 8.54 | 52.0 ± 10.28 | 51.9 ± 10.28 | ||
| Range | 23.0–70.0 | 18.0–84.0 | 18.0–84.0 | ||
| Median | 48.0 | 53.0 | 53.0 | ||
| Gender | Female | 26 (19.3%) | 16,337 (33.9%) | 16,363 (33.9%) | 0.0003 |
| Male | 109 (80.7%) | 31,862 (66.1%) | 31,971 (66.1%) | ||
| Ethnicity | Asian | 1 (0.7%) | 2,038 (4.2%) | 2,039 (4.2%) | <0.0001 |
| Black | 29 (21.5%) | 4,012 (8.3%) | 4,041 (8.4%) | ||
| Hispanic | 15 (11.1%) | 5,862 (12.2%) | 5,877 (12.2%) | ||
| Other | 0 (0.0%) | 498 (1.0%) | 498 (1.0%) | ||
| White | 90 (66.7%) | 35,789 (74.3%) | 35,879 (74.2%) | ||
| Diagnosis group | HBV | 14 (10.4%) | 1,414 (2.9%) | 1,428 (3.0%) | <0.0001 |
| HCC | 17 (12.6%) | 4,871 (10.1%) | 4,888 (10.1%) | ||
| HCV | 59 (43.7%) | 16,230 (33.7%) | 16,289 (33.7%) | ||
| Other | 45 (33.3%) | 25,684 (53.3%) | 25,729 (53.2%) |
HIV-Positive Liver Transplant Recipients
Transplantation trends during the study period for the HIV-positive patients are illustrated in Fig. 1. There was a steady increase in the number of LT recipients with HIV over time with the exception of a notable decline in 2008. The exact reason for the decrease in the number of LT recipients with HIV during the 2008 period is unknown, but a downward trend was also observed in the non-HIV group. Figure 2 shows the wide variation in geographic location (UNOS region) of the HIV-positive LT recipients during the study period. Though center-specific data was not available for analysis, we found approximately a sevenfold increase in the number of states with centers performing LT in HIV-positive patients during the study period (4–27 US states). The 1-, 3-, and 5-year patient and graft survival for the HIV-positive patients was 79, 65, 53 and 72, 57 and 53%, respectively. With stratification based on underlying liver diagnosis (HBV, HCV, HCC, and others), there was no significant difference in patient and graft survival (p = 0.22 and 0.13, respectively). Furthermore, there were no ethnic differences in patient and graft survival.
Fig 1.
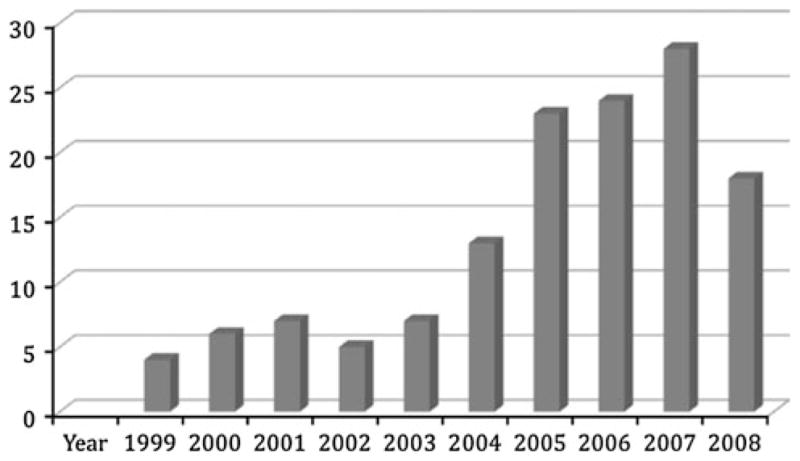
Liver transplantation trends for HIV-positive recipients (1999–2008) (p ≤ 0.001)
Fig 2.
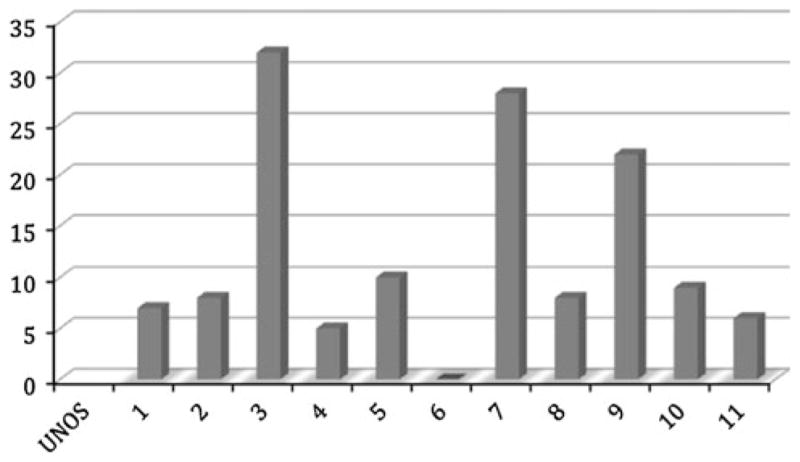
Liver transplantation trends for HIV based on UNOS regions (p ≤ 0.001)
In our study cohort, ethnic minorities accounted for 33.3% (n = 45) of the LT recipients with HIV infection. Among the ethnic minorities, African Americans represent 64.4% in contrast to 34.4% in the non-HIV group. Table 2 shows the demographics and clinical characteristics of the HIV-positive LT recipients. Though there were no differences in gender and underlying liver disease among ethnic groups, a significant difference in age and insurance payer was observed (p = 0.008).
Table 2.
Demographics and clinical characteristics of HIV-positive recipients
| Variable | Black | Hispanic | White | Total | P value | |
|---|---|---|---|---|---|---|
| Age | N | 29 | 15 | 90 | 134 | 0.008 |
| Mean ± SD | 51.2 ± 8.88 | 49.9 ± 7.17 | 46.1 ± 8.32 | 47.6 ± 8.57 | ||
| Range | 23.0–67.0 | 41.0–63.0 | 24.0–70.0 | 23.0–70.0 | ||
| Median | 51.0 | 51.0 | 47.0 | 48.5 | ||
| Gender | Female | 10 (52.6%) | 4 (26.7%) | 12 (13.3%) | 26 (19.4%) | 0.0333 |
| Male | 19 (47.4%) | 11 (73.3%) | 78 (86.7%) | 108 (80.6%) | ||
| Diagnosis group | HBV | 0 (0.0%) | 2 (13.3%) | 12 (13.3%) | 14 (10.4%) | 0.1785 |
| HCC | 3 (10.4%) | 3 (20%) | 11 (12.2%) | 17 (12.7%) | ||
| HCV | 13 (44.8%) | 4 (26.7%) | 41 (45.5%) | 58 (43.3%) | ||
| Other | 13 (44.8%) | 6 (40%) | 26 (29%) | 45 (33.6%) | ||
| Insurance | Private | 9 (31%) | 7 (47%) | 43(48%) | 59 (44%) | 0.016 |
| Medicaid | 12(41%) | 5 (33%) | 15(17%) | 32 (24%) | ||
| Non-Medicaid | 7 (24%) | 3 (20%) | 29(32%) | 39 (29%) | ||
| Other | 1(4%) | – | 3(3%) | 4 (3%) |
Discussion
In the US, the use of liver transplantation as a therapeutic option for the HIV-infected individual with ESLD has evolved from an absolute contraindication to an indication for those that meet the commonly accepted eligibility criteria of a CD4 count ≥100 cells/mm3 with undetectable plasma HIV RNA and a stable HAART regimen [7]. Despite recent reports of excellent post-transplant survival in HIV-positive recipients, very few ESLD patients with HIV (ESLD-HIV) have received liver transplantation.
In the present study, only 0.3% (n = 135) of the patients who received LT in the past 10 years (1999–2008) were documented as being HIV-positive. The reason for the discordance between recipients with HIV and the ESLD-HIV patients in need of LT is unclear. According to the recent Centers for Disease Control and Prevention estimates, there are about 1.2 million individuals in the US living with HIV. While the exact prevalence of HCV/HIV and HBV/HIV coinfection is unknown, various estimates suggest that more than 250,000 have HCV/HIV coinfection and more than 150,000 have HBV coinfection [8, 9]. Furthermore, the estimated prevalence of advanced fibrosis and cirrhosis in this cohort is 20–30%, with a much higher likelihood of progression to HCC in comparison to the non-HIV population [10–14]. With the reported rapid progression of fibrosis and higher rate of hepatic decompensation in this population, the number of HIV-positive LT recipients in the US probably likely represents a small proportion of those in need [15]. The factors contributing to this underrepresentation have not yet been explored, but may be related to a referral bias from HIV caregivers to transplant centers, a transplant workup bias, or a failure of patients to survive long enough to get to transplant centers.
Disparities in access to transplantation have been described within the entire transplant cohort. Inequitable access to organ transplant centers has been attributed to several factors including delayed referral, socioeconomic status, type of insurance payer (public or private), and geographic location [16–21]. Although this study was not designed to address accessibility to transplantation in the HIV population, we found significant variations in insurance status and geographic location of the LT recipients. In the non-HIV transplant population, non-private insurance has been associated with delayed referral for transplantation and lower likelihood of wait-listing or receipt of organ transplant [17, 22–24]. In our study cohort, a higher proportion of the recipients had non-private insurance (56%) compared to reports from the non-HIV transplant population (~28%) [17, 25]. It is uncertain if non-private insurance poses a similar barrier to LT in ESLD-HIV as has been described in the non-HIV population. In a recent study by Heslin et al. [26], the investigators report that in contrast to non-private insurance, those with private insurance have a higher likelihood of access to physicians with HIV expertise. It is not known if provision of care by an infectious disease specialist directly improves access to transplantation in those with ESLD-HIV. Therefore, studies to further examine the association between type of insurance and access to LT in ESLD-HIV will greatly improve our understanding of this complex interaction.
The exact reason for the observed differences in geographic location of LT recipients with HIV in our study is unknown. Barriers at the organ transplant center can indirectly contribute to geographic variation. At the institutional level, the lack of an LT center with expertise in HIV care and/or participation in the US-based multi-site HIVTR study may impede accessibility at different geographic locations [27]. Similarly, at the provider-level, variations in determination of LT eligibility for ESLD-HIV could influence accessibility at different geographic locations. Published surveys targeting program directors, HIV specialists, and transplant surgeons report a wide variation (33–50%) in the acceptance of ESLD-HIV as appropriate transplant candidates [28–30]. The geographic impact of the reported variations in practice and policies at LT centers is unknown. Identification of the factors contributing to geographic variation in LT accessibility for ESLD-HIV will provide useful insights into the obstacles that are unique to this population.
The role of patient-specific characteristics such as age, gender, and ethnicity on accessibility to LT has been extensively studied in the non-HIV population [31–33]. In a recent study by Moylan et al. [32], the investigators report female candidates as being less likely to receive LT in the MELD era. We show a similar disparity in LT recipients among HIV-positive females in our study cohort. With the recent reports from epidemiologic data showing a rise in the incidence of new HIV infections among females, particularly of African American ethnicity and a trend towards increasing liver-related deaths among women, studies to elucidate the specific barriers encountered in this group are necessary [34–36]. Consistent with reports from non-transplant settings, several studies have documented ethnic disparities in access, utilization and survival in LT population [33, 37, 38]. However, there is limited information on ethnicity and LT in the HIV population. Though there were no ethnic differences in age, gender, or liver disease among the HIV-positive LT recipients, there was a significant difference in insurance payer. African Americans were less likely to be covered by private insurance compared to the other ethnic groups. In particular they had a higher proportion of Medicaid coverage when compared to others. Despite this difference in insurance coverage, African Americans were not under-represented among the HIV LT recipients as opposed to the non-HIV LT population. Since African Americans are disproportionately affected with HIV, our findings may be a reflection of the current epidemiologic trend. Regarding post-transplant outcome, though there was a trend towards a decreased survival among African Americans, this was not statistically different. Future studies to determine if the reported lower post-LT survival among African Americans in the non-HIV population exists in recipients with HIV infection will provide invaluable information to the transplant community [38, 39].
Other patient-related factors not addressed in this study include the restrictive eligibility criteria and waiting list mortality for the ESLD-HIV population. In the HIV Organ Sharing and Transplantation (HOST) study, only 18.2% of the UNOS status 2A/B candidates with ESLD-HIV were eligible for LT based on an inclusion criteria of a CD4 count ≥200 cells/mm3 with undetectable plasma HIV RNA [40]. Though the CD4 level in the latter study was higher than the traditional cut-off (≥100 cells/mm3) used in US transplant centers, the findings provide insight into the viral-related barriers to wait list addition. Another barrier contributing to the inequitable LT access, which was not addressed in this study, is the reported higher waiting-list mortality observed in ESLD-HIV compared to the non-HIV population (36.2% vs. 15.5%) [41].
The primary limitations of this study are related to the retrospective nature and circumscribed reporting characteristics of the UNOS database. Despite these limitations, this study provides insight into the trends and patterns of LT utilization for the ESLD-HIV population in the US. The observed variation in patient demographics and geographic location identifies specific areas for future implementation of strategies to improve accessibility and availability of LT, in anticipation of the expected burden of liver disease in this population. Furthermore, with the higher liver-related mortality in the ESLD-HIV population and recent reports of improved post-LT survival in those with and without concomitant HCC, it is imperative for the transplant community to provide a uniform guideline on criteria for transplant eligibility [42, 43]. The guidelines for transplant candidacy in the HIV population differs by transplant centers and also by respective countries. Though transplant programs mandate an undetectable HIV viral load, they differ in the requirement for CD4 levels and opportunistic infections. The US transplant centers primarily reflect the criteria based on the HIVTR study (CD4 count ≥100 or ≥200 cells/mm3 in those with a history of opportunistic infections) [7]. This differs from the Spanish group, which excludes candidates with a history of opportunistic infections and uses a cut-off CD4 count of ≥100 cells/mm3, while the British HIV Association recommends a CD4 count ≥200 or ≥100 cells/mm3 in the presence of portal hypertension in contrast to the Italian criteria of a CD4 count ≥200 cells/mm3 in candidates who have never been on ART therapy or ≥100 cells/mm3 in patients without hepatic decompensation or those that are intolerant to ART therapy [44–47]. This variation in inclusion criteria impairs access to transplantation and makes it difficult to compare LT outcomes across geographic locations. Despite the issues highlighted above, the past decade has witnessed an increase in the use of liver transplantation as a modality for management of end-stage liver disease and HCC in HIV-infected individuals. Many key questions will potentially be answered when data from prospective trials of HIV transplantation in the US and Europe are reported in full.
Acknowledgments
Dr. Kenneth E. Sherman is supported by a National Institute of Diabetes, Digestive and Kidney Diseases Mid-career Investigator award in Patient-Oriented Research (K24 DK070528).
Contributor Information
Nyingi M. Kemmer, Email: Nyingi.Kemmer@uc.edu, University of Cincinnati Academic Health Center, 231 Albert Sabin Way, MSB Room 6363, Cincinnati, OH 45267-0595, USA, Cincinnati VA Medical Center, Cincinnati, OH, USA
Kenneth E. Sherman, University of Cincinnati Academic Health Center, 231 Albert Sabin Way, MSB Room 6363, Cincinnati, OH 45267-0595, USA
References
- 1.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 2.Pineda JA, Romero-Gomez M, Diaz-Garcia F, et al. HIV coinfection shortens the survival of patients with hepatitis c virus-related decompensated cirrhosis. Hepatology. 2005;41:779–789. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 3.Mindikoglu AL, Regev A, Magder LS. Impact of human immunodeficiency virus on survival after liver transplantation: analysis of United Network of Organ Sharing database. Transplantation. 2008;85:359–368. doi: 10.1097/TP.0b013e3181605fda. [DOI] [PubMed] [Google Scholar]
- 4.Norris S, Taylor C, Muiesan P, et al. Outcomes of liver transplantation in HIV-infected individuals: the impact of HCV and HBV infection. Liver Transpl. 2004;10:1271–1278. doi: 10.1002/lt.20233. [DOI] [PubMed] [Google Scholar]
- 5.Duclos-Vallee JC, Teicher E, Vittecoq D, Samuel D. Liver transplantation for patients infected with both HIV and HCV or HIV and HBV. Med Sci (Paris) 2007;23:723–728. doi: 10.1051/medsci/20072389723. [DOI] [PubMed] [Google Scholar]
- 6.Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. Am J Transpl. 2008;8:355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 7.Stock PG, Roland ME. Evolving clinical strategies for transplantation in the HIV-positive recipient. Transplantation. 2007;84:563–571. doi: 10.1097/01.tp.0000279190.96029.77. [DOI] [PubMed] [Google Scholar]
- 8.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Sherman KE, Peters M, Koziel MJ. HIV and liver disease forum: conference proceedings. Hepatology. 2007;45:1566–1577. doi: 10.1002/hep.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004;164:2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 11.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 12.Castellares C, Barreiro P, Martin-Carbonero L, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat. 2008;15:165–172. doi: 10.1111/j.1365-2893.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Torres M, Govindarajan S, Diago M, et al. Hepatic steatosis in patients with chronic hepatitis C virus genotype 2 or 3 does not affect viral response in patients treated with peginterferon alpha-2a (40 kD) (PEGASYS) plus ribavirin (COPEGUS) for 16 or 24 weeks. Liver Int. 2009;29:237–241. doi: 10.1111/j.1478-3231.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Carbonero L, de Ledinghen V, Moreno A, et al. Liver fibrosis in patients with chronic hepatitis C and persistently normal liver enzymes: influence of HIV infection. J Viral Hepat. 2009;16:790–795. doi: 10.1111/j.1365-2893.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 16.Yoo HY, Galabova V, Edwin D, Thuluvath PJ. Socioeconomic status does not affect the outcome of liver transplantation. Liver Transpl. 2002;8:1133–1137. doi: 10.1053/jlts.2002.37000. [DOI] [PubMed] [Google Scholar]
- 17.Kemmer N, Zacharias V, Kaiser TE, Neff GW. Access to liver transplantation in the meld era: role of ethnicity and insurance. Dig Dis Sci. 2008;54:1794–1797. doi: 10.1007/s10620-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 18.Thamer M, Henderson SC, Ray NF, Rinehart CS, Greer JW, Danovitch GM. Unequal access to cadaveric kidney transplantation in California based on insurance status. Health Serv Res. 1999;34:879–900. [PMC free article] [PubMed] [Google Scholar]
- 19.Winkelmayer WC, Glynn RJ, Levin R, Owen W, Jr, Avorn J. Late referral and modality choice in end-stage renal disease. Kidney Int. 2001;60:1547–1554. doi: 10.1046/j.1523-1755.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 20.Cass A, Cunningham J, Snelling P, Ayanian JZ. Late referral to a nephrologist reduces access to renal transplantation. Am J Kidney Dis. 2003;42:1043–1049. doi: 10.1016/j.ajkd.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Kemmer N, Safdar K, Kaiser T, Zacharias V, Neff GW. Impact of geographic location on access to liver transplantation among ethnic minorities. Transplantation. 2008;85:166–170. doi: 10.1097/TP.0b013e31816223f8. [DOI] [PubMed] [Google Scholar]
- 22.Muir AJ, Sanders LL, Heneghan MA, Kuo PC, Wilkinson WE, Provenzale D. An examination of factors predicting prioritization for liver transplantation. Liver Transpl. 2002;8:957–961. doi: 10.1053/jlts.2002.35545. [DOI] [PubMed] [Google Scholar]
- 23.McCormick PA, O’Rourke M, Carey D, Laffoy M. Ability to pay and geographical proximity influence access to liver transplantation even in a system with universal access. Liver Transpl. 2004;10:1422–1427. doi: 10.1002/lt.20276. [DOI] [PubMed] [Google Scholar]
- 24.Gill JS, Hussain S, Rose C, Hariharan S, Tonelli M. Access to kidney transplantation among patients insured by the United States department of veterans affairs. J Am Soc Nephrol. 2007;18:2592–2599. doi: 10.1681/ASN.2007010050. [DOI] [PubMed] [Google Scholar]
- 25.Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl. 2004;10:235–243. doi: 10.1002/lt.20069. [DOI] [PubMed] [Google Scholar]
- 26.Heslin KC, Andersen RM, Ettner SL, Cunningham WE. Racial and ethnic disparities in access to physicians with HIV-related expertise. J Gen Intern Med. 2005;20:283–289. doi: 10.1111/j.1525-1497.2005.40109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.https://secure.emmes.com/emmesweb/projects/hivtr: HIVTR study.
- 28.Kroeker KI, Bain VG, Shaw-Stiffel T, Fong TL, Yoshida EM. Adult liver transplant survey: policies towards eligibility criteria in Canada and the United States 2007. Liver Int. 2008;28:1250–1255. doi: 10.1111/j.1478-3231.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 29.Cooper CL, DeForest J, Gill J, Lalonde R. Barriers preventing liver transplantation in Canadians with HIV-infection–perceptions of HIV specialists. Can J Gastroenterol. 2007;21:179–182. doi: 10.1155/2007/769752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern SD, Asch DA, Shaked A, Stock PG, Blumberg E. Determinants of transplant surgeons’ willingness to provide organs to patients infected with HBV, HCV or HIV. Am J Transpl. 2005;5:1319–1325. doi: 10.1111/j.1600-6143.2005.00812.x. [DOI] [PubMed] [Google Scholar]
- 31.Kemmer N, Safdar K, Kaiser TE, Zacharias V, Neff GW. Liver transplantation trends for older recipients: regional and ethnic variations. Transplantation. 2008;86:104–107. doi: 10.1097/TP.0b013e318176b4c1. [DOI] [PubMed] [Google Scholar]
- 32.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the meld score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid AE, Resnick M, Chang Y, Buerstatte N, Weissman JS. Disparity in use of orthotopic liver transplantation among blacks and whites. Liver Transpl. 2004;10:834–841. doi: 10.1002/lt.20174. [DOI] [PubMed] [Google Scholar]
- 34.French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmore SK, Satcher AJ, Hu S. Epidemiology of HIV/aids among non-Hispanic black women in the United States. J Natl Med Assoc. 2005;97:19S–24S. [PMC free article] [PubMed] [Google Scholar]
- 36.Arya M, Behforouz HL, Viswanath K. African American women and HIV/aids: a national call for targeted health communication strategies to address a disparity. AIDS Read. 2009;19:79–84. [PMC free article] [PubMed] [Google Scholar]
- 37.Smith AK, Ladner D, McCarthy EP. Racial/ethnic disparities in liver transplant surgery and hospice use: parallels, differences, and unanswered questions. Am J Hosp Palliat Care. 2008;25:285–291. doi: 10.1177/1049909108315914. [DOI] [PubMed] [Google Scholar]
- 38.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002;359:287–293. doi: 10.1016/S0140-6736(02)07494-9. [DOI] [PubMed] [Google Scholar]
- 39.Ananthakrishnan AN, Saeian K. Racial differences in liver transplantation outcomes in the meld era. Am J Gastroenterol. 2008;103:901–910. doi: 10.1111/j.1572-0241.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 40.Costigliola P, Tumietto F, Zagnoli A, Chiodo F. Need for liver transplant in HIV-positive patients: first results of a specific survey in Italy, project host. AIDS. 2003;17:2119–2121. doi: 10.1097/01.aids.0000088180.01779.9b. [DOI] [PubMed] [Google Scholar]
- 41.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretransplant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver Transpl. 2005;11:1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 42.Vibert E, Duclos-Vallee JC, Ghigna MR, et al. Liver transplantation for hepatocellular carcinoma: the impact of human immunodeficiency virus infection. Hepatology. 2011;53:475–482. doi: 10.1002/hep.24062. [DOI] [PubMed] [Google Scholar]
- 43.Baccarani U, Adani GL, Tavio M, Viale P. Liver transplantation for hepatocellular carcinoma: the impact of human immunodeficiency virus infection–21 plus 13. Hepatology. 2011;53:2138. doi: 10.1002/hep.24287. author reply 2138–2139. [DOI] [PubMed] [Google Scholar]
- 44.Miro JM, Torre-Cisnero J, Moreno A, et al. GESIDA/GESITRASEIMC, PNS and ONT consensus document on solid organ transplant (SOT) in HIV-infected patients in Spain (March, 2005) Enferm Infecc Microbiol Clin. 2005;23:353–362. doi: 10.1157/13076175. [DOI] [PubMed] [Google Scholar]
- 45.O’Grady J, Taylor C, Brook G. Guidelines for liver transplantation in patients with HIV infection (2005) HIV Med. 2005;6:149–153. doi: 10.1111/j.1468-1293.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- 46.Baccarani U, Adani GL, Bragantini F, et al. Long-term outcomes of orthotopic liver transplantation in human immunodeficiency virus-infected patients and comparison with human immunodeficiency virus-negative cases. Transpl Proc. 2011;43:1119–1122. doi: 10.1016/j.transproceed.2011.01.124. [DOI] [PubMed] [Google Scholar]
- 47.Di Benedetto F, Tarantino G, De Ruvo N, et al. University of Modena experience in HIV-positive patients undergoing liver transplantation. Transpl Proc. 2011;43:1114–1118. doi: 10.1016/j.transproceed.2011.03.017. [DOI] [PubMed] [Google Scholar]


