Abstract
The state-of-the-art of biocomposites and hybrid biomaterials based on calcium orthophosphates that are suitable for biomedical applications is presented in this review. Since these types of biomaterials offer many significant and exciting possibilities for hard tissue regeneration, this subject belongs to a rapidly expanding area of biomedical research. Through successful combinations of the desired properties of matrix materials with those of fillers (in such systems, calcium orthophosphates might play either role), innovative bone graft biomaterials can be designed. Various types of biocomposites and hybrid biomaterials based on calcium orthophosphates, either those already in use or being investigated for biomedical applications, are extensively discussed. Many different formulations, in terms of the material constituents, fabrication technologies, structural and bioactive properties as well as both in vitro and in vivo characteristics, have already been proposed. Among the others, the nanostructurally controlled biocomposites, those containing nanodimensional compounds, biomimetically fabricated formulations with collagen, chitin and/or gelatin as well as various functionally graded structures seem to be the most promising candidates for clinical applications. The specific advantages of using biocomposites and hybrid biomaterials based on calcium orthophosphates in the selected applications are highlighted. As the way from the laboratory to the hospital is a long one, and the prospective biomedical candidates have to meet many different necessities, this review also examines the critical issues and scientific challenges that require further research and development.
Keywords: biocomposites, biomedical applications, bone grafts, calcium orthophosphates, hybrid biomaterials, hydroxyapatite, tissue engineering
Introduction
The fracture of bones due to various traumas or natural aging is a typical type of a tissue failure. An operative treatment frequently requires implantation of a temporary or a permanent prosthesis, which is still a challenge for orthopedic surgeons, especially in the case of large bone defects. A rapidly aging population and serious drawbacks to using natural bone grafts make the situation even worse; therefore, there is a high clinical demand for bone substitutes. Unfortunately, medical application of xenografts (e.g., bovine bone) is generally associated with potential viral infections. In addition, xenografts have a low osteogenicity, an increased immunogenicity and usually resorb more rapidly than autogenous bone. Similar limitations are also valid for human allografts (i.e., tissue transplantation between individuals of the same species but of nonidentical genetic composition), where the concerns about potential risks of transmitting tumor cells, a variety of bacterial and viral infections as well as immunological and blood group incompatibility are even stronger.1-3 Moreover, harvesting and conservation of allografts (exogenous bones) are additional limiting factors. Autografts (endogenous bones) are still the “golden standard” among any substitution materials, because they are osteogenic, osteoinductive, osteoconductive, completely biocompatible, non-toxic and do not cause any immunological problems (non-allergenic). They contain viable osteogenic cells, bone matrix proteins and support bone growth. Usually, autografts are well accepted by the body and rapidly integrate into the surrounding bone tissues. For these reasons, they are routinely used for a long period with good clinical results,3-6 and it is fair to say that complications mostly arose in the past.7,8 Unfortunately, a limited number of donor sites restrict the quantity of autografts harvested from the iliac crest or other locations of the patient’s own body. In addition, their medical application always involves additional traumas and scars resulting from the extraction of a donor tissue during a superfluous surgical operation, which requires further healing at the donation site and can involve long-term postoperative pain.1,8-11 Thus, any types of biologically derived transplants appear to be imperfect solutions, mainly due to a restricted quantity of donor tissues, donor site morbidity as well as potential risks of an immunological incompatibility and disease transfer.9,11,12 In this light, man-made materials (alloplastic or synthetic bone grafts) stand out as a reasonable option, because they are easily available and might be processed and modified to suit the specific needs of a given application.13-15 What’s more, there are no concerns about potential infections, immunological incompatibility, sterility or donor site morbidity. Therefore, investigations on artificial materials for bone tissue repair appear to be one of the key subjects in the field of biomaterials research for clinical applications.16
Currently, there are several classes of synthetic bone grafting biomaterials for in vivo applications.17-21 Examples include natural coral, coral-derived materials, bovine porous demineralized bone, human demineralized bone matrix, bioactive glasses, glass-ceramics and calcium orthophosphates.11 All of these biomaterials are biocompatible and osteoconductive, guiding bone tissue from the edges toward the center of the defect, and aim to provide a scaffold of interconnected pores, with pore dimensions ranging from 200 µm22,23 to 2 mm,24 to facilitate tissue and vessel ingrowths. Among them, porous bioceramics made of calcium orthophosphates appear very promising due to both excellent biocompatibility and their ability to bond to living bone in the body. This is directly related to the fact that the inorganic material of mammalian calcified tissues, i.e., of bones and teeth, consists of calcium orthophosphates.25-27 For this reason, other artificial materials are normally encapsulated by fibrous tissue when implanted in body defects, while calcium orthophosphates are not.28 Many types of calcium orthophosphate-based bioceramics with different chemical composition are already on the market. Unfortunately, as with any ceramic material, calcium orthophosphate bioceramics alone lack the mechanical and elastic properties of calcified tissues. Namely, scaffolds made of calcium orthophosphates suffer from a low elasticity, a high brittleness, a poor tensile strength, a low mechanical reliability and fracture toughness, which leads to various concerns about their mechanical performance after implantation.29-31 In addition, in many cases, it is difficult to form calcium orthophosphate bioceramics into the desired shapes.
The superior strength and partial elasticity of biological calcified tissues (e.g., bones) are due to the presence of bioorganic polymers (mainly, collagen type I fibers32) rather than to a natural ceramic (mainly, a poorly crystalline, ion-substituted CDHA, often referred to as “biological apatite”) phase.34,35 The elastic collagen fibers are aligned along the main stress directions in bone. The biochemical composition of bones is given in Table 1.36 A decalcified bone becomes very flexible and is easily twisted, whereas a bone without collagen is very brittle; thus, the inorganic, nano-sized crystals of biological apatite provide hardness and stiffness, while the bioorganic fibers are responsible for the elasticity and toughness.26,37 In bones, both types of materials integrate with each other on a nanometric scale in such a way that the crystallite size, fiber orientation, short-range order between the components, etc. determine its nanostructure and, therefore, the function and mechanical properties of the entire composite.33,38-42 From the mechanical point of view, bone is a tough material at low strain rates but fractures more like a brittle material at high strain rates; generally, it is rather weak in tension and shear, particularly along the longitudinal plane. Besides, bone is an anisotropic material, because its properties are directionally dependent.25,26,31
Table 1. The biochemical composition* of bones.36 .
| Inorganic phases | wt. % | Bioorganic phases | wt. % |
|---|---|---|---|
| calcium orthophosphates (biological apatite) | ~60 | collagen type I | ~20 |
| water | ~9 | non-collagenous proteins: osteocalcin, osteonectin, osteopontin, thrombospondin, morphogenetic proteins, sialoprotein, serum proteins | ~3 |
| carbonates | ~4 | other traces: polysaccharides, lipids, cytokines | balance |
| citrates | ~0.9 | primary bone cells: osteoblasts, osteocytes, osteoclasts | balance |
| sodium | ~0.7 | ||
| magnesium | ~0.5 | ||
| other traces: Cl-, F-, K+ Sr2+, Pb2+, Zn2+, Cu2+, Fe2+ | balance |
The composition is varied from species to species and from bone to bone.
It remains a great challenge to design the ideal bone graft, one that emulates nature’s own structures or functions. Certainly, the successful design requires an appreciation of the structure of bone. According to expectations, the ideal bone graft should be benign, available in a variety of forms and sizes, all with sufficient mechanical properties for use in load-bearing sites, form a chemical bond at the bone/implant interface as well as be osteogenic, osteoinductive, osteoconductive, biocompatible, completely biodegradable, at the expense of bone growth, and moldable to fill and restore bone defects.29,40,43 Further, it should resemble the chemical composition of bones (thus, the presence of calcium orthophosphates is mandatory), exhibit contiguous porosity to encourage invasion by the live host tissue as well as possess both viscoelastic and semi-brittle behavior, as bones do.44-47 Moreover, the degradation kinetics of the ideal implant should be adjusted to the healing rate of the human tissue, with absence of any chemical or biological irritation and/or toxicity caused by substances that are released due to corrosion or degradation. Ideally, the combined mechanical strength of the implant and the ingrowing bone should remain constant throughout the regenerative process. Furthermore, the substitution implant material should not significantly disturb the stress environment of the surrounding living tissue.48 Finally, there is the opinion that, in the case of a serious trauma, the bone should fracture rather than the implant.29 A good sterilizability, storability and processability as well as a relatively low cost are also of a great importance to permit clinical application. Unfortunately, no artificial biomaterial is yet available that embodies all these requirements, and it is unlikely that one will appear in the near future. To date, most of the available biomaterials appear to be either predominantly osteogenic or osteoinductive or else purely osteoconductive.2
Careful consideration of the bone type and mechanical properties are needed to design bone substitutes. Indeed, in high load-bearing bones, such as the femur, the stiffness of the implant needs to be adequate: not too stiff to result in strain shielding, but rigid enough to present stability. However, in relatively low load-bearing applications such as cranial bone repairs, it is more important to have stability and the correct three-dimensional shapes for aesthetic reasons. One of the most promising alternatives is to apply materials with similar composition and nanostructure to that of bone tissue.40 Mimicking the structure of calcified tissues and addressing the limitations of the individual materials in the development of organic-inorganic hybrid biomaterials provides excellent possibilities for improving conventional bone implants. In this sense, suitable biocomposites tailored to physical, biological and mechanical properties and predictable degradation behavior can be prepared by combining biologically relevant calcium orthophosphates with bioresorbable polymers.49,50 As a rule, the general behavior of these bioorganic/calcium orthophosphate biocomposites is dependent on nature, structure and relative contents of the constitutive components, although other parameters, such as the preparation conditions, also determine the properties of the final materials. Currently, biocomposites with calcium orthophosphates incorporated as either a filler or a coating (or both) and either into or onto a biodegradable polymer matrix in the form of particles or fibers are increasingly considered for use as bone tissue engineering scaffolds due to their improved physical, biological and mechanical properties.51-57 In addition, such biocomposites could set out general requirements for the next generation of biomaterials; they should combine bioactive and bioresorbable properties to activate in vivo mechanisms of tissue regeneration, stimulating the body to heal itself and leading to replacement of the implants by the regenerating tissue.50,58,59 Thus, through the successful combinations of ductile polymer matrixes with hard and bioactive particulate bioceramic fillers, optimal materials can be designed, and, ideally, this approach could lead to a superior construction to be used as either implants or posterior dental restorative material.60
A lint-reinforced plaster was the first composite used in clinical orthopedics as an external immobilizer (bandage) in the treatment of bone fracture by Mathijsen in 1852,61 followed by Dreesman in 1892.62 A great progress in the clinical application of various types of composite materials has been achieved since then. Based on past experience and newly gained knowledge, various composite materials with tailored mechanical and biological performance can now be manufactured and used to meet various clinical requirements.63 This review presents only a brief history as well as advances in the field of calcium orthophosphate-based biocomposites and hybrid biomaterials suitable for biomedical application. The majority of the reviewed literature is restricted to the recent publications; a limited number of papers published in the 20th century have been cited. Various aspects of the material constituents, fabrication technologies, structural and bioactive properties as well as phase interactions have been considered and discussed in detail. Finally, several critical issues and scientific challenges that are needed for further advancement are outlined.
General Information on Composites and Biocomposites
According to Wikipedia, the free encyclopedia, “composite materials” (or “composites” for short) are engineered materials made from two or more constituent materials with significantly different physical or chemical properties, which remain separate and distinct on a macroscopic level within the finished structure.”64 Thus, composites are always heterogeneous. Furthermore, the phases of any composite retain their identities and properties and are bonded, which is why an interface is maintained between them. This provides improved specific or synergistic characteristics that cannot be obtained by any of the original phases alone.65 Following the point of view of some predecessors, we also consider that, “for the purpose of this review, composites are defined as those having a distinct phase distributed through their bulk, as opposed to modular or coated components.”66 For this reason, with a few important exceptions, the structures obtained by soaking various materials in supersaturated solutions containing ions of calcium and orthophosphate (reviewed in ref.67–73), those obtained by coating of various materials by calcium orthophosphates (reviewed in ref.74–82) as well as calcium orthophosphates coated by other compounds83-87 have not been considered; however, composite coatings have been considered. Occasionally, porous calcium orthophosphate scaffolds filled by cells inside the pores88-91 as well as calcium orthophosphates impregnated by biologically active substances92,93 are also defined as composites and/or hybrids; nevertheless, such structures have not been considered in this review either.
In any composite, there are two major categories of constituent materials: a matrix (or a continuous phase) and (a) dispersed phase(s). To create a composite, at least one portion of each type is required. General information on the major fabrication and processing techniques may be found elsewhere.66,94 The continuous phase is responsible for filling the volume as well as surrounding and supporting the dispersed material(s) by maintaining their relative positions. The dispersed phase(s) is(are) usually responsible for enhancing one or more properties of the matrix. Most of the composites target an enhancement of mechanical properties of the matrix, such as stiffness and strength; however, other properties, such as erosion stability, transport properties (electrical or thermal), radiopacity, density or biocompatibility might also be of a great interest. This synergism produces properties that are unavailable from the individual constituent materials.94,95 What’s more, by controlling the volume fractions and local and global arrangement of the dispersed phase, the properties and design of composites can be varied and tailored to suit the necessary conditions. For example, in the case of ceramics, the dispersed phase serves to impede crack growth. In this case, it acts as reinforcement. A number of methods, including deflecting crack tips, forming bridges across crack faces, absorbing energy during pullout and causing a redistribution of stresses in regions, adjacent to crack tips, can be used to accomplish this.96 Other factors to be considered in composites include the volume fraction of (a) dispersed phase(s), its(their) orientation and homogeneity of the overall composite. For example, higher volume fractions of reinforcement phases tend to improve the mechanical properties of the composites, while continuous and aligned fibers best prevent crack propagation, with the added property of anisotropic behavior. Furthermore, the uniform distribution of the dispersed phase is also desirable, as it imparts consistent properties to the composite.64,94,95
In general, composites might be simple, complex, graded or hierarchical. The term “a simple composite” refers to composites that result from the homogeneous dispersion of one dispersed phase throughout a matrix. The term “a complex composite” refers to composites that result from the homogeneous dispersion of several dispersed phases throughout one matrix. The term “a graded composite” refers to composites that result from the intentionally structurally inhomogeneous dispersion of one or several dispersed phases throughout one matrix. The term “a hierarchical composite” refers to those cases in which fine entities of either a simple or a complex composite are somehow aggregated to form coarser ones (e.g., granules or particles), which afterwards are dispersed inside another matrix to produce the second hierarchical scale of the composite structure. There is another set of four types of composites: (1) fibrous composites, where the fibers are in a matrix; (2) laminar composites, in which the phases are in layers; (3) particulate composites, where the particles or flakes are in a matrix and (4) hybrid composites, which are combinations of any of the above. Yet another classification system of the available composites is based on the matrix materials (metals, ceramics and polymers).63
In most cases, three interdependent factors must be considered in designing of any composite: (1) the selection of a suitable matrix and dispersed materials, (2) the choice of appropriate fabrication and processing methods and (3) both internal and external design of the device itself.66 Furthermore, any composite must be formed to shape. To do this, the matrix material can be added before or after the dispersed material has been placed into a mold cavity or onto the mold surface. The matrix material experiences a melding event that, depending upon the nature of the matrix material, can occur in various ways, such as chemical polymerization, setting, curing or solidification from a melted state. Due to a general inhomogeneity, the physical properties of many composite materials are not isotropic, but rather orthotropic (i.e., there are different properties or strengths in different orthogonal directions).64,94,95
In order to prepare any type of a composite, at least two different materials must be mixed. Thus, a phase miscibility phenomenon appears to be of paramount importance.97,98 Furthermore, the interfacial strength among the phases is a very important factor, because a lack of adhesion among the phases will result in an early failure at the interface and thus in a decrease in the mechanical properties, especially the tensile strength. From a chemical point of view, we can distinguish several types of interactions among the composite components: materials with strong (covalent, coordination, ionic) interactions; those with weak interactions (van der Waals forces, hydrogen bonds, hydrophilic-hydrophobic balance) and those without chemical interactions among the components.99 Wetting is also important in bonding or adherence of the materials. It depends on the hydrophilicity or polarity of the filler(s) and the available polar groups of the matrix.
Biocomposites are defined as non-toxic composites that are able to interact well with the human body in vivo and, ideally, contain one or more component(s) that stimulate(s) the healing process and uptake of the implant.100 Thus, for biocomposites, biological compatibility appears to be more important than any other type of compatibility.63,101,102 Interestingly, according to the databases, the first paper with the term “biocomposite” in the title was published in 1987,103 and the first one containing a combination of the terms “biocomposite” and HA in the title was published in 1991.104 Thus, this subject appears to be quite new. The most common properties from the bioorganic and inorganic domains to be combined in biocomposites have been summarized in Table 2.40 For general advantages of the modern calcium orthophosphate-based biocomposites over calcium orthophosphate bioceramics and bioresorbable polymers individually, interested readers are advised to see the “Composite Materials Strategy” section of reference 50.
Table 2. General respective properties from the bioorganic and inorganic domains, to be combined in various composites and hybrid materials40 .
| inorganic | bioorganic |
|---|---|
| hardness, brittleness | elasticity, plasticity |
| high density | low density |
| thermal stability | permeability |
| hydrophilicity | hydrophobicity |
| high refractive index | selective complexation |
| mixed valence slate (red-ox) | chemical reactivity |
| strength | bioactivity |
The Major Constituents of Biocomposites and Hybrid Biomaterials for Bone Grafting
Calcium orthophosphates
The main driving force behind the use of calcium orthophosphates as bone substitute materials is their chemical similarity to the mineral component of mammalian bones and teeth.25-27 As a result, in addition to being non-toxic, they are biocompatible, not recognized as foreign materials in the body and, most importantly, exhibit both bioactive behavior and the ability to integrate into living tissue by the same processes active in remodeling healthy bone. This leads to an intimate physicochemical bond between the implants and bone, termed osteointegration.105 More to the point, calcium orthophosphates are also known to support osteoblast adhesion and proliferation.106,107 Even so, the major limitations to the use of calcium orthophosphates as load-bearing biomaterials are their mechanical properties; namely, they are brittle with poor fatigue resistance.29-31 Their poor mechanical behavior is even more evident for highly porous ceramics and scaffolds. Because porosity greater than 100 µm is the requirement for proper vascularization and bone cell colonization,108-110 in biomedical applications, calcium orthophosphates are used primarily as fillers and coatings.27
The complete list of known calcium orthophosphates, including their standard abbreviations and major properties, is given in Table 3, while the detailed information on calcium orthophosphates, their synthesis, structure, chemistry, other properties and biomedical application has been comprehensively reviewed recently in reference.27 Even more thorough information on calcium orthophosphates might be found in special books and monographs.111-117
Table 3. Existing calcium orthophosphates and their major properties27 .
| Ca/P molar ratio | Compound | Formula | Solubility at 25°C, -log(Ks) |
Solubility at 25°C, g/L |
pH stability range in aqueous solutions at 25°C |
|---|---|---|---|---|---|
| 0.5 | Monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 1.14 | ~18 | 0.0 – 2.0 |
| 0.5 | Monocalcium phosphate anhydrous (MCPA or MCP) | Ca(H2PO4)2 | 1.14 | ~17 | [c] |
| 1.0 | Dicalcium phosphate dihydrate (DCPD), mineral brushite | CaHPO4·2H2O | 6.59 | ~0.088 | 2.0 – 6.0 |
| 1.0 | Dicalcium phosphate anhydrous (DCPA or DCP), mineral monetite | CaHPO4 | 6.90 | ~0.048 | [c] |
| 1.33 | Octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4·5H2O | 96.6 | ~0.0081 | 5.5 – 7.0 |
| 1.5 | α-Tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 25.5 | ~0.0025 | [a] |
| 1.5 | β-Tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | 28.9 | ~0.0005 | [a] |
| 1.2 – 2.2 | Amorphous calcium phosphates (ACP) | CaxHy(PO4)z·nH2O, n = 3 – 4.5; 15 – 20% H2O | [b] | [b] | ~5 – 12 [d] |
| 1.5 – 1.67 | Calcium-deficient hydroxyapatite (CDHA or Ca-def HA)[e] | Ca10-x(HPO4)x(PO4)6-x(OH)2-x (0 < x < 1) | ~85 | ~0.0094 | 6.5 – 9.5 |
| 1.67 | Hydroxyapatite (HA, HAp or OHAp) | Ca10(PO4)6(OH)2 | 116.8 | ~0.0003 | 9.5 – 12 |
| 1.67 | Fluorapatite (FA or FAp) | Ca10(PO4)6F2 | 120.0 | ~0.0002 | 7 – 12 |
| 1.67 | Oxyapatite (OA, OAp or OXA)[f] | Ca10(PO4)6O | ~69 | ~0.087 | [a] |
| 2.0 | Tetracalcium phosphate (TTCP or TetCP), mineral hilgenstockite | Ca4(PO4)2O | 38 – 44 | ~0.0007 | [a] |
[a] These compounds cannot be precipitated from aqueous solutions. [b] Cannot be measured precisely. However, the following values were found: 25.7 ± 0.1 (pH = 7.40), 29.9 ± 0.1 (pH = 6.00), 32.7 ± 0.1 (pH = 5.28). The comparative extent of dissolution in acidic buffer is: ACP > > α-TCP > > β-TCP > CDHA > > HA > FA. [c] Stable at temperatures above 100°C. [d] Always metastable. [e] Occasionally, it is called “precipitated HA (PHA).” [f] Existence of OA remains questionable.
Polymers
Polymers are a class of materials consisting of large molecules, often containing many thousands of small units, or monomers, joined together chemically to form one giant chain, thus creating very ductile materials. In this respect, polymers are comparable with major functional components of the biological environment: lipids, proteins and polysaccharides. They differ from each other in chemical composition, molecular weight, polydispersity, crystallinity, hydrophobicity, solubility and thermal transitions. Their properties can be fine-tuned over a wide range by varying the type of polymer or chain length as well as by copolymerization or blending of two or more polymers.118-120 Unlike ceramics, polymers exhibit substantial viscoelastic properties and easily can be fabricated into complex structures, such as sponge-like sheets, gels or complex structures with intricate porous networks and channels.121 Being X-ray transparent and non-magnetic, polymeric materials are fully compatible with modern diagnostic methods, such as CT and magnetic resonance imaging. Unfortunately, most of them are unable to meet the strict demands of the in vivo physiological environment. Namely, the main requirements for polymers suitable for biomedical applications are that they must be biocompatible, not elicit an excessive or chronic inflammatory response upon implantation and, for those that degrade, they must breakdown into non-toxic products only. Unfortunately, polymers, for the most part, lack rigidity, ductility and, ultimately, the mechanical properties required in load-bearing applications. Thus, despite their good biocompatibility, many of the polymeric materials are mainly used for soft tissue replacements (such as skin, blood vessel, cartilage, ligament replacement, etc.). Moreover, the sterilization processes (autoclave, ethylene oxide and 60 Co irradiation) may affect the polymer properties.122
There are a variety of biocompatible polymers suitable for biomedical applications.123,124 For example, polyacrylates, poly(acrylonitrile-co-vinylchloride) and polylysine have been investigated for cell encapsulation and immunoisolation.125,126 Polyorthoesters and PCL have been investigated as drug delivery devices, the latter for long-term sustained release because of its slow degradation rates.127 PCL is a hydrolytic polyester with an appropriate resorption period that releases non-toxic byproducts upon degradation.128 Other polyesters and PTFE are used for vascular tissue replacement. Polyurethanes are in use as coatings for pacemakers’ lead insulation and have been investigated for reconstruction of the meniscus.129,130 Polymers considered for orthopedic purposes include polyanhydrides, which have also been investigated as delivery devices (due to their rapid and well-defined surface erosion) and for bone augmentation or replacement, since they can be photopolymerized in situ.127,131,132 To overcome their poor mechanical properties, they have been copolymerized with imides or formulated to be cross-linkable in situ.132 Other polymers, such as polyphosphazenes, can have their properties (e.g., degradation rate) easily modified by varying the nature of their side groups and have been shown to support osteoblast adhesion, which makes them candidate materials for skeletal tissue regeneration.132 PPF has emerged as a good bone replacement material, exhibiting good mechanical properties (comparable to trabecular bone), possessing the capability to cross-link in vivo through the C = C bond and being hydrolytically degradable. It has also been examined as a material for drug delivery devices.127,131-134 Polycarbonates have been suggested as suitable materials to make scaffolds for bone replacement and have been modified with tyrosine-derived amino acids to render them biodegradable.127 Polydioxanone has been also tested for biomedical applications.135 PMMA is widely used in orthopedics as a bone cement for implant fixation as well as to repair certain fractures and bone defects, for example, osteoporotic vertebral bodies.136,137 However, PMMA sets via a polymerization of toxic monomers, which also produces a significant amount of heat that damages tissues. Moreover, it is neither degradable nor bioactive, does not bond chemically to bones and might generate particulate debris, leading to an inflammatory foreign body response.131,138 A number of other nondegradable polymers applied in orthopedic surgery include PE in its different modifications, such as low density PE, HDPE and UHMWPE (used as the articular surface of total hip replacement implants139,140), polyethylene terepthalate, PP and PTFE, which are applied to repair knee ligaments.141 PolyactiveTM, a block copolymer of PEG and PBT, has also been considered for biomedical application.142-147 Cellulose148,149 and its esters150,151 are also popular. Finally, and importantly, polyethylene oxide, PHB and blends thereof have also been tested for biomedical applications.50
Nonetheless, the most popular synthetic polymers used in medicine are the linear aliphatic poly(α-hydroxyesters), such as PLA, PGA and their copolymers, PLGA (Table 4). These materials have been extensively studied; they appear to be the only synthetic and biodegradable polymers with an extensive FDA approval history.50,132,152-156 They are biocompatible, mostly non-inflammatory and can degrade in vivo through hydrolysis and, possibly, enzymatic action into products that are removed from the body by regular metabolic pathways.49,127,132,156-161 They might also be used for drug delivery purposes.162 Poly(α-hydroxyesters) have been investigated as scaffolds for replacement and regeneration of a variety of tissues, cell carriers, controlled delivery devices for drugs or proteins (e.g., growth factors), membranes or films, screws, pins and plates for orthopedic applications.127,132,153,154,156,163-165 Additionally, the degradation rate of PLGA can be adjusted by varying the amounts of the two component monomers (Table 4), which in orthopedic applications can be exploited to create materials that degrade in concert with bone ingrowth.160,166 Furthermore, PLGA is known to support osteoblast migration and proliferation,59,132,157,167 which is a necessity for bone tissue regeneration. Unfortunately, such polymers on their own, though they reduce the effect of stress shielding, are too weak to be used in load-bearing situations and are only recommended in certain clinical indications, such as ankle and elbow fractures.156,161 In addition, they exhibit bulk degradation, leading to both a loss in mechanical properties and lowering of the local solution pH, which further accelerates degradation in an autocatalytic manner. As the body is unable to cope with the vast amounts of implant degradation products, this might lead to an inflammatory foreign body response.132,156,163 Finally, poly(α-hydroxyesters) do not possess the bioactive and osteoconductive properties of calcium orthophosphates.153,168
Table 4. Major properties of several FDA approved biodegradable polymers.152 .
| polymer | thermal properties*, °C | tensile modulus, GРa | degradation time, months |
|---|---|---|---|
| polyglycolic acid (PGA) | tg = 35 – 40 tm = 225 – 230 |
7.06 | 6 – 12 (strength loss within 3 weeks) |
| L-polylactic acid (LPLA) | tg = 60 – 65 tm = 173 – 178 |
2.7 | > 24 |
| D,L-polylactic acid (DLPLA) | tg = 55 – 60 amorphous |
1.9 | 12 – 16 |
| 85/15 D,L-polylactic-co-glycolic acid (85/15 DLPLGA) | tg = 50 – 55 amorphous |
2.0 | 5 – 6 |
| 75/25 D,L-polylactic-co-glycolic acid (75/25 DLPLGA) | tg = 50 – 55 amorphous |
2.0 | 4 – 5 |
| 65/35 D,L-polylactic-co-glycolic acid (65/35 DLPLGA) | tg = 45 – 50 amorphous |
2.0 | 3 – 4 |
| 50/50 D,L-polylactic-co-glycolic acid (50/50 DLPLGA) | tg = 45 – 50 amorphous |
2.0 | 1 – 2 |
| poly(ε-caprolactone) (PCL) | tg = (– 60) – (– 65) tm = 58 – 63 |
0.4 | > 24 |
tg – glass transition temperature; tm – melting point.
Several classifications of the biomedically relevant polymers are possible. For example, some authors distinguish between synthetic polymers, like PLA, PGA or their copolymers, and PCL and polymers of biological origin like polysaccharides (starch, alginate, chitin/chitosan,169-171 gelatin, cellulose, hyaluronic acid derivatives), proteins (soy, collagen, fibrin,11 silk) and a variety of biofibers, such as lignocellulosic natural fibers.10,172,173 Natural polymers often possess highly organized structures and may contain an extracellular substance, called ligand, which is necessary to bind with cell receptors. However, they always contain various impurities that should be gotten rid of prior to use. As synthetic polymers can be produced under controlled conditions, in general, they exhibit predictable and reproducible mechanical and physical properties, such as tensile strength, elastic modulus and degradation rate. Control of impurities is a further advantage of synthetic polymers. Other authors differentiate between resorbable or biodegradable [e.g., poly(α-hydroxyesters), polysaccharides and proteins] and non-resorbable (e.g., PE, PP, PMMA and cellulose) polymers.60,173 Furthermore, polymeric materials can be broadly classified as thermoplastics and thermosets. HDPE and PEEK are examples of thermoplastics, while polydimethylsiloxane and PMMA are the examples of thermosets.122 The list of synthetic biodegradable polymers used for biomedical application as scaffold materials is available as Table 1 in reference ,173 while further details on polymers suitable for biomedical applications are available in the literature (refs.122,165,174–183), where interested readers are referred. Good reviews on the synthesis of different biodegradable polymers184 as well as on the experimental trends in polymer composites185 are available elsewhere.
Inorganic materials and compounds
Metals
Titanium (Ti) is one of the best biocompatible metals and is used most widely as an implant.16,186,187 Besides Ti, there are other metallic implants made of pure Zr, Hf, V, Nb, Ta, Re,186 Ni, Fe, Cu,188-190 Ag, stainless steels and various alloys190 suitable for biomedical application. Recent studies revealed an even greater biomedical potential for porous metals.191-194 Metallic implants provide the necessary strength and toughness required in load-bearing parts of the body, and, due to these advantages, metals will continue to play an important role as orthopedic biomaterials in the future, even though there are concerns with regard to the release of certain ions from and corrosion products of metallic implants. Of course, neither metals nor alloys are biomimetic (the term biomimetic can be defined as a processing technique that either mimics or inspires the biological mechanism, in part or whole195) in terms of chemical composition, because there are no elemental metals in the human body. In addition, even biocompatible metals are bioinert; although they are not rejected by the human body, metallic implants cannot actively interact with the surrounding tissues. Nevertheless, in some cases (especially when they are coated by calcium orthophosphates; however, that is another story), the metallic implants show a reasonable biocompatibility.196 Only permanent implants are made of metals and alloys, in which degradation or corrosion is not desirable. However, in recent years, a number of magnesium implants have been proposed which are aimed to degrade in the body in order to make room for ingrowing bones.193,197,198
Glasses and glass-ceramics
Special types of glasses and glass-ceramics are also suitable materials for biomedical applications,199-201 and a special Na2O-CaO-SiO2-P2O5 glass, named Bioglass®,13,28,30,31,202,203 is the most popular among them. They are produced via standard glass production techniques and require pure raw materials. Bioglass® is a biocompatible and osteoconductive biomaterial. It bonds to bone without an intervening fibrous connective tissue interface and, due to these properties, it has been widely used for filling bone defects.204 The primary shortcoming of Bioglass® is mechanical weakness and low fracture toughness due to an amorphous two-dimensional glass network. The bending strength of most Bioglass® compositions is in the range of 40–60 MPa, which is not suitable for major load-bearing applications. Making porosity in Bioglass®-based scaffolds is beneficial, even for better resorption and bioactivity.205
By heat treatment, a suitable glass can be converted into glass-crystal composites containing crystalline phase(s) of controlled sizes and contents. The resultant glass-ceramics can have superior mechanical properties to the parent glass as well as to sintered crystalline ceramics. The bioactive A-W glass-ceramics are made from the parent glass in the pseudoternary system 3CaO·P2O5-CaO·SiO2-MgO·CaO·2SiO2, which is produced by a conventional melt-quenching method. The bioactivity of A-W glass-ceramics is much higher than that of sintered HA. They possess excellent mechanical properties and have, therefore, been used clinically for iliac and vertebrae prostheses and as intervertebral spacers.16,206-208
Ceramics
Metal oxide ceramics, such as alumina (Al2O3, high purity, polycrystalline, fine grained), zirconia (ZrO2) and some other oxides (e.g., TiO2, SiO2) have been widely studied due to their bioinertness, excellent tribological properties, high wear resistance, fracture toughness and strength as well as relatively low friction.16,209 Unfortunately, due to transformation from the tetragonal to the monoclinic phase, a volume change occurs when pure zirconia is cooled down, which causes cracking of the zirconia ceramics. Therefore, additives such as calcia (CaO), magnesia (MgO) and yttria (Y2O3) must be mixed with zirconia to stabilize the material in either the tetragonal or the cubic phase. Such material is called PSZ.210-212 However, the brittle nature of ceramics has limited their scope of clinical applications, and hence, more research needs to be conducted to improve their properties.
Carbon
Due to its bioinertness, excellent tribological properties, fracture toughness and strength as well as low friction, elemental carbon has been used as a biomaterial at least since 1972.213 Applications include orthopedic prostheses, vitreous carbon roots for replacement teeth, structural skeletal extensions, bone bridges and hip prostheses. Biomedical properties of amorphous carbon were studied as well.214 However, current trends primarily represent investigations on biomedical applications of carbon nanotubes.215,216
Carbon nanotubes, with their small dimensions, high aspect (length to diameter) ratio as well as exceptional mechanical properties, including extreme flexibility and strength, significant resistance to bending, high resilience and the ability to reverse any buckling of the tube, have excellent potential for accomplishing the necessary mechanical properties.217 Recent studies have even suggested that they may possess some bioactivity.218-221 However, non-functionalized carbon nanotubes tend to agglomerate and form bundles. Besides, they are soluble in neither water nor organic solvents. Luckily, chemical functionalization82,222 allows carbon nanotubes to be dispersed more easily, which can improve interfacial bonding with other components of the composites. Furthermore, functionalization of carbon nanotubes with carboxylic groups was found to confer a capacity to induce calcification similar to woven bones.223 Interestingly, carbon nanotubes might be functionalized by in situ deposition of CDHA on their surface.224
Biocomposites and Hybrid Biomaterials Based on Calcium Orthophosphates
Generally, the available biocomposites and hybrid biomaterials based on calcium orthophosphates might be divided into several (partly overlapping) broad areas:
biocomposites with polymers,
self-setting formulations and concretes,
formulations based on nanodimensional calcium orthophosphates and nanodimensional biocomposites,
biocomposites with collagen,
formulations with other bioorganic compounds and/or biological macromolecules,
injectable bone substitutes (IBS),
biocomposites with glasses, inorganic compounds, carbon and metals,
functionally graded formulations and
biosensors
The details on each subject are discussed below.
Biocomposites with polymers
Typically, the polymeric components of biocomposites and hybrid biomaterials comprise polymers that have shown both a good biocompatibility and are routinely used in surgical applications. In general, since polymers have a low modulus (2–7 GPa, as the maximum) as compared with that of bone (3–30 GPa), calcium orthophosphate bioceramics need to be loaded at a high weight % ratio. Besides, general knowledge on composite mechanics suggests that any high aspect ratio particles, such as whiskers or fibers, significantly improve the modulus at a lower loading.179 Thus, some attempts have already been made to prepare biocomposites containing whisker-like225-229 or needle-like230-232 calcium orthophosphates as well as calcium orthophosphate fibers.49,233
The history of implantable polymer-calcium orthophosphate biocomposites and hybrid biomaterials started in 1981234 with the pioneering study by Prof. William Bonfield and colleagues performed on HA/PE formulations.236,237 That initial study introduced a bone analog concept, in which proposed biocomposites comprised a polymer ductile matrix of PE and a ceramic stiff phase of HA that was substantially extended and developed in further investigations by that research group.102,238-254 More recent studies have included investigations on the influence of surface topography of HA/PE composites on cell proliferation and attachment.255-261 The material is composed of a particular combination of HA particles at a volume loading of ~40% uniformly dispensed in a HDPE matrix. Alternatively, PP might be used instead of PE.262-264 The idea was to mimic bones by using a polymeric matrix that can develop a considerable anisotropic character through adequate orientation techniques, reinforced with a bone-like bioceramic material that assures both a mechanical reinforcement and a bioactive character of the composite. Following FDA approval in 1994, in 1995 this material became commercially available under the trade name HAPEXTM (Smith and Nephew, Richards), and, to date, it has been implanted in over 300,000 patients with successful results. It remains the only clinically successful bioactive composite and appeared to be a major step in the implant field.31,265 The major production stages of HAPEXTM include blending, compounding and centrifugal milling. A bulk material or device is then created from this powder by compression and injection molding.63 Alternatively, HA/HDPE biocomposites might be prepared by a hot rolling technique that facilitates uniform dispersion and blending of the reinforcements in the matrix.266
A mechanical interlock between the two phases of HAPEXTM is formed by the shrinkage of HDPE onto the HA particles during cooling.102,267 Both HA particle size and their distribution in the HDPE matrix are recognized as important parameters affecting the mechanical behavior of HAPEXTM.247 Smaller HA particles, for example, were found to lead to stiffer composites due to the increase in interfaces between the polymer and the ceramics. In addition, rigidity of HAPEXTM was found to be proportional to HA volume fraction.239 Coupling agents, e.g., 3-trimethoxysiyl propylmethacrylate for HA and acrylic acid for HDPE, might be used to improve bonding (by both chemical adhesion and mechanical coupling) between HA and HDPE.268,269 Obviously, other calcium orthophosphates might be used instead of HA in biocomposites with PE.270 Indeed, attempts were made to improve the mechanical properties of HAPEXTM by incorporating other ceramic phases into the polymer matrix, such as PSZ271 and alumina.272 A partial replacement of HA filler particles by PSZ particles was found to lead to an increase in the strength and fracture toughness of HA/HDPE biocomposites. The compressive stress, set up by the volume expansion associated with the tetragonal-to-monoclinic phase transformation of PSZ, inhibits or retards the crack propagation within the composite. This results in an enhanced fracture toughness of the HA/ZrO2/HDPE biocomposite.271
Various studies revealed that HAPEXTM attached directly to bones by chemical bonding (a bioactive fixation) rather than by forming fibrous encapsulation (a morphological fixation). Initial clinical applications of HAPEXTM came in orbital reconstruction,273 but since 1995, the main uses of this composite have been in the shafts of middle ear implants for the treatment of conductive hearing loss.274,275 In both applications, HAPEXTM offers the advantage of in situ shaping, so a surgeon can make final alterations to optimize the fit of the prosthesis to the bone of a patient, and subsequent activity requires only limited mechanical loading with virtually no risk of failure from insufficient tensile strength.102,202 As compared with cortical bones, HA/ PE composites have a superior fracture toughness for HA concentrations below ~40% and similar fracture toughness in the 45–50% range. Their Young’s modulus is in the range of 1–8 GPa, which is quite close to that of bone. The examination of the fracture surfaces revealed that only a mechanical bond occurs between HA and PE. Unfortunately, the HA/PE composites are not biodegradable, the available surface area of HA is low, and the presence of bioinert PE decreases the ability to bond to bones. Furthermore, HAPEXTM has been designed with a maximized density to increase its strength, but the resulting lack of porosity limits the ingrowth of osteoblasts when the implant is placed into the body.29,203 Further details on HAPEXTM are available elsewhere.102 In addition to HAPEXTM, other types of HA/PE biocomposites are also known.276-282
Both linear and branched PE were used as a matrix, and the biocomposites with the former were found to give a higher modulus.277 The reinforcing mechanisms in calcium orthophosphate/polymer biocomposites have yet to be convincingly disclosed. Generally, if a poor filler choice is made, the polymeric matrix might be affected by the filler through reduction of molecular weight during composite processing, formation of an immobilized shell of polymer around the particles (transcrystallization, surface-induced crystallization or epitaxial growth) and changes in conformation of the polymer due to particle surfaces and inter-particle spacing.102 On the other hand, the reinforcing effect of calcium orthophosphate particles might depend on the molding technique employed: a higher orientation of the polymeric matrix was found to result in a higher mechanical performance of the composite.282,283
Many other blends of calcium orthophosphates with various polymers are possible, including rather unusual formulations with dendrimers.284 Even light-curable polymer/calcium orthophosphate formulations are known.285 The list of the appropriate calcium orthophosphates is shown in Table 3 (except MCPM and MCPA, as both are too acidic and, therefore, are not biocompatible;27 however, to overcome this drawback, they might be mixed with basic compounds, such as HA, TTCP, CaCO3, CaO, etc.). Many biomedically suitable polymers have been listed above. The combination of calcium orthophosphates and polymers into biocomposites has a 2-fold purpose. The desirable mechanical properties of polymers compensate for a poor mechanical behavior of calcium orthophosphate bioceramics, while, in turn, the desirable bioactive properties of calcium orthophosphates improve those of polymers, expanding the possible uses of each material within the body.158-160,286-290 Namely, polymers have been added to calcium orthophosphates in order to improve their mechanical strength,158,286 and calcium orthophosphate fillers have been blended with polymers to improve their compressive strength and modulus in addition to increasing their osteoconductive properties.52,160,168,291-295 Furthermore, biocompatibility of such biocomposites is enhanced, because calcium orthophosphate fillers induce an increased initial flash spread of serum proteins compared with the more hydrophobic polymer surfaces.296 What’s more, experimental results of these biocomposites indicate favorable cell-material interactions with increased cell activities as compared with each polymer alone.288 As a rule, with increasing of calcium orthophosphate content, both Young’s modulus and bioactivity of the biocomposites increase, while the ductility decreases.29,291 Furthermore, such formulations can provide a sustained release of calcium and orthophosphate ions into the milieus, which is important for mineralized tissue regeneration.287 Indeed, a combination of two different materials draws on the advantages of each one to create a superior biocomposite with respect to the materials on their own.
It is logical to assume that the proper biocomposite of a calcium orthophosphate (for instance, CDHA) with a bioorganic polymer (for instance, collagen) would yield physical, chemical and mechanical properties similar to those of human bones. Different methods for bringing these two components together into biocomposites have already been realized, including mechanical blending, compounding, ball milling, dispersion of ceramic fillers into a polymer-solvent solution, a melt extrusion of a ceramic/polymer powder mixture, coprecipitation and electrochemical co-deposition.36,63,297-299 Three methods for preparing a homogeneous blend of HA with PLLA were compared.297 First, a dry process, consisting of mixing ceramic powder and polymer pellets before a compression molding step, was used. The second technique was based on the dispersion of ceramic fillers into a polymer-solvent solution. The third method was a melt extrusion of a ceramic/polymer powder mixture. Mixing dry powders led to a ceramic particle network around the polymer pellets, whereas the solvent and melt methods produced a homogeneous dispersion of HA in the matrix. The main drawback of the solvent casting method is the risk of potentially toxic organic solvent residues. The melt extrusion method was shown to be a good way to prepare homogeneous ceramic/polymer blends.297
There is also in situ formation, which involves either synthesizing the reinforcement inside a preformed matrix material or synthesizing the matrix material around the reinforcement.63,300,301 This is one of the most attractive routes, since it avoids extensive particle agglomeration. Several papers have reported that the in situ formation technique has produced various composites of apatites with carbon nanotubes.302-308 Other appoaches include using amino acid-capped nano-sized gold particles as scaffolds to grow CDHA309 and in preparation of nano-sized HA/polyamide biocomposites.310,311 In certain cases, a mechanochemical route,312 emulsions,313-316 freeze-drying317 and freeze-thawing techniques,318 flame-sprayed technique319 or gel-templated mineralization320 might be applied to produce calcium othophosphates-based biocomposites. Various fabrication procedures are well described elsewhere in references 36, 63 and 297 where the interested readers are referred.
The interfacial bonding between a calcium orthophosphate and a polymer is an important issue for any biocomposite. Four types of mutual arrangements of nanodimensional particles to polymer chains have been classified by Kickelbick (Fig. 1): (1) inorganic particles embedded in inorganic polymer, (2) incorporation of particles by bonding to the polymer backbone, (3) an interpenetrating network with chemical bonds and (4) an inorganic-organic hybrid polymer.321 If adhesion among the phases is poor, the mechanical properties of a biocomposite suffer. To solve the problem, various approaches have been already introduced. For example, a diisocyanate coupling agent was used to bind PEG/PBT (PolyactiveTM) block copolymers to HA filler particles. Using surface-modified HA particles as a filler in a PEG/PBT matrix significantly improved the elastic modulus and strength of the polymer as compared with the polymers filled with ungrafted HA.293,322 Another group used processing conditions to achieve a better adhesion of the filler to the matrix. Ignjatovic et al. prepared PLLA/HA composites by pressing blends of varying PLLA and HA content at different temperatures and pressures.158,159,323 They found that maximum compressive strength was achieved at ~15 wt% of PLLA. By using blends with 20 wt% of PLLA, the authors also established that increasing the pressing temperature and pressure improved the mechanical properties. The former was explained by a decrease in viscosity of the PLLA associated with a temperature increase, hence leading to improved wettability of HA particles. The latter was explained by increased compaction and penetration of pores at higher pressure in conjunction with a greater fluidity of the polymer at higher temperatures. The combination of high pressures and temperatures was found to decrease porosity and guarantee a close apposition of a polymer to the particles, thereby improving the compressive strength286 and fracture energy324 of the biocomposites. The PLLA/HA biocomposites’ scaffolds were found to improve cell survival over plain PLLA scaffolds.325
Figure 1.
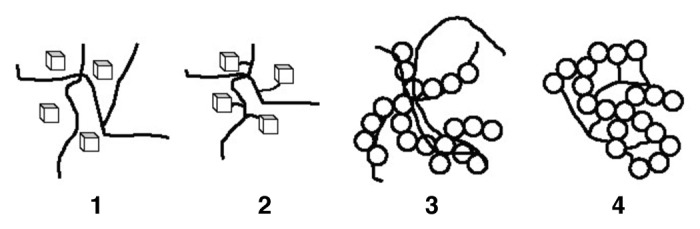
Four types of mutual arrangements of nano-sized particles to a polymer chain: (1) inorganic particles embedded in an inorganic polymer, (2) incorporation of particles by bonding to the polymer backbone, (3) interpenetrating network with chemical bonds, (4) inorganic-organic hybrid polymer. Reprinted from reference 321 with permission.
It is also possible to introduce porosity into calcium orthophosphate-based biocomposites, which is advantageous for most applications as bone substitution material. The porosity facilitates migration of osteoblasts from surrounding bones to the implant site.160,326,327 Various material processing strategies to prepare composite scaffolds with interconnected porosity comprise thermally induced phase separation, solvent casting and particle leaching, solid freeform fabrication techniques, microsphere sintering and coating.173,328-330 A supercritical gas foaming technique might be used as well.297,331,332
Apatite-based formulations
A biological apatite is known to be the major inorganic phase of mammalian calcified tissues.25,26 Consequently, CDHA, HA, carbonateapatite (both with and without dopants) and, occasionally, FA have been applied to prepare biocomposites with other compounds, usually with the aim of improving the bioactivity. For example, PS composed with HA can be used as a starting material for long-term implants.333-335 Retrieved in vivo, HA/PS biocomposite-coated samples from rabbit distal femurs demonstrated direct bone apposition to the coatings as compared with the fibrous encapsulation that occurred when uncoated samples were used.333 The resorption time of such biocomposites is a very important factor, which depends on polymer’s microstructure and the presence of modifying phases.334
Various apatite-containing biocomposites with PVA,318,336-344 PVAP345 and several other polymeric components346-358 have already been developed. Namely, PVA/CDHA biocomposite blocks were prepared by precipitation of CDHA in aqueous solutions of PVA.318 An artificial cornea consisting of a porous nano-sized HA/PVA hydrogel skirt and a transparent center of PVA hydrogel has been prepared as well. The results displayed good biocompatibility and interlocking between artificial cornea and host tissues.340,341 PVAP has been chosen as a polymer matrix, because its phosphate groups can act as a coupling/anchoring agent with a higher affinity toward the HA surface.345 Greish and Brown developed HA/Ca poly(vinyl phosphonate) biocomposites.349-351 A template-driven nucleation and mineral growth process for the high-affinity integration of CDHA with PHEMA hydrogel scaffold has been developed as well.358
PEEK225,227,359-365,367 and HIPS366 were applied to create biocomposites with HA because of their potential for clinical use in load-bearing applications. The study on reinforcing PEEK with thermally sprayed HA particles revealed that the mechanical properties increased monotonically with the reinforcement concentration, with a maximum value in the study of ~40% volume fraction of HA particles.361-363 The reported ranges of stiffness within 2.8–16.0 GPa and strength within 45.5–69 MPa exceeded the lower values for human bone (7–30 GPa and 50–150 MPa, respectively).362 Modeling of the mechanical behavior of HA/ PEEK biocomposites is available elsewhere.364
Biodegradable poly(α-hydroxyesters) are well established in clinical medicine. Currently, they provide a good choice when a suitable polymeric filler material is sought. For example, HA/ PLGA composites have been developed that appear to possess a cellular compatibility suitable for bone tissue regeneration.368-376 Zhang and Ma seeded highly porous PLLA foams with HA particles in order to improve the osteoconductivity of polymer scaffolds for bone tissue engineering.52,292 They pointed out that hydration of the foams prior to incubation in simulated body fluid increased the amount of carbonated CDHA material due to an increase of COOH and OH groups on the polymer surface, which apparently acted as nucleation sites for apatite. The following values of Young’s modulus, compressive, bending and tensile strengths for PLLA/HA composites have been achieved: 5–12 GPa, 78–137 MPa, 44–280 MPa and 10–30 MPa, respectively.377 However, these data do not appear to be in a good agreement with HA/PLLA biocomposite unit cell model predictions.378
On their own, PGA and PLA are known to degrade to acidic products (glycolic and lactic acids, respectively) that both catalyze polymer degradation and cause inflammatory reactions of the surrounding tissues.379 However, in biocomposites of poly(α-hydroxyesters) with calcium orthophosphates, the presence of slightly basic compounds (HA, TTCP) neutralizes the acid molecules to some extent and provides a weak pH-buffering effect at the polymer surface, therefore more or less compensating for their drawbacks.168,380-382 However, additives of even more basic chemicals (e.g., CaO, CaCO3) might be necessary.173,381,383,384 Extensive cell culture experiments on pH-stabilized composites of PGA and carbonateapatite were reported, which afterwards were supported by extensive in vitro pH studies.385 A consequent development of this approach has led to the designing of functionally graded composite skull implants consisting of polylactides, carbonateapatite and CaCO3.386,387 Besides the pH-buffering effect, inclusion of calcium orthophosphates was found to modify both surface and bulk properties of the biodegradable poly(α-hydroxyesters) by increasing the hydrophilicity and water absorption of the polymer matrix, thus altering the scaffold degradation kinetics. For example, polymer biocomposites filled with HA particles were found to hydrolyze homogeneously due to water penetrating into interfacial regions.388
Biocomposites of poly(α-hydroxyesters) with calcium orthophosphates are prepared mainly by incorporating the inorganic phase into a polymeric solution followed by drying under vacuum. The resulting solid biocomposites might be shaped using different processing techniques. One can also prepare these biocomposites by mixing HA particles with L-lactide prior to the polymerization380 or by a combination of a slip-casting technique and hot pressing.389 Addition of a surfactant (surface active agent) might be useful to keep the suspension homogenous.390 Furthermore, HA/PLA314,315 and HA/PLGA316 microspheres might be prepared by a microemulsion technique. More complex carbonated FA/PLA391 and PLGA/carbon nanotubes/HA392 porous biocomposite scaffolds are also known. An interesting list of references assigned to the different ways of preparing HA/poly(α-hydroxyesters) biodegradable composites might be found in publications by Durucan and Brown.53,393,394 The authors prepared CDHA/PLA and CDHA/PLGA biocomposites using a solvent casting technique with a subsequent hydrolysis of α-TCP to CDHA in aqueous solutions. The presence of both polymers was found to inhibit α-TCP hydrolysis compared with that of single-phase α-TCP alone; what’s more, the inhibiting effect of PLA exceeded that of PLGA.53,393,394 The physical interactions between calcium orthophosphates and poly(α-hydroxyesters) might be easily seen in Figure 2.53 Another set of good pictures might be found in reference 87. Nevertheless, it should not be forgotten that, typically, non-melt-based routes lead to the development of composites with lower mechanical performance and often times require the use of toxic solvents and intensive hand labor.178
Figure 2.
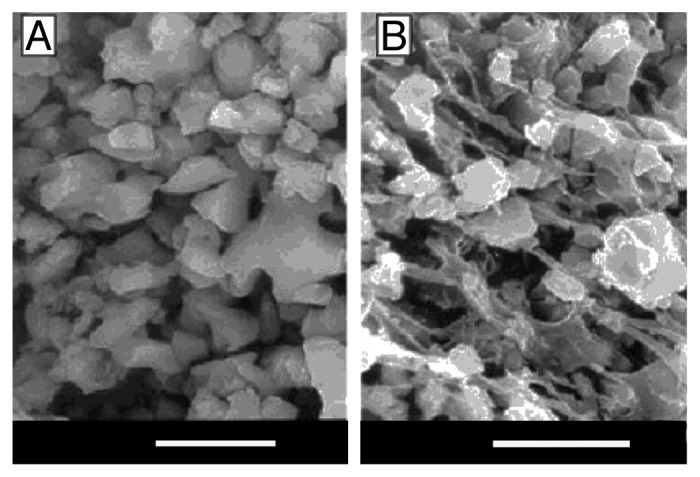
SEM micrographs of (A) α-TCP compact; (B) α-TCP/PLGA biocomposite (bars = 5 µm). Reprinted from reference 53 with permission.
The mechanical properties of poly(α-hydroxyesters) could be substantially improved by the addition of calcium orthophosphates.395,396 Shikinami and Okuno developed CDHA/PLLA composites with very high mechanical properties;168 mini-screws and mini-plates made of these composites have been manufactured and tested.388 They have shown easy handling and shaping according to the implant site geometry, total resorbability, good ability to bond directly to the bone tissue without interposed fibrous tissue, osteoconductivity, biocompatibility and high stiffness that can be retained for the period necessary to achieve bone union.388 The initial bending strength of ~280 MPa exceeded that of cortical bone (120–210 MPa), while the modulus was as high as 12 GPa.168 The strength could be maintained above 200 MPa up to 25 weeks in phosphate-buffered saline solution. Such biocomposites were obtained from precipitation of a PLLA/dichloromethane solution, where small granules of uniformly distributed CDHA microparticles (average size of 3 µm) could be prepared.167 Porous scaffolds of PDLLA and HA have been manufactured as well.332,397,398 Upon implantation into rabbit femora, a newly formed bone was observed, and biodegradation was significantly enhanced compared with single-phase HA bioceramics. This might be due to a local release of lactic acid, which, in turn, dissolves HA. In other studies, PLA and PGA fibers were combined with porous HA scaffolds. Such reinforcement did not hinder bone ingrowth into the implants, which supported further development of such biocomposites as bone graft substitutes.50,51,377,399,400
Blends (named SEVA-C) of EVOH with starch filled with 10–30 wt% HA have been fabricated to yield biocomposites with moduli up to ~7 GPa and a 30% HA loading.401-406 The incorporation of bioactive fillers, such as HA into SEVA-C, aimed to insure the bioactive behavior of the composite and to provide the necessary stiffness within the typical range of human cortical bone properties. These biocomposites exhibited a strong in vitro bioactivity, which was supported by the polymer’s water-uptake capability.407 However, the reinforcement of SEVA-C by HA particles was found to affect the rheological behavior of the blend. A degradation model of these biocomposites has been developed.408
Higher homologs poly(3-hydroxybutyrate), 3-PHB and poly(3-hydroxyvalerate), 3-PHV, show almost no biodegradation. Nevertheless, biocomposites of these polymers with calcium orthophosphates show a good biocompatibility both in vitro and in vivo.102,409-415 Both bioactivity and mechanical properties of these biocomposites can be tailored by varying the volume percentage of calcium orthophosphates. Similarly, biocomposites of PHBHV with both HA and amorphous carbonated apatite (almost ACP) appeared to have promising potential for repair and replacement of damaged bones.416-419
Along these lines, PCL is used as a slowly biodegradable but good biocompatible polymer. PCL/HA and PCL/CDHA biocomposites have already been discussed as suitable materials for substitution, regeneration and repair of bone tissues.328,420-433 For example, biocomposites were obtained by infiltration of Σ-caprolactone monomer into porous apatite blocks and by in situ polymerization.423 The composites were found to be biodegradable and might be applied as cancellous or trabecular bone replacement material or for cartilage regeneration. Both the mechanical performance and biocompatibility in osteoblast cell culture of PCL were shown to be strongly increased when HA was added.434 Several preparation techniques of PCL/HA biocomposites are known. For example, to make biocomposite fibers of PCL with nanodimensional HA, the desired amount of nanodimensional HA powder was dispersed in a solvent using magnetic stirrer, followed by ultrasonication for 30 min. Then, PCL was dissolved in this suspension, followed by solvent evaporation.435 The opposite preparation order has also been used: PCL was initially dissolved in chloroform at room temperature (7–10% weight/volume), then HA (~10 µm particle size) was suspended in the solution, sonicated for 1 min, followed by solvent evaporation160 or salt-leaching.436 The mechanical properties obtained by this technique were about one-third that of trabecular bone. In a comparative study, PCL and biological apatite were mixed in a 19:1 ratio in an extruder.437 At the end of the preparation, the mixture was cooled in an atmosphere of nitrogen. The authors observed that the presence of biological apatite improved the modulus, while concurrently increasing the hydrophilicity of the polymeric substrate. In addition, an increase in apatite concentration was found to increase both the modulus and yield stress of the composite, which indicated good interfacial interactions between the biological apatite and PCL. It was also observed that the presence of biological apatite stimulated osteoblasts’ attachment to the biomaterial and cell proliferation.437 In another study, a PCL/HA biocomposite was prepared by blending in melt form at 120°C until the torque reached equilibrium in the rheometer that was attached to the blender.438 Then the sample was compression molded and cut into specimens of appropriate size for testing. It was observed that the composite containing 20 wt% HA had the highest strength.438 However, a direct grafting of PCL onto the surface of HA particles seems to be the most interesting preparation technique.420 In another study, HA porous scaffolds were coated by a PCL/HA composite coating.54 In this system, PCL as a coating component was able to improve the brittleness and low strength of the HA scaffolds, while the particles in the coating improved the osteoconductivity and bioactivity of the coating layer. More complex formulations, such as PDLLA/PCL/HA,439 PLLA/PCL/HA440 and supramolecular PCL/functionalized HA441 biocomposites, have been prepared as well. Further details on both the PCL/HA biocomposites and the processing methodologies thereof might be found in reference 328.
A spread of human osteoblasts attached to PLA and PCL films reinforced with CDHA and sintered HA was shown to have higher stength than the polymers alone.184 Moreover, biochemical assays relating cell activity to DNA content allowed for the conclusion that cell activity was more intense for the composite films.184 Kim et al. coated porous HA blocks with PCL from dichloromethane solution and performed drug release studies. The antibiotic tetracycline hydrochloride was added into this layer, yielding a bioactive implant with drug release for longer than a week.54
Yoon et al. investigated the highest mechanical and chemical stability of FA by preparing FA/collagen biocomposites and studying their effect on osteoblast-like cell culture.442 The researchers found an increased cellular activity in FA composites compared with HA composites. This finding was confirmed in another study by means of variations in the fluoride content for FA-HA/PCL composites.443 An interesting phenomenon of fractal growth of FA/gelatin composite crystals (Fig. 3) was achieved by diffusion of calcium- and orthophosphate+ fluoride solutions from opposite sides into a tube filled with a gelatin gel.444-453 The reasons for this phenomenon are not quite clear yet; besides, up to now, nothing has yet been reported on a possible biomedical application for such very unusual structural composites.
Figure 3.
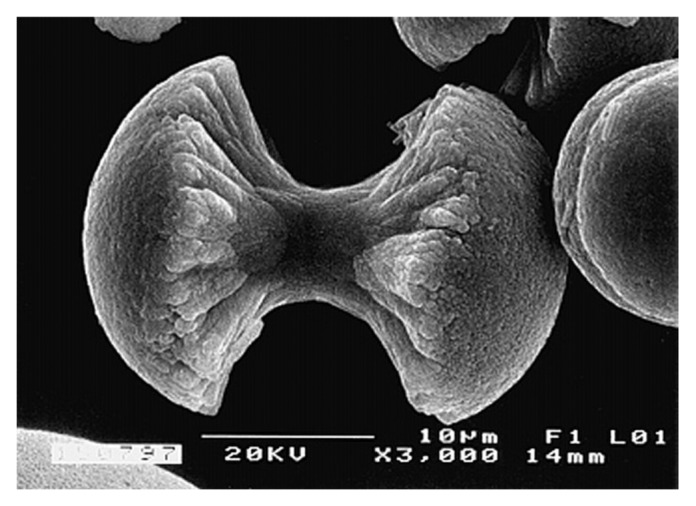
A biomimetically grown aggregate of FA that was crystallized in a gelatin matrix. Its shape can be explained and simulated by a fractal growth mechanism. Scale bar: 10 µm. Reprinted from reference 444 with permission.
TCP-based formulations
Both α-TCP and β-TCP have a higher solubility than HA (Table 3), and they are resorbed more quickly in vivo.454 Therefore, these calcium orthophosphates were widely used instead of apatites to prepare completely biodegradable biocomposites.456-479 For example, a biodegradable and osteoconductive biocomposite made of β-TCP particles and gelatin was proposed.466 This material was tested in vivo with good results. It was found to be biocompatible, osteoconductive and biodegradable, with no need for a second surgical operation to remove the device after healing occurred. Both herbal extracts467 and K2HPO4468 might be added to this formulation. Another research group prepared biocomposites of cross-linked gelatin with β-TCP, and both a good biocompatibility and bone formation upon subcutaneous implantation in rats were found.469 Yang et al.474 extended this to porous (porosity ~75%) β-TCP/gelatin biocomposites that also contained BMP-4. Porous β-TCP/alginate-gelatin hybrid scaffolds that were cell-compatible and possessing some osteoinductive properties were aso prepared and successfully tested in vitro.471 Biocomposites of β-TCP with PLLA462-464 and PLGC465 were prepared as well. Although β-TCP was able to counter the acidic degradation of the polyester to some extent, it did not prevent a pH drop down to ~6. Nevertheless, implantation of this biocomposite in beagles’ mandibular bones was successful.465 α-TCP/gelatin formulations are known as well.477
Based on a self-reinforcement concept, biocomposites of TCP with polylactides were prepared and studied using conventional mechanical testing.480 Resorbable scaffolds were fabricated from such biocomposites.481 Chitosan was used as the matrix for the incorporation of β-TCP by a solid/liquid phase separation of the polymer solution and subsequent sublimation of the solvent. Due to complexation of the functional groups of chitosan with calcium ions of β-TCP, these biocomposites had a high compressive modulus and strength.482 PCL/β-TCP biocomposites were developed in other studies,483-486 and their in vitro degradation behavior was systematically monitored by immersion in simulated body fluid at 37°C.485 To extend this topic further, PCL/β-TCP biocomposites might be loaded by drugs.486
Cell culture tests on β-TCP/PLLA biocomposites were reported; the biocomposites showed no cytotoxicity and evidenced good cell attachment to its surface.456 An in vitro study with primary rat calvarial osteoblasts showed an increased cellular activity in the BMP-loaded samples.474 Other researchers investigated BMP-2-loaded porous β-TCP/gelatin biocomposites (porosity ~95%, average pore size 180–200 µm)487 and confirmed the results of the previous study. Biocomposites of β-TCP and glutaraldehyde cross-linked gelatin were manufactured and tested in vitro to measure the material cytotoxicity.470 The experimental results revealed that the amount of glutaraldehyde cross-linking agent should be less than 8% to decrease toxicity on the osteoblasts and to avoid inhibition of cellular growth caused by the release of residual or un-cross-linked glutaraldehyde. A long-term implantation study of PDLLA/ α -TCP composites in a loaded sheep implant model showed good results after 12 mo but a strong osteolytic reaction after 24 mo. This was ascribed to the almost complete dissolution of α -TCP at this time and an adverse reaction of the remaining PDLLA.488
More complex calcium orthophosphate-based formulations are known as well. For example, there is a biocomposite consisting of three interpenetrating networks: TCP, CDHA and PLGA.489 First, a porous TCP network was produced by coating a polyurethane foam with a hydrolysable α-TCP slurry. Then, a CDHA network was derived from a calcium orthophosphate cement and used to fill in the porous TCP network. Finally, the remaining open pore network in the CDHA/ α-TCP structures was infiltrated with PLGA. This biocomposite consists of three phases with different degradation behaviors. It was postulated that bone would grow on the fastest degrading network of PLGA, while the remaining calcium orthophosphate phases would remain intact, thus maintaining their geometry and load-bearing capability.489
Formulations based on other calcium orthophosphates
The number of research publications devoted to formulations based on other calcium orthophosphates is substantially less than those devoted to apatites and TCP. Biphasic calcium phosphate (BCP), which is a solid composite of HA and β-TCP (however, similar formulations of HA and α-TCP, as well as of α-TCP and β-TCP, are possible as well) appears to be most popular among the remaining calcium orthophosphates. For example, collagen-coated BCP ceramics were studied, and their biocompatibility toward osteoblasts was found to increase upon coating with collagen.490 Another research group created porous PDLLA/BCP scaffolds and coated them with a hydrophilic PEG/vancomycin composite for both drug delivery purposes and surface modification.491 More relevantly, both PLGA/BCP492,493 and PLLA/BCP494 biocomposites were fabricated, and their cytotoxicity and fibroblast properties were found to be acceptable for natural bone tissue reparation, filling and augmentation.495,496 PCL/BCP497 and gelatin/BCP498,499 biocomposites are known as well.
A choice of DCPD-based biocomposites of DCPD, albumin and duplex DNA was prepared by a water/oil/water interfacial reaction method.313 Core-shell-type DCPD/chitosan biocomposite fibers were prepared by a wet spinning method in another study.500 The energy-dispersive X-ray spectroscopy analysis indicated that Ca and P atoms were mainly distributed on the outer layer of the composite fibers; however, a small number of P atoms remained inside the fibers. This indicated that the composite fibers formed a unique core-shell structure with a shell of calcium orthophosphate and core of chitosan.500 A similar formulation was prepared for further applications in bone cement biocomposites.501 DCPA/BSA biocomposites were synthesized through the coprecipitation of BSA on the nanodimensional particles of DCPA performed in ethanol.502 Nanodimensional DCPA was synthesized and incorporated into dental resins to form dental biocomposites.503-505 As an aside, it is interesting to mention that some DCPD/polymer composites could be used as proton conductors in battery devices.506,507 Nothing has been reported on their biocompatibility yet, but perhaps sometime, improved formulations will be used to fabricate biocompatible batteries for implantable electronic devices.
Various ACP-based biocomposites and hybrid formulations for dental applications have been developed,508-511 and several ACP-based formulations have been investigated as potential biocomposites for bone grafting419,512-514 and drug delivery.515 ACP/PPF biocomposites were prepared by in situ precipitation,513 while PHB/carbonated ACP and PHBHV/carbonated ACP biocomposites appeared to be well-suited as slowly biodegradable bone substitution materials.419 Another example is hybrid nanodimensional capsules, ~50–70 nm in diameter, which were fabricated by ACP mineralization of shell cross-linked polymer micelles and nano-sized cages.514 These nano-sized capsules consisted of a continuous ultrathin inorganic surface layer that infiltrated the outer cross-linked polymeric domains. They might be used as structurally robust, pH-responsive biocompatible hybrid nanostructures for drug delivery, bioimaging and therapeutic applications.514
Self-setting formulations and concretes
Inorganic self-setting calcium orthophosphate cements, which harden in the body, were introduced by LeGeros et al.516 and Brown and Chow517,518 in the early 1980s.519 Since then, these cements have been broadly studied, and many formulations have been proposed. The cements set and harden due to various chemical interactions among calcium orthophosphates, which finally lead to formation of a monolithic body consisting of either CHDA or DCPD, with possible admixtures of other phases. Unfortunately, because of their ceramic natures, calcium orthophosphate cements are brittle after hardening, and the setting time is sometimes unsuitable for clinical procedures.519 Therefore, various attempts have been made to transform the cements into biocomposites, e.g., by adding hydroxylcarboxylic acids to control the setting time,520 gelatin to improve both the mechanical properties and the setting time473,521-523 or osteocalcin/collagen to increase the bioactivity.524 More to the point, various reinforcement additives of different shapes and nature are widely used to improve the mechanical properties of calcium orthophosphate cements. Even carbon nanotubes were used for this purpose!525 Although the biomaterials community does not use this term, a substantial amount of the reinforced cement formulations might be defined as calcium orthophosphate-based concretes.526 The idea behind the concretes is simple: if a strong filler is present in the matrix, it might stop crack propagation.
Various apatite-containing biocomposite formulations based on PMMA527-540 and PEMA102,541,542 have been already developed. Such biocomposites might be prepared by dispersion of apatite powder into a PMMA viscous fluid543 and could be used for drug delivery purposes.544 When the mechanical properties of the concretes composed of PMMA matrix and HA particles of various sizes were tested, the tensile results showed that strength was independent of particle size. In addition, up to 40% more weight in HA could be added without impairing the mechanical properties.530,531 After immersion into Ringer’s solution, the tensile strength was not altered, whereas the fatigue properties were significantly reduced. The biocompatibility of PMMA/HA biocomposites was tested in vivo, and enhanced osteogenic properties of the implants compared with single-phase PMMA was observed.528,532-535 It was shown that not only the mechanical properties of PMMA were improved, but the osteoblast response of PMMA was also enhanced with the addition of HA.532 Thereby, by adding of calcium orthophosphates, a non-biodegradable PMMA was made more bioactive and osteoconductive, yielding a well-processible biocomposite concrete. As a drawback, the PMMA/HA formulations possess a low flexural, compressive and tensile strength.
A biocomposite made from HA granules and bis-phenol- α-glycidylmethacrylate-based resin appeared to possess comparable mechanical and biological properties to typical PMMA cement, leading to potential uses for implant fixation.545 To improve the mechanical properties of calcium orthophosphate cements and stabilize them at the implant site, various researchers have resorted to formulations that set in situ, primarily through cross-linking reactions of the polymeric matrix. For example, TTCP reacting with PAA formed a cross-linked CDHA/calcium polyacrylate biocomposite.546 In aqueous solutions, TTCP hydrolyzes to CDHA,27 and the liberated calcium cations react with PAA, forming the cross-linked network.546 Reed et al. synthesized a dicarboxy polyphosphazene that can be cross-linked by calcium cations, and cement-based (TTCP + DCPD) CDHA/polyphosphazene biocomposites with a compressive strength ~10 MPa and of ~65% porosity were prepared as a result.547 To mimic PMMA cements, PFF/β-TCP biocomposites were prepared with the addition of vinyl monomer to cross-link PPF. As a result, quick-setting and degradable biocomposite cements with a low heat output and compressive strengths in the range of 1–12 MPa could be prepared by varying the molecular weight of PPF as well as the contents of the monomer, β-TCP initiator and NaCl as a porogen.548,549 An acrylic cement with Sr-containing HA as a filler,138 an injectable polydimethylsiloxane/HA cement,550 biocomposites consisting of PLGA microspheres and a calcium orthophosphate cement551,552 as well as a hybrid cement formulation of chitosan oligosaccharide/gelatin/calcium orthophosphate553 were prepared as well.
In order to improve the mechanical properties of self-setting formulations, numerous researchers blended various polymers with the cements. For example, gelatin might be added to calcium orthophosphate cement formulations primarily to stabilize the paste in aqueous solution before it develops adequate rigidity and, second, to improve the compressive strength.473,521,554 Adding rod-like fillers to the self-setting formulations also caused an improvement in the mechanical properties.554 For example, PAA and PVA were successfully used to improve the mechanical properties of a TTCP + DCPD cement but, unfortunately, with an inevitable and unacceptable reduction of both workability and setting time.555,556 Similar findings were reported in the presence of sodium alginate and sodium polyacrylate.557 Other polymers, such as polyphosphazene, might be used instead.558-560 Other examples of polymer/calcium orthophosphate cement formulations might be found elsewhere.561,562
Porous calcium orthophosphate scaffolds with interconnected macropores (~1 mm), micropores (~5 µm) and of high porosity (~80%) were prepared by coating polyurethane foams with a TTCP + DCPA cement, followed by firing at 1,200°C. In order to improve the mechanical properties of the scaffolds, the open micropores of the struts were then infiltrated by a PLGA solution to achieve an interpenetrating bioactive ceramic/biodegradable polymer composite structure. The PLGA-filled struts were further coated with a 58S bioactive glass/PLGA composite coating. The complex porous biocomposites obtained could be used as tissue engineering scaffolds for low load-bearing applications.563 A more complicated construction, in which the PLGA macroporous phase has been reinforced with a bioresorbable TTCP + DCPA cement, followed by surface coating of the entire construct by a non-stoichiomentic CDHA layer, has been designed as well.564 The latter approach has culminated in a unique, three-phase biocomposite that is simple to fabricate, osteoconductive and completely biodegradable.
A porosity level of 42–80% was introduced into calcium orthophosphate cement/chitosan biocomposites by the addition of the water-soluble mannitol.565 Chitosan significantly improved the mechanical strength of the entire biocomposite.566 A similar approach was used by other researchers who studied the effect of the addition of PLGA microparticles567-570 (which can also be loaded with drugs or growth factors571-573) to calcium orthophosphate cements. These biocomposites were implanted into cranial defects of rats, and a content of ~30 wt% of the microparticles was found to give the best results,567 while the addition of a growth factor to the biocomposites significantly increased bone contact at 2 weeks and enhanced new bone formation at 8 weeks.573 The in vivo rabbit femur implant tests showed that PLGA/calcium orthophosphate cement formulations exhibited outstanding biocompatibility and bioactivity as well as a better osteoconduction and degradability than pure calcium orthophosphate cements.568
Formulations based on nanodimensional calcium orthophosphates and nanodimensional biocomposites
Nanodimensional and nanophasic materials are materials that have particles or grain sizes less than 100 nm, respectively. Thus, one should clearly differentiate between nanodimensional composites and composites based on nanodimensional compounds. The former might be any type of composite that has been disintegrated to particles with dimensions < 100 nm, while the latter are made up of two or more materials, in which at least one of the materials is of a nanometer scale.
Nanodimensional and nanophasic materials have different mechanical and optical properties than large grained materials of the same chemical composition. In particular, they possess unique surface properties, such as an increased number of atoms, grain boundaries and defects at the surface, huge surface area and altered electronic structure compared with conventional micron-sized materials. For example, nanodimensional HA (size ~67 nm) has a higher surface roughness of 17 nm compared with 10 nm for the conventional submicron size HA (~180 nm), while the contact angles (a quantitative measure of the wetting of a solid by a liquid) are significantly lower for nanodimensional HA (6.1) if compared with the conventional HA (11.51). Additionally, the diameter of individual pores in nanodimensional HA compacts is five times smaller (pore diameter ~6.6 Å) than that in the conventional grain-sized HA compacts (pore diameter within 19.8–31.0 Å).574-576 Besides, nanodimensional HA promotes osteoblast cell adhesion, differentiation and proliferation, osteointegration and deposition of calcium-containing minerals on its surface better than microcrystalline HA, thus enhancing formation of a new bone tissue within a short period.574-576 More to the point, nanodimensional HA was found to cause apoptosis of the leukemia P388 cells.577
Natural bones and teeth are hierarchical biocomposites of biological origin based on nanodimensional compounds, because they consist of nano-sized blade-like crystals of biological apatite grown in intimate contact with the organic matrix, which is rich in bioorganic fibers and organized in complicated hierarchical structures. Given the fact that the major bioorganic phase of bones is collagen, i.e., a natural polymer (Table 1), it is obvious that a composite of a nanodimensional calcium orthophosphate with a biodegradable polymer should be advantageous as bone substitution material. The inorganic nanodimensional phase would be responsible for the mechanical strength (hardness) and bioactivity, while the polymeric phase would provide the elasticity. In addition, the solubility of calcium orthophosphates depends on their crystallite size (smaller crystals have a higher solubility) and on their carbonate content (higher carbonate content increases the solubility).578 To the author’s best knowledge, among the calcium orthophosphates listed in Table 3, only apatites (CDHA, HA and, perhaps, FA) were available in a nanodimensional state until very recently. However, recently, nano-sized DCPA503-505 and nano-sized MCPM579 have been synthesized and applied to prepare biocomposites with strong ionic release to combat tooth caries. Presumably, all the calcium orthophosphates in Table 3 might be manufactured in a nanodimensional and/or nanocrystalline state; however, not all of them have been prepared yet.
A number of investigations have been conducted recently to determine the mineralization, biocompatibility and mechanical properties of biocomposites based on various (bio)polymers and nanodimensional HA. Unfortunately, in the majority of the papers that have been published, it is unclear whether “nanodimensional HA,” in fact, represented the nanodimensional stoichiometric HA or a nanodimensional non-stoichiometric CDHA. These studies covered biocomposites with PLA332,580-589 and its copolymer with PGA,590-593 collagen,594-607 collagen + PLA,607-615 collagen + PVA,616 collagen + alginate,617,618 gelatin,619-624 PPF,625-627 polyamide,310,311,628-639 PVA,340,341,640-642 PVAP,345 poly(ethylene-co-acrylic) acid,643,644 chitosan645-651 and its derivatives,652 konjac glucomannan + chitosan,653 PHEMA + PCL,654 PCL,390,435,655,656 cellulose,70,71,657-659 Ti,660-662 PCL semi-interpenetrating biocomposites663 and many other biocompatible hybrid formulations.279,320,335,417,664-683 Furthermore, each of the aforementioned formulations might be covered by a layer of nanodimensional calcium orthophosphate, as was done by Zandi et al., who coated a biocomposite of nano-sized rods HA with gelatin by nano-sized HA. Several nanodimensional biocomposites were found to be applicable as carriers for delivery of drugs and growth factors38,685-687 and were promising as vectors with ultra high gene loading and transfection efficiency.688 Data are available on the excellent biocompatibility of such biocomposites.605 The dispersion state of nano-sized particles appears to be the critical parameter in controlling the mechanical properties of nanodimensional biocomposites, as nano-sized particles always tend to aggregate owing to their high surface energy.417 A comparison was made of the mechanical properties of biocomposites with nano-sized and micron-sized HA with a polyamide. The results showed that the bending and tensile strengths of the biocomposite increased with increasing content of nanodimensional HA but decreased with increasing micron-sized HA content.310 A SEM image of the mineralized collagen fibrils demonstrating homogeneity of the nanodimensional biocomposite and the close interaction between the mineral phase and the reconstituted collagen fibrils is shown in Figure 4.689
Figure 4.
Scanning electron microscopy image of reconstituted mineralized collagen I fibrils. An example of an organic-inorganic nanostructural composite, mimicking the extracellular matrix of bone tissue on the nanometer scale. Reprinted from reference 689 with permission.
Porous (porosity ~85%) biocomposites of nano-sized HA with collagen and PLA have been prepared by precipitation and freeze-drying; these biocomposites did not show a pH drop upon in vitro degradation.608-610 They were implanted in the radius of rabbits and showed a high biocompatibility and partial resorption after 12 weeks. Nano-sized HA/chitosan biocomposites with improved mechanical stability were prepared from HA/chitosan nano-sized rods.690 Nano-sized HA/PLLA biocomposites of high porosity (~90%) were prepared using thermally induced phase separation.691 Nanodimensional HA was also used to prepare biocomposites with PAA, and the nanostructure of the resulting nano-sized crystals exhibited a core-shell configuration.692,693
Nanodimensional crystals of HA appear to be suitable for intraosseous implantation and offer the potential to formulate enhanced biocomposites for clinical applications.694 For example, the biocompatibility of chitosan in osteoblast cell culture was significantly improved by the addition of nano-sized HA.695 Similar findings are valid for nanodimensional HA/polyamide biocomposites.630 Further details on nanodimensional biocomposites might be found in an excellent review in reference.36 More to the point, a more general review on applications of nanodimensional biomaterials in orthopedics is also available,696 where interested readers are referred.
Biocomposites with collagen
The main constituent of the bioorganic matrix of bones is type I collagen (Table 1) with molecules about 300 nm in length. The structural and biochemical properties of collagens have been widely investigated, and over 25 collagen subtypes have been identified.697,698 This protein is conducive to crystal formation in the associated inorganic matrix. It is easily degraded and resorbed by the body and allows good attachment to cells. Collagen alone is not effective as an osteoinductive material, but it becomes osteoconductive in combination with calcium orthophosphates.699 Both collagen type I and HA were found to enhance osteoblast differentiation,700 but together, they were shown to accelerate osteogenesis. However, this tendency is not so straightforward: in the available data, implanted HA/collagen biocomposites enhanced regeneration of calvaria bone defects in young rats but postponed the regeneration of calvaria bone in aged rats.701 Finally, the addition of calcium orthophosphates to collagen sheets was found to give a higher stability and an increased resistance to 3D swelling compared with collagen.702 Therefore, a bone analog based on these two constituents should possess remarkable properties. Furthermore, addition of bone marrow constituents gives osteogenic and osteoinductive properties to calcium orthophosphate/collagen biocomposites.1
The unique characteristics of bones originate from the spatial orientation between the nanodimentional crystals of biological apatite and collagen macromolecules at the nano scale,39 where the crystals (about 50 nm length) are aligned parallel to the collagen fibrils,25,26,35,42 which is believed to be the source of the mechanical strength of bones. The collagen molecules and the crystals of biological apatite assembled into mineralized fibrils are approximately 6 nm in diameter and 300 nm long.35,39,42,609,703 Although the complete mechanisms involved in the bone building strategy are still unclear, the strengthening effect of nanodimentional crystals of biological apatite in calcified tissues might be explained by the fact that the collagen matrix is a load-transfer medium and thus transfers the load to the intrinsically rigid inorganic crystals. Furthermore, the crystals of biological apatite located in between tangled fibrils cross-link the fibers, either through mechanical interlocking or by forming calcium ion bridges, thus increasing deformation resistance of the collagenous fiber network.704
When calcium orthophosphates are combined with collagen in a laboratory, the prepared biocomposites appear to be substantially different from natural bone tissue due to a lack of real interaction between the two components, i.e., the interactions that are able to modify the intrinsic characteristics of the singular components themselves. The main characteristic of the route by which the mineralized hard tissues are formed in vivo is that the organic matrix is laid down first, and the inorganic reinforcing phase grows within this organic matrix.25,26,35,42 Although, to date, neither the elegance of the biomineral assembly mechanisms nor the intricate composite nano-sized architectures have been duplicated by nonbiological methods, the best way to mimic bone is to copy the way it is formed, namely by nucleation and growth of CDHA nano-sized crystals from a supersaturated solution both onto and within the collagen fibrils.705-707 Such syntheses were denoted as “biologically inspired” which means they reproduce an ordered pattern and an environment very similar to natural ones.708-710 The biologically inspired biocomposites of collagen and calcium orthophosphates (mainly, apatites) for bone substitute have a long history,33,442,597,711-730 which began with the pioneering study by Mittelmeier and Nizard,731 who mixed calcium orthophosphate granules with a collagen web. Such combinations were found to be bioactive, osteoconductive, osteoinductive,33,699,732-734 and, in general, artificial grafts manufactured from this type of biocomposites are likely to behave similarly to bones and be of more use in surgery than those prepared from any other materials. Indeed, data are available on the superiority of calcium orthophosphate/collagen biocomposite scaffolds over the artificial polymeric and calcium orthophosphate bioceramic scaffolds individually.735
It has been found that calcium orthophosphates may be successfully precipitated onto a collagen substrate of whatever form or source.33,40,597,736,737 However, adherence of calcium orthophosphate crystals to collagen does depend on how much the collagen had been denatured: the more fibrillar the collagen, the greater attachment. Clarke et al. first reported the production of a biocomposite produced by precipitation of DCPD onto a collagen matrix with the aid of phosphorylated amino acids commonly associated with fracture sites.716 Apatite cements (DCPD + TTCP) have been mixed with a collagen suspension, hydrated and allowed to set. CDHA crystals were found to nucleate on the collagen fibril network, producing a material with weaker mechanical properties than those reported for bone. Even more significantly, the prepared biocomposites were without a nanostructure similar to that of bone.713,738 The oriented growth of OCP crystals on collagen was achieved by an experimental device in which Ca2+ and PO43- ions diffused into a collagen disc from the opposite directions.737,739,740 Unfortunately, these experiments were designed to simulate the mechanism of in vivo precipitation of biological apatite only; for this reason, the mechanical properties of the biocomposites were not tested.741
Conventionally, collagen/calcium orthophosphate biocomposites have been prepared by blending or mixing of collagen and calcium orthophosphates, as well as by biomimetic methods.33,36,38,41,594,597,609,686,703,708-710,713,736,745-757 For example, Tampieri et al.710 produced and compared artificial bone like tissue apatite/collagen biocomposites prepared using two different methodologies: (1) dispersion of apatite in a collagen aqueous suspension followed by freeze-drying and (2) direct nucleation of an apatitic phase on assembling collagen fibrils. Biocomposites obtained using first method were similar to uncalcified natural collagen. The crystallite sizes were not uniform and were often aggregated and randomly distributed into the matrix, proving that there was no real interaction between apatite and collagen fibers. However, the second method allowed the direct nucleation of nano-sized crystals of apatite on self-assembled collagen fibers. In this case, the two components (CDHA and collagen) exhibited strong interactions, highlighted by several analysis techniques, which showed a complete analogy of the composite with calcified natural tissue.710 Other production techniques are also possible. For example, using a polymer-induced, liquid-precursor process, collagen/apatite biocomposites mimicking the nanostructure of bones, wherein nano-sized crystals of apatite were embedded within the collagen fibrils, were prepared.757 More complicated formulations, such as magnetite-enriched HA/collagen758 and HA/collagen/PVA759 biocomposites, have been developed as well.
Furthermore, collagen might be incorporated into various calcium orthophosphate cements.713,738,760-764 Typically, a type I collagen sponge is presoaked in a PO43--containing, highly basic aqueous solution and then immersed into a Ca2+-containing solution to allow mineral deposition. Alternatively, collagen I fibers might be dissolved in acetic acid, then this solution added to phosphoric acid, followed by a neutralization synthesis (performed at 25°C and solution pH within 9–10) between an aqueous suspension of Ca(OH)2 and the H3PO4/collagen solution.708,709 To ensure the quality of the final product, it is necessary to control the Ca/P ionic ratio in the reaction solution. One way to do this is to dissolve a commercial calcium orthophosphate in an acid; another is to add Ca2+ and PO43- ions in a certain ratio to the solution and, after that, induce the reaction.39 Biomimetically, one can achieve an oriented growth of CDHA crystals onto dissolved collagen fibrils in aqueous solutions via a self-organization mechanism.747 A number of authors produced calcium orthophosphate/collagen biocomposites by mixing preformed ceramic particles with a collagen suspension.765-767 However, in all blended composites, the crystallite sizes of calcium orthophosphates were not uniform, and the crystals were often aggregated and randomly distributed within a fibrous matrix of collagen. Therefore, no structural similarity to natural bone was obtained, and only a compositional similarity to that of natural bone was achieved. Instead, CDHA crystallization from aqueous solutions might be performed in the presence of a previously dispersed collagen,33,597 or, more to the point, collagen might be first dispersed in an acidic solution, followed by addition of calcium and orthophosphate ions; then, coprecipitation of collagen and CDHA might be induced by either increasing the solution pH or adding mixing agents.41 Although it resulted in biocomposites with poor mechanical properties, pressing of the apatite/collagen mixtures at 40°C under 200 MPa for several days is also possible.768 Attempts have been made to create a computer simulation of the apatite/collagen composite formation process.769 It is interesting to note that such biocomposites were found to possess some piezoelectric properties.770
As the majority of the collagen/HA biocomposites are conventionally processed by anchoring micron-sized HA particles into a collagen matrix, it is quite difficult to obtain a uniform and homogeneous composite graft. Besides, such biocomposites have inadequate mechanical properties. Over and above, the proper pore sizes have not been achieved either. Further, microcrystalline HA, in contrast to nanocrystalline bone apatite, might take a longer time to be remodeled into a new bone tissue upon implantation. In addition, some of the biocomposites exhibit very poor mechanical properties, probably due to a lack of strong interfacial bonding between the constituents. The aforementioned data clearly demonstrate that a chemical composition similar to bone is insufficient for manufacturing the proper grafts; both the mechanical properties and mimetic bone nanostructure are necessary for a graft to function as bone in recipient sites. There is a chance for improving osteointegration by reducing the grain size of HA crystals by activating ultrafine apatite growth into the matrix. This may lead to the enhancement of mechanical properties and osteointegration, with improved biological and biochemical affinity to the host bone. Besides, porosity was found to have a positive influence on the ingrowth of the surrounding tissues into the pores of collagen/HA biocomposites.771,772
Another approach is to mix bovine collagen with calcium orthophosphates. These biocomposites are marketed commercially as bone graft substitutes and can further be combined with bone marrow aspirated from the iliac crest of the site of the fracture. Collagraft®, BioOss® and Healos® are several examples of the commercially available grafts for clinical use.36 Application of these materials was compared with autografts for the management of acute fractures of long bones with defects that had been stabilized by internal or external fixation.773,774 These biocomposites are osteogenic, osteoinductive and osteoconductive; however, they lack structural strength and require a harvest of the patient’s bone marrow. Although no transmission of diseases has been recorded yet, the use of bovine collagen might be a source of concern.2
Collagen sponges with an open porosity (30–100 µm) were prepared by a freeze-drying technique, and then their surface was coated by a 10 µm layer of biomimetic apatite precipitated from simulated body fluid.775 The researchers found a good in vitro performance with fibroblast cell culture. Other preparation techniques are also possible.776 Collagen/HA microspheres or gel beads have been prepared with the intention of making injectable bone fillers.777,778 Liao et al. succeeded in mimicking the bone structure by blending carbonateapatite with collagen.779 A similar material (mineralized collagen) was implanted into femur of rats, and excellent clinical results were observed after 12 weeks.780 Collagen/HA biocomposites were prepared, and their mechanical performance was increased by cross-linking the collagen fibers with glutaraldehyde.598,600,601 These biocomposites were tested in rabbits and showed a good biological performance, osteoconductivity and biodegradation. A similar approach was selected to prepare HA/collagen microspheres (diameter ~5 µm) by a water-oil emulsion technique in which the surface was also cross-linked by glutaraldehyde.778 That material showed a good in vitro performance with osteoblast cell culture. A porous bone graft substitute was formed from a nano-sized HA/collagen biocomposite combined with PLA by a freeze-drying method; the resulting material was found to mimic natural bones at several hierarchical levels.609 Subsequent in vitro experiments confirmed a good adhesion, proliferation and migration of osteoblasts into this composite.608 A further increase in biocompatibility might be achieved by the addition of various dopants. For example, to enhance bone substitution, Si-substituted HA/collagen composites have been developed, with silicon located preferentially in the collagen phase.599 Porous (porosity level ~95% with interconnected pores of 50–100 µm) biocomposites of collagen (cross-linked with glutaraldehyde) and β-TCP have been prepared by a freeze-drying technique, followed by sublimation of the solvent; the biocomposites showed a good biocompatibility upon implantation in the rabbit jaw.781
Biocomposites of calcium orthophosphates with collagen were found to be useful for drug delivery purposes.618,721,764,782-784 Namely, an HA/collagen-alginate (20 µl) with rh-BMP2 (100 µg/ml, 15 µl) showed bone formation throughout the implant 5 weeks after implantation without obvious deformation of the material.618 Gotterbarm et al. developed a two-layered collagen/β-TCP implant augmented with chondral inductive growth factors for repair of osteochondral defects in the trochlear groove of minipigs. This approach might be a promising new option for the treatment of deep osteochondral defects in joint surgery.783
To conclude this part, one should note that biocomposites of apatites with collagen are a very hot topic in research, and, up to now, just a few papers are devoted to biocomposites of other calcium orthophosphates with collagen.742-744,783,785-788 These biomaterials mimic natural bones to some extent, while their subsequent biological evaluation suggests that they are readily incorporated into the bone metabolism in a way similar to bone remodeling instead of acting as permanent implant.609,731 However, the performance of these biocomposites depends on the source of the collagen from which it was processed. Several attempts have been made to simulate the collagen-HA interfacial behavior in real bone by means of cross-linking agents, such as glutaraldehyde,598,600,601,736,778,781 with the intention of improving the mechanical properties of these biocomposites. Unfortunately, further progress in this direction is restricted by high cost, the difficulty of controlling cross-infection, a poor definition of commercial sources of collagens as well as by a lack of appropriate technology for fabricating bone-resembling microstructures. Further details on calcium orthophosphate/collagen biocomposites might be found elsewhere.36,725
Formulations with other bioorganic compounds and/or biological macromolecules
The biggest practical problems with collagen type I are its cost and the poor definition of commercial sources of this material, which makes it difficult to follow up on well-controlled processing. Therefore, collagen type I can be replaced by other compounds. One should notice that, besides collagen, both human and mammalian bodies contain dozens types of various bioorganic compounds, proteins and biological macromolecules. The substantial amount of them potentially might be used to prepare biocomposites with calcium orthophosphates. For example, a biologically strong adhesion (to prevent invasion of bacteria) between teeth and the surrounding epithelial tissues is attributed to a cell-adhesive protein, laminin.789 In order to mimic nature, a laminin/apatite biocomposite layer was successfully created on the surface of both titanium790 and EVOH791,792 using the biomimetic approach. A more complicated laminin/DNA/apatite biocomposite layer was found to be an efficient gene transfer system.793
Calcium orthophosphate/gelatin biocomposites have been widely investigated as potential bone replacement biomaterials.317,336-338,444-452,466-474,487,521-523,554,619-624,794-807 For example, gelatin foams were successfully mechanically reinforced by HA and then cross-linked by a carbodiimide derivative.317 Such foams were shown to be good carriers for antibiotic tetracycline.798 Several biocomposites of calcium orthophosphates with alginates have been prepared.471,617,618,622,709,808,809 For example, porous HA/alginate composites based on hydrogels were prepared both biomimetically709 and using a freeze-drying technique.808 Another research group succeeded in preparing biphasic but monolithic scaffolds using a similar preparation route.810 Their biocompatibility in cell culture experiments and in vitro biodegradability were high; however, their mechanical strength could be better.
Various biocomposites of calcium orthophosphates with chitosan298,482,500,512,527,565,645-653,674,675,690,695,799,807,811-828 and chitin232,476,612,829-833 are also very popular. For example, a solution-based method was developed to combine HA powders with chitin, in which the ceramic particles were uniformly dispersed.829,830 Unfortunately, it was difficult to obtain uniform dispersions. The mechanical properties of the final biocomposites were not very good; due to a poor adhesion between the filler and the matrix, both the tensile strength and modulus were found to decrease with increase of the amount of HA. Microscopic examination revealed that HA particles were intervened between the polymer chains, weakening their interactions and decreasing the overall strength.829,830 Other manufacturing techniques might be found in the aforementioned references; I just would like to mention an interesting approach in which a HA/chitosan biocomposite was produced by a hydrothermal process from the natural CaCO3/chitosan biocomposite of crab shells.827 Biocomposites of natural HA with chitosan were found to possess both a good hard tissue biocompatibility and an excellent osteoconductivity, which is suitable for artificial bone implants and frame materials for tissue engineering.823 Data are available that show the addition of HA into chitosan improved cell attachment and provided a higher cell proliferation and well-spread morphology when compared with chitosan alone.650 More complex formulations, such as silk fibers, reinforced HA/chitosan834 and HA/collagen/chitosan835 biocomposites, have been studied as well. Interestingly, hybrid biocomposites of nano-sized HA with chitin/chitosan might be used for removal of Fe(III)836 and fluorides837,838 from aqueous solutions. Further details on the biocomposites and hybrid biomaterials of calcium orthophosphates with chitosan are available in the literature.836
Biocomposites of CDHA with water-soluble proteins, such as BSA, might be prepared by a precipitation method.561,839-842 In such biocomposites, BSA is not strongly fixed to solid CDHA, which is useful for a sustained release. However, this is not the case if a water/oil/water interfacial reaction route has been used.313 To extend this subject, inclusion of DNA into CDHA/BSA biocomposites was claimed.313,843-845 Furthermore, nanodimentional biocomposites of an unspecified calcium orthophosphate with DNA846 as well as biocomposites of nano-sized crystals of biomimetic apatite with C60 and Au-DNA nano-sized particles847 were also prepared.
Akashi and coworkers developed a procedure for preparing calcium orthophosphate-based biocomposites by soaking hydrogels in solutions supersaturated by Ca2+ and PO43- ions in order to precipitate CDHA in the hydrogels (up to 70% more weight in CDHA could be added to these biocomposites).848 This procedure was applied to chitosan; the 3D shape of the resulting biocomposite was controlled by the shape of the starting chitosan hydrogel.849 Another research group developed biocomposites based on in situ calcium orthophosphate mineralization of self-assembled supramolecular hydrogels.850 Other experimental approaches are also possible.851
Various biocomposites of CDHA with glutamic and aspartic amino acids as well as poly-glutamic and poly-aspartic amino acids have been prepared and investigated by Bigi et al.346,347,852-855 These (poly)amino acids were quantitatively incorporated into CDHA crystals, provoking a reduction of the coherent length of the crystalline domains and decreasing the crystal sizes. The relative amounts of the (poly)amino acid content in the solid phase, determined through HPLC analysis, increased with their concentration in solution up to a maximum of about 7.8 wt% for CDHA/aspartic acid and 4.3 wt% for CDHA/glutamic acid biocomposites. The small crystal dimensions, which implied a great surface area, and the presence of (poly)amino acids were suggested to be relevant for possible application of these biocomposites for hard tissues replacement.346,347,852-855 A schematic description of a biocomposite formation from amino acids and ACP is shown in Figure 5.856
Figure 5.
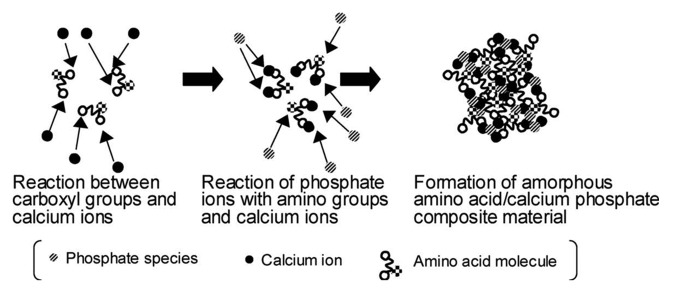
A proposed mechanism for the formation of ACP/amino acid biocomposites in aqueous solutions. Reprinted from reference 856 with permission.
Furthermore, BCP (HA + β-TCP)/agarose macroporous scaffolds with controlled and complete interconnection, high porosity, thoroughly open pores and tailored pore size were prepared for tissue engineering applications.857,858 Agarose, a biodegradable polymer, was selected as the organic matrix, because it is a biocompatible hydrogel that acts as gelling agent, leading to strong gels and fast room temperature polymerization. Porous scaffolds with the designed architecture were manufactured by combining a low temperature shaping method with stereo-lithography and two drying techniques. The biocompatibility of this BCP/agarose system was tested with mouse L929 fibroblasts and human SAOS-2 osteoblasts during different colonization times.859
Fibrin sealants are non-cytotoxic, fully resorbable biological matrices that simulate the last stages of a natural coagulation cascade, forming a structured fibrin clot similar to a physiological clot.860 Biocomposites of calcium orthophosphates with fibrin sealants might help develop the clinical applications of bone substitutes. The 3D mesh of fibrin sealant interpenetrates the macro- and microporous structure of calcium orthophosphate ceramics.11 The physical, chemical and biological properties of calcium orthophosphate bioceramics and the fibrin glue might cumulate in biocomposites suitable for preparation of advanced bone grafts.861-873
Furthermore, there are biocomposites of calcium orthophosphates with bisphosphonates,874 silk fibroin (that is a hard protein extracted from silk cocoon),312,670-672,677,678,875-881 chitosan + silk fibroin,882 fibronectin,883 chondroitin sulfate,299,733,884 casein phosphopeptides885 and vitamins.886 The reader’s attention is also drawn to an interesting approach to crystallizing CDHA inside poly(allylamine)/poly(styrene sulfonate) polyelectrolyte capsules, resulting in empty biocomposite spheres of micron size.887 Depending on the amount of precipitated CDHA, the thickness of the shell of biocomposite spheres can be varied between 25 and 150 nm. These biocomposite capsules might find application as medical agents for bone repairing and catalytic microreactors.887
Injectable bone substitutes (IBS)
With the development of minimally invasive surgical methods, for example, percutaneous surgery, directly injectable biomaterials are needed. The challenge is to place a biomaterial at the site of surgery by the least invasive method. In this regard, IBS appear to be a convenient alternative to solid bone-filling materials. They represent ready-to-use suspensions of calcium orthophosphate microspheres,888,889 nano-sized rods890 or powder(s) in a liquid carrier phase. However, addition of other phases, such as calcium sulfate,891 is also possible. They look like opaque viscous pastes with rheological properties sufficient to inject them into bone defects by means of surgical syringes and needles. Besides, IBS could be easily produced in a sterile stage. Their stable composition and mechanical properties are suitable for reproducibility of the biological response.892 All types of IBS are divided into two major groups: self-setting formulations and those that do not set. Cements and concretes belong to the former (see the “Self-setting formulations and concretes” section above), while those that fall into the latter are described here.
IBS requires suitable rheological properties to ensure bonding of the mineral phase in situ with good cell permeability. Usually, the necessary level of viscosity is created by addition of water-soluble polymers.131,893,894 Therefore, the majority of calcium orthophosphate-based IBS formulations might be considered a subgroup of calcium orthophosphate/polymer biocomposites. For example, an IBS was described that involved a silanized hydroxyethylcellulose carrier with BCP (HA + β-TCP).895 The suspension is liquid at a pH within 10–12, but it gels quickly at a pH < 9. Injectable composites can be formed with β-TCP to improve mechanical integrity.548 Similarly, Bennett et al. showed that a polydioxanone-co-glycolide-based biocomposite reinforced with HA or β-TCP can be used as an injectable or moldable putty.896 During the cross-linking reaction following injection, carbon dioxide is released, allowing the formation of interconnected pores. Furthermore, HA/poly(L-lactide-co-Σ-caprolactone) biocomposite microparticles were fabricated as an injectable scaffold via the Pickering emulsion route in the absence of any molecular surfactants. A stable injectable oil-in-water emulsion was obtained using water-dispersed HA nano-sized crystals as the particulate emulsifier and a dichloromethane solution of poly(L-lactide-co-Σ-caprolactone) as an oil phase.897
Daculsi et al. developed viscous IBS biocomposites based on BCP (60% HA + 40% β-TCP) and a 2% aqueous solution of HPMC that was said to be perfectly biocompatible, resorbable and easily fitted to bone defects (due to an initial plasticity).108,894,898-905 The best ratio BCP/HPMC aqueous solution was found to be at ~65/35 w/w. To extend this subject further, IBS might be loaded by cells,906,907 radiopaque elements908 or microparticles909 as well as be functionalized by nucleic acids.890 Self-hardening formulations based on Si-HPMC hydrogel are known as well.906 The list of the commercially available calcium orthophosphate-based IBS formulations is presented in Table 5.910
Table 5. A list of some commercial non-setting calcium orthophosphate IBS and pastes with indication of producer, product name, composition (when available) and form.910 .
| Producer | Product name | Composition | Form |
|---|---|---|---|
| ApaTech (UK) | Actifuse™ | HA, polymer and aqueous solution | pre-mixed |
| Actifuse™ Shape Actifuse™ ABX | Si-substituted calcium orthophosphate and a polymer | pre-mixed | |
| Baxter (US) | TricOs Τ TricOs |
BCP (60% HA, 40% β-TCP) granules and Tissucol (fibrin glue) | to be mixed |
| Berkeley Advanced Biomaterials | Bi-Ostetic Putty | not disclosed | not disclosed |
| BioForm (US) | Calcium hydroxylapatite implant | HA powder embedded in a mixture of glycerine, water and carboxymethylcellulose | pre-mixed |
| Biomatlante (FR) | MBCP Gel® | BCP granules (60% HA, 40% β-TCP; 0.08 – 0.2 mm) and 2% HPMC | pre-mixed |
| Hydr’Os | BCP granules (60% HA, 40% β-TCP; micro- and nano-sized particles) and saline solution | pre-mixed | |
| Degradable solutions (CH) | Easy graft™ | β-TCP or BCP granules (0.45 – l.0 mm) coated with 10 μm PLGA, N-methyl-2-pyrrolydone | to be mixed |
| Dentsply (US) | Pepgen P-15® flow | HA (0.25 – 0.42 mm), P-15 peptide and aqueous Na hyaluronate solution | to be mixed |
| DePuy Spine (US) | Healos® Fx | HA (20 – 30%) and collagen | to be mixed |
| Fluidinova (P) | nanoXIM TCP | β-TCP (5 or 15%) and water | pre-mixed |
| nanoXIM HA | HA (5, 15, 30 or 40%) and water | pre-mixed | |
| Integra LifeSciences (US) | Mozaik Osteoconductive Scaffold | β-TCP (80%) and type 1 collagen (20%) | to be mixed |
| Mathys Ltd (CH) | Ceros® Putty / cyclOS® Putty | β-TCP granules (0.125 – 0.71 mm; 94%) and recombinant Na hyaluronate powder (6%) | to be mixed |
| Medtronic (US) | Mastergraft® | BCP (85% HA, 15% β-TCP) and bovine collagen | to be mixed |
| Osartis / ΑΑΡ (GER) | Ostim® | Nanocrystalline HA (35%) and water (65%) | pre-mixed |
| Smith and Nephew (US) | JAXTCP | β-TCP granules and an aqueous solution of 1.75% carboxymethylcellulose and 10% glycerol | to be mixed |
| Stryker (US) | Calstrux™ | β-TCP granules and carboxymethylcellulose | to be mixed |
| Teknimed (FR) | Nanogel | HA (100 – 200 nm) (30%) and water (70%) | pre-mixed |
| Therics (US) | Therigraft™ Putty | β-TCP granules and polymer | pre-mixed |
| Zimmer (US) | Collagraft | BCP granules (65% HA, 35% β-TCP; 0.5 – 1.0 mm), bovine collagen and bone marrow aspirate | to be mixed |
The advanced characteristics of IBS come from their good rheological properties and biocompatibility and the ease of tissue regeneration. Although the fabrication of IBS biocomposites, in most cases, improved the mechanical properties of the system and provided the material with resistance to fluids penetration, these achievements were limited by the amount of polymer that can be added to the paste. For instance, Mickiewicz et al. reported that after a critical concentration (which depended on the type and molecular weight of the polymer but was always around 10%), the polymer started forming a thick coating on the crystal clusters, preventing them from interlocking, originating plastic flow and, as a consequence, decreasing their mechanical properties.561 More to the point, Fujishiro et al. reported a decrease in mechanical properties when using higher amounts of gel, which was attributed to formation of pores due to leaching of gelatin in solution.554 Therefore, it seems that mechanical properties, although improved by the addition of polymers, are still a limitation for the application of calcium orthophosphate-based IBS formulations in load-bearing sites.178 Further details on IBS might be found in a recent review in reference.892
Biocomposites with glasses, inorganic materials, carbon and metals
To overcome the problem of poor mechanical properties of calcium orthophosphate bioceramics, suitable biocomposites of calcium orthophosphates reinforced by various inorganic materials, glasses and metals have been developed. Such biocomposites are mainly prepared by common ceramic processing techniques, such as thermal treatment after kneading,911-913 powder slurry coating914 and metal-sol mixing.915 For example, HA was combined with Bioglass® (Novabone Products, Alachua, FL)916,917 and with other glasses918 to form glass-ceramic biocomposites. Other reinforcement materials for calcium orthophosphates are differentiated either by shape of the fillers, namely, particles,919,920 platelets,921,922 whiskers,579,923-925 fibers926-930 or by their chemical composition, zirconia and/or PSZ,313,911-914,923,931-966 alumina,313,919,922,965,967-996 other oxides,925,997–1004 silica and/or glasses,1005–1014 wollastonite,206,1015–1025 mullite,1026,1027 various metals and alloys,540,928,967,997,1028–1045 calcium sulfate,1046–1049 calcium carbonate,1050,1051 silicon carbide,683,924 barium titanate,1052 zeolite,1053 boron nitride1054 and several other materials.335,1055–1057 More complicated formulations, such as HA/aluminum oxide/carbon nanotubes,1058 have been developed as well. All these materials have been added to calcium orthophosphate bioceramics to improve their reliability. Unfortunately, significant amounts of the reinforcing phases are needed to achieve the desired properties and, as these materials are either bioinert, significantly less bioactive than calcium orthophosphates or not bioresorbable, the ability of the biocomposites to form a stable interface with bone is poor compared with calcium orthophosphate bioceramics alone. Due to the presence of bioinert compounds, such formulations might be called bioinert/bioactive composites.1005 The ideal reinforcement material would impart mechanical integrity to a biocomposite at low loadings without diminishing its bioactivity.
There are several types of HA/glass biocomposites. The first one is also called bioactive glass-ceramics. A dense and homogeneous biocomposite was obtained after a heat treatment of the parent glass, which comprised ~38 wt% oxy-FAP (Ca10(PO4)6(O,F)2) and ~34 wt% β-wollastonite (CaO·SiO2) crystals, 50–100 nm in size in a MgO-CaO-SiO2 glassy matrix.206,1015–1025 A-W glass-ceramics are an assembly of small apatite particles effectively reinforced by wollastonite. The bending strength, fracture toughness and Young’s modulus of A-W glass-ceramics are the highest among bioactive glass and glass-ceramics, enabling them to be used in some major compression load-bearing applications, such as vertebral prostheses and iliac crest replacement. They combine a high bioactivity with suitable mechanical properties.1059 β-TCP/wollastonite biocomposites are also known.1060–1062 More complicated formulations have been developed as well. For example, (A-W)/HDPE composite (AWPEX) biomaterials have been designed to match the mechanical strength of human cortical bone and to provide favorable bioactivity, with potential use in many orthopedic applications.1063–1066 Other examples include wollastonite-reinforced HA/Ca polycarboxylate,1067 glass-reinforced HA/polyacrylate1068 as well as collagen1069 and gelatin1070 calcium phosphate silicate/wollastonite biocomposites.
HA/glass biocomposites can be prepared by simple sintering of appropriate HA/glass powder mixtures.1071–1074 If sintering is performed below 1,000°C, HA does not react with the bioactive glass1072,1073 or the reaction is limited.1074 Besides, reactions between HA and glasses depend on the glass composition. In another approach, small quantities of bioactive glass have been added to HA bioceramics in order to improve densification and/ or mechanical properties.29 Biocomposites might also be sintered from HA and silica.1005 In general, bioactive glass-ceramics maintain a high strength for a longer time than HA bioceramics under both in vitro and in vivo conditions.1012,1019
Due to a huge difference in shapes, it is a challenge to prepare homogeneous mixtures of calcium orthophosphates and carbon nanotubes: “one can imagine something similar to achieving a homogeneous mixture of peas and spaghetti.”217 Nevertheless, different strategies might be employed to prepare calcium orthophosphate/carbon nanotube biocomposites. For example, apatites might be chemically synthesized by using carboxyl functionalized carbon nanotubes as a matrix.302-308 Physicochemical characterization of these biocomposites showed that nucleation of CDHA is initiated through the carboxyl group.302 Hot pressing,1075 plasma spraying,1076 laser surface alloying,1077–1079 spark plasma sintering1080 and precipitation1081 techniques might be applied as well. Due to carbon oxidation at elevated temperature, sintering of calcium orthophosphate/carbon nanotube biocomposites must be performed in a deoxidizing atmosphere.1082 The research on calcium orthophosphate (up to now, only apatites)/carbon nanotube biocomposites is in its early stages, with the first papers published in 2004.307,525 For this reason, the mechanical property data for such biocomposites have been reported only in a few papers; however, these results are encouraging. For example, Chen et al. performed nano-indentation tests on biocomposite coatings to give hardness and Young’s modulus values.1079 They found that the higher the loading of the nanotubes, the better the mechanical properties. Namely, at 20 wt% loading, hardness was increased by ~43% and Young’s modulus by ~21% over a single-phase HA coating.1079 Scratching test results indicated that alloyed HA biocomposite coatings exhibited improved wear resistance and a lower friction coefficient when the amount of carbon nanotubes in the precursor material powders was increased.1078 Additionally, measurements of the elastic modulus and hardness of the biocomposite coatings indicated that the mechanical properties were also affected by the amount of carbon nanotubes.1077 Another research group performed compression tests on bulk HA/carbon nanotubes biocomposites and found an increase in strength over single-phase HA.307 However, the highest compressive strength they achieved for any material was only 102 MPa, which is similar to that of cortical bone but much lower than the typical values for dense HA.217 More complex formulations, such as poly-l-lysine/HA/carbon nanotube hybrid biocomposites, have also been developed.1083 Furthermore, calcium orthophosphate/carbon nanotube biocomposites might be immobilized by hemoglobin.1084 Unfortunately, carbon nanotubes are very stable substances; they are neither bioresorbable nor biodegradable. Therefore, during in vivo bioresorption, the nanotubes will get into the human body from the biocomposite matrix and might cause uncertain health problems. Certainly, this problem must be solved. To conclude the carbon subject, one should mention the application of carbon fibers of microscopic dimensions,1085–1087 nanodimensional diamonds1088 and C60847 to reinforce HA bioceramics.
As clearly seen from the amount of references, apatite/zirconia biocomposites are the most popular among researchers. The main disadvantage of HA reinforced by PSZ is degradation of zirconia in wet environments.923,932,933,955 Transformation of the tetragonal ZrO2 to the monoclinic phase on the surface results in the formation of microcracks and, consequently, lowers the strength of the implant.1089,1090 Interestingly, though, Fe3O4/HA composites possess photocatalytic properties.1003,1004
Various biocomposites of calcium orthophosphates with metals and alloys have been fabricated as well.540,928,967,997,1028–1045 For example, an HA-based biocomposite reinforced with 20 vol.% of Ti particles was fabricated by hot pressing.1030 Calcium orthophosphate/Ti biocomposites might be prepared by powder metallurgy processing.1032–1034 At high temperatures, the presence of Ti metal phase was found to promote dehydration and decomposition of HA into β-TCP and TTCP1030,1032 or partial formation of β-TCP and calcium titanate instead of HA.661,1033,1034 Compared with pure HA bioceramics manufactured under the same conditions, the HA/Ti biocomposites possessed a higher fracture toughness, bending strength, work of fracture, porosity and lower elastic modulus, which makes them more suitable for biomedical applications. However, the mechanical properties appear not to be high enough to use HA/Ti biocomposites in load-bearing applications. Luckily, the histological evaluations revealed that HA/Ti biocomposites could be partially integrated with newborn bone tissues after 3 weeks and fully osteointegrated at 12 weeks in vivo.1030 Similar findings had earlier been made for HA bioceramics reinforced by the addition of silver particulates (5–30 vol. %) and subsequent sintering of the HA/Ag powder compacts.1028,1029 The addition of silver also imparts an antimicrobial activity.1042 Other studies on calcium orthophosphate/Ti biocomposites are available elsewhere in reference 1035–1038.
To conclude this section, biocomposites consisting only of calcium orthophosphates should be briefly described. First, all multiphasic and polyphasic calcium orthophosphates should be mentioned. For example, ca. 1980, BCP was described as “TCP ceramics complexed with HA,”1091 Even nowadays BCP is occasionally called a “nanocomposite,”1092 Furthermore, fluoridated HA [described by a chemical formula Ca10(PO4)6(OH)2 - xFx, where 0 < x < 2] might be mentioned as a composite;1093 however, the applicability of the term “composite” for such systems is doubtful. One should better consider 70% HA-powder + 30% HA-whisker biocomposites, which were fabricated by pressureless sintering, hot pressing and hot isostatic pressing. These biocomposites were found to exhibit an improved toughness, attaining the lower fracture toughness limit of bone without a decrease of bioactivity and biocompatibility.1094,1095 A dual HA biocomposite that combined two HA materials with different porosities: HA with 75% porosity, for bone ingrowth, and HA with 0% porosity, for load bearing, was also manufactured. This dual HA biocomposite appeared to be suitable for use as an implant material for spinal interbody fusion as a substitute for iliac bone grafts, which could eliminate the disadvantages associated with autograft harvesting.1096 A biodegradable biocomposite porous scaffold comprised of a β-TCP matrix and nano-sized fibers of HA was developed and studied for load-bearing bone tissue engineering. The nano-sized fibers of HA were prepared by a biomimetic precipitation method, the inclusion of which significantly enhanced the mechanical property of the scaffold, attaining a compressive strength of 9.87 MPa, comparable to the high-end value (2–10 MPa) of cancellous bone.1097 Finally, it is interesting to mention a successful reinforcement of carbonateapatite porous blocks by newly prepared carbonateapatite crystals (i.e., by the same compound; thus, a biocomposite of two different carbonateapatites was obtained).1098 First, a calcium salt was introduced to micropores of carbonateapatite blocks. Then, the calcium salt was carbonated to form calcite inside the micropores of the carbonateapatite blocks by exposing the blocks to carbon dioxide. For the third step, the blocks were immersed in a Na2HPO4 aqueous solution. In this process, calcite inside the micropores of the carbonateapatite blocks was transformed to carbonateapatite and the newly formed crystals of carbonateapatite entangled on those of the existing carbonateapatite blocks. Due to bonding between the newly formed carbonateapatite crystals and the existing ones in the carbonateapatite blocks, the mechanical strength of the blocks became ~1.5 times higher compared with that before the treatment.1098
Functionally graded formulations
Although, in most cases, the homogeneous distribution of filler(s) inside a matrix is required,426 there are composites where this is not the case. For example, functionally graded materials (commonly referred to as FGM) might be characterized by the intentional variations in composition and/or structured gradually over volume, resulting in corresponding changes in the properties of the composite. The main feature of such materials is the almost continuously graded composition, which results in two different properties at the two ends of the structure. Such composites can be designed for specific functions and applications. Various approaches based on bulk (particulate) processing, preform processing, layer processing and melt processing are used to fabricate the functionally graded materials.
Bone is a biologically formed composite with variable density ranging from very dense and stiff (cortical bone) to a soft and foamed structure (trabecular bone). Normally the outer part of long bones consists of cortical bone, with the density decreasing toward the core, where the trabecular bone is found. The trabecular bone is porous, and the pores are filled with osseous medulla.25,26 This brief description clearly indicates that bones are natural functionally graded composites.
The concept of FGM has been increasingly used for biomaterial design and, currently, it remains an important area of research. For example, many studies have been performed to fabricate porosity-graded calcium orthophosphate bioceramics in attempts to mimic the porous structure of bones.1099–1102 This is a structural approach to fabricating FGM. Besides that, there is a compositional approach. For example, powder metallurgy methods have been used to fabricate HA/Ti functionally graded biocomposite dental implants, offering the biocompatible HA on the tissue side and titanium on the outer side for mechanical strength.1103–1105 The graded structure in the longitudinal direction contains more Ti in the upper section and more HA in the lower section. Actually, in the upper section, the occlusal force is directly applied, and Ti offers the required mechanical performance; in the lower part, which is implanted inside the bone, the HA confers bioactive and osteoconductive properties to the material.1103 Since the optimum conditions of sintering for Ti and HA are very different, HA/Ti functionally graded biocomposites are difficult to fabricate, and the sintering conditions for their mixtures are obliged to compromise. The expected properties of this implant are shown in Figure 6.1104 Such biocomposites might be both symmetrical1106 and asymmetrical.1107 Furthermore, functionally graded HA/Ti biocomposite coatings might be prepared by RF plasma spraying.1108 More to the point, a Ti alloy substrate has been combined with HA granules that could be spread over the surface.1109
Figure 6.
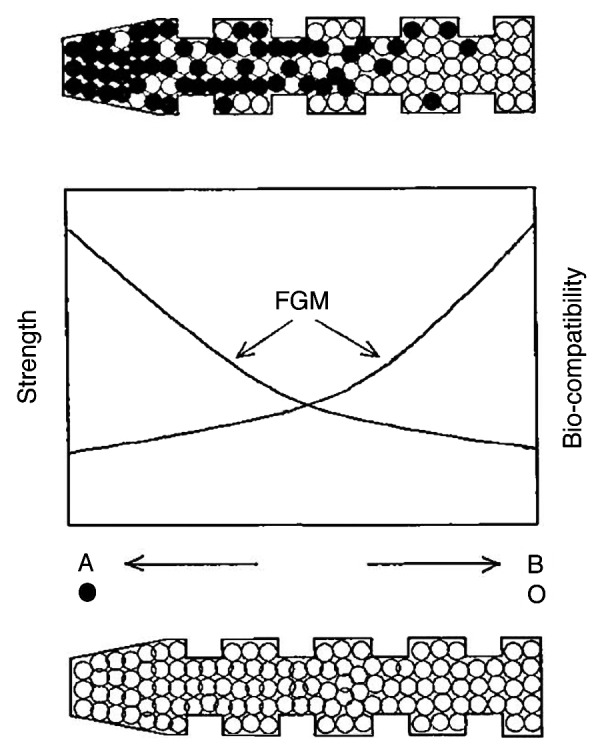
Expected properties of functionally graded biocomposite dental implant. For comparison, the upper drawing shows a functionally graded implant and the lower one shows a conventional uniform implant. The properties are shown in the middle. The implant with the composition changed from a biocompatible metal (Ti) at one end (left in the figure), increasing the concentration of bioceramics (HA) toward 100% HA at the other end (right in the figure), could control both mechanical properties and biocompatibility without an abrupt change due to the formation of discrete boundary. This FGM biocomposite was designed to provide more titanium for the upper part where occlusal force is directly applied and more HA for the lower part, which is implanted inside the jawbone. Reprinted from reference 1104 with permission.
A series of functionally graded HA coatings incorporated with various percentages of silver were deposited on titanium substrates using ion beam-assisted deposition. The analysis of the coating’s cross-section revealed a decreased crystallinity as well as a distribution of nano-sized (10–50 nm) silver particles from the coating/substrate interface to top surface.1110 A functionally graded HA/PMMA biocomposite was developed based on sedimentary HA distributions in a PMMA viscous fluid, using a centrifuge to avoid stress convergence on the interface. The stress-strain curves of this biocomposite showed sufficient strength for biomedical applications along with the relaxation of brittleness and fragility.543 A compositionally graded collagen/nanodimensional HA biocomposite scaffold might be prepared by an in situ diffusion method.1111 Chemical and microstructural analysis revealed a gradient of the Ca-to-P ratio across the width of the scaffold template, resulting in the formation of a Ca-rich side and a Ca-depleted side of the scaffold. The Ca-rich side featured low porosity and agglomerates of the nanodimensional HA crystallites, while the Ca-depleted side featured higher porosity and nanodimensional HA crystallites integrated with collagen fibrils to form a porous network structure.1111 A three-layered, graded biocomposite membrane with one face of 8% nanodimensional carbonateapatite/collagen/PLGA porous membrane, the opposite face of pure PLGA non-porous membrane and the middle layer of 4% nanodimensional carbonateapatite/collagen/PLGA as the transition was prepared using the layer-by-layer casting method.611 Functionally graded non-woven meshes of PCL incorporated by nano-sized particles of β-TCP were prepared using a hybrid twin-screw extrusion/electrospinning process.1112 A functionally graded HA/silk fibroin biocomposite was prepared by pulse electric current sintering.1113 HA/glass FGM layers were coated on titanium alloy (Ti-6Al-4V) substrates. The design of these layers and the use of the glass were meant to achieve a strong bond between the FGM layered coatings and the substrates.1114,1115
Functionally graded β-TCP/FA biocomposites combine the biostability of FA with the bioresorbable properties of β-TCP.1116 An interesting multilayered (each layer 1 mm thick) structure consisting of β-TCP/FA biocomposites with different molar ratios has been prepared, giving rise to formation of an FGM (Fig. 7). After implantation, the preferential dissolution of the β-TCP phase would result in functionally gradient porosity for bone ingrowth.1116 Functionally graded fluoridated HA with a gradient of fluoride1117 and carbonated HA with a gradient of carbonate1118 were synthesized as well. HA/zirconia graded biocomposites were fabricated to enhance the mechanical properties of HA while retaining its bone bonding property.963 TiO2 and HA were found to be a good combination for FGM, providing both a gradient of bioactivity and good mechanical strength.1119 In addition, graded HA/CaCO3 biocomposite structures for bone ingrowth were also developed.1120 Functionally graded composite skull implants consisting of polylactides, carbonateapatite and CaCO3 are known as well.386,387 Thus, the research in this field is quite promising, but currently, the mechanical properties of the available biocomposites do not match the corresponding properties of bones.179
Figure 7.
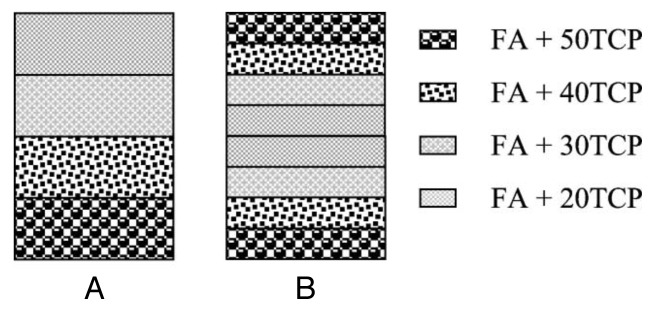
A schematic diagram showing the arrangement of the FA/β-TCP biocomposite layers. (A) A non-symmetric functionally gradient material (FGM); (B) symmetric FGM. Reprinted from reference 1116 with permission.
Biosensors
A biosensor is a device for detection of an analyte that combines a biological component with a physicochemical detector component. Very briefly, it consists of three parts: a sensitive biological element, a transducer or a detector element, which transforms the signal resulting from the interaction of the analyte and the biological element into another signal, and the associated electronics, which are primarily responsible for the display of the results in a user-friendly way.1121
The surface of biologically relevant calcium orthophosphates (CDHA, HA, α-TCP, β-TCP, DCPD, DCPA) has an excellent capacity for adsorption of functional biomolecules, such as proteins, albumins, DNA as well as some other types of chemicals. Therefore, several calcium orthophosphate-based biocomposites and hybrid biomaterials were found to be applicable for biosensor manufacturing.354,642,1041,1083,1122–1127 For example, formation of poly-l-lysine/HA/carbon nanotube hybrid nanodimensional particles was described, and a general design strategy for an immunosensing platform was proposed based on adsorption of antibodies onto this biocomposite.1083 In another paper, a hybrid material formed by assembling nanodimensional particles of gold onto nano-sized HA was employed for the interface design of a piezoelectric immunosensor on which the antibodies were bound. The sensing interface that was developed appeared to possess some advantages, such as activation-free immobilization and high antigen-binding activities of antibodies, over using nano-sized either HA or gold alone.1041 A novel tyrosinase biosensor based on nano-sized HA/chitosan composites has been developed for the detection of phenolic compounds.1125 Further details on the subject are available in the aforementioned references.
To date, not many papers have been published on the biosensor application of calcium orthophosphate-based biocomposites and hybrid biomaterials. Presumably, this subject will be further developed in future, and, perhaps sometime, implantable biosensors will be designed to perform the continuous concentration monitoring of the important biological macromolecules in vivo. Possibly, those implantable biocencors will be able to use electric power generated by DCPD/polymer composite-based battery devices.506,507
Interaction Among the Phases in Calcium Orthophosphate-Based Formulations
An important aspect that should be addressed in detail is a mutual interaction among calcium orthophosphates and other phases in biocomposites and hybrid biomaterials. In general, an interaction among the phases in any composite can be either mechanical, when it results from radial compression forces exerted by the matrix on the filler particles (for example, developed during cooling due to thermal contraction), or chemical, when the reactivity of the filler toward the matrix has an important role. In the latter case, it is important to distinguish a physical interaction from chemical bonding.282 According to Wypych,1128 physical interaction is more or less temporary, implicating hydrogen bonding or van der Waals forces, whereas chemical bonding is stronger and more permanent, involving covalent bond formation. Thus, a chemical interfacial bond among the phases is preferred to achieve higher strength in a composite. The magnitude of the interfacial bond among the phases determines how well a weak matrix transmits stress to the strong fibers. However, while a bond among the matrix and reinforcements must exist for the purpose of stress transfer, it should not be so strong that it prevents toughening mechanisms, such as debonding and fiber pullout.217
There is still doubt as to the exact bonding mechanism among bone minerals (biological apatite) and bioorganics (collagen), which undoubtedly plays a critical role in determining the mechanical properties of bones. Namely, bone minerals are not bonded directly to collagen but through non-collagenous proteins that make up ~3% of bones (Table 1) and provide with active sites for biomineralization and for cellular attachment.36 In bones, the interfacial bonding forces are mainly ionic bonds, hydrogen bonds and hydrophobic interactions, which give the bones their unique composite behavior.53 There is an opinion that, in contrast to bones, there is no sign of chemical bonding among the phases in conventional calcium orthophosphate/collagen biocomposites, probably due to a lack of suitable interfacial bonding during mixing.39 However, this is not the case for phosphorylated collagens.753 Interested readers are directed to a density functional theory study of the interaction of collagen peptides with hydroxyapatite surfaces.1129
Anyway, the Fourier-transformed infrared (FTIR) spectra of some calcium orthophosphate-based composites and collagen films were measured and transformed into absorption spectra, using the Kramers-Kronig equation to demonstrate energy shifts of residues on the apatite/collagen interface. After comparing FTIR spectra of biocomposites and collagen films in detail, red shifts of the absorption bands for C-O bonds were observed in the spectra of the biocomposites. These red shifts were described as a decrease of bonding energies of C-O bonds and assumed to be caused by an interaction with Ca2+ ions located on the surfaces of apatite nano-sized crystals as shown in Figure 8.747 Another proof of a chemical interaction between apatite and collagen was also evaluated in FTIR spectra of CDHA/collagen biocomposites, in which a shift of the band corresponding to -COO- stretching from 1,340 to 1,337 cm−1 was observed.708,709 More to the point, nucleation of apatite crystals onto collagen through a chemical interaction with carboxylate groups of collagen macromolecules has been reported in references 1130–1132.
Figure 8.
A schematic drawing of the relation between self-organization (directional deposition of HA on collagen) and interfacial interaction in biocomposites. Direction of interaction between HA and collagen is restricted by covalent bonding between COO and Ca(2) to maintain regular coordination number of 7. Reprinted from reference 747 with permission.
FTIR spectroscopy seems to be the major tool for studying a possible chemical bonding among the phases in calcium orthophosphate-based biocomposites and hybrid biomaterials.276,310,345,353,355,462,513,600,616,629,635,638,641,644,653,664,673,678,709,753,802,803,847,882,1133–1136 For example, the characteristic bands at 2,918, 2,850 and 1,472 cm−1 for the hydrocarbon backbone of PE appeared to have zero shift in an HA/PE biocomposite. However, in the case of polyamide, several FTIR bands indicated that the polar groups shifted significantly: the bands at 3,304, 1,273 and 692 cm−1 derived from stretching of N-H, stretching of C-N-H and vibrating of N-H moved to 3,306, 1,275 and 690 cm−1, respectively, in the HA/polyamide biocomposites. Furthermore, both stretching (3,568 cm−1) and vibrating (692 cm−1) modes of hydroxide in HA moved to 3,570 and 690 cm−1 in the HA/polyamide biocomposites, respectively, indicating the formation of hydrogen bonds. In addition, bands at 1,094 and 1,031 cm−1 of PO4 modes also shifted to 1,093 and 1,033 cm−1 in the HA/polyamide biocomposite. That the bands shifted in a fingerprint area indicated that the hydroxide and orthophosphate on the surface of HA might interact with plentiful carboxyl and amino groups of polyamide through nucleophilic addition.276 Comparable conclusions were made for HA/PVA,641 CDHA/alginate,709 ACP/PPF,513 HA/maleic anhydride355 and β-TCP/PLLA462 biocomposites, in which weak chemical bonds were considered to form between Ca2+ ions located on the HA, CDHA, ACP or β-TCP surface, respectively, and slightly polarized O atoms of C = O bonds in the surrounding bioorganic compounds. The data obtained suggest that crystallization of calcium orthophosphates in chitosan-containing solutions is substantially modulated by a chemical interaction of the components; apparently, a part of calcium is captured by chitosan and does not participate in the formation of the main mineral phase.1136 This type of the chemical interaction is shown schematically in Figure 9.709
Figure 9.
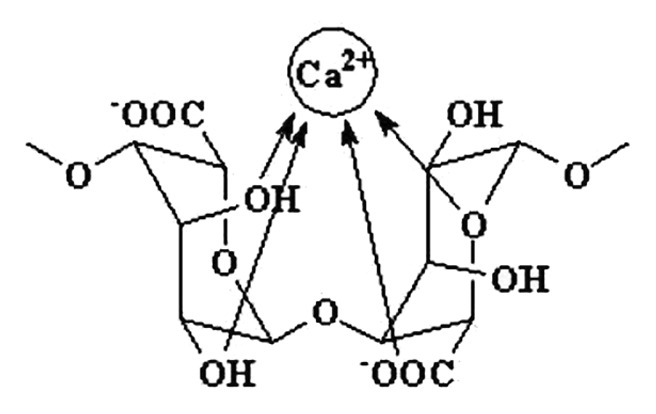
A schematic diagram of Ca2+ ion binding with alginate chains. Reprinted from reference 709 with permission.
Besides FTIR spectroscopy, other measurement techniques are also able to show some evidence of a chemical interaction among the phases in calcium orthophosphate-based biocomposites and hybrid biomaterials.345,462,635,638,641,1134–1138 For example, for nano-sized crystals of CDHA/alendronate such evidence was obtained by thermogravimetric analysis: DTG plots of the crystals appeared to be quite different from those obtained from mechanical mixtures of CDHA and calcium alendronate with similar compositions.1137 Analogous DTG results were obtained for nano-sized HA/PVA biocomposites.641 In the case of biocomposites of nano-sized HA with polyamide, a hydrogen bonding among the phases was detected by a differential scanning calorimetry technique.635 Another example comprises application of dynamic mechanical analysis to investigate the softening mechanism of β-TCP/PLLA biocomposites.462 As to biocomposites of nano-sized HA with PVAP, some indirect evidence of chemical bonding among the phases was found by X-ray diffraction and thermogravimetric analysis.345 A strong structural correlation between the orientation of FA crystallites and gelatin within the FA/gelatin composite spheres was discovered, indicating a substantial reorganization of the macromolecular matrix within the area of a growing aggregate.444 Recently, chemical interactions between HA and organic molecules have been elucidated using ab initio calculation methods.1139
By means of the X-ray photoelectronic spectroscopy (XPS) technique, binding energies of Ca, P and O atoms were found to vary between nano-sized HA (Ca: 350.5 and 345.5; O: 530.2; P: 132.5 eV) and nano-sized HA/konjac glucomannan/chitosan biocomposite (Ca: 352.1 and 347.4; O: 531.2; P: 133.4 eV).653 Further measurements by FTIR and X-ray diffraction revealed that nano-sized HA was mainly linked to konjac glucomannan and chitosan by hydrogen bonding among OH- and PO43- ions of HA and -C = O and -NH groups of konjac glucomannan and chitosan copolymer, and there was a stable interface formed among the three phases in the biocomposite. Meanwhile, coordinate bonding might be formed between Ca2+ and -NH. Stable interfaces have been formed among the three phases in a biocomposite.653 In HA/collagen biocomposites, a covalent bond formation between Ca2+ ions of HA and RCOO- groups of collagen molecules was found by XPS.601 Similar XPS observations were also made for several other calcium orthophosphate-based biocomposites and hybrid biomaterials.629,664,673
The interaction and adhesion between calcium orthophosphate fillers and their respective matrices have significant effects on the properties of particulate-filled, reinforced materials, as these forces are essential to load transfer among the phases and thus to improving the mechanical performance of the biocomposites.353 However, for a substantial amount of the aforementioned formulations, the interaction among the phases is mechanical in nature. This is because the matrix often consists of compounds with no functional groups or unsaturated bonds that can form ionic complexes with the constituents of calcium orthophosphates. Obviously, less coupling exists between non-polar polymers and calcium orthophosphate ceramic particles. Therefore, polymers with functional groups pendant to the polymer backbone, which can act as sites for bridging to calcium orthophosphates, are more promising in this respect.53
In order to improve the situation, various supplementary reagents are applied. If the primary effect of a processing additive is to increase the interaction between the phases, such additives can be regarded as coupling agents.1140 These agents establish chemical bridges between the matrix and the fillers, promoting adhesion among the phases. In many cases, their effect is not unique; they might, for example, also influence rheology of the composites.282 In the case of calcium orthophosphates, a hexamethylene diisocyanate coupling agent was used to bind PEG/PBT (PolyactiveTM) block copolymers293 and other polymers1133 to HA filler particles. Thermogravimetric and infrared analysis demonstrated that the polymers were chemically bonded to the HA particles through the isocyanate groups, making it a suitable approach to improve adhesion.1133 Other researchers used glutaraldehyde as a cross-linked reagent.470,474,598,600,601,619,624,699,736,778,781,1141 Alternatively, the interfacial bonding among calcium orthophosphates and other components might be induced by silanes,242,268,269,293,406,640,1142–1145 zirconates,282,406,408,1146,1147 titanates,282,406,1146 phosphoric acid,643 alkaline pretreatment,877,880 polyacids143,144,293 and some other chemicals. Furthermore, some polymers might be grafted onto the surface of calcium orthophosphate particles.656 Structural modifications of the polymeric matrices, for instance, with introduction of acrylic acid,245,268,269,293 have also proved to be effective. For example, application of polyacids as a bonding agent for HA/PolyactiveTM composites caused the surface modified HA particles to maintain better contact with polymers at the fracture and improved mechanical properties.143,144,293 The use of titanate and zirconate coupling agents appeared to be very dependent on the molding technique employed.282 Silane-coupled HA powders were tested before applying them as fillers in biodegradable composites.1143–1145 This treatment allowed HA to withstand the attack of water without impairing overall bioactivity. Besides that, a chemically modified reinforcement phase-matrix interface was found to improve the mechanical properties of the biocomposites. The examples include chemically coupled HA/PE,268,269 chemically formed HA/Ca poly(vinylphosphonate)349 and PLA/HA fibers.233 These biocomposites are able to consume a large amount of energy at the fracture.
The action of some coupling agents was found to combine two distinct mechanisms: (1) cross-linking of the polymeric matrix (valid for zirconate and titanate coupling agents) and (2) improvement of the interfacial interactions among the major phases of the biocomposites. This interfacial adhesion improvement appeared to be much dependent on the chemical nature (pH and type of metallic center) of the coupling agents.406 Several works claimed that silanes do interact with HA.242,268,269,1143–1145 It was shown that a silicon-containing interphase existed between HA and PE that promoted chemical adhesion between the HA particles and the polymer. A silane-coupling agent also facilitated penetration of PE into cavities of individual HA particles, which resulted in enhanced mechanical interlocking at the matrix-reinforcement interface.268,269
Thus, the optimization of biocomposite properties by coupling agents is currently an important area of the research. The control and development of molecular-level associations of polymers with calcium orthophosphates is suggested to be significant for the resulting mechanical responses in biocomposites. It appears that a fundamental molecular understanding of the interfacial behavior in biocomposites is an area not sufficiently addressed in the literature. Various experimental characterization techniques using electron microscopy, vibrational spectroscopy, X-ray diffraction, scanning probe microscopy and others are used routinely to characterize these materials beyond mechanical property characterization. In addition, atomic scale models for simulating phase interaction and predicting responses in the novel material systems, where nanostructures and nano-interfaces are included, are important to understand and predict load deformation behavior.179
In addition to the aforementioned, the surface of calcium orthophosphates might be modified as well.144,509,510,656,1147–1154 An interesting approach for HA surface modification was described by Lee et al.1154 First, in situ synthesis of surface thiol-functionalized HA (HA-SH) was realized by adding 3-mercaptopropionic acid during hydrothermal synthesis of HA (Fig. 10A). This was followed by grafting polymerization of ethylene glycol methacrylate phosphate by radical chain transfer, generating the sulfur-centered radicals on the HA surfaces (Fig. 10B), which initiated the surface grafting polymerization of ethylene glycol methacrylate phosphate (Fig. 10C).1154 Other examples might be found in the literature.144,509,510,656,1147–1153 In general, the purpose of surface modifying is not only to guarantee the even distribution of calcium orthophosphate particles at a high loading level in the matrix, but also to prevent or delay the debonding process of calcium orthophosphate particles from the matrix. Obviously, all surface modifiers must satisfy several biomedical requirements, such as no toxicity, good biocompatibility and no changes in the biological or physicochemical properties of the fillers.
Figure 10.
Surface modification of HA particles by grafting polymerization according to Lee et al.1154 (A) surface thiol functionalized HA, (B) sulfur-centered radical on HA surface, (C) surface grafting polymerization of ethylene glycol methacrylate phosphate. Reprinted from reference 99 with permission.
Addition of adhesion-promoting agents might be an alternative to improve the interaction between the fillers and the matrix. For example, Morita et al. incorporated 4-methacryloyloxyethyl trimellitate anhydride to promote adhesion of the polymer to HA.1155 In another study, phosphoric ester was added to the liquid component of the formulation.1156 Both the strength and the affinity index of biocomposites were found to increase, probably due to the effects of co-polymerization.
Possible interactions between BCP and HPMC have been investigated in IBS composites.900,901,1157 After mixing, there was a decrease in the mean diameter of BCP granules, and this influenced the viscosity of the paste. Dissolution of grain boundaries of β-TCP crystals and precipitation of CDHA on the HA crystal surface were found during the interaction. Both phenomena were responsible for the observed granulometric changes;900,901 however, within the sensitivity of the employed measurement techniques, no chemical bonding between BCP and HPMC was detected.1157
A coprecipitation technique was used to prepare CDHA/chitosan biocomposites.811 Growth of CDHA crystals was inhibited by organic acids with more than two carboxyl groups, which strongly bind to CDHA surfaces via COO-Ca bonds. Transmission electron microscopy images revealed that CDHA formed elliptic aggregates with chemical interactions (probably coordination bond) between Ca on its surface and amino groups of chitosan; the nano-sized crystals of CDHA were found to align along the chitosan molecules, with the amino groups working as the nucleation sites.811 Formation of calcium cross-linked polymer carboxylate salts was suggested during setting of calcium orthophosphate cement (TTCP + DCPA)/polyphosphazane biocomposites; a chemical involvement of the polymer in the cement setting was determined based on the results of pH monitoring.558-560
A chemical bond between the phases was presumed in PCL/ HA composites prepared by the grafting technique;420 unfortunately, no strong experimental evidences were provided. In another study, CDHA/poly(α-hydroxyester) composites were prepared by a low temperature chemical route.393 In that study, pre-composite structures were prepared by combining α-TCP with PLA, PLGA and copolymers thereof. The final biocomposite was achieved by in situ hydrolysis of α-TCP to CDHA performed at 56°C either in solvent cast or pressed pre-composites. That transformation occurred without any chemical reaction between the polymer and calcium orthophosphates, as determined by FTIR spectroscopy.393
In nearly every study on HA/carbon nanotubes biocomposites, the nanotubes were functionalized before combining them with HA. Most researchers did this by oxidation,303-307 although noncovalent functionalizing with sodium dodecylsulfate307 and coating the nanotubes by a polymer1158 before combining them with HA were also reported. Several studies by transmission electron microscopy revealed evidence that the functionalization enhanced interaction between carbon nanotubes and HA.306,307,1159
For calcium orthophosphate-based biocomposites able to sustain high-temperature sintering (valid for the formulations consisting of inorganic components only), an interdiffusion of chemical elements might take place among the phases. Such an effect was detected by energy-dispersive X-ray spectroscopy in HA/TiO2 biocomposite particles with partial formation of calcium titanates; this process was found to be favorable to enhancing the cohesive strength of particles in the composite coating.997 A similar high-temperature interaction between HA and zirconia911,940 as well as between HA and Ti661,1030,1032–1034 was also detected. Namely, lower Ti content composites sintered at 1,200°C showed main crystalline phases as CaTiO3, CaO and TixPy, while an increase in Ti content to 50 vol.% revealed Ti2O and residual α-Ti as additional phases. Thus, the chemical reactions between HA and Ti were expressed by the following unbalanced illustrative equation (Eqn. 1):1032
Besides, partial decomposition of HA and formation of different calcium aluminates were detected in HA/Al2O3 biocomposites after sintering at 1,200–1,300°C. This has been attributed to the diffusion of Ca2+ from HA into the alumina matrix, and the depletion of Ca2+ from HA leads to the decomposition of HA into β-TCP.968,974-976 Presumably, all these processes influence the mechanical strength of the biocomposites.
Bioactivity and Biodegradation of Calcium Orthophosphate-Based Formulations
The continuous degradation of an implant causes a gradual load transfer to the healing tissue, preventing stress shielding atrophy, and stimulates the healing and remodeling of bones. Some requirements must be fulfilled by the ideal prosthetic biodegradable materials, such as biocompatibility, adequate initial strength and stiffness, retention of mechanical properties long enough to assure its biofunctionality and the non-toxicity of the degradation by-products.178 Generally speaking, bioactivity (i.e., ability of bonding to bones) of biologically relevant calcium orthophosphates reinforced by other materials is usually lower than that of pure calcium orthophosphates.30,31,1160
In general, both bioactivity and biodegradability of any biocomposite and/or hybrid biomaterial are determined by the same properties of the constituents. Both processes are very multi-factorial, because during implantation, the surface of any graft comes into contact with biological fluids and, shortly afterwards, is colonized by cells. Much more biology than chemistry and material science together is involved in these very complex processes, and many specific details still remain unknown. To simplify the task, the biodegradability of the biologically relevant calcium orthophosphates might be described by a chemical dissolution in slightly acidic media (calcium orthophosphates are almost insoluble in alkaline solutions111-117), which, in the case of CDHA, might be described as a sequence of four successive chemical equations (Eqns. 2–5):519,1161,1162
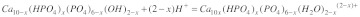 |
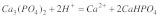 |
Biodegradability of polymers generally depends on the following factors: (1) chemical stability of the polymer backbone, (2) hydrophobicity of the monomer, (3) morphology of the polymer, (4) initial molecular weight, (5) fabrication processes, (6) geometry of the implant and (7) properties of the scaffold, such as porosity and pore diameter.328 A summary on degradation of PLA and PGA as well as that of SEVA-C is available in the literature (reviewed in ref. 178 p. 798 and p. 803, respectively), where the interested readers are referred. Biodegradation of HA/PLLA and CDHA/PLLA biocomposite rods in subcutis and medullary cavities of rabbits were investigated mechanically and histologically; the degradation was found to be faster when using uncalcinated CDHA instead of calcinated HA.1163 In a more detailed study, new bone formation was detected at 2 weeks after implantation, especially for formulations with a high HA content.1164 More to the point, direct contact between bones and these composites without intervening fibrous tissue was detected in this case.1164,1165 Both SEVA-C and SEVA-C/ HA biocomposites were found to exhibit non-cytotoxic behavior,1166,1167 inducing a satisfactory tissue response when implanted as shown by in vivo studies.1167 Furthermore, SEVA-C/HA biocomposites induce a positive response in osteoblast-like cells for what concerns cell adhesion and proliferation.1166 An in vivo study on biodegradation of microspheres [PLGA, gelatin and poly(trimethylene carbonate) were used]/calcium orthophosphate cement biocomposites revealed that they exhibited microsphere degradation after 12 weeks of subcutaneous implantation, which was accompanied by a decrease in compression strength.1168 Interestingly, though, the amount of calcium orthophosphates in biocomposites was found to have a greater effect on the early stages of osteoblast behavior (cell attachment and proliferation) rather than the immediate and late stages (proliferation and differentiation).1169
Both in vitro (the samples were immersed into 1% trypsin/phosphate-buffered saline solution at 37°C) and in vivo (implantation of samples into the posterolateral lumbar spine of rabbits) biodegradation have been investigated for nano-sized HA/collagen/PLA biocomposites.610 The results demonstrated that weight loss increased continuously in vitro, with a reduction in mass of ~20% after 4 weeks. During the experimental period in vitro, a relative rate of reduction of the three components in this material was shown to differ greatly: collagen decreased the fastest, from 40% weight to ~20% in the composite; HA content increased from 45 to ~60%; PLA changed little. In vivo, the collagen/HA ratio appeared to be slightly higher near the transverse process than in the central part of the intertransverse process.610 Hasegawa et al.1170 performed an in vivo study, spanning a period of 5–7 y, on high-strength HA/PLLA biocomposite rods for the internal fixation of bone fractures. In that work, both uncalcined CDHA and calcined HA were used as reinforcing phases in a PLLA matrix. Those composites were implanted in the femur of 25 rabbits. It was found that the implanted materials were resorbed after 6 y of implantation. The presence of remodeled bone and trabecular bone bonding was the significant outcome. These data clearly demonstrate the biodegradation independence of various components of biocomposites.
Some Challenges and Critical Issues
The scientific information summarized in this review represents the recent developments of calcium orthophosphate-based biocomposites and hybrid biomaterials from a variety of approaches, starting from conventional ones to tissue engineering. Such formulations combined with osteoconductive or osteoinductive factors and/or osteogenic cells have gained much interest as a new and versatile class of biomaterials and are perceived to be beneficial in many aspects as bone grafts.36,1171 However, current applications of these biomaterials in medicine and surgery are still remarkably less than might be expected. In many biomedical applications, research and testing of such formulations have been introduced and highly developed, but only in a very few cases have industrial production and commercial distribution of medical devices partially or entirely made of biocomposites been started. The medical application of biocomposites and hybrid biomaterials requires a better understanding of the objectives and limitations involved. Recently, the main critical issues have been summarized as follows:265
There are not enough reliable experimental and clinical data supporting the long-term performance of biocomposites with respect to monolithic traditional materials.
The design of biocomposites and hybrid biomaterials is far more complex than that of conventional monolithic materials because of the large number of additional design variables that must be considered.
The available fabrication methods may limit the possible reinforcement configurations, may be time consuming, expensive and may require special cleaning and sterilization processes as well as highly skilled personnel.
There are no satisfactory standards yet for biocompatibility testing of the biocomposite implants, because the ways in which the different components of any biocomposite interact with living tissues are not completely understood.
There are no adequate standards for the assessment of biocomposite fatigue performance, because the fatigue behavior of such materials is far more complex and difficult to predict than that of traditional materials.265
On the other hand, in spite of an enormous progress in biocomposite processing, to achieve the desired characteristics, researchers still need to develop more advanced technologies to fabricate a bone-resembling hierarchical organization over several length scales. Development of novel grafting materials depends on the progress of research into the structure of natural bones. The key issues are not only to understand the fundamentals of biomineralization but also to translate such knowledge into practical synthetic pathways to produce better bone grafts. Unfortunately, when it comes to the fabrication of biocomposites mimicing natural bones, from the nanometer to the micrometer dimensions, there are many key issues, including control of morphology, incorporation of foreign ions, interaction with biomolecules and assembly of the organic and inorganic phases, which are still not well understood. A processing gap between the lower-level building units and the higher-order architecture could severely limit the practical application of current calcium orthophosphate-based biocomposites and hybrid biomaterials. Therefore, further substantial research efforts have been outlined to address the following key challenges:36,41
Optimization of biocomposite processing conditions.
Optimization of interfacial bonding and strength equivalent to natural bone.
Optimization of the surface properties and pore size to maximize bone growth.
Maintaining the adequate volume of the construct in vivo to allow bone formation to take place.
Withstanding the load-bearing conditions.
Matching the bioresorbability of the grafts and their biomechanical properties while forming new bone.
Understanding the molecular mechanisms by which the cells and the biocomposite matrix interact with each other in vivo to promote bone regeneration.
Supporting angiogenesis and vascularization for the growth of healthy bone cells and subsequent tissue formation and remodeling.36,41
The aforementioned critical issues have to be solved before a widespread commercial use of calcium orthophosphate-based biocomposites and hybrid biomaterials can be made in surgery and medicine.
Conclusions
All types of calcified tissues of humans and mammals appear to possess a complex hierarchical biocomposite structure. Their mechanical properties are outstanding (considering the weak constituents from which they are assembled) and far beyond those, that can be achieved using the same synthetic materials with present technologies. This is because biological organisms produce biocomposites that are organized in terms of both composition and structure, containing both brittle calcium orthophosphates and ductile bioorganic components in very complex structures, hierarchically organized at the nano-, micro- and meso levels. Additionally, the calcified tissues are always multifunctional. For example, bone provides structural support for the body plus blood cell formation. The third defining characteristic of biological systems, in contrast with current synthetic systems, is their self-healing ability, which is nearly universal in nature. These complex structures, which have risen from millions of years of evolution, inspire materials scientists in the design of novel biomaterials.1172
Obviously, no single-phase biomaterial is able to provide all the essential features of bones and/or other calcified tissues, and therefore, there is a great need to engineer multi-phase biomaterials (biocomposites) with a structure and composition mimicking those of natural bones. The studies summarized in this review have shown that the proper combination of a ductile matrix with a brittle, hard and bioactive calcium orthophosphate filler offers many advantages for biomedical applications. Namely, the desirable properties of some components can compensate for a poor mechanical behavior of calcium orthophosphate bioceramics, while, in turn, the desirable bioactive properties of calcium orthophosphates improve those of other phases, thus expanding the possible application of each material within the body.102 However, the reviewed literature clearly indicates that, among possible types of calcium orthophosphate-based biocomposites and hybrid biomaterials, only simple, complex and graded ones, as well as fibrous, laminar and particulate ones (see classification types of the composites in the “General Information on Composites and Biocomposites” section) have been investigated. Presumably, future progress in this subject will require concentrating efforts on elaboration and development of both hierarchical and hybrid biocomposites. Furthermore, following the modern tendency of tissue engineering, a novel generation of calcium orthophosphate-based biocomposites and hybrid biomaterials should also contain a living biological part.
To conclude, the future of the calcium orthophosphate-based biocomposites and hybrid biomaterials is now directly dependent on the formation of multidisciplinary teams composed of experts but, primarily, experts ready to work in close collaboration with others and thus be able to deal efficiently with the complexity of the human organism. The physical chemistries of solids, solid surfaces, polymer dispersion and solutions as well as material-cell interactions are among the phenomena to be tackled. Furthermore, much work remains to be done on the long way from laboratory to clinic, and success depends on the effective cooperation of clinicians, chemists, biologists, bioengineers and materials scientists.
Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Br. 2001;83:3–8. doi: 10.1302/0301-620X.83B1.11952.
Chau AMT, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18:449–64. doi: 10.1007/s00586-008-0878-4.
Kaveh K, Ibrahim R, Bakar MZA, Ibrahim TA. Bone grafting and bone graft substitutes. J Anim Vet Adv. 2010;9:1055–67. doi: 10.3923/javaa.2010.1055.1067.
Murugan R, Ramakrishna S. Bioactive nanomaterials in bone grafting and tissue engineering. In: Handbook of nanostructured biomaterials and their applications in nanobiotechnology, Nalwa HS (Ed.); American Scientific Publishers: Stevenson Ranch USA 2005; 2:141-8.
Tazaki J, Murata M, Yuasa T, Akazawa T, Ito K, Hino J, et al. Autograft of human tooth and demineralized dentin matrices for bone augmentation. J Ceram Soc Jpn. 2010;118:442–5. doi: 10.2109/jcersj2.118.442.
Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41:75–84. doi: 10.1016/j.ocl.2009.07.006.
Keller EE, Triplett WW. Iliac bone grafting: review of 160 consecutive cases. J Oral Maxillofac Surg. 1987;45:11–4. doi: 10.1016/0278-2391(87)90079-6.
Laurie SW, Kaban LB, Mulliken JB, Murray JE. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933–8. doi: 10.1097/00006534-198406000-00014.
Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5. doi: 10.1097/00005131-198909000-00002.
Neumann M, Epple M. Composites of calcium phosphate and polymers as bone substitution materials. Eur J Trauma. 2006;32:125–31. doi: 10.1007/s00068-006-6044-y.
Le Guéhennec L, Layrolle P, Daculsi G. A review of bioceramics and fibrin sealant. Eur Cell Mater. 2004;8:1–10, discussion 10-1. doi: 10.22203/ecm.v008a01.
Fuchs JR, Nasseri BA, Vacanti JP. Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg. 2001;72:577–91. doi: 10.1016/S0003-4975(01)02820-X.
Hench LL, Wilson J. Surface-active biomaterials. Science. 1984;226:630–6. doi: 10.1126/science.6093253.
Rose FRAJ, Oreffo ROC. Breakthroughs and views bone tissue engineering: hope vs. hype. Biochem Biophys Res. 2002;292:1–7. doi: 10.1006/bbrc.2002.6519.
Beaman FD, Bancroft LW, Peterson JJ, Kransdorf MJ. Bone graft materials and synthetic substitutes. Radiol Clin North Am. 2006;44:451–61. doi: 10.1016/j.rcl.2006.01.001.
Kokubo T, Kim HM, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161–75. doi: 10.1016/S0142-9612(03)00044-9.
Rueger JM. Bone replacement materials—state of the art and the way ahead. Orthopade. 1998;27:72–9. doi: 10.1007/PL00003481.
Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN, American Academy of Orthopaedic Surgeons. The Committee on Biological Implants Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001;83-A(Suppl 2 Pt 2):98–103. doi: 10.2106/00004623-200100022-00007.
Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A:454–64. doi: 10.2106/00004623-200203000-00020.
Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7. doi: 10.1016/j.injury.2005.07.029.
Bohner M. Resorbable biomaterials as bone graft substitutes. Mater Today. 2010;13:24–30. doi: 10.1016/S1369-7021(10)70014-6.
Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–89. doi: 10.1089/107632701753337645.
Burg KJL, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–59. doi: 10.1016/S0142-9612(00)00102-2.
Holy CE, Shoichet MS, Davies JE. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: investigating initial cell-seeding density and culture period. J Biomed Mater Res. 2000;51:376–82. doi: 10.1002/1097-4636(20000905)51:3<376::AID-JBM11>3.0.CO;2-G.
Lowenstam HA, Weiner S. On biomineralization, Oxford University Press, New York USA 1989; 324.
Weiner S, Wagner HD. The material bone: structure-mechanical function relations. Annu Rev Mater Sci. 1998;28:271–98. doi: 10.1146/annurev.matsci.28.1.271.
Dorozhkin SV. Calcium orthophosphates in nature, biology and medicine. Materials. 2009;2:399–498. doi: 10.3390/ma2020399.
Hench LL, Wilson J. In: An introduction to bioceramics, Hench LL, Wilson J, (Eds.); Advanced series in ceramics. World Scientific, Singapore 1993; 1:1.
Suchanek W, Yoshimura M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res. 1998;13:94–117. doi: 10.1557/JMR.1998.0015.
Hench LL. Bioceramics: from a concept to clinics. J Am Ceram Soc. 1991;74:1487–510. doi: 10.1111/j.1151-2916.1991.tb07132.x.
Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–28. doi: 10.1111/j.1151-2916.1998.tb02540.x.
One molecule of collagen type I is a triple helix with 338 repetitions of amino acid residues and is about 300 nm in length [33]. Additionally, bone contains small quantities of other bioorganic materials, such as proteins, polysaccharides and lipids, as well as bone contains cells and blood vessels.
Itoh S, Kikuchi M, Koyama Y, Takakuda K, Shinomiya K, Tanaka J. Development of an artificial vertebral body using a novel biomaterial, hydroxyapatite/collagen composite. Biomaterials. 2002;23:3919–26. doi: 10.1016/S0142-9612(02)00126-6.
Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Bone indentation recovery time correlates with bond reforming time. Nature. 2001;414:773–6. doi: 10.1038/414773a.
Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–23. doi: 10.1039/b402005g.
Murugan R, Ramakrishna S. Development of nanocomposites for bone grafting. Compos Sci Technol. 2005;65:2385–406. doi: 10.1016/j.compscitech.2005.07.022.
Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31:8–11. doi: 10.1016/S8756-3282(02)00815-3.
Itoh S, Kikuchi M, Koyama Y, Matumoto HN, Takakuda K, Shinomiya K, et al. Development of a novel biomaterial, hydroxyapatite/collagen (HAp/Col) composite for medical use. Biomed Mater Eng. 2005;15:29–41.
Cui FZ, Li Y, Ge J. Self-assembly of mineralized collagen composites. Mater Sci Eng Rep. 2007;57:1–27. doi: 10.1016/j.mser.2007.04.001.
Vallet-Regi M, Arcos D. Nanostructured hybrid materials for bone tissue regeneration. Curr Nanosci. 2006;2:179–89.
Chan CK, Kumar TSS, Liao S, Murugan R, Ngiam M, Ramakrishnan S. Biomimetic nanocomposites for bone graft applications. Nanomedicine (Lond) 2006;1:177–88. doi: 10.2217/17435889.1.2.177.
Olszta MJ, Cheng XG, Jee SS, Kumar BR, Kim YY, Kaufman MJ, et al. Bone structure and formation: a new perspective. Mater Sci Eng Rep. 2007;58:77–116. doi: 10.1016/j.mser.2007.05.001.
Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. doi: 10.1097/00003086-200002000-00003.
Athanasiou KA, Zhu CF, Lanctot DR, Agrawal CM, Wang X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000;6:361–81. doi: 10.1089/107632700418083.
Zioupos P. Recent developments in the study of solid biomaterials and bone: “fracture” and “pre-fracture” toughness. Mater Sci Eng C. 1998;6:33–40. doi: 10.1016/S0928-4931(98)00033-2.
Doblaré M, Garcia JM, Gómez MJ. Modelling bone tissue fracture and healing: a review. Eng Fract Mech. 2004;71:1809–40. doi: 10.1016/j.engfracmech.2003.08.003.
Vallet-Regí M. Revisiting ceramics for medical applications. Dalton Trans. 2006:5211–20. doi: 10.1039/b610219k.
Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–6. doi: 10.1038/35015116.
Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials. 1998;19:1935–43. doi: 10.1016/S0142-9612(98)00097-0.
Boccaccini AR, Blaker JJ. Bioactive composite materials for tissue engineering scaffolds. Expert Rev Med Devices. 2005;2:303–17. doi: 10.1586/17434440.2.3.303.
Verheyen CCPM, de Wijn JR, van Blitterswijk CA, de Groot K, Rozing PM. Hydroxylapatite/poly(L-lactide) composites: an animal study on push-out strengths and interface histology. J Biomed Mater Res. 1993;27:433–44. doi: 10.1002/jbm.820270404.
Zhang RY, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–55. doi: 10.1002/(SICI)1097-4636(19990315)44:4<446::AID-JBM11>3.0.CO;2-F.
Durucan C, Brown PW. Biodegradable hydroxyapatite-polymer composites. Adv Eng Mater. 2001;3:227–31. doi: 10.1002/1527-2648(200104)3:4<227::AID-ADEM227>3.0.CO;2-1.
Kim HW, Knowles JC, Kim HE. Hydroxyapatite/poly(epsilon-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials. 2004;25:1279–87. doi: 10.1016/j.biomaterials.2003.07.003.
Hutmacher DW, Schantz JT, Lam CXF, Tan KC, Lim TC. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245–60. doi: 10.1002/term.24.
Guarino V, Causa F, Ambrosio L. Bioactive scaffolds for bone and ligament tissue. Expert Rev Med Devices. 2007;4:405–18. doi: 10.1586/17434440.4.3.405.
Yunos DM, Bretcanu O, Boccaccini AR. Polymer-bioceramic composites for tissue engineering scaffolds. J Mater Sci. 2008;43:4433–42. doi: 10.1007/s10853-008-2552-y.
Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. doi: 10.1126/science.1067404.
Crane GM, Ishaug SL, Mikos AG. Bone tissue engineering. Nat Med. 1995;1:1322–4. doi: 10.1038/nm1295-1322.
LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988;2:164–80. doi: 10.1177/08959374880020011101.
Mathijsen A. Nieuwe Wijze van Aanwending van het Gips-Verband bij Beenbreuken J.B. van Loghem, Haarlem 1852.
Dreesman H. Über Knochenplombierung. Beitr Klin Chir. 1892;9:804–10.
Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24:2133–51. doi: 10.1016/S0142-9612(03)00037-1.
http://en.wikipedia.org/wiki/Composite_material (assessed in November 2010).
Gibson RF. A review of recent research on mechanics of multifunctional composite materials and structures. Compos Struct. 2010;92:2793–810. doi: 10.1016/j.compstruct.2010.05.003.
Evans SL, Gregson PJ. Composite technology in load-bearing orthopaedic implants. Biomaterials. 1998;19:1329–42. doi: 10.1016/S0142-9612(97)00217-2.
Habibovic P, Barrère F, van Blitterswijk CA, de Groot K, Layrolle P. Biomimetic hydroxyapatite coating on metal implants. J Am Ceram Soc. 2002;85:517–22. doi: 10.1111/j.1151-2916.2002.tb00126.x.
Zhang RY, Ma PX. Biomimetic polymer/apatite composite scaffolds for mineralized tissue engineering. Macromol Biosci. 2004;4:100–11. doi: 10.1002/mabi.200300017.
Oliveira AL, Mano JF, Reis RL. Nature-inspired calcium phosphate coatings: present status and novel advances in the science of mimicry. Curr Opin Solid State Mater Sci. 2003;7:309–18. doi: 10.1016/j.cossms.2003.10.009.
Wan YZ, Hong L, Jia SR, Huang Y, Zhu Y, Wang YL, et al. Synthesis and characterization of hydroxyapatite-bacterial cellulose nanocomposites. Compos Sci Technol. 2006;66:1825–32. doi: 10.1016/j.compscitech.2005.11.027.
Wan YZ, Huang Y, Yuan CD, Raman S, Zhu Y, Jiang HJ, et al. Biomimetic synthesis of hydroxyapatite/bacterial cellulose nanocomposites for biomedical applications. Mater Sci Eng C. 2007;27:855–64. doi: 10.1016/j.msec.2006.10.002.
Ohtsuki C, Kamitakahara M, Miyazaki T. Coating bone-like apatite onto organic substrates using solutions mimicking body fluid. J Tissue Eng Regen Med. 2007;1:33–8. doi: 10.1002/term.3.
Oyane A. Development of apatite-based composites by a biomimetic process for biomedical applications. J Ceram Soc Jpn. 2010;118:77–81. doi: 10.2109/jcersj2.118.77.
de Groot K, Geesink RGT, Klein CPAT, Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res. 1987;21:1375–81. doi: 10.1002/jbm.820211203.
de Groot K, Wolke JGC, Jansen JA. Calcium phosphate coatings for medical implants. Proc Inst Mech Eng H. 1998;212:137–47. doi: 10.1243/0954411981533917.
Ignjatović NL, Liu CZ, Czernuszka JT, Uskoković DP. Micro- and nano-injectable composite biomaterials containing calcium phosphate coated with poly(DL-lactide-co-glycolide) Acta Biomater. 2007;3:927–35. doi: 10.1016/j.actbio.2007.04.001.
Manso M, Langlet M, Fernandez M, Vasquez L, Martinez-Duart JM. Surface and interface analysis of hydroxyapatite/TiO2 biocompatible structures. Mater Sci Eng C. 2003;23:451–4. doi: 10.1016/S0928-4931(02)00320-X.
Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001;58:570–92. doi: 10.1002/jbm.1056.
Song J, Malathong V, Bertozzi CR. Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone. J Am Chem Soc. 2005;127:3366–72. doi: 10.1021/ja043776z.
Yoshida K, Hashimoto K, Toda Y, Udagawa S, Kanazawa T. Fabrication of structure-controlled hydroxyapatite/zirconia composite. J Eur Ceram Soc. 2006;26:515–8. doi: 10.1016/j.jeurceramsoc.2005.07.047.
Madhumathi K, Binulal NS, Nagahama H, Tamura H, Shalumon KT, Selvamurugan N, et al. Preparation and characterization of novel beta-chitin-hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 2009;44:1–5. doi: 10.1016/j.ijbiomac.2008.09.013.
Lee M, Ku SH, Ryu J, Park CB. Mussel-inspired functionalization of carbon nanotubes for hydroxyapatite mineralization. J Mater Chem. 2010;20:8848–53. doi: 10.1039/c0jm01339k.
Planeix JM, Jaunky W, Duhoo T, Czernuszka JT, Hosseini MW, Brès EF. A molecular tectonics-crystal engineering approach for building organic-inorganic composites. Potential application to the growth control of hydroxyapatite crystals. J Mater Chem. 2003;13:2521–4. doi: 10.1039/b303029f.
Zhao J, Guo LY, Yang XB, Weng J. Preparation of bioactive porous HA/PCL composite scaffolds. Appl Surf Sci. 2008;255:2942–6. doi: 10.1016/j.apsusc.2008.08.056.
Dorozhkin S, Ajaal T. Toughening of porous bioceramic scaffolds by bioresorbable polymeric coatings. Proc Inst Mech Eng H. 2009;223:459–70. doi: 10.1243/09544119JEIM513.
Woo AS, Jang JL, Liberman RF, Weinzweig J. Creation of a vascularized composite graft with acellular dermal matrix and hydroxyapatite. Plast Reconstr Surg. 2010;125:1661–9. doi: 10.1097/PRS.0b013e3181d52830.
Zhao J, Duan K, Zhang JW, Lu X, Weng J. The influence of polymer concentrations on the structure and mechanical properties of porous polycaprolactone-coated hydroxyapatite scaffolds. Appl Surf Sci. 2010;256:4586–90. doi: 10.1016/j.apsusc.2010.02.053.
Dong J, Uemura T, Kojima H, Kikuchi M, Tanaka J, Tateishi T. Application of low-pressure system to sustain in vivo bone formation in osteoblast/porous hydroxyapatite composite. Mater Sci Eng C. 2001;17:37–43. doi: 10.1016/S0928-4931(01)00333-2.
Zerbo IR, Bronckers ALJJ, de Lange G, Burger EH. Localisation of osteogenic and osteoclastic cells in porous beta-tricalcium phosphate particles used for human maxillary sinus floor elevation. Biomaterials. 2005;26:1445–51. doi: 10.1016/j.biomaterials.2004.05.003.
Mikán J, Villamil M, Montes T, Carretero C, Bernal C, Torres ML, et al. Porcine model for hybrid material of carbonated apatite and osteoprogenitor cells. Mater Res Innovations. 2009;13:323–6. doi: 10.1179/143307509X440659.
Oe K, Miwa M, Nagamune K, Sakai Y, Lee SY, Niikura T, et al. Nondestructive evaluation of cell numbers in bone marrow stromal cell/beta-tricalcium phosphate composites using ultrasound. Tissue Eng Part C Methods. 2010;16:347–53. doi: 10.1089/ten.tec.2008.0564.
Krout A, Wen HB, Hippensteel E, Li P. A hybrid coating of biomimetic apatite and osteocalcin. J Biomed Mater Res A. 2005;73:377–87. doi: 10.1002/jbm.a.30310.
Kundu B, Soundrapandian C, Nandi SK, Mukherjee P, Dandapat N, Roy S, et al. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: a systematic approach for in vitro and in vivo animal trial. Pharm Res. 2010;27:1659–76. doi: 10.1007/s11095-010-0166-y.
Kickelbick G, ed. Hybrid materials. Synthesis, characterization and applications. Wiley-VCH Verlag: Weinheim, Germany 2007; 498.
Matthews FL, Rawlings RD. Composite materials: engineering and science. CRC Press: Boca Raton FL, USA 2000; 480.
Xia Z, Riester L, Curtin WA, Li H, Sheldon BW, Liang J, et al. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta Mater. 2004;52:931–44. doi: 10.1016/j.actamat.2003.10.050.
Tavares MIB, Ferreira O, Preto M, Miguez E, Soares IL, da Silva EP. Evaluation of composites miscibility by low field NMR. Int J Polym Mater. 2007;56:1113–8. doi: 10.1080/00914030701283063.
Kiran E. Polymer miscibility, phase separation, morphological modifications and polymorphic transformations in dense fluids. J Supercrit Fluids. 2009;47:466–83. doi: 10.1016/j.supflu.2008.11.010.
Supová M. Problem of hydroxyapatite dispersion in polymer matrices: a review. J Mater Sci Mater Med. 2009;20:1201–13. doi: 10.1007/s10856-009-3696-2.
Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615–21. doi: 10.1016/S0142-9612(00)00129-0.
John MJ, Thomas S. Biofibres and biocomposites. Carbohydr Polym. 2008;71:343–64. doi: 10.1016/j.carbpol.2007.05.040.
Rea SM, Bonfield W. Biocomposites for medical applications. J Aust Ceram Soc. 2004;40:43–57.
Gravitis YaA, Tééyaér RE, Kallavus UL, Andersons BA, Ozol’-Kalnin VG, Kokorevich AG, et al. Biocomposite structure of wood cell membranes and their destruction by explosive autohydrolysis. Mechanics Composite Mater. 1987;22:721–5. doi: 10.1007/BF00605309.
Bernard SL, Picha GJ. The use of coralline hydroxyapatite in a “biocomposite” free flap. Plast Reconstr Surg. 1991;87:96–105, discussion 106-7. doi: 10.1097/00006534-199101000-00015.
Ong JL, Chan DCN. Hydroxyapatite and their use as coatings in dental implants: a review. Crit Rev Biomed Eng. 2000;28:667–707. doi: 10.1615/critrevbiomedeng.v28.i56.10.
Davies JE. In vitro modeling of the bone/implant interface. Anat Rec. 1996;245:426–45. doi: 10.1002/(SICI)1097-0185(199606)245:2<426::AID-AR21>3.0.CO;2-Q.
Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–81. doi: 10.1016/S0142-9612(99)00242-2.
Gauthier O, Bouler JM, Weiss P, Bosco J, Daculsi G, Aguado E. Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calcium-phosphate bone substitutes. J Biomed Mater Res. 1999;47:28–35. doi: 10.1002/(SICI)1097-4636(199910)47:1<28::AID-JBM4>3.0.CO;2-P.
Hing KA, Best SM, Bonfield W. Characterization of porous hydroxyapatite. J Mater Sci Mater Med. 1999;10:135–45. doi: 10.1023/A:1008929305897.
Carotenuto G, Spagnuolo G, Ambrosio L, Nicolais L. Macroporous hydroxyapatite as alloplastic material for dental applications. J Mater Sci Mater Med. 1999;10:671–6. doi: 10.1023/A:1008952111545.
LeGeros RZ. Calcium phosphates in oral biology and medicine, Monographs in oral science. Myers HM, (Ed.); Karger: Basel, Switzerland 1991; 15:201.
Elliott JC. Structure and chemistry of the apatites and other calcium orthophosphates, Studies in inorganic chemistry. Elsevier: Amsterdam, Netherlands 1994; 18:389.
Brown PW, Constantz B, eds. Hydroxyapatite and related materials. CRC Press: Boca Raton FL, USA 1994; 343.
Amjad Z, ed. Calcium phosphates in biological and industrial systems, Kluwer: Boston MA, USA 1997; 529.
Hughes JM, Kohn M, Rakovan J, eds. Phosphates: geochemical, geobiological and materials importance. Series: Reviews in mineralogy and geochemistry, Mineralogical Society of America: Washington DC, USA 2002; 48:742.
Chow LC, Eanes ED, eds. Octacalcium phosphate, Monographs in oral science. S. Karger: Basel, Switzerland 2001; 18:168.
Brès E, Hardouin P, eds. Les matériaux en phosphate de calcium. Aspects fondamentaux./Calcium phosphate materials. Fundamentals, Sauramps Medical: Montpellier, France 1998; 176.
Walton DJ, Lorimer JP. Polymers, Oxford University Press: New York USA 2001; 160.
Carraher CE Jr. Introduction to Polymer Chemistry. CRC Press: Boca Raton FL, USA 2010; 2:534.
Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc Chem Res. 2000;33:94–101. doi: 10.1021/ar9800993.
Thomson RC, Ak S, Yaszemski MJ, Mikos AG. Polymer scaffold processing. In: Principles of Tissue Engineering, Academic Press: NY USA 2000; 251-62.
Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–224. doi: 10.1016/S0266-3538(00)00241-4.
Shastri VP. Non-degradable biocompatible polymers in medicine: past, present and future. Curr Pharm Biotechnol. 2003;4:331–7. doi: 10.2174/1389201033489694.
Chen H, Yuan L, Song W, Wu Z, Li D. Biocompatible polymer materials: role of protein-surface interactions. Prog Polym Sci. 2008;33:1059–87. doi: 10.1016/j.progpolymsci.2008.07.006.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529.
Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14:1107–11. doi: 10.1038/nbt0996-1107.
Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–50. doi: 10.1002/1097-4636(200105)55:2<141::AID-JBM1000>3.0.CO;2-J.
Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee HS, et al. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials. 2003;24:801–8. doi: 10.1016/S0142-9612(02)00370-8.
de Groot JH, de Vrijer R, Pennings AJ, Klompmaker J, Veth RPH, Jansen HWB. Use of porous polyurethanes for meniscal reconstruction and meniscal prostheses. Biomaterials. 1996;17:163–73. doi: 10.1016/0142-9612(96)85761-9.
Resiak I, Rokicki G. Modified polyurethanes for biomedical applications. Polimery. 2000;45:592–602.
Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–12. doi: 10.1016/S0142-9612(00)00108-3.
Behravesh E, Yasko AW, Engel PS, Mikos AG. Synthetic biodegradable polymers for orthopaedic applications. Clin Orthop Relat Res. 1999;367(Suppl):S118–29. doi: 10.1097/00003086-199910001-00012.
Lewandrowski KU, Gresser JD, Wise DL, White RL, Trantolo DJ. Osteoconductivity of an injectable and bioresorbable poly(propylene glycol-co-fumaric acid) bone cement. Biomaterials. 2000;21:293–8. doi: 10.1016/S0142-9612(99)00180-5.
Peter SJ, Miller MJ, Yaszemski MJ, Mikos AG. Poly(propylene fumarate). In: Handbook of biodegradable polymers, Domb AJ, Kost J, Wiseman DM, (Eds.); Harwood Academic: Amsterdam, Netherlands 1997; 87-97.
Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 2005;1:115–23. doi: 10.1016/j.actbio.2004.09.003.
Gilbert JL. Acrylics in biomedical engineering. In: Encyclopedia of materials: science and technology, Elsevier: Amsterdam, Netherlands 2001; 11-8.
Frazer RQ, Byron RT, Osborne PB, West KP. PMMA: an essential material in medicine and dentistry. J Long Term Eff Med Implants. 2005;15:629–39. doi: 10.1615/jlongtermeffmedimplants.v15.i6.60.
Li YW, Leong JCY, Lu WW, Luk KDK, Cheung KMC, Chiu KY, et al. A novel injectable bioactive bone cement for spinal surgery: a developmental and preclinical study. J Biomed Mater Res. 2000;52:164–70. doi: 10.1002/1097-4636(200010)52:1<164::AID-JBM21>3.0.CO;2-R.
Mckellop H, Shen F, Lu B, Campbell P, Salovey R. Development of an extremely wear resistant UHMW polyethylene for total hip replacements. J Orthop Res. 1999;17:157–67. doi: 10.1002/jor.1100170203.
Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20:1659–88. doi: 10.1016/S0142-9612(99)00053-8.
Laurencin CT, Ambrosio AM, Borden MD, Cooper JA., Jr. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19.
Jansen JA, de Ruijter JE, Janssen PT, Paquay YG. Histological evaluation of a biodegradable Polyactive/hydroxyapatite membrane. Biomaterials. 1995;16:819–27. doi: 10.1016/0142-9612(95)94142-8.
Liu Q, de Wijn JR, Bakker D, van Blitterswijk CA. Surface modification of hydroxyapatite to introduce interfacial bonding with PolyactiveTM 70/30 in a biodegradable composite. J Mater Sci Mater Med. 1996;7:551–7. doi: 10.1007/BF00122178.
Liu Q, de Wijn JR, Bakker D, van Toledo M, van Blitterswijk CA. Polyacids as bonding agents in hydroxyapatite polyester-ether (Polyactive 30/70) composites. J Mater Sci Mater Med. 1998;9:23–30. doi: 10.1023/A:1008826410395.
Meijer GJ, Cune MS, van Dooren M, de Putter C, van Blitterswijk CA. A comparative study of flexible (Polyactive) versus rigid (hydroxylapatite) permucosal dental implants. I. Clinical aspects. J Oral Rehabil. 1997;24:85–92. doi: 10.1046/j.1365-2842.1997.d01-264.x.
Meijer GJ, Dalmeijer RA, de Putter C, van Blitterswijk CA. A comparative study of flexible (Polyactive) versus rigid (hydroxylapatite) permucosal dental implants. II. Histological aspects. J Oral Rehabil. 1997;24:93–101. doi: 10.1046/j.1365-2842.1997.00475.x.
Waris E, Ashammakhi N, Lehtimäki M, Tulamo RM, Törmälä P, Kellomäki M, et al. Long-term bone tissue reaction to polyethylene oxide/polybutylene terephthalate copolymer (Polyactive) in metacarpophalangeal joint reconstruction. Biomaterials. 2008;29:2509–15. doi: 10.1016/j.biomaterials.2008.02.013.
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M, et al. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials. 2005;26:419–31. doi: 10.1016/j.biomaterials.2004.02.049.
Rampinelli G, di Landro L, Fujii T. Characterization of biomaterials based on microfibrillated cellulose with different modifications. J Reinforced Plastics Compos. 2010;29:1793–803. doi: 10.1177/0731684409335453.
Granja PL, Barbosa MA, Pouysége L, de Jéso B, Rouais F, Baquuey C. Cellulose phosphates as biomaterials. Mineralization of chemically modified regenerated cellulose hydrogels. J Mater Sci. 2001;36:2163–72. doi: 10.1023/A:1017587815583.
Granja PL, Jéso BD, Bareille R, Rouais F, Baquey C, Barbosa MA. Cellulose phosphates as biomaterials. In vitro biocompatibility studies. React Funct Polym. 2006;66:728–39. doi: 10.1016/j.reactfunctpolym.2005.10.027.
Thomas V, Dean DR, Vohra YK. Nanostructured biomaterials for regenerative medicine. Curr Nanosci. 2006;2:155–77.
Li SM, Garreau H, Vert M. Structure-property relationships in the case of the degradation of massive aliphatic poly(α -hydroxyacids) in aqueous media. Part 1: poly(D,L-lactic acid) J Mater Sci Mater Med. 1990;1:123–30. doi: 10.1007/BF00700871.
Daniels AU, Adriano KP, Smuts WP, Chang MKO, Keller J. Evaluation of absorbable poly(orthoesters) for use in surgical implants. J Biomed Mater Res B Appl Biomater. 1994;5:51–64. doi: 10.1002/jab.770050108.
Adriano KP, Pohjonen T, Törmällä P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Biomed Mater Res B Appl Biomater. 1994;5:133–40. doi: 10.1002/jab.770050206.
Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1.
Dee KC, Bizios R. Mini-review: Proactive biomaterials and bone tissue engineering. Biotechnol Bioeng. 1996;50:438–42. doi: 10.1002/(SICI)1097-0290(19960520)50:4<438::AID-BIT11>3.0.CO;2-F.
Ignjatović N, Tomić S, Dakić M, Miljković M, Plavsić M, Uskoković D. Synthesis and properties of hydroxyapatite/poly-L-lactide composite biomaterials. Biomaterials. 1999;20:809–16. doi: 10.1016/S0142-9612(98)00234-8.
Ignjatović N, Savić V, Najman S, Plavgić M, Uskoković D. A study of HAp/PLLA composite as a substitute for bone powder, using FT-IR spectroscopy. Biomaterials. 2001;22:571–5. doi: 10.1016/S0142-9612(00)00215-5.
Marra KG, Szem JW, Kumta PN, DiMilla PA, Weiss LE. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324–35. doi: 10.1002/(SICI)1097-4636(19991205)47:3<324::AID-JBM6>3.0.CO;2-Y.
Ashammakhi N, Rokkanen P. Absorbable polyglycolide devices in trauma and bone surgery. Biomaterials. 1997;18:3–9. doi: 10.1016/S0142-9612(96)00107-X.
Boyan B, Lohmann C, Somers A, Neiderauer G, Wozney J, Dean D, et al. Potential of porous poly-D,L-lactide-co-glycolide particles as a carrier for recombinant human bone morphogenetic protein-2 during osteoinduction in vivo. J Biomed Mater Res 1999; 46:51-9; PMID: 10357135; DOI: 10.1002/(SICI)1097-4636(199907)46:1<51::AID-JBM6 >3.0.CO;2-I.
Hofmann GO. Biodegradable implants in traumatology: a review on the state-of-the-art. Arch Orthop Trauma Surg. 1995;114:123–32. doi: 10.1007/BF00443385.
Hollinger JO, Leong K. Poly(alpha-hydroxy acids): carriers for bone morphogenetic proteins. Biomaterials. 1996;17:187–94. doi: 10.1016/0142-9612(96)85763-2.
Griffith LG. Polymeric biomaterials. Acta Mater. 2000;48:263–77. doi: 10.1016/S1359-6454(99)00299-2.
Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. J Biomed Mater Res. 1998;43:422–7. doi: 10.1002/(SICI)1097-4636(199824)43:4<422::AID-JBM9>3.0.CO;2-1.
Ishaug SL, Payne RG, Yaszemski MJ, Aufdemorte TB, Bizios R, Mikos AG. Osteoblast migration on poly(alpha-hydroxy esters) Biotechnol Bioeng. 1996;50:443–51. doi: 10.1002/(SICI)1097-0290(19960520)50:4<443::AID-BIT12>3.0.CO;2-K.
Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials. 1999;20:859–77. doi: 10.1016/S0142-9612(98)00241-5.
Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–49. doi: 10.1016/S0142-9612(03)00026-7.
Ishihara M, Nakanishi K, Ono K, Sato M, Kikuchi M, Saito Y, et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23:833–40. doi: 10.1016/S0142-9612(01)00189-2.
Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016.
Piskin E, Bölgen N, Egri S, Isoglu IA. Electrospun matrices made of poly(alpha-hydroxy acids) for medical use. Nanomedicine (Lond) 2007;2:441–57. doi: 10.2217/17435889.2.4.441.
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–31. doi: 10.1016/j.biomaterials.2006.01.039.
Mohanty AK, Misra M, Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng. 2000;277:1–24. doi: 10.1002/(SICI)1439-2054(20000301)276:1<1::AID-MAME1>3.0.CO;2-W.
Kohane DS, Langer R. Polymeric biomaterials in tissue engineering. Pediatr Res. 2008;63:487–91. doi: 10.1203/01.pdr.0000305937.26105.e7.
Seal BL, Otero TC, Panitch A. Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng Rep. 2001;34:147–230. doi: 10.1016/S0927-796X(01)00035-3.
An YH, Woolf SK, Friedman RJ. Pre-clinical in vivo evaluation of orthopaedic bioabsorbable devices. Biomaterials. 2000;21:2635–52. doi: 10.1016/S0142-9612(00)00132-0.
Mano JF, Sousa RA, Boesel LF, Neves NM, Reis RL. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos Sci Technol. 2004;64:789–817. doi: 10.1016/j.compscitech.2003.09.001.
Katti KS. Biomaterials in total joint replacement. Colloids Surf B Biointerfaces. 2004;39:133–42. doi: 10.1016/j.colsurfb.2003.12.002.
Hayashi T. Biodegradable polymers for biomedical uses. Prog Polym Sci. 1994;19:663–702. doi: 10.1016/0079-6700(94)90030-2.
Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–46. doi: 10.1016/S0142-9612(00)00101-0.
Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–98. doi: 10.1016/j.addr.2007.08.041.
Coombes AG, Meikle MC. Resorbable synthetic polymers as replacements for bone graft. Clin Mater. 1994;17:35–67. doi: 10.1016/0267-6605(94)90046-9.
Okada M. Chemical syntheses of biodegradable polymers. Prog Polym Sci. 2002;27:87–133. doi: 10.1016/S0079-6700(01)00039-9.
Jordan J, Jacob KI, Tannenbaum R, Sharaf MA, Jasiuk I. Experimental trends in polymer nanocomposites—a review. Mater Sci Eng A. 2005;393:1–11. doi: 10.1016/j.msea.2004.09.044.
Matsuno H, Yokoyama A, Watari F, Uo M, Kawasaki T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials. 2001;22:1253–62. doi: 10.1016/S0142-9612(00)00275-1.
Hanawa T. Biofunctionalization of titanium for dental implant. Jpn Dent Sci Rev. 2010;46:93–101. doi: 10.1016/j.jdsr.2009.11.001.
Uo M, Watari F, Yokoyama A, Matsuno H, Kawasaki T. Dissolution of nickel and tissue response observed by X-ray scanning analytical microscopy. Biomaterials. 1999;20:747–55. doi: 10.1016/S0142-9612(98)00224-5.
Uo M, Watari F, Yokoyama A, Matsuno H, Kawasaki T. Visualization and detectability of rarely contained elements in soft tissue by X-ray scanning analytical microscopy and electron probe micro analysis. Biomaterials. 2001;22:1787–94. doi: 10.1016/S0142-9612(00)00349-5.
Uo M, Watari F, Yokoyama A, Matsuno H, Kawasaki T. Tissue reaction around metal implants observed by X-ray scanning analytical microscopy. Biomaterials. 2001;22:677–85. doi: 10.1016/S0142-9612(00)00230-1.
Ryan G, Pandit A, Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials. 2006;27:2651–70. doi: 10.1016/j.biomaterials.2005.12.002.
Shimko DA, Shimko VF, Sander EA, Dickson KF, Nauman EA. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J Biomed Mater Res B Appl Biomater. 2005;73:315–24. doi: 10.1002/jbm.b.30229.
Wen CE, Yamada Y, Shimojima K, Chino Y, Hosokawa H, Mabuchi M. Compressibility of porous magnesium foam: dependency on porosity and pore size. Mater Lett. 2004;58:357–60. doi: 10.1016/S0167-577X(03)00500-7.
Levine B. A new era in porous metals: applications in orthopaedics. Adv Eng Mater. 2008;10:788–92. doi: 10.1002/adem.200800215.
Green D, Walsh D, Mann S, Oreffo ROC. The potential of biomimesis in bone tissue engineering: lessons from the design and synthesis of invertebrate skeletons. Bone. 2002;30:810–5. doi: 10.1016/S8756-3282(02)00727-5.
Kokubo T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater. 1998;46:2519–27. doi: 10.1016/S1359-6454(98)80036-0.
Witte F, Reifenrath J, Müller PP, Crostack HA, Nellesen J, Bach FW, et al. Cartilage repair on magnesium scaffolds used as a subchondral bone replacement. Mater.-Wiss. u. Werkstofflech. 2006;37:504–8. doi: 10.1002/mawe.200600027.
Gu XN, Zhou WR, Zheng YF, Liu Y, Li YX. Degradation and cytotoxicity of lotus-type porous pure magnesium as potential tissue engineering scaffold material. Mater Lett. 2010;64:1871–4. doi: 10.1016/j.matlet.2010.06.015.
Uo M, Mizuno M, Kuboki Y, Makishima A, Watari F. Properties and cytotoxicity of water soluble Na2O-CaO-P2O5 glasses. Biomaterials. 1998;19:2277–84. doi: 10.1016/S0142-9612(98)00136-7.
Imai T, Watari F, Yamagata S, Kobayashi M, Nagayama K, Toyoizumi Y, et al. Mechanical properties and aesthetics of FRP orthodontic wire fabricated by hot drawing. Biomaterials. 1998;19:2195–200. doi: 10.1016/S0142-9612(98)00127-6.
Watari F, Yamagata S, Imai T, Nakamura S, Kobayashi M. The fabrication and properties of aesthetic FRP wires for use in orthodontics. J Mater Sci. 1998;33:5661–4. doi: 10.1023/A:1004484703341.
Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967–78. doi: 10.1007/s10856-006-0432-z.
Cao W, Hench LL. Bioactive materials. Ceram Int. 1996;22:493–507. doi: 10.1016/0272-8842(95)00126-3.
Vogel M, Voigt C, Gross UM, Müller-Mai CM. In vivo comparison of bioactive glass particles in rabbits. Biomaterials. 2001;22:357–62. doi: 10.1016/S0142-9612(00)00191-5.
De Aza PN, Luklinska ZB, Santos C, Guitian F, De Aza S. Mechanism of bone-like formation on a bioactive implant in vivo. Biomaterials. 2003;24:1437–45. doi: 10.1016/S0142-9612(02)00530-6.
Ikeda N, Kawanabe K, Nakamura T. Quantitative comparison of osteoconduction of porous, dense A-W glass-ceramic and hydroxyapatite granules (effects of granule and pore sizes) Biomaterials. 1999;20:1087–95. doi: 10.1016/S0142-9612(99)00005-8.
Weizhong Y, Dali Z, Guangfu Y. Research and development of A-W bioactive glass ceramic. J Biomed Eng. 2003;20:541–5.
Encinas-Romero MA, Aguayo-Salinas S, Valenzuela-García JL, Payán SR, Castillón-Barraza FF. Mechanical and bioactive behavior of hydroxyapatite-wollastonite sintered composites. Int J Appl Ceram Technol. 2010;7:164–77. doi: 10.1111/j.1744-7402.2009.02377.x.
Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/S0142-9612(98)00010-6.
Christel P, Meunier A, Heller M, Torre JP, Peille CN. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J Biomed Mater Res. 1989;23:45–61. doi: 10.1002/jbm.820230105.
Garvie RC, Urban D, Kennedy DR, McMeuer JC. Biocompatibility of magnesium partially stabilized zirconia (Mg-PSZ ceramics) J Mater Sci. 1984;19:3224–8. doi: 10.1007/BF00549808.
Burger W, Richter HG, Piconi C, Vatteroni R, Cittadini A, Boccalari M. New Y-TZP powders for medical grade zirconia. J Mater Sci Mater Med. 1997;8:113–8. doi: 10.1023/A:1018562917779.
Benson J. Elemental carbon as a biomaterial. J Biomed Mater Res. 1972;5:41–7. doi: 10.1002/jbm.820050607.
Olborska A, Swider M, Wolowiec R, Niedzielski P, Rylski A, Mitura S. Amorphous carbon—biomaterial for implant coatings. Diamond Related Materials. 1994;3:899–901. doi: 10.1016/0925-9635(94)90296-8.
Saito N, Usui Y, Aoki K, Narita N, Shimizu M, Hara K, et al. Carbon nanotubes: biomaterial applications. Chem Soc Rev. 2009;38:1897–903. doi: 10.1039/b804822n.
Vila M, Manzano M, Vallet-Regí M.Carbon nanotubes: a solution for processing smart biomaterials. Key Eng Mater 2010; 441:3-29; .
White AA, Best SM, Kinloch IA. Hydroxyapatite-carbon nanotube composites for biomedical applications: a review. Int J Appl Ceram Technol. 2007;4:1–13. doi: 10.1111/j.1744-7402.2007.02113.x.
Chlopek J, Czajkowska B, Szaraniec B, Frackowiak E, Szostak K, Beguin F. In vitro studies of carbon nanotubes biocompatibility. Carbon. 2006;44:1106–11. doi: 10.1016/j.carbon.2005.11.022.
Price RL, Waid MC, Haberstroh KM, Webster TJ. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24:1877–87. doi: 10.1016/S0142-9612(02)00609-9.
Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6:562–7. doi: 10.1021/nl051861e.
Saito N, Usui Y, Aoki K, Narita N, Shimizu M, Ogiwara N, et al. Carbon nanotubes for biomaterials in contact with bone. Curr Med Chem. 2008;15:523–7. doi: 10.2174/092986708783503140.
Banerjee S, Kahn MGC, Wong SS. Rational chemical strategies for carbon nanotube functionalization. Chemistry. 2003;9:1898–908. doi: 10.1002/chem.200204618.
Beuvelot J, Bergeret C, Mallet R, Fernandez V, Cousseau J, Baslé MF, et al. In vitro calcification of chemically functionalized carbon nanotubes. Acta Biomater. 2010;6:4110–7. doi: 10.1016/j.actbio.2010.05.011.
Xiao Y, Gong T, Zhou S. The functionalization of multi-walled carbon nanotubes by in situ deposition of hydroxyapatite. Biomaterials. 2010;31:5182–90. doi: 10.1016/j.biomaterials.2010.03.012.
Converse GL, Yue W, Roeder RK. Processing and tensile properties of hydroxyapatite-whisker-reinforced polyetheretherketone. Biomaterials. 2007;28:927–35. doi: 10.1016/j.biomaterials.2006.10.031.
Yue W, Roeder RK. Micromechanical model for hydroxyapatite whisker reinforced polymer biocomposites. J Mater Res. 2006;21:2136–45. doi: 10.1557/jmr.2006.0263.
Converse GL, Roeder RK. Tensile properties of hydroxyapatite whisker reinforced polyetheretherketone. Mater Res Soc Symp Proc 2005; 898:44-9.
Mizutani Y, Hattori M, Okuyama M, Kasuga T, Nogami M.Preparation of porous composites with a porous framework using hydroxyapatite whiskers and poly(L-lactic acid) short fibers. Key Eng Mater 2006; 311:1079-82; .
Choi WY, Kim HE, Kim MJ, Kim UC, Kim JH, Koh YH. Production and characterization of calcium phosphate (CaP) whisker-reinforced poly(∑-caprolactone) composites as bone regenerative. Mater Sci Eng C. 2010;30:1280–4. doi: 10.1016/j.msec.2010.07.018.
Watanabe T, Ban S, Ito T, Tsuruta S, Kawai T, Nakamura H. Biocompatibility of composite membrane consisting of oriented needle-like apatite and biodegradable copolymer with soft and hard tissues in rats. Dent Mater J. 2004;23:609–12. doi: 10.4012/dmj.23.609.
Li H, Chen Y, Xie Y. Photo-cross-linking polymerization to prepare polyanhydride/needle-like hydroxyapatite biodegradable nanocomposite for orthopedic application. Mater Lett. 2003;57:2848–54. doi: 10.1016/S0167-577X(02)01386-1.
Peng Q, Weng J, Li X, Gu Z.Manufacturing porous blocks of nano-composite of needle-like hydroxyapatite crystallites and chitin for tissue engineering. Key Eng Mater 2005; 288-289:199-202; .
Kasuga T, Ota Y, Nogami M, Abe Y. Preparation and mechanical properties of polylactic acid composites containing hydroxyapatite fibers. Biomaterials. 2001;22:19–23. doi: 10.1016/S0142-9612(00)00091-0.
However, a more general topic “ceramic-plastic material as a bone substitute” is, at least, 18 years older.
Smith L. Ceramic-plastic material as a bone substitute. Arch Surg. 1963;87:653–61. doi: 10.1001/archsurg.1963.01310160115023.
Bonfield W, Grynpas MD, Tully AE, Bowman J, Abram J. Hydroxyapatite reinforced polyethylene--a mechanically compatible implant material for bone replacement. Biomaterials. 1981;2:185–6. doi: 10.1016/0142-9612(81)90050-8.
Bonfield W, Bowman J, Grynpas MD. Composite material for use in orthopaedics. UK Patent 8032647, 1981.
Bonfield W. Composites for bone replacement. J Biomed Eng. 1988;10:522–6. doi: 10.1016/0141-5425(88)90110-0.
Wang M, Porter D, Bonfield W. Processing, characterisation and evaluation of hydroxyapatite reinforced polyethylene composites. Br Ceram Trans. 1994;93:91–5.
Guild FJ, Bonfield W. Predictive character of hydroxyapatite-polyethelene HAPEXTM composite. Biomaterials. 1993;14:985–93. doi: 10.1016/0142-9612(93)90190-D.
Huang J, Di Silvio L, Wang M, Tanner KE, Bonfield W. In vitro mechanical and biological assessment of hydroxyapatite-reinforced polyethylene composite. J Mater Sci Mater Med. 1997;8:775–9. doi: 10.1023/A:1018516813604.
Deb S, Wang M, Tanner KE, Bonfield W. Hydroxyapatite-polyethylene composites: effect of grafting and surface treatment of hydroxyapatite. J Mater Sci Mater Med. 1996;7:191–3. doi: 10.1007/BF00119729.
Wang M, Joseph R, Bonfield W. Hydroxyapatite-polyethylene composites for bone substitution: effects of ceramic particle size and morphology. Biomaterials. 1998;19:2357–66. doi: 10.1016/S0142-9612(98)00154-9.
Suwanprateeb J, Tanner KE, Turner S, Bonfield W. Influence of Ringer’s solution on creep resistance of hydroxyapatite reinforced polyethylene composites. J Mater Sci Mater Med. 1997;8:469–72. doi: 10.1023/A:1018522025474.
Ladizesky NH, Ward IM, Bonfield W. Hydroxyapatite/high-performance polyethylene fiber composites for high load bearing bone replacement materials. J Appl Polym Sci. 1997;65:1865–82. doi: 10.1002/(SICI)1097-4628(19970906)65:10<1865::AID-APP3>3.0.CO;2-D.
Ladizesky NH, Pirhonen EM, Appleyard DB, Ward IM, Bonfield W. Fibre reinforcement of ceramic/polymer composites for a major load-bearing bone substitute material. Compos Sci Technol. 1998;58:419–34. doi: 10.1016/S0266-3538(97)00140-1.
Nazhat SN, Joseph R, Wang M, Smith R, Tanner KE, Bonfield W. Dynamic mechanical characterization of hydroxyapatite reinforced polyethylene: effect of particle size. J Mater Sci Mater Med. 2000;11:621–8. doi: 10.1023/A:1008957729512.
Ladizesky NH, Ward IM, Bonfield W. Hydrostatic extrusion of polyethylene filled with hydroxyapatite. Polym Adv Technol. 1996;8:496–504. doi: 10.1002/(SICI)1099-1581(199708)8:8<496::AID-PAT676>3.0.CO;2-R.
Guild FJ, Bonfield W. Predictive modelling of the mechanical properties and failure processes in hydroxyapatite- polyethylene (Hapex) composite. J Mater Sci Mater Med. 1998;9:497–502. doi: 10.1023/A:1008831720198.
Di Silvio L, Dalby M, Bonfield W. In vitro response of osteoblasts to hydroxyapatite-reinforced polyethylene composites. J Mater Sci Mater Med. 1998;9:845–8. doi: 10.1023/A:1008900312950.
Wang M, Ladizesky NH, Tanner KE, Ward IM, Bonfield W. Hydrostatically extruded HAPEXTM. J Mater Sci. 2000;35:1023–30. doi: 10.1023/A:1004731315328.
That PT, Tanner KE, Bonfield W. Fatigue characterization of a hydroxyapatite-reinforced polyethylene composite. I. Uniaxial fatigue. J Biomed Mater Res. 2000;51:453–60. doi: 10.1002/1097-4636(20000905)51:3<453::AID-JBM20>3.0.CO;2-Q.
Ton That PT, Tanner KE, Bonfield W. Fatigue characterization of a hydroxyapatite-reinforced polyethylene composite. II. Biaxial fatigue. J Biomed Mater Res. 2000;51:461–8. doi: 10.1002/1097-4636(20000905)51:3<461::AID-JBM21>3.0.CO;2-M.
Bonner M, Saunders LS, Ward IM, Davies GW, Wang M, Tanner KE, et al. Anisotropic mechanical properties of oriented HAPEXTM. J Mater Sci. 2002;37:325–34. doi: 10.1023/A:1013652312670.
Dalby MJ, Di Silvio L, Davies GW, Bonfield W. Surface topography and HA filler volume effect on primary human osteoblasts in vitro. J Mater Sci Mater Med. 2000;11:805–10. doi: 10.1023/A:1008957630020.
Di Silvio L, Dalby MJ, Bonfield W. Osteoblast behaviour on HA/PE composite surfaces with different HA volumes. Biomaterials. 2002;23:101–7. doi: 10.1016/S0142-9612(01)00084-9.
Dalby MJ, Kayser MV, Bonfield W, Di Silvio L. Initial attachment of osteoblasts to an optimised HAPEX topography. Biomaterials. 2002;23:681–90. doi: 10.1016/S0142-9612(01)00156-9.
Dalby MJ, Di Silvio L, Gurav N, Annaz B, Kayser MV, Bonfield W. Optimizing HAPEX topography influences osteoblast response. Tissue Eng. 2002;8:453–67. doi: 10.1089/107632702760184718.
Zhang Y, Tanner KE, Gurav N, Di Silvio L. In vitro osteoblastic response to 30 vol% hydroxyapatite-polyethylene composite. J Biomed Mater Res A. 2007;81:409–17. doi: 10.1002/jbm.a.31078.
Rea SM, Best SM, Bonfield W. Bioactivity of ceramic-polymer composites with varied composition and surface topography. J Mater Sci Mater Med. 2004;15:997–1005. doi: 10.1023/B:JMSM.0000042685.63383.86.
Rea SM, Brooks RA, Schneider A, Best SM, Bonfield W. Osteoblast-like cell response to bioactive composites-surface-topography and composition effects. J Biomed Mater Res B Appl Biomater. 2004;70:250–61. doi: 10.1002/jbm.b.30039.
Bonner M, Ward IM, McGregor W, Tanner KE, Bonfield W. Hydroxyapatite/polypropylene composite: a novel bone substitute material. J Mater Sci Lett. 2001;20:2049–52. doi: 10.1023/A:1013594125371.
Suppakarn N, Sanmaung S, Ruksakulpiwa Y, Sutapun W.Effect of surface modification on properties of natural hydroxyapatite/polypropylene composites. Key Eng Mater 2008; 363:511-4; .
Younesi M, Bahrololoom ME. Formulating the effects of applied temperature and pressure of hot pressing process on the mechanical properties of polypropylene-hydroxyapatite bio-composites by response surface methodology. Mater Des. 2010;31:4621–30. doi: 10.1016/j.matdes.2010.05.037.
Salernitano E, Migliaresi C. Composite materials for biomedical applications: a review. J Appl Biomater Biomech. 2003;1:3–18.
Pandey A, Jan E, Aswath PB. Physical and mechanical behavior of hot rolled HDPE/HA composites. J Mater Sci. 2006;41:3369–76. doi: 10.1007/s10853-005-5350-9.
Sousa RA, Reis RL, Cunha AM, Bevis MJ. Processing and properties of bone analogue biodegradable and bioinert polymeric composites. Compos Sci Technol. 2003;63:389–402. doi: 10.1016/S0266-3538(02)00213-0.
Wang M, Deb S, Bonfield W. Chemically coupled hydroxyapatite-polyethylene composites: processing and characterisation. Mater Lett. 2000;44:119–24. doi: 10.1016/S0167-577X(00)00026-4.
Wang M, Bonfield W. Chemically coupled hydroxyapatite-polyethylene composites: structure and properties. Biomaterials. 2001;22:1311–20. doi: 10.1016/S0142-9612(00)00283-0.
Homaeigohar SSh, Shokrgozar MA, Khavandi A, Sadi AY. In vitro biological evaluation of beta-TCP/HDPE--A novel orthopedic composite: a survey using human osteoblast and fibroblast bone cells. J Biomed Mater Res A. 2008;84:491–9. doi: 10.1002/jbm.a.31473.
Sadi AY, Homaeigohar SSh, Khavandi AR, Javadpour J. The effect of partially stabilized zirconia on the mechanical properties of the hydroxyapatite-polyethylene composites. J Mater Sci Mater Med. 2004;15:853–8. doi: 10.1023/B:JMSM.0000036272.28022.3a.
Nath S, Bodhak S, Basu B. HDPE-Al2O3-HAp composites for biomedical applications: processing and characterizations. J Biomed Mater Res B Appl Biomater. 2009;88:1–11. doi: 10.1002/jbm.b.31050.
Downes RN, Vardy S, Tanner KE, Bonfield W. Hydroxyapatite-polyethylene composite in orbital surgery. Bioceramics. 1991;4:239–46.
Dornhoffer JL. Hearing results with the Dornhoffer ossicular replacement prostheses. Laryngoscope. 1998;108:531–6. doi: 10.1097/00005537-199804000-00013.
Swain RE, Wang M, Beale B, Bonfield W. HAPEXTM for otologic applications. Biomed Eng Appl Basis Commun. 1999;11:315–20.
Yi Z, Li Y, Jidong L, Xiang Z, Hongbing L, Yuanyuan W, et al. Novel bio-composite of hydroxyapatite reinforced polyamide and polyethylene: composition and properties. Mater Sci Eng A. 2007;453:512–7. doi: 10.1016/j.msea.2006.11.138.
Unwin AP, Ward IM, Ukleja P, Weng J. The role of pressure annealing in improving the stiffness of polyethylene/hydroxyapatite composites. J Mater Sci. 2001;36:3165–77. doi: 10.1023/A:1017926100999.
Fang LM, Leng Y, Gao P. Processing and mechanical properties of HA/UHMWPE nanocomposites. Biomaterials. 2006;27:3701–7. doi: 10.1016/j.biomaterials.2006.02.023.
Fang LM, Gao P, Leng Y. High strength and bioactive hydroxyapatite nano-particles reinforced ultrahigh molecular weight polyethylene. Composites B. 2007;38:345–51. doi: 10.1016/j.compositesb.2006.05.004.
Fang LM, Leng Y, Gao P. Processing of hydroxyapatite reinforced ultrahigh molecular weight polyethylene for biomedical applications. Biomaterials. 2005;26:3471–8. doi: 10.1016/j.biomaterials.2004.09.022.
Selvin TP, Seno J, Murukan B, Santhosh AA, Sabu T, Weimin Y, et al. Poly(ethylene-co-vinyl acetate)/calcium phosphate nanocomposites: thermo mechanical and gas permeability measurements. Polym Compos. 2010;31:1011–9.
Sousa RA, Reis RL, Cunha AM, Bevis MJ. Structure development and interfacial interactions in high-density polyethylene/hydroxyapatite (HDPE/HA) composites molded with preferred orientation. J Appl Polym Sci. 2002;86:2873–86. doi: 10.1002/app.11301.
Reis RL, Cunha AM, Oliveira MJ, Campos AR, Bevis MJ. Relationship between processing and mechanical properties of injection molded high molecular mass polyethylene + hydroxyapatite composites. Mater Res Innovat. 2001;4:263–72. doi: 10.1007/s100190000103.
Donners JJJM, Nolte RJM, Sommerdijk NA. Dendrimer-based hydroxyapatite composites with remarkable materials properties. Adv Mater (Deerfield Beach Fla) 2003;15:313–6. doi: 10.1002/adma.200390076.
Schneider OD, Stepuk A, Mohn D, Luechinger NA, Feldman K, Stark WJ. Light-curable polymer/calcium phosphate nanocomposite glue for bone defect treatment. Acta Biomater. 2010;6:2704–10. doi: 10.1016/j.actbio.2010.01.033.
Ignjatovic NL, Plavsic M, Miljkovic MS, Zivkovic LM, Uskokovic DP. Microstructural characteristics of calcium hydroxyapatite/poly-L-lactide based composites. J Microsc. 1999;196:243–8. doi: 10.1046/j.1365-2818.1999.00623.x.
Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stand Technol. 2003;108:167–82. doi: 10.6028/jres.108.017.
Rizzi SC, Heath DJ, Coombes AGA, Bock N, Textor M, Downes S. Biodegradable polymer/hydroxyapatite composites: surface analysis and initial attachment of human osteoblasts. J Biomed Mater Res. 2001;55:475–86. doi: 10.1002/1097-4636(20010615)55:4<475::AID-JBM1039>3.0.CO;2-Q.
Kato K, Eika Y, Ikada Y. In situ hydroxyapatite crystallization for the formation of hydroxyapatite/polymer composites. J Mater Sci. 1997;32:5533–43. doi: 10.1023/A:1018616306104.
Navarro M, Planell JA. Bioactive composites based on calcium phosphates for bone regeneration. Key Eng Mater 2010; 441:203-33; .
Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307.
Zhang RY, Ma PX. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J Biomed Mater Res. 1999;45:285–93. doi: 10.1002/(SICI)1097-4636(19990615)45:4<285::AID-JBM2>3.0.CO;2-2.
Liu Q, de Wijn JR, van Blitterswijk CA. Composite biomaterials with chemical bonding between hydroxyapatite filler particles and PEG/PBT copolymer matrix. J Biomed Mater Res. 1998;40:490–7. doi: 10.1002/(SICI)1097-4636(19980605)40:3<490::AID-JBM20>3.0.CO;2-M.
Cerrai P, Guerra GD, Tricoli M, Krajewski A, Ravaglioli A, Martinetti R, et al. Periodontal membranes from composites of hydroxyapatite and bioresorbable block copolymers. J Mater Sci Mater Med. 1999;10:677–82. doi: 10.1023/A:1008904229292.
Roeder RK, Sproul MM, Turner CH. Hydroxyapatite whiskers provide improved mechanical properties in reinforced polymer composites. J Biomed Mater Res A. 2003;67:801–12. doi: 10.1002/jbm.a.10140.
Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/S0142-9612(00)00121-6.
Mathieu LM, Bourban PE, Manson JAE. Processing of homogeneous ceramic/polymer blends for bioresorbable composites. Compos Sci Technol. 2006;66:1606–14. doi: 10.1016/j.compscitech.2005.11.012.
Redepenning J, Venkataraman G, Chen J, Stafford N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J Biomed Mater Res A. 2003;66:411–6. doi: 10.1002/jbm.a.10571.
Rhee SH, Tanaka J. Synthesis of a hydroxyapatite/collagen/chondroitin sulfate nanocomposite by a novel precipitation method. J Am Ceram Soc. 2001;84:459–61. doi: 10.1111/j.1151-2916.2001.tb00679.x.
Pezzotti G, Asmus SMF. Fracture behavior of hydroxyapatite/polymer interpenetrating network composites prepared by in situ polymerization process. Mater Sci Eng A. 2001;316:231–7. doi: 10.1016/S0921-5093(01)01250-3.
Weickmann H, Gurr M, Meincke O, Thomann R, Mülhaupt R. A versatile solvent-free “one-pot” route to polymer nanocomposites and the in situ formation of calcium phosphate/layered silicate hybrid nanoparticles. Adv Funct Mater. 2010;20:1778–86. doi: 10.1002/adfm.201000041.
Aryal S, Bhattarai SR, Bahadur KCR, Khil MS, Lee DR, Kim HY. Carbon nanotubes assisted biomimetic synthesis of hydroxyapatite from simulated body fluid. Mater Sci Eng A. 2006;426:202–7. doi: 10.1016/j.msea.2006.04.004.
Kealley C, Ben-Nissan B, van Riessen A, Elcombe M.Development of carbon nanotube reinforced hydroxyapatite bioceramics. Key Eng Mater 2006; 309-311:597-600; .
Kealley C, Elcombe M, van Riessen A, Ben-Nissan B. Development of carbon nanotube reinforced hydroxyapatite bioceramics. Physica B. 2006;385-386:496–8. doi: 10.1016/j.physb.2006.05.254.
Aryal S, Bahadur KCR, Dharmaraj N, Kim KW, Kim HY. Synthesis and characterization of hydroxyapatite using carbon nanotubes as a nano-matrix. Scr Mater. 2006;54:131–5. doi: 10.1016/j.scriptamat.2005.09.050.
Wei Q, Yang XP, Chen GQ, Tang JT, Deng XL. The ultrasonic assisted synthesis of nano-hydroxyapatite and MWNT/hydroxyapatite composites. New Carbon Mater. 2005;20:164–70.
Zhao LP, Gao L. Novel in situ synthesis of MWNT-hydroxyapatite composites. Carbon. 2004;42:423–60. doi: 10.1016/j.carbon.2003.10.024.
Bai Y, Neupane MP, Park IS, Lee MH, Bae TS, Watari F, et al. Electrophoretic deposition of carbon nanotubes-hydroxyapatite nanocomposites on titanium substrate. Mater Sci Eng C. 2010;30:1043–9. doi: 10.1016/j.msec.2010.05.007.
Rautaray D, Mandal S, Sastry M. Synthesis of hydroxyapatite crystals using amino acid-capped gold nanoparticles as a scaffold. Langmuir. 2005;21:5185–91. doi: 10.1021/la048541f.
Wang XJ, Li Y, Wei J, de Groot K. Development of biomimetic nano-hydroxyapatite/poly(hexamethylene adipamide) composites. Biomaterials. 2002;23:4787–91. doi: 10.1016/S0142-9612(02)00229-6.
Wei J, Li Y. Tissue engineering scaffold material of nano-apatite crystals and polyamide composite. Eur Polym J. 2004;40:509–15. doi: 10.1016/j.eurpolymj.2003.10.028.
Memoto R, Nakamura S, Isobe T, Senna M. Direct synthesis of hydroxyapatite-silk fibroin nano-composite sol via a mechanochemical route. J Sol-Gel Sci Technol. 2001;21:7–12. doi: 10.1023/A:1011245213742.
Fujiwara M, Shiokawa K, Morigaki K, Tatsu Y, Nakahara Y. Calcium phosphate composite materials including inorganic powders, BSA or duplex DNA prepared by W/O/W interfacial reaction method. Mater Sci Eng C. 2008;28:280–8. doi: 10.1016/j.msec.2007.01.006.
Nagata F, Miyajima T, Yokogawa Y. A method to fabricate hydroxyapatite/poly(lactic acid) microspheres intended for biomedical application. J Eur Ceram Soc. 2006;26:533–5. doi: 10.1016/j.jeurceramsoc.2005.06.006.
Russias J, Saiz E, Nalla RK, Tomsia AP. Microspheres as building blocks for hydroxyapatite/polylactide biodegradable composites. J Mater Sci. 2006;41:5127–33. doi: 10.1007/s10853-006-0449-1.
Khan YM, Cushnie EK, Kelleher JK, Laurencin CT. In situ synthesized ceramic-polymer composites for bone tissue engineering: bioactivity and degradation studies. J Mater Sci. 2007;42:4183–90. doi: 10.1007/s10853-006-0636-0.
Kim HW, Knowles JC, Kim HE. Hydroxyapatite and gelatin composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J Biomed Mater Res A. 2005;72:136–45. doi: 10.1002/jbm.a.30168.
Sinha A, Das G, Sharma BK, Roy RP, Pramanick AK, Nayar S. Poly(vinyl alcohol)-hydroxyapatite biomimetic scaffold for tissue regeneration. Mater Sci Eng C. 2007;27:70–4. doi: 10.1016/j.msec.2006.02.008.
Su L, Berndt CC, Gross KA. Hydroxyapatite/polymer composite flame-sprayed coatings for orthopedic applications. J Biomater Sci Polym Ed. 2002;13:977–90. doi: 10.1163/156856202760319135.
Sugawara A, Yamane S, Akiyoshi K. Nanogel-templated mineralization: polymer-calcium phosphate hybrid nanomaterials. Macromol Rapid Commun. 2006;27:441–6. doi: 10.1002/marc.200500778.
Kickelbick G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog Polym Sci. 2003;28:83–114. doi: 10.1016/S0079-6700(02)00019-9.
Liu Q, de Wijn JR, van Blitterswijk CA. Nano-apatite/polymer composites: mechanical and physicochemical characteristics. Biomaterials. 1997;18:1263–70. doi: 10.1016/S0142-9612(97)00069-0.
Uskokovic PS, Tang CY, Tsui CP, Ignjatovic N, Uskokovic DP. Micromechanical properties of a hydroxyapatite/poly-L-lactide biocomposite using nanoindentation and modulus mapping. J Eur Ceram Soc. 2007;27:1559–64. doi: 10.1016/j.jeurceramsoc.2006.04.122.
Todo M, Kagawa T. Improvement of fracture energy of HA/PLLA biocomposite material due to press processing. J Mater Sci. 2008;43:799–801. doi: 10.1007/s10853-007-2308-0.
Woo KM, Seo J, Zhang RY, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28:2622–30. doi: 10.1016/j.biomaterials.2007.02.004.
Ma PX, Zhang R, Xiao G, Franceschi R. Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds. J Biomed Mater Res. 2001;54:284–93. doi: 10.1002/1097-4636(200102)54:2<284::AID-JBM16>3.0.CO;2-W.
Wang M, Chen LJ, Ni J, Weng J, Yue CY. Manufacture and evaluation of bioactive and biodegradable materials and scaffolds for tissue engineering. J Mater Sci Mater Med. 2001;12:855–60. doi: 10.1023/A:1012899318688.
Baji A, Wong SC, Srivatsan TS, Njus GO, Mathur G. Processing methodologies for polycaprolactone-hydroxyapatite composites: a review. Mater Manuf Process. 2006;21:211–8. doi: 10.1081/AMP-200068681.
Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res A. 2006;78:306–15. doi: 10.1002/jbm.a.30704.
Guan L, Davies JE. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J Biomed Mater Res A. 2004;71:480–7. doi: 10.1002/jbm.a.30173.
Teng XR, Ren J, Gu SY. Preparation and characterization of porous PDLLA/HA composite foams by supercritical carbon dioxide technology. J Biomed Mater Res B Appl Biomater. 2007;81:185–93. doi: 10.1002/jbm.b.30652.
Ren J, Zhao P, Ren T, Gu S, Pan K. Poly (D,L-lactide)/nano-hydroxyapatite composite scaffolds for bone tissue engineering and biocompatibility evaluation. J Mater Sci Mater Med. 2008;19:1075–82. doi: 10.1007/s10856-007-3181-8.
Wang M, Yue CY, Chua B. Production and evaluation of hydroxyapatite reinforced polysulfone for tissue replacement. J Mater Sci Mater Med. 2001;12:821–6. doi: 10.1023/A:1017933220894.
Chlopek J, Rosol P, Morawska-Chochol A. Durability of polymer-ceramics composite implants determined in creep tests. Compos Sci Technol. 2006;66:1615–22. doi: 10.1016/j.compscitech.2005.11.016.
Szaraniec B, Rosol P, Chlopek J. Carbon composite material and polysulfone modified by nano-hydroxyapatite. e-Polymers 2005; 30:1-7.
Nayar S, Sinha A. Systematic evolution of a porous hydroxyapatite-poly(vinylalcohol)-gelatin composite. Colloids Surf B Biointerfaces. 2004;35:29–32. doi: 10.1016/j.colsurfb.2004.01.013.
Chang MC, Ko CC, Douglas WH. Modification of hydroxyapatite/gelatin composite by polyvinylalcohol. J Mater Sci. 2005;40:505–9. doi: 10.1007/s10853-005-6115-1.
Chang MC, Ko CC, Douglas WH. Modification of hydroxyapatite/gelatin composite by polyvinylalcohol. J Mater Sci. 2005;40:2723–7. doi: 10.1007/s10853-005-2116-3.
You C, Miyazaki T, Ishida E, Ashizuka M, Ohtsuki C, Tanihara M. Fabrication of poly(vinyl alcohol)-apatite hybrids through biomimetic process. J Eur Ceram Soc. 2007;27:1585–8. doi: 10.1016/j.jeurceramsoc.2006.04.055.
Fenglan X, Yubao L, Xiaoming Y, Hongbing L, Li Z. Preparation and in vivo investigation of artificial cornea made of nano-hydroxyapatite/poly (vinyl alcohol) hydrogel composite. J Mater Sci Mater Med. 2007;18:635–40. doi: 10.1007/s10856-007-2313-5.
Xu F, Li Y, Deng Y, Xiong J. Porous nano-hydroxyapatite/poly(vinyl alcohol) composite hydrogel as artificial cornea fringe: characterization and evaluation in vitro. J Biomater Sci Polym Ed. 2008;19:431–9. doi: 10.1163/156856208783719473.
Nayar S, Pramanick AK, Sharma BK, Das G, Ravi Kumar B, Sinha A. Biomimetically synthesized polymer-hydroxyapatite sheet like nano-composite. J Mater Sci Mater Med. 2008;19:301–4. doi: 10.1007/s10856-007-3129-z.
Poursamar SA, Orang F, Bonakdar S, Savar MK. Preparation and characterisation of poly vinyl alcohol/hydroxyapatite nanocomposite via in situ synthesis: a potential material as bone tissue engineering scaffolds. Int J Nanomanuf. 2010;5:330–4.
Guha A, Nayar S, Thatoi HN. Microwave irradiation enhances kinetics of the biomimetic process of hydroxyapatite nanocomposites. Bioinspir Biomim. 2010;5:024001. doi: 10.1088/1748-3182/5/2/024001.
Pramanik N, Biswas SK, Pramanik P. Synthesis and characterization of hydroxyapatite/poly(vinyl alcohol phosphate) nanocomposite biomaterials. Int J Appl Ceram Technol. 2008;5:20–8. doi: 10.1111/j.1744-7402.2008.02179.x.
Bigi A, Boanini E, Gazzano M, Rubini K. Structural and morphological modifications of hydroxyapatite-polyaspartate composite crystals induced by heat treatment. Cryst Res Technol. 2005;40:1094–8. doi: 10.1002/crat.200410493.
Bertoni E, Bigi A, Falini G, Panzavolta S, Roveri N. Hydroxyapatite polyacrylic acid nanocrystals. J Mater Chem. 1999;9:779–82. doi: 10.1039/a807890d.
Qiu HJ, Yang J, Kodali P, Koh J, Ameer GA. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials. 2006;27:5845–54. doi: 10.1016/j.biomaterials.2006.07.042.
Greish YE, Brown PW. Chemically formed HAp-Ca poly(vinyl phosphonate) composites. Biomaterials. 2001;22:807–16. doi: 10.1016/S0142-9612(00)00243-X.
Greish YE, Brown PW. Preparation and characterization of calcium phosphate-poly(vinyl phosphonic acid) composites. J Mater Sci Mater Med. 2001;12:407–11. doi: 10.1023/A:1011292819246.
Greish YE, Brown PW. Formation and properties of hydroxyapatite-calcium poly(vinyl phosphonate) composites. J Am Ceram Soc. 2002;85:1738–44. doi: 10.1111/j.1151-2916.2002.tb00345.x.
Nakahira A, Tamai M, Miki S, Pezotti G. Fracture behavior and biocompatibility evaluation of nylon-infiltrated porous hydroxyapatite. J Mater Sci. 2002;37:4425–30. doi: 10.1023/A:1020681309572.
Sailaja GS, Velayudhan S, Sunny MC, Sreenivasan K, Varma HK, Ramesh P. Hydroxyapatite filled chitosan-polyacrylic acid polyelectrolyte complexes. J Mater Sci. 2003;38:3653–62. doi: 10.1023/A:1025689701309.
Zhang H, Xu JJ, Chen HY. Electrochemically deposited 2D nanowalls of calcium phosphate-PDDA on a glassy carbon electrode and their applications in biosensing. J Phys Chem C. 2007;111:16564–70. doi: 10.1021/jp073212s.
Piticescu RM, Chitanu GC, Albulescu M, Giurginca M, Popescu ML, Łojkowski W. Hybrid HAp-maleic anhydride copolymer nanocomposites obtained by in-situ functionalisation. Solid State Phenomena 2005; 106:47-56; .
Enlow D, Rawal A, Kanapathipillai M, Schmidt-Rohr K, Mallapragada S, Lo CT, et al. Synthesis and characterization of self-assembled block copolymer templated calcium phosphate nanocomposite gels. J Mater Chem. 2007;17:1570–8. doi: 10.1039/b613760a.
Kaito T, Myoui A, Takaoka K, Saito N, Nishikawa M, Tamai N, et al. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials. 2005;26:73–9. doi: 10.1016/j.biomaterials.2004.02.010.
Song J, Saiz E, Bertozzi CR. A new approach to mineralization of biocompatible hydrogel scaffolds: an efficient process toward 3-dimensional bonelike composites. J Am Chem Soc. 2003;125:1236–43. doi: 10.1021/ja028559h.
Meenan BJ, McClorey C, Akay M. Thermal analysis studies of poly(etheretherketone)/hydroxyapatite biocomposite mixtures. J Mater Sci Mater Med. 2000;11:481–9. doi: 10.1023/A:1013005707430.
Fan JP, Tsui CP, Tang CY, Chow CL. Modeling of the mechanical behavior of HA/PEEK biocomposite under quasi-static tensile load. Biomaterials. 2004;25:5363–73. doi: 10.1016/j.biomaterials.2003.12.050.
Abu Bakar MS, Cheng MHW, Tang SM, Yu SC, Liao K, Tan CT, et al. Tensile properties, tension-tension fatigue and biological response of polyetheretherketone-hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials. 2003;24:2245–50. doi: 10.1016/S0142-9612(03)00028-0.
Abu Bakar MS, Cheang P, Khor KA. Mechanical properties of injection molded hydroxyapatite-polyetheretherketone biocomposites. Compos Sci Technol. 2003;63:421–5. doi: 10.1016/S0266-3538(02)00230-0.
Abu Bakar MS, Cheang P, Khor KA. Tensile properties and microstructural analysis of spheroidized hydroxyapatite-poly(etheretherketone) biocomposites. Mater Sci Eng A. 2003;345:55–63. doi: 10.1016/S0921-5093(02)00289-7.
Fan JP, Tsui CP, Tang CY. Modeling of the mechanical behavior of HA/PEEK biocomposite under quasi-static tensile load. Mater Sci Eng A. 2004;382:341–50. doi: 10.1016/j.msea.2004.04.078.
Yu S, Hariram KP, Kumar R, Cheang P, Aik KK. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials. 2005;26:2343–52. doi: 10.1016/j.biomaterials.2004.07.028.
Gong XH, Tang CY, Hu HC, Zhou XP, Xie XL. Improved mechanical properties of HIPS/hydroxyapatite composites by surface modification of hydroxyapatite via in-situ polymerization of styrene. J Mater Sci Mater Med. 2004;15:1141–6. doi: 10.1023/B:JMSM.0000046397.09060.15.
Wang L, Weng L, Song S, Sun Q. Mechanical properties and microstructure of polyetheretherketone-hydroxyapatite nanocomposite materials. Mater Lett. 2010;64:2201–4. doi: 10.1016/j.matlet.2010.06.067.
Laurencin CT, Attawia MA, Elgendy HE, Herbert KM. Tissue engineered bone-regeneration using degradable polymers: the formation of mineralized matrices. Bone. 1996;19(Suppl):93S–9S. doi: 10.1016/S8756-3282(96)00132-9.
Laurencin CT, Attawia MA, Lu LQ, Borden MD, Lu HH, Gorum WJ, et al. Poly(lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: a regional gene therapy approach to bone regeneration. Biomaterials. 2001;22:1271–7. doi: 10.1016/S0142-9612(00)00279-9.
Kim SS, Ahn KM, Park MS, Lee JH, Choi CY, Kim BS. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J Biomed Mater Res A. 2007;80:206–15. doi: 10.1002/jbm.a.30836.
Oliveira JM, Miyazaki T, Lopes MA, Ohtsuki C, Santos JD. Bonelike/PLGA hybrid materials for bone regeneration: preparation route and physicochemical characterisation. J Mater Sci Mater Med. 2005;16:253–9. doi: 10.1007/s10856-005-6687-y.
Kim S, Kim SS, Lee SH, Eun Ahn S, Gwak SJ, Song JH, et al. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-co-glycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29:1043–53. doi: 10.1016/j.biomaterials.2007.11.005.
Petricca SE, Marra KG, Kumta PN. Chemical synthesis of poly(lactic-co-glycolic acid)/hydroxyapatite composites for orthopaedic applications. Acta Biomater. 2006;2:277–86. doi: 10.1016/j.actbio.2005.12.004.
Sato M, Slamovich EB, Webster TJ. Enhanced osteoblast adhesion on hydrothermally treated hydroxyapatite/titania/poly(lactide-co-glycolide) sol-gel titanium coatings. Biomaterials. 2005;26:1349–57. doi: 10.1016/j.biomaterials.2004.04.044.
Gu SY, Zhan H, Ren J, Zhou XY. Sol-gel synthesis and characterisation of nano-sized hydroxyapatite powders and hydroxyapatite/poly(D,L-lactide-co-glycolide) composite scaffolds. Polymers & Polym Compos. 2007;15:137–44.
Aboudzadeh N, Imani M, Shokrgozar MA, Khavandi A, Javadpour J, Shafieyan Y, et al. Fabrication and characterization of poly(D,L-lactide-co-glycolide)/hydroxyapatite nanocomposite scaffolds for bone tissue regeneration. J Biomed Mater Res A. 2010;94:137–45. doi: 10.1002/jbm.a.32673.
Verheyen CCPM, de Wijn JR, van Blitterswijk CA, de Groot K. Evaluation of hydroxylapatite/poly(L-lactide) composites: mechanical behavior. J Biomed Mater Res. 1992;26:1277–96. doi: 10.1002/jbm.820261003.
Balać I, Uskoković PS, Aleksić R, Uskoković D. Predictive modeling of the mechanical properties of particulate hydroxyapatite reinforced polymer composites. J Biomed Mater Res. 2002;63:793–9. doi: 10.1002/jbm.10407.
Dawes E, Rushton N. The effects of lactic acid on PGE2 production by macrophages and human synovial fibroblasts: a possible explanation for problems associated with the degradation of poly(lactide) implants? Clin Mater. 1994;17:157–63. doi: 10.1016/0267-6605(94)90031-0.
Verheyen CCPM, Klein CPAT, de Blieck-Hogervorst JMA, Wolke JGC, de Wijin JR, van Blitterswijk CA, et al. Evaluation of hydroxylapatite poly(L-lactide) composites-physicochemical properties. J Mater Sci Mater Med. 1993;4:58–65. doi: 10.1007/BF00122979.
Li H, Chang J. pH-compensation effect of bioactive inorganic fillers on the degradation of PLGA. Compos Sci Technol. 2005;65:2226–32. doi: 10.1016/j.compscitech.2005.04.051.
Agrawal CM, Athanasiou KA. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res. 1997;38:105–14. doi: 10.1002/(SICI)1097-4636(199722)38:2<105::AID-JBM4>3.0.CO;2-U.
Peter SJ, Miller ST, Zhu G, Yasko AW, Mikos AG. In vivo degradation of a poly(propylene fumarate)/beta-tricalcium phosphate injectable composite scaffold. J Biomed Mater Res. 1998;41:1–7. doi: 10.1002/(SICI)1097-4636(199807)41:1<1::AID-JBM1>3.0.CO;2-N.
Ara M, Watanabe M, Imai Y. Effect of blending calcium compounds on hydrolytic degradation of poly(DL-lactic acid-co-glycolic acid) Biomaterials. 2002;23:2479–83. doi: 10.1016/S0142-9612(01)00382-9.
Linhart W, Peters F, Lehmann W, Schwarz K, Schilling AF, Amling M, et al. Biologically and chemically optimized composites of carbonated apatite and polyglycolide as bone substitution materials. J Biomed Mater Res. 2001;54:162–71. doi: 10.1002/1097-4636(200102)54:2<162::AID-JBM2>3.0.CO;2-3.
Schiller C, Epple M. Carbonated apatites can be used as pH-stabilizing filler for biodegradable polyesters. Biomaterials. 2003;24:2037–43. doi: 10.1016/S0142-9612(02)00634-8.
Schiller C, Rasche C, Wehmöller M, Beckmann F, Eufinger H, Epple M, et al. Geometrically structured implants for cranial reconstruction made of biodegradable polyesters and calcium phosphate/calcium carbonate. Biomaterials. 2004;25:1239–47. doi: 10.1016/j.biomaterials.2003.08.047.
Shikinami Y, Okuno M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly L-lactide (PLLA). Part II: practical properties of miniscrews and miniplates. Biomaterials. 2001;22:3197–211. doi: 10.1016/S0142-9612(01)00072-2.
Russias J, Saiz E, Nalla RK, Gryn K, Ritchie RO, Tomsia AP. Fabrication and mechanical properties of PLA/HA composites: a study of in vitro degradation. Mater Sci Eng C. 2006;26:1289–95. doi: 10.1016/j.msec.2005.08.004.
Kim HW, Lee HH, Knowles JC. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly(lactic acid) for bone regeneration. J Biomed Mater Res A. 2006;79:643–9. doi: 10.1002/jbm.a.30866.
Gross KA, Rodríguez-Lorenzo LM. Biodegradable composite scaffolds with an interconnected spherical network for bone tissue engineering. Biomaterials. 2004;25:4955–62. doi: 10.1016/j.biomaterials.2004.01.046.
Zhang H, Chen Z. Fabrication and characterization of electrospun PLGA/MWNTs/hydroxyapatite biocomposite scaffolds for bone tissue engineering. J Bioact Compat Polym. 2010;25:241–59. doi: 10.1177/0883911509359486.
Durucan C, Brown PW. Low temperature formation of calcium-deficient hydroxyapatite-PLA/PLGA composites. J Biomed Mater Res. 2000;51:717–25. doi: 10.1002/1097-4636(20000915)51:4<717::AID-JBM21>3.0.CO;2-Q.
Durucan C, Brown PW. Calcium-deficient hydroxyapatite-PLGA composites: mechanical and microstructural investigation. J Biomed Mater Res. 2000;51:726–34. doi: 10.1002/1097-4636(20000915)51:4<726::AID-JBM22>3.0.CO;2-L.
Ignjatovic N, Suljovrujic E, Budinski-Simendic J, Krakovsky I, Uskokovic D. Evaluation of hot-pressed hydroxyapatite/poly-L-lactide composite biomaterial characteristics. J Biomed Mater Res B Appl Biomater. 2004;71:284–94. doi: 10.1002/jbm.b.30093.
Nazhat SN, Kellomäki M, Törmälä P, Tanner KE, Bonfield W. Dynamic mechanical characterization of biodegradable composites of hydroxyapatite and polylactides. J Biomed Mater Res. 2001;58:335–43. doi: 10.1002/jbm.1026.
Hasegawa S, Tamura J, Neo M, Goto K, Shikinami Y, Saito M, et al. In vivo evaluation of a porous hydroxyapatite/poly-DL-lactide composite for use as a bone substitute. J Biomed Mater Res A. 2005;75:567–79. doi: 10.1002/jbm.a.30460.
Hasegawa S, Neo M, Tamura J, Fujibayashi S, Takemoto M, Shikinami Y, et al. In vivo evaluation of a porous hydroxyapatite/poly-DL-lactide composite for bone tissue engineering. J Biomed Mater Res A. 2007;81:930–8. doi: 10.1002/jbm.a.31109.
Higashi S, Yamamuro T, Nakamura T, Ikada Y, Hyon SH, Jamshidi K. Polymer-hydroxyapatite composites for biodegradable bone fillers. Biomaterials. 1986;7:183–7. doi: 10.1016/0142-9612(86)90099-2.
Ylinen P. Filling of bone defects with porous hydroxyapatite reinforced with polylactide or polyglycolide fibres. J Mater Sci Mater Med. 1994;5:522–8. doi: 10.1007/BF00124884.
Reis RL, Cunha AM, Bevis MJ. Using nonconventional processing routes to develop anisotropic and biodegradable composites of starch-based thermoplastics reinforced with bone-like ceramics. J Appl Med Polym. 1998;2:49–53.
Reis RL, Cunha AM. New degradable load-bearing biomaterials composed of reinforced starch based blends. J Appl Med Polym. 2000;4:1–5.
Sousa RA, Mano JF, Reis RL, Cunha AM, Bevis MJ. Mechanical performance of starch based bioactive composites moulded with preferred orientation for potential medical applications. Polym Eng Sci. 2002;42:1032–45. doi: 10.1002/pen.11010.
Marques AP, Reis RL. Hydroxyapatite reinforcement of different starch-based polymers affects osteoblast-like cells adhesion/spreading and proliferation. Mater Sci Eng C. 2005;25:215–29. doi: 10.1016/j.msec.2005.01.013.
Reis RL, Cunha AM, Allan PS, Bevis MJ. Structure development and control of injection-molded hydroxylapatite-reinforced starch/EVOH composites. J Polym Adv Tech. 1997;16:263–77. doi: 10.1002/(SICI)1098-2329(199711)16:4<263::AID-ADV2>3.0.CO;2-T.
Vaz CM, Reis RL, Cunha AM. Use of coupling agents to enhance the interfacial interactions in starch-EVOH/hydroxylapatite composites. Biomaterials. 2002;23:629–35. doi: 10.1016/S0142-9612(01)00150-8.
Leonor IB, Ito A, Onuma K, Kanzaki N, Reis RL. In vitro bioactivity of starch thermoplastic/hydroxyapatite composite biomaterials: an in situ study using atomic force microscopy. Biomaterials. 2003;24:579–85. doi: 10.1016/S0142-9612(02)00371-X.
Vaz CM, Reis RL, Cunha AM. Degradation model of starch-EVOH + HA composites. Mater Res Innovat. 2001;4:375–80. doi: 10.1007/s100190000112.
Boeree NR, Dove J, Cooper JJ, Knowles JC, Hastings GW. Development of a degradable composite for orthopaedic use: mechanical evaluation of an hydroxyapatite-polyhydroxybutyrate composite material. Biomaterials. 1993;14:793–6. doi: 10.1016/0142-9612(93)90046-5.
Doyle C, Tanner ET, Bonfield W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials. 1991;12:841–7. doi: 10.1016/0142-9612(91)90072-I.
Chen LJ, Wang M. Production and evaluation of biodegradable composites based on PHB-PHV copolymer. Biomaterials. 2002;23:2631–9. doi: 10.1016/S0142-9612(01)00394-5.
Ni J, Wang M. In vitro evaluation of hydroxyapatite reinforced polyhydroxybutyrate composite. Mater Sci Eng C. 2002;20:101–9. doi: 10.1016/S0928-4931(02)00019-X.
Knowles JC, Hastings GW, Ohta H, Niwa S, Boeree N. Development of a degradable composite for orthopaedic use: in vivo biomechanical and histological evaluation of two bioactive degradable composites based on the polyhydroxybutyrate polymer. Biomaterials. 1992;13:491–6. doi: 10.1016/0142-9612(92)90099-A.
Luklinska ZB, Bonfield W. Morphology and ultrastructure of the interface between hydroxyapatite-polyhydroxybutyrate composite implant and bone. J Mater Sci Mater Med. 1997;8:379–83. doi: 10.1023/A:1018589018205.
Reis ECC, Borges APB, Fonseca CC, Martinez MMM, Eleotério RB, Morato GO, et al. Biocompatibility, osteointegration, osteoconduction and biodegradation of a hydroxyapatite-polyhydroxybutyrate composite. Braz Arch Biol Technol. 2010;53:817–26. doi: 10.1590/S1516-89132010000400010.
Chen DZ, Tang CY, Chan KC, Tsui CP, Yu PHF, Leung MCP, et al. Dynamic mechanical properties and in vitro bioactivity of PHBHV/HA nanocomposite. Compos Sci Technol. 2007;67:1617–26. doi: 10.1016/j.compscitech.2006.07.034.
Rai B, Noohom W, Kithva PH, Grøndahl L, Trau M. Bionanohydroxyapatite/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composites with improved particle dispersion and superior mechanical properties. Chem Mater. 2008;20:2802–8. doi: 10.1021/cm703045u.
Wang YW, Wu Q, Chen J, Chen GQ. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials. 2005;26:899–904. doi: 10.1016/j.biomaterials.2004.03.035.
Linhart W, Lehmann W, Siedler M, Peters F, Schilling AF, Schwarz K, et al. Composites of amorphous calcium phosphate and poly(hydroxybutyrate) and poly(hydroxybutyrate-co-hydroxyvalerate) for bone substitution: assessment of the biocompatibility. J Mater Sci. 2006;41:4806–13. doi: 10.1007/s10853-006-0023-x.
Azevedo MC, Reis RL, Claase MB, Grijpma DW, Feijen J. Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J Mater Sci Mater Med. 2003;14:103–7. doi: 10.1023/A:1022051326282.
Choi D, Marra KG, Kumta PN. Chemical synthesis of hydroxyapatite/poly(∑-caprolactone) composites. Mater Res Bull. 2004;39:417–32. doi: 10.1016/j.materresbull.2003.10.013.
Hao J, Yuan M, Deng X. Biodegradable and biocompatible nanocomposites of poly(∑-caprolactone) with hydroxyapatite nanocrystals: thermal and mechanical properties. J Appl Polym Sci. 2003;86:676–83. doi: 10.1002/app.10955.
Walsh D, Furuzono T, Tanaka J. Preparation of porous composite implant materials by in situ polymerization of porous apatite containing epsilon-caprolactone or methyl methacrylate. Biomaterials. 2001;22:1205–12. doi: 10.1016/S0142-9612(00)00268-4.
Cerrai P, Guerra GD, Tricoli M, Krajewski A, Guicciardi S, Ravaglioli A, et al. New composites of hydroxyapatite and bioresorbable macromolecular material. J Mater Sci Mater Med. 1999;10:283–9. doi: 10.1023/A:1008905529461.
Verma D, Katti K, Katti D. Bioactivity in in situ hydroxyapatite-polycaprolactone composites. J Biomed Mater Res A. 2006;78:772–80. doi: 10.1002/jbm.a.30774.
Kim HW. Biomedical nanocomposites of hydroxyapatite/polycaprolactone obtained by surfactant mediation. J Biomed Mater Res A. 2007;83:169–77. doi: 10.1002/jbm.a.31247.
Kim HW, Knowles JC, Kim HE. Development of hydroxyapatite bone scaffold for controlled drug release via poly(epsilon-caprolactone) and hydroxyapatite hybrid coatings. J Biomed Mater Res B Appl Biomater. 2004;70:240–9. doi: 10.1002/jbm.b.30038.
Guerra GD, Cerrai P, Tricoli M, Krajewski A, Ravaglioli A, Mazzocchi M, et al. Composites between hydroxyapatite and poly(epsilon-caprolactone) synthesized in open system at room temperature. J Mater Sci Mater Med. 2006;17:69–79. doi: 10.1007/s10856-006-6331-5.
Bianco A, di Federico E, Moscatelli I, Camaioni A, Armentano I, Campagnolo L, et al. Electrospun poly(∑-caprolactone)/Ca-deficient hydroxyapatite nanohybrids: microstructure, mechanical properties and cell response by murine embryonic stem cells. Mater Sci Eng C. 2009;29:2063–71. doi: 10.1016/j.msec.2009.04.004.
Chuenjitkuntaworn B, Inrung W, Damrongsri D, Mekaapiruk K, Supaphol P, Pavasant P. Polycaprolactone/hydroxyapatite composite scaffolds: preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J Biomed Mater Res A. 2010;94:241–51. doi: 10.1002/jbm.a.32657.
Di Foggia M, Corda U, Plescia E, Taddei P, Torreggiani A. Effects of sterilisation by high-energy radiation on biomedical poly-(epsilon-caprolactone)/hydroxyapatite composites. J Mater Sci Mater Med. 2010;21:1789–97. doi: 10.1007/s10856-010-4046-0.
Lebourg M, Suay Antón J, Gomez Ribelles JL. Hybrid structure in PCL-HAp scaffold resulting from biomimetic apatite growth. J Mater Sci Mater Med. 2010;21:33–44. doi: 10.1007/s10856-009-3838-6.
Makarov C, Gotman I, Jiang X, Fuchs S, Kirkpatrick CJ, Gutmanas EY. In situ synthesis of calcium phosphate-polycaprolactone nanocomposites with high ceramic volume fractions. J Mater Sci Mater Med. 2010;21:1771–9. doi: 10.1007/s10856-010-4039-z.
Causa F, Netti PA, Ambrosio L, Ciapetti G, Baldini N, Pagani S, et al. Poly-epsilon-caprolactone/hydroxyapatite composites for bone regeneration: in vitro characterization and human osteoblast response. J Biomed Mater Res A. 2006;76:151–62. doi: 10.1002/jbm.a.30528.
Thomas V, Jagani S, Johnson K, Jose MV, Dean DR, Vohra YK, et al. Electrospun bioactive nanocomposite scaffolds of polycaprolactone and nanohydroxyapatite for bone tissue engineering. J Nanosci Nanotechnol. 2006;6:487–93. doi: 10.1166/jnn.2006.097.
Dunn AS, Campbell PG, Marra KG. The influence of polymer blend composition on the degradation of polymer/hydroxyapatite biomaterials. J Mater Sci Mater Med. 2001;12:673–7. doi: 10.1023/A:1011204106373.
Calandrelli L, Immirzi B, Malinconico M, Volpe M, Oliva A, Ragione F. Preparation and characterization of composites based on biodegradable polymers for in vivo application. Polymer (Guildf) 2000;41:8027–33. doi: 10.1016/S0032-3861(00)00165-8.
Chen B, Sun K. Poly(∑-caprolactone)/hydroxyapatite composites: effects of particle size, molecular weight distribution and irradiation on interfacial interaction and properties. Polym Test. 2005;24:64–70. doi: 10.1016/j.polymertesting.2004.07.010.
Ural E, Kesenci K, Fambri L, Migliaresi C, Piskin E. Poly(D,L-lactide/epsilon-caprolactone)/hydroxyapatite composites. Biomaterials. 2000;21:2147–54. doi: 10.1016/S0142-9612(00)00098-3.
Takayama T, Todo M. Improvement of fracture properties of hydroxyapatite particle filled poly(L-lactide)/poly(∑-caprolactone) biocomposites using lysine tri-isocyanate. J Mater Sci. 2010;45:6266–70. doi: 10.1007/s10853-010-4822-8.
Shokrollahi P, Mirzadeh H, Scherman OA, Huck WTS. Biological and mechanical properties of novel composites based on supramolecular polycaprolactone and functionalized hydroxyapatite. J Biomed Mater Res A. 2010;95:209–21. doi: 10.1002/jbm.a.32828.
Yoon BH, Kim HW, Lee SH, Bae CJ, Koh YH, Kong YM, et al. Stability and cellular responses to fluorapatite-collagen composites. Biomaterials. 2005;26:2957–63. doi: 10.1016/j.biomaterials.2004.07.062.
Kim HW, Lee EJ, Kim HE, Salih V, Knowles JC. Effect of fluoridation of hydroxyapatite in hydroxyapatite-polycaprolactone composites on osteoblast activity. Biomaterials. 2005;26:4395–404. doi: 10.1016/j.biomaterials.2004.11.008.
Busch S, Dolhaine H, DuChesne A, Heinz S, Hochrein O, Laeri F, et al. Biomimetic morphogenesis of fluorapatite-gelatin composites: fractal growth, the question of intrinsic electric fields, core/shell assemblies, hollow spheres and reorganization of denatured collagen. Eur J Inorg Chem. 1999:1643–53. doi: 10.1002/(SICI)1099-0682(199910)1999:10<1643::AID-EJIC1643>3.0.CO;2-J.
Busch S, Schwarz U, Kniep R. Chemical and structural investigations of biomimetically grown fluorapatite-gelatin composite aggregates. Adv Funct Mater. 2003;13:189–98. doi: 10.1002/adfm.200390029.
Simon P, Carrillo-Cabrera W, Formanek P, Göbel C, Geiger D, Ramlau R, et al. On the real-structure of biomimetically grown hexagonal pristamic seed of fluoroapatite-gelatin-composites: TEM investigations along. J Mater Chem. 2004;14:2218–24. doi: 10.1039/b402627f. [001]
Göbel C, Simon P, Buder J, Tlatlik H, Kniep R. Phase formation and morphology of calcium phosphate-gelatin-composites grown by double diffusion technique: the influence of fluoride. J Mater Chem 2004; 14:2225-30; DOI: 10.1039/b403503 h.
Simon P, Schwarz U, Kniep R. Hierarchical architecture and real structure in a biomimetic nano-composite of fluorapatite with gelatine: a model system for steps in dentino- and osteogenesis? J Mater Chem. 2005;15:4992–6. doi: 10.1039/b504977f.
Tlatlik H, Simon P, Kawska A, Zahn D, Kniep R. Biomimetic fluorapatite-gelatine nanocomposites: pre-structuring of gelatine matrices by ion impregnation and its effect on form development. Angew Chem Int Ed Engl. 2006;45:1905–10. doi: 10.1002/anie.200503610.
Simon P, Zahn D, Lichte H, Kniep R. Intrinsic electric dipole fields and the induction of hierarchical form developments in fluorapatite-gelatine nanocomposites: a general principle for morphogenesis of biominerals? Angew Chem Int Ed Engl. 2006;45:1911–5. doi: 10.1002/anie.200504465.
Kniep R, Simon P. Fluorapatite-gelatin-nanocomposites: self-organized morphogenesis, real structure and relations to natural hard materials. In: Biomineralization I, crystallization and self-organization process, Naka K. (Ed.); Topics in Current Chemistry, Springer: Berlin, Germany 2007; 270:73-125.
Kniep R, Simon P. “Hidden” hierarchy of microfibrils within 3D-periodic fluorapatite-gelatine nanocomposites: development of complexity and form in a biomimetic system. Angew Chem Int Ed Engl. 2008;47:1405–9. doi: 10.1002/anie.200702532.
Brickmann J, Paparcone R, Kokolakis S, Zahn D, Duchstein P, Carrillo-Cabrera W, et al. Fluorapatite-gelatine nanocomposite superstructures: new insights into a biomimetic system of high complexity. Chemphyschem. 2010;11:1851–3. doi: 10.1002/cphc.201090041.
However, there are some reports about a lack of TCP biodegradation after implantation in calvarial defects.
Handschel J, Wiesmann HP, Stratmann U, Kleinheinz J, Meyer U, Joos U. TCP is hardly resorbed and not osteoconductive in a non-loading calvarial model. Biomaterials. 2002;23:1689–95. doi: 10.1016/S0142-9612(01)00296-4.
Kikuchi M, Tanaka J, Koyama Y, Takakuda K. Cell culture test of TCP/CPLA composite. J Biomed Mater Res. 1999;48:108–10. doi: 10.1002/(SICI)1097-4636(1999)48:2<108::AID-JBM2>3.0.CO;2-F.
Yaszemski MJ, Payne RG, Hayes WC, Langer R, Mikos AG. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials. 1996;17:2117–30. doi: 10.1016/0142-9612(96)00008-7.
Wang M, Wang J, Ni J. Developing tricalcium phosphate/polyhydroxybutyrate composite as a new biodegradable material for clinical applications. Biomechanics. 2000;192:741–4.
Kikuchi M, Koyama Y, Takakuda K, Miyairi H, Shirahama N, Tanaka J. In vitro change in mechanical strength of beta-tricalcium phosphate/copolymerized poly-L-lactide composites and their application for guided bone regeneration. J Biomed Mater Res. 2002;62:265–72. doi: 10.1002/jbm.10248.
Ignatius AA, Augat P, Claes LE. Degradation behavior of composite pins made of tricalcium phosphate and poly(L,DL-lactide) J Biomater Sci Polym Ed. 2001;12:185–94. doi: 10.1163/156856201750180915.
Ignatius AA, Wolf S, Augat P, Claes LE. Composites made of rapidly resorbable ceramics and poly(lactide) show adequate mechanical properties for use as bone substitute materials. J Biomed Mater Res. 2001;57:126–31. doi: 10.1002/1097-4636(200110)57:1<126::AID-JBM1151>3.0.CO;2-M.
Kikuchi M, Tanaka J. Chemical interaction in β-tricalcium phosphate/copolymerized poly-L-lactide composites. J Ceram Soc Jpn. 2000;108:642–5. doi: 10.2109/jcersj.108.1259_642.
Aunoble S, Clément D, Frayssinet P, Harmand MF, Le Huec JC. Biological performance of a new beta-TCP/PLLA composite material for applications in spine surgery: in vitro and in vivo studies. J Biomed Mater Res A. 2006;78:416–22. doi: 10.1002/jbm.a.30749.
Haaparanta AM, Haimi S, Ellä V, Hopper N, Miettinen S, Suuronen R, et al. Porous polylactide/beta-tricalcium phosphate composite scaffolds for tissue engineering applications. J Tissue Eng Regen Med. 2010;4:366–73. doi: 10.1002/term.249.
Kikuchi M, Koyama Y, Yamada T, Imamura Y, Okada T, Shirahama N, et al. Development of guided bone regeneration membrane composed of beta-tricalcium phosphate and poly (L-lactide-co-glycolide-co-epsilon-caprolactone) composites. Biomaterials. 2004;25:5979–86. doi: 10.1016/j.biomaterials.2004.02.001.
Chen TM, Yao CH, Wang HJ, Chou GH, Lee TW, Lin FH. Evaluation of a novel malleable, biodegradable osteoconductive composite in a rabbit cranial defect model. Mater Chem Phys. 1998;55:44–50. doi: 10.1016/S0254-0584(98)00049-2.
Dong GC, Chen HM, Yao CH. A novel bone substitute composite composed of tricalcium phosphate, gelatin and drynaria fortunei herbal extract. J Biomed Mater Res A. 2008;84:167–77. doi: 10.1002/jbm.a.31261.
Ji J, Yuan X, Xia Z, Liu P, Chen J.Porous β-tricalcium phosphate composite scaffold reinforced by K2HPO4 and gelatin. Key Eng Mater 2010; 434:620-3; .
Yao CH, Liu BS, Hsu SH, Chen YS, Tsai CC. Biocompatibility and biodegradation of a bone composite containing tricalcium phosphate and genipin crosslinked gelatin. J Biomed Mater Res A. 2004;69:709–17. doi: 10.1002/jbm.a.30045.
Lin FH, Yao CH, Sun JS, Liu HC, Huang CW. Biological effects and cytotoxicity of the composite composed by tricalcium phosphate and glutaraldehyde cross-linked gelatin. Biomaterials. 1998;19:905–17. doi: 10.1016/S0142-9612(97)00202-0.
Eslaminejad MB, Mirzadeh H, Mohamadi Y, Nickmahzar A. Bone differentiation of marrow-derived mesenchymal stem cells using beta-tricalcium phosphate-alginate-gelatin hybrid scaffolds. J Tissue Eng Regen Med. 2007;1:417–24. doi: 10.1002/term.49.
Takahashi Y, Yamamoto M, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26:3587–96. doi: 10.1016/j.biomaterials.2004.09.046.
Bigi A, Cantelli I, Panzavolta S, Rubini K. Alpha-Tricalcium phosphate-gelatin composite cements. J Appl Biomater Biomech. 2004;2:81–7.
Yang SH, Hsu CK, Wang KC, Hou SM, Lin FH. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein--a viable scaffold for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74:468–75. doi: 10.1002/jbm.b.30200.
Kato M, Namikawa T, Terai H, Hoshino M, Miyamoto S, Takaoka K. Ectopic bone formation in mice associated with a lactic acid/dioxanone/ethylene glycol copolymer-tricalcium phosphate composite with added recombinant human bone morphogenetic protein-2. Biomaterials. 2006;27:3927–33. doi: 10.1016/j.biomaterials.2006.03.013.
Muramatsu K, Oba K, Mukai D, Hasegawa K, Masuda S, Yoshihara Y. Subacute systemic toxicity assessment of beta-tricalcium phosphate/carboxymethyl-chitin composite implanted in rat femur. J Mater Sci Mater Med. 2007;18:513–22. doi: 10.1007/s10856-007-2012-2.
Panzavolta S, Fini M, Nicoletti A, Bracci B, Rubini K, Giardino R, et al. Porous composite scaffolds based on gelatin and partially hydrolyzed alpha-tricalcium phosphate. Acta Biomater. 2009;5:636–43. doi: 10.1016/j.actbio.2008.08.017.
Uchino T, Kamitakahara M, Otsuka M, Ohtsuki C. Hydroxyapatite-forming capability and mechanical properties of organic-inorganic hybrids and α -tricalcium phosphate porous bodies. J Ceram Soc Jpn. 2010;118:57–61. doi: 10.2109/jcersj2.118.57.
Bogun M, Rabiej S. The influence of fiber formation conditions on the structure and properties of nanocomposite alginate fibers containing tricalcium phosphate or montmorillonite. Polym Compos. 2010;31:1321–31.
Bleach NC, Tanner KE, Kellomäki M, Törmälä P. Effect of filler type on the mechanical properties of self-reinforced polylactide-calcium phosphate composites. J Mater Sci Mater Med. 2001;12:911–5. doi: 10.1023/A:1012884310027.
Liu L, Xiong Z, Yan YN, Hu YY, Zhang RJ, Wang SG. Porous morphology, porosity, mechanical properties of poly(alpha-hydroxy acid)-tricalcium phosphate composite scaffolds fabricated by low-temperature deposition. J Biomed Mater Res A. 2007;82:618–29. doi: 10.1002/jbm.a.31177.
Zhang Y, Zhang MQ. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res. 2001;55:304–12. doi: 10.1002/1097-4636(20010605)55:3<304::AID-JBM1018>3.0.CO;2-J.
Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–48. doi: 10.1016/j.biomaterials.2004.09.052.
Rai B, Teoh SH, Ho KH, Hutmacher DW, Cao T, Chen F, et al. The effect of rhBMP-2 on canine osteoblasts seeded onto 3D bioactive polycaprolactone scaffolds. Biomaterials. 2004;25:5499–506. doi: 10.1016/j.biomaterials.2004.01.007.
Lei Y, Rai B, Ho KH, Teoh SH. In vitro degradation of novel bioactive polycaprolactone—20% tricalcium phosphate composite scaffolds for bone engineering. Mater Sci Eng C. 2007;27:293–8. doi: 10.1016/j.msec.2006.05.006.
Miyai T, Ito A, Tamazawa G, Matsuno T, Sogo Y, Nakamura C, et al. Antibiotic-loaded poly-epsilon-caprolactone and porous beta-tricalcium phosphate composite for treating osteomyelitis. Biomaterials. 2008;29:350–8. doi: 10.1016/j.biomaterials.2007.09.040.
Takahashi Y, Yamamoto M, Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26:4856–65. doi: 10.1016/j.biomaterials.2005.01.012.
Ignatius AA, Betz O, Augat P, Claes LE. In vivo investigations on composites made of resorbable ceramics and poly(lactide) used as bone graft substitutes. J Biomed Mater Res. 2001;58:701–9. doi: 10.1002/jbm.10024.
Miao X, Lim WK, Huang X, Chen Y. Preparation and characterization of interpenetrating phased TCP/HA/PLGA composites. Mater Lett. 2005;59:4000–5. doi: 10.1016/j.matlet.2005.07.062.
Brodie JC, Goldie E, Connel G, Merry J, Grant MH. Osteoblast interactions with calcium phosphate ceramics modified by coating with type I collagen. J Biomed Mater Res A. 2005;73:409–21. doi: 10.1002/jbm.a.30279.
Zhang LF, Sun R, Xu L, Du J, Xiong ZC, Chen HC, et al. Hydrophilic poly (ethylene glycol) coating on PDLLA/BCP bone scaffold for drug delivery and cell culture. Mater Sci Eng C. 2008;28:141–9. doi: 10.1016/j.msec.2007.01.005.
Ignjatovic N, Ninkov P, Ajdukovic Z, Konstantinovic V, Uskokovic D.Biphasic calcium phosphate/poly-(D,L-lactide-co-glycolide) biocomposite as filler and blocks for reparation of bone tissue. Mater Sci Forum 2005; 494:519-24; .
Ignjatovic N, Ninkov P, Ajdukovic Z, Vasiljevic-Radovic D, Uskokovic D. Biphasic calcium phosphate coated with poly-D,L-lactide-co-glycolide biomaterial as a bone substitute. J Eur Ceram Soc. 2007;27:1589–94. doi: 10.1016/j.jeurceramsoc.2006.04.104.
Yang W, Yin G, Zhou D, Gu J, Li Y. In vitro characteristics of surface-modified biphasic calcium phosphate/poly(L-Lactide) biocomposite. Adv Eng Mater. 2010;12:128–32. doi: 10.1002/adem.200980025.
Ignjatović N, Ninkov P, Kojić V, Bokurov M, Srdić V, Krnojelac D, et al. Cytotoxicity and fibroblast properties during in vitro test of biphasic calcium phosphate/poly-dl-lactide-co-glycolide biocomposites and different phosphate materials. Microsc Res Tech. 2006;69:976–82. doi: 10.1002/jemt.20374.
Ajduković Z, Ignjatović N, Petrović D, Uskoković D. Substitution of osteoporotic alveolar bone by biphasic calcium phosphate/poly-DL-lactide-co-glycolide biomaterials. J Biomater Appl. 2007;21:317–28. doi: 10.1177/0885328207073760.
Kim HW, Knowles JC, Kim HE. Effect of biphasic calcium phosphates on drug release and biological and mechanical properties of poly(epsilon-caprolactone) composite membranes. J Biomed Mater Res A. 2004;70:467–79. doi: 10.1002/jbm.a.30100.
Bakhtiari L, Rezai HR, Hosseinalipour SM, Shokrgozar MA. Investigation of biphasic calcium phosphate/gelatin nanocomposite scaffolds as a bone tissue engineering. Ceram Int. 2010;36:2421–6. doi: 10.1016/j.ceramint.2010.07.012.
Bakhtiari L, Rezai HR, Hosseinalipour SM, Shokrgozar MA. Preparation of porous Biphasic calcium phosphate-gelatin nanocomposite for bone tissue engineering. J Nano Res 2010; 11:67-72; .
Matsuda A, Ikoma T, Kobayashi H, Tanaka J. Preparation and mechanical property of core-shell type chitosan/calcium phosphate composite fiber. Mater Sci Eng C. 2004;24:723–8. doi: 10.1016/j.msec.2004.08.047.
Rattanachan S, Lorprayoon C, Boonphayak P. Synthesis of chitosan/brushite powders for bone cement composites. J Ceram Soc Jpn. 2008;116:36–41. doi: 10.2109/jcersj2.116.36.
Ohsawa H, Ito A, Sogo Y, Yamazaki A, Ohno T.Synthesis of albumin/DCP nano-composite particles. Key Eng Mater 2007; 332:239-42; .
Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC, et al. Nano DCPA-whisker composites with high strength and Ca and PO(4) release. J Dent Res. 2006;85:722–7. doi: 10.1177/154405910608500807.
Xu HHK, Weir MD, Sun L, Takagi S, Chow LC. Effects of calcium phosphate nanoparticles on Ca-PO4 composite. J Dent Res. 2007;86:378–83. doi: 10.1177/154405910708600415.
Xu HHK, Weir MD, Sun L. Nanocomposites with Ca and PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–91. doi: 10.1016/j.dental.2007.01.002.
Tortet L, Gavarri JR, Nihoul G, Dianoux AJ. Proton mobilities in brushite and brushite/polymer composites. Solid State Ion. 1997;97:253–6. doi: 10.1016/S0167-2738(97)00047-7.
Tortet L, Gavarri JR, Musso J, Nihoul G, Sarychev AK. Percolation and modeling of proton conduction in polymer/brushite composites. J Solid State Chem. 1998;141:392–403. doi: 10.1006/jssc.1998.7960.
Park MS, Eanes ED, Antonucci JM, Skrtic D. Mechanical properties of bioactive amorphous calcium phosphate/methacrylate composites. Dent Mater. 1998;14:137–41. doi: 10.1016/S0109-5641(98)00020-7.
Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53:381–91. doi: 10.1002/1097-4636(2000)53:4<381::AID-JBM12>3.0.CO;2-H.
Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials. 2004;25:1141–50. doi: 10.1016/j.biomaterials.2003.08.001.
Skrtic D, Antonucci JM. Effect of bifunctional comonomers on mechanical strength and water sorption of amorphous calcium phosphate- and silanized glass-filled Bis-GMA-based composites. Biomaterials. 2003;24:2881–8. doi: 10.1016/S0142-9612(03)00119-4.
Gutierrez MC, Jobbágy M, Ferrer ML, del Monte F. Enzymatic synthesis of amorphous calcium phosphate-chitosan nanocomposites and their processing into hierarchical structures. Chem Mater. 2008;20:11–3. doi: 10.1021/cm7020164.
Hakimimehr D, Liu DM, Troczynski T. In-situ preparation of poly(propylene fumarate)--hydroxyapatite composite. Biomaterials. 2005;26:7297–303. doi: 10.1016/j.biomaterials.2005.05.065.
Perkin KK, Turner JL, Wooley KL, Mann S. Fabrication of hybrid nanocapsules by calcium phosphate mineralization of shell cross-linked polymer micelles and nanocages. Nano Lett. 2005;5:1457–61. doi: 10.1021/nl050817w.
Wang KW, Zhu YJ, Chen F, Cao SW. Calcium phosphate/block copolymer hybrid porous nanospheres: preparation and application in drug delivery. Mater Lett. 2010;64:2299–301. doi: 10.1016/j.matlet.2010.07.060.
LeGeros RZ, Chohayeb A, Shulman A. Apatitic calcium orthophosphates: possible dental restorative materials. J Dent Res. 1982;61:343.
Brown WE, Chow LC. A new calcium orthophosphate setting cement. J Dent Res. 1983;62:672.
Brown WE, Chow LC. Dental restorative cement pastes. US Patent No. 4518430. May 21, 1985.
Dorozhkin SV. Calcium orthophosphate cements and concretes. Materials. 2009;2:221–91. doi: 10.3390/ma2010221.
Barralet JA, Hofmann M, Grover LM, Gbureck U. High-strength apatitic cement by modification with α -hydroxyacid salts. Adv Mater (Deerfield Beach Fla) 2003;15:2091–4. doi: 10.1002/adma.200305469.
Bigi A, Bracci B, Panzavolta S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials. 2004;25:2893–9. doi: 10.1016/j.biomaterials.2003.09.059.
Bigi A, Panzavolta S, Sturba L, Torricelli P, Fini M, Giardino R. Normal and osteopenic bone-derived osteoblast response to a biomimetic gelatin-calcium phosphate bone cement. J Biomed Mater Res A. 2006;78:739–45. doi: 10.1002/jbm.a.30765.
Panzavolta S, Torricelli P, Sturba L, Bracci B, Giardino R, Bigi A. Setting properties and in vitro bioactivity of strontium-enriched gelatin-calcium phosphate bone cements. J Biomed Mater Res A. 2008;84:965–72. doi: 10.1002/jbm.a.31412.
Rammelt S, Neumann M, Hanisch U, Reinstorf A, Pompe W, Zwipp H, et al. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J Biomed Mater Res A. 2005;73:284–94. doi: 10.1002/jbm.a.30263.
Wang X, Ye J, Wang Y, Chen L. Reinforcement of calcium phosphate cement by bio-mineralized carbon nanotube. J Am Ceram Soc. 2007;90:962–4. doi: 10.1111/j.1551-2916.2006.01460.x.
According to Wikipedia, the free encyclopedia: “Concrete is a construction material that consists of a cement (commonly Portland cement), aggregates (generally gravel and sand) and water. It solidifies and hardens after mixing and placement due to a chemical process known as hydration. The water reacts with the cement, which bonds the other components together, eventually creating a stone-like material”. http://en.wikipedia.org/wiki/Concrete (accessed in December 2010).
Kim SB, Kim YJ, Yoon TL, Park SA, Cho IH, Kim EJ, et al. The characteristics of a hydroxyapatite-chitosan-PMMA bone cement. Biomaterials. 2004;25:5715–23. doi: 10.1016/j.biomaterials.2004.01.022.
Vallo CI, Montemartini PE, Fanovich MA, Porto López JM, Cuadrado TR. Polymethylmethacrylate-based bone cement modified with hydroxyapatite. J Biomed Mater Res. 1999;48:150–8. doi: 10.1002/(SICI)1097-4636(1999)48:2<150::AID-JBM9>3.0.CO;2-D.
Sogal A, Hulbert SF. Mechanical properties of a composite bone cement: polymethylmethacrylate and hydroxyapatite. Bioceramics. 1992;5:213–24.
Harper EJ, Behiri JC, Bonfield W. Flexural and fatigue properties of a bone cement based upon polymethylmethacrylate and hydroxyapatite. J Mater Sci Mater Med. 1995;6:799–803. doi: 10.1007/BF00134320.
Harper EJ, Braden M, Bonfield W. Mechanical properties of hydroxyapatite reinforced poly(methylmethacrylate) bone cement after immersion in a physiological solution: influence of a silane coupling agent. J Mater Sci Mater Med. 2001;11:491–7. doi: 10.1023/A:1013057724268.
Moursi AM, Winnard AV, Winnard PL, Lannutti JJ, Seghi RR. Enhanced osteoblast response to a PMMA-HA composite. Biomaterials. 2002;23:133–44. doi: 10.1016/S0142-9612(01)00088-6.
Dalby MJ, Di Silvio L, Harper EJ, Bonfield W. Initial interaction of osteoblasts with the surface of a hydroxyapatite-poly(methylmethacrylate) cement. Biomaterials. 2001;22:1739–47. doi: 10.1016/S0142-9612(00)00334-3.
Dalby MJ, Di Silvio L, Harper EJ, Bonfield W. Increasing hydroxyapatite incorporation into poly(methylmethacrylate) cement increases osteoblast adhesion and response. Biomaterials. 2002;23:569–76. doi: 10.1016/S0142-9612(01)00139-9.
Itokawa H, Hiraide T, Moriya M, Fujimoto M, Nagashima G, Suzuki R, et al. A 12 month in vivo study on the response of bone to a hydroxyapatite-polymethylmethacrylate cranioplasty composite. Biomaterials. 2007;28:4922–7. doi: 10.1016/j.biomaterials.2007.08.001.
Cheang P, Khor KA. Effect of particulate morphology on the tensile behaviour of polymer-hydroxyapatite composites. Mater Sci Eng A. 2003;345:47–54. doi: 10.1016/S0921-5093(02)00284-8.
Dalby MJ, Di Silvio L, Harper EJ, Bonfield W. In vitro evaluation of a new polymethylmethacrylate cement reinforced with hydroxyapatite. J Mater Sci Mater Med. 1999;10:793–6. doi: 10.1023/A:1008907218330.
Tham WL, Chow WS, Ishak ZAM. The effect of 3-(trimethoxysilyl) propyl methacrylate on the mechanical, thermal and morphological properties of poly(methyl methacrylate)/hydroxyapatite composites. J Appl Polym Sci. 2010;118:218–28.
Tham WL, Chow WS, Mohd Ishak ZA. Flexural and morphological properties of Poly(Methyl Methacrylate)/hydroxyapatite composites: effects of planetary ball mill grinding time. J Reinf Plastics Compos. 2010;29:2065–75. doi: 10.1177/0731684409344899.
Pattanayak DK, Rao BT, Mohan TRR. Calcium phosphate bioceramics and bioceramic composites. J Sol-Gel Sci Technol. 2011 doi: 10.1007/s10971-010-2354-y.
Deb S, Braden M, Bonfield W. Water absorption characteristics of modified hydroxyapatite bone cements. Biomaterials. 1995;16:1095–100. doi: 10.1016/0142-9612(95)98906-U.
Borzacchiello A, Ambrosio L, Nicolais L, Harper EJ, Tanner KE, Bonfield W. Comparison between the polymerization behavior of a new bone cement and a commercial one: modeling and in vitro analysis. J Mater Sci Mater Med. 1998;9:835–8. doi: 10.1023/A:1008996112042.
Ohgaki M, Yamashita K. Preparation of polymethylmethacrylate-reinforced functionally graded hydroxyapatite composites. J Am Ceram Soc. 2003;86:1440–2. doi: 10.1111/j.1151-2916.2003.tb03492.x.
del Real RP, Padilla S, Vallet-Regí M. Gentamicin release from hydroxyapatite/poly(ethyl methacrylate)/poly(methyl methacrylate)composites. J Biomed Mater Res. 2000;52:1–7. doi: 10.1002/1097-4636(200010)52:1<1::AID-JBM1>3.0.CO;2-R.
Saito M, Maruoka A, Mori T, Sugano N, Hino K. Experimental studies on a new bioactive bone cement: hydroxyapatite composite resin. Biomaterials. 1994;15:156–60. doi: 10.1016/0142-9612(94)90266-6.
Watson KE, Tenhuisen KS, Brown PW. The formation of hydroxyapatite-calcium polyacrylate composites. J Mater Sci Mater Med. 1999;10:205–13. doi: 10.1023/A:1008902027278.
Reed CS, Ten Huisen KS, Brown PW, Allcock HR. Thermal stability and compressive strength of calcium-deficient hydroxyapatite—poly[bis(carboxylatophenoxy)phosphazene] composites. Chem Mater. 1996;8:440–7. doi: 10.1021/cm9503644.
Peter SJ, Kim P, Yasko AW, Yaszemski MJ, Mikos AG. Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J Biomed Mater Res. 1999;44:314–21. doi: 10.1002/(SICI)1097-4636(19990305)44:3<314::AID-JBM10>3.0.CO;2-W.
He S, Yaszemski MJ, Yasko AW, Engel PS, Mikos AG. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials. 2000;21:2389–94. doi: 10.1016/S0142-9612(00)00106-X.
Ignjatović N, Jovanović J, Suljovrujić E, Uskoković D. Injectable polydimethylsiloxane-hydroxyapatite composite cement. Biomed Mater Eng. 2003;13:401–10.
Fei Z, Hu Y, Wu D, Wu H, Lu R, Bai J, et al. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med. 2008;19:1109–16. doi: 10.1007/s10856-007-3050-5.
Link DP, van den Dolder J, van den Beucken JJJP, Cuijpers VM, Wolke JGC, Mikos AG, et al. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A. 2008;87:760–9. doi: 10.1002/jbm.a.31831.
Chiang TY, Ho CC, Chen DCH, Lai MH, Ding SJ. Physicochemical properties and biocompatibility of chitosan oligosaccharide/gelatin/calcium phosphate hybrid cements. Mater Chem Phys. 2010;120:282–8. doi: 10.1016/j.matchemphys.2009.11.007.
Fujishiro Y, Takahashi K, Sato T. Preparation and compressive strength of alpha-tricalcium phosphate/gelatin gel composite cement. J Biomed Mater Res. 2001;54:525–30. doi: 10.1002/1097-4636(20010315)54:4<525::AID-JBM80>3.0.CO;2-#.
Miyazaki K, Horibe T, Antonucci JM, Takagi S, Chow LC. Polymeric calcium phosphate cements: analysis of reaction products and properties. Dent Mater. 1993;9:41–5. doi: 10.1016/0109-5641(93)90104-X.
Miyazaki K, Horibe T, Antonucci JM, Takagi S, Chow LC. Polymeric calcium phosphate cements: setting reaction modifiers. Dent Mater. 1993;9:46–50. doi: 10.1016/0109-5641(93)90105-Y.
dos Santos LA, De Oliveria LC, Rigo ECS, Carrodeguas RG, Boschi AO, De Arruda ACF. Influence of polymeric additives on the mechanical properties of alpha-tricalcium phosphate cement. Bone. 1999;25(Suppl):99S–102S. doi: 10.1016/S8756-3282(99)00143-X.
Greish YE, Brown PW, Bender JD, Allcockm HR, Lakshmim S, Laurencin CT. Hydroxyapatite-polyphosphazane composites prepared at low temperatures. J Am Ceram Soc. 2007;90:2728–34. doi: 10.1111/j.1551-2916.2007.01780.x.
Greish YE, Bender JD, Lakshmi S, Brown PW, Allcock HR, Laurencin CT. Formation of hydroxyapatite-polyphosphazene polymer composites at physiologic temperature. J Biomed Mater Res A. 2006;77:416–25. doi: 10.1002/jbm.a.30145.
Greish YE, Bender JD, Lakshmi S, Brown PW, Allcock HR, Laurencin CT. Low temperature formation of hydroxyapatite-poly(alkyl oxybenzoate)phosphazene composites for biomedical applications. Biomaterials. 2005;26:1–9. doi: 10.1016/j.biomaterials.2004.02.016.
Mickiewicz RA, Mayes AM, Knaack D. Polymer--calcium phosphate cement composites for bone substitutes. J Biomed Mater Res. 2002;61:581–92. doi: 10.1002/jbm.10222.
Carey LE, Xu HHK, Simon CG, Jr., Takagi S, Chow LC. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials. 2005;26:5002–14. doi: 10.1016/j.biomaterials.2005.01.015.
Miao X, Tan LP, Tan LS, Huang X. Porous calcium phosphate ceramics modified with PLGA—bioactive glass. Mater Sci Eng C. 2007;27:274–9. doi: 10.1016/j.msec.2006.05.008.
Lickorish D, Guan L, Davies JE. A three-phase, fully resorbable, polyester/calcium phosphate scaffold for bone tissue engineering: Evolution of scaffold design. Biomaterials. 2007;28:1495–502. doi: 10.1016/j.biomaterials.2006.11.025.
Xu HHK, Simon CG., Jr. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials. 2005;26:1337–48. doi: 10.1016/j.biomaterials.2004.04.043.
Zhang L, Li Y, Zhou G, Lu GY, Zuo Y. Setting mechanism of nano-hydroxyapatite/chitosan bone cement. J Inorg Mater. 2006;21:1197–202.
Ruhé PQ, Hedberg EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG. Biocompatibility and degradation of poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composites. J Biomed Mater Res A. 2005;74:533–44. doi: 10.1002/jbm.a.30341.
Dagang G, Haoliang S, Kewei X, Yong H. Long-term variations in mechanical properties and in vivo degradability of CPC/PLGA composite. J Biomed Mater Res B Appl Biomater. 2007;82:533–44. doi: 10.1002/jbm.b.30759.
Habraken WJEM, Wolke JGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17:1057–74. doi: 10.1163/156856206778366004.
Ruhé PQ, Hedberg-Dirk EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789–800. doi: 10.1089/ten.2006.12.789.
Ruhé PQ, Hedberg EL, Padron NT, Spauwen PHM, Jansen JA, Mikos AG. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A(Suppl 3):75–81. doi: 10.2106/00004623-200300003-00013.
Ruhé PQ, Boerman OC, Russel FGM, Spauwen PHM, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–71. doi: 10.1016/j.jconrel.2005.04.018.
Plachokova A, Link D, van den Dolder J, van den Beucken J, Jansen JA. Bone regenerative properties of injectable PGLA-CaP composite with TGF-beta1 in a rat augmentation model. J Tissue Eng Regen Med. 2007;1:457–64. doi: 10.1002/term.59.
Webster TJ, Siegel RW, Bizios R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999;20:1221–7. doi: 10.1016/S0142-9612(99)00020-4.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475–83. doi: 10.1002/1097-4636(20000905)51:3<475::AID-JBM23>3.0.CO;2-9.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–10. doi: 10.1016/S0142-9612(00)00075-2.
Li G, Huang J, Li Y, Zhang R, Deng B, Zhang J, et al. In vitro study on influence of a discrete nano-hydroxyapatite on leukemia P388 cell behavior. Biomed Mater Eng. 2007;17:321–7.
Tadic D, Peters F, Epple M. Continuous synthesis of amorphous carbonated apatites. Biomaterials. 2002;23:2553–9. doi: 10.1016/S0142-9612(01)00390-8.
Xu HHK, Sun L, Weir MD, Takagi S, Chow LC, Hockey B. Effects of incorporating nanosized calcium phosphate particles on properties of whisker-reinforced dental composites. J Biomed Mater Res B Appl Biomater. 2007;81:116–25. doi: 10.1002/jbm.b.30644.
Deng X, Hao JY, Wang CS. Preparation and mechanical properties of nanocomposites of poly(D,L-lactide) with Ca-deficient hydroxyapatite nanocrystals. Biomaterials. 2001;22:2867–73. doi: 10.1016/S0142-9612(01)00031-X.
Hong Z, Zhang PB, He CL, Qiu XY, Liu AX, Chen L, et al. Nano-composite of poly(L-lactide) and surface grafted hydroxyapatite: mechanical properties and biocompatibility. Biomaterials. 2005;26:6296–304. doi: 10.1016/j.biomaterials.2005.04.018.
Deng C, Weng J, Cheng QY, Zhou SB, Lu X, Wan JX, et al. Choice of dispersants for the nano-apatite filler of polylactide-matrix composite biomaterial. Curr Appl Phys. 2007;7:679–82. doi: 10.1016/j.cap.2007.03.005.
Deng C, Weng J, Lu X, Zhou SB, Wan JX, Qu SX, et al. Mechanism of ultrahigh elongation rate of poly(D,L-lactide)-matrix composite biomaterial containing nano-apatite fillers. Mater Lett. 2008;62:607–10. doi: 10.1016/j.matlet.2007.06.014.
Kothapalli CR, Shaw MT, Wei M. Biodegradable HA-PLA 3-D porous scaffolds: effect of nano-sized filler content on scaffold properties. Acta Biomater. 2005;1:653–62. doi: 10.1016/j.actbio.2005.06.005.
Hong Z, Qiu X, Sun J, Deng M, Chen X, Jing X. Grafting polymerization of L-lactide on the surface of hydroxyapatite nano-crystals. Polymer (Guildf) 2004;45:6699–706. doi: 10.1016/j.polymer.2004.07.036.
Xiao Y, Li D, Fan H, Li X, Gu Z, Zhang X. Preparation of nano-HA/PLA composite by modified-PLA for controlling the growth of HA crystals. Mater Lett. 2007;61:59–62. doi: 10.1016/j.matlet.2006.04.005.
Qiu X, Han Y, Zhuang X, Chen X, Li Y, Jing X. Preparation of nano-hydroxyapatite/poly(L-lactide) biocomposite microspheres. J Nanopart Res. 2007;9:901–8. doi: 10.1007/s11051-006-9158-6.
Nejati E, Firouzdor V, Eslaminejad MB, Bagheri F. Needle-like nano hydroxyapatite/poly(l-lactide acid) composite scaffold for bone tissue engineering application. Mater Sci Eng C. 2009;29:942–9. doi: 10.1016/j.msec.2008.07.038.
Deplaine H, Ribelles JLG, Ferrer GG. Effect of the content of hydroxyapatite nanoparticles on the properties and bioactivity of poly(L-lactide)—hybrid membranes. Compos Sci Technol. 2010;70:1805–12. doi: 10.1016/j.compscitech.2010.06.009.
Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–409. doi: 10.1016/j.biomaterials.2005.08.016.
Hong Z, Zhang P, Liu A, Chen L, Chen X, Jing X. Composites of poly(lactide-co-glycolide) and the surface modified carbonated hydroxyapatite nanoparticles. J Biomed Mater Res A. 2007;81:515–22. doi: 10.1002/jbm.a.31038.
Huang YX, Ren J, Chen C, Ren TB, Zhou XY. Preparation and properties of poly(lactide-co-glycolide) (PLGA)/ nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J Biomater Appl. 2008;22:409–32. doi: 10.1177/0885328207077632.
Xue D, Zheng Q, Zong C, Li Q, Li H, Qian S, et al. Osteochondral repair using porous poly(lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J Biomed Mater Res A. 2010;94:259–70. doi: 10.1002/jbm.a.32691.
Du C, Cui FZ, Zhu XD, de Groot K. Three-dimensional nano-HAp/collagen matrix loading with osteogenic cells in organ culture. J Biomed Mater Res. 1999;44:407–15. doi: 10.1002/(SICI)1097-4636(19990315)44:4<407::AID-JBM6>3.0.CO;2-T.
Wang RZ, Cui FZ, Lu HB, Wen HB, Ma CL, Li HD. Synthesis of nanophase hydroxyapatite/collagen composite. J Mater Sci Lett. 1995;14:490–2. doi: 10.1007/BF00665911.
Du C, Cui FZ, Feng QL, Zhu XD, de Groot K. Tissue response to nano-hydroxyapatite/collagen composite implants in marrow cavity. J Biomed Mater Res. 1998;42:540–8. doi: 10.1002/(SICI)1097-4636(19981215)42:4<540::AID-JBM9>3.0.CO;2-2.
Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001;22:1705–11. doi: 10.1016/S0142-9612(00)00305-7.
Kikuchi M, Matsumoto HN, Yamada T, Koyama Y, Takakuda K, Tanaka J. Glutaraldehyde cross-linked hydroxyapatite/collagen self-organized nanocomposites. Biomaterials. 2004;25:63–9. doi: 10.1016/S0142-9612(03)00472-1.
Lynn AK, Nakamura T, Patel N, Porter AE, Renouf AC, Laity PR, et al. Composition-controlled nanocomposites of apatite and collagen incorporating silicon as an osseopromotive agent. J Biomed Mater Res A. 2005;74:447–53. doi: 10.1002/jbm.a.30373.
Chang MC, Tanaka J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials. 2002;23:4811–8. doi: 10.1016/S0142-9612(02)00232-6.
Chang MC, Tanaka J. XPS study for the microstructure development of hydroxyapatite-collagen nanocomposites cross-linked using glutaraldehyde. Biomaterials. 2002;23:3879–85. doi: 10.1016/S0142-9612(02)00133-3.
Murugan R, Ramakrishna S. In situ formation of recombinant humanlike collagen-hydroxyapatite nanohybrid through bionic approach. Appl Phys Lett. 2006;88:193124–7. doi: 10.1063/1.2202138.
Wang Y, Yang C, Chen X, Zhao N. Biomimetic formation of hydroxyapatite/collagen matrix composite. Adv Eng Mater. 2006;8:97–100. doi: 10.1002/adem.200500220.
Thomas V, Dean DR, Jose MV, Mathew B, Chowdhury S, Vohra YK. Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules. 2007;8:631–7. doi: 10.1021/bm060879w.
Fukui N, Sato T, Kuboki Y, Aoki H. Bone tissue reaction of nano-hydroxyapatite/collagen composite at the early stage of implantation. Biomed Mater Eng. 2008;18:25–33.
Kim TG, Park SH, Chung HJ, Yang DY, Park TG. Microstructured scaffold coated with hydroxyapatite/collagen nanocomposite multilayer for enhanced osteogenic induction of human mesenchymal stem cells. J Mater Chem. 2010;20:8927–33. doi: 10.1039/c0jm01062f.
Liao SS, Tamura K, Zhu Y, Wang W, Uo M, Akasaka T, et al. Human neutrophils reaction to the biodegraded nano-hydroxyapatite/collagen and nano-hydroxyapatite/collagen/poly(L-lactic acid) composites. J Biomed Mater Res A. 2006;76:820–5. doi: 10.1002/jbm.a.30582.
Liao S, Cui FZ, Zhu Y. Osteoblasts adherence and migration through three-dimensional porous mineralized collagen based composite: nHAC/PLA. J Bioact Compat Polym. 2004;19:117–30. doi: 10.1177/0883911504042643.
Liao SS, Cui FZ, Zhang W, Feng QL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res B Appl Biomater. 2004;69:158–65. doi: 10.1002/jbm.b.20035.
Liao SS, Cui FZ. In vitro and in vivo degradation of mineralized collagen-based composite scaffold: nanohydroxyapatite/collagen/poly(L-lactide) Tissue Eng. 2004;10:73–80. doi: 10.1089/107632704322791718.
Liao S, Wang W, Uo M, Ohkawa S, Akasaka T, Tamura K, et al. A three-layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane for guided tissue regeneration. Biomaterials. 2005;26:7564–71. doi: 10.1016/j.biomaterials.2005.05.050.
Li X, Feng Q, Cui FZ. In vitro degradation of porous nano-hydroxyapatite/collagen/PLLA scaffold reinforced by chitin fibres. Mater Sci Eng C. 2006;26:716–20. doi: 10.1016/j.msec.2005.06.062.
Zhou DS, Zhao KB, Li Y, Cui FZ, Lee IS. Repair of segmental defects with nano-hydroxyapatite/collagen/PLA composite combined with mesenchymal stem cells. J Bioact Compat Polym. 2006;21:373–84. doi: 10.1177/0883911506068554.
Zhang C, Hu YY, Cui FZ, Zhang SM, Ruan DK. A study on a tissue-engineered bone using rhBMP-2 induced periosteal cells with a porous nano-hydroxyapatite/collagen/poly(L-lactic acid) scaffold. Biomed Mater. 2006;1:56–62. doi: 10.1088/1748-6041/1/2/002.
Liao S, Watari F, Zhu Y, Uo M, Akasaka T, Wang W, et al. The degradation of the three layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane in vitro. Dent Mater. 2007;23:1120–8. doi: 10.1016/j.dental.2006.06.045.
Degirmenbasi N, Kalyon DM, Birinci E. Biocomposites of nanohydroxyapatite with collagen and poly(vinyl alcohol) Colloids Surf B Biointerfaces. 2006;48:42–9. doi: 10.1016/j.colsurfb.2006.01.002.
Zhang SM, Cui FZ, Liao SS, Zhu Y, Han L. Synthesis and biocompatibility of porous nano-hydroxyapatite/collagen/alginate composite. J Mater Sci Mater Med. 2003;14:641–5. doi: 10.1023/A:1024083309982.
Sotome S, Uemura T, Kikuchi M, Chen J, Itoh S, Tanaka J, et al. Synthesis and in vivo evaluation of a novel hydroxyapatite/collagen-alginate as a bone filler and a drug delivery carrier of bone morphogenetic protein. Mater Sci Eng C. 2004;24:341–7. doi: 10.1016/j.msec.2003.12.003.
Chang MC, Ko CC, Douglas WH. Conformational change of hydroxyapatite/gelatin nanocomposite by glutaraldehyde. Biomaterials. 2003;24:3087–94. doi: 10.1016/S0142-9612(03)00150-9.
Kim HW, Kim HE, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials. 2005;26:5221–30. doi: 10.1016/j.biomaterials.2005.01.047.
Chang MC, Ikoma T, Tanaka J. Cross-linkage of hydroxyapatite/gelatin nanocomposite using EGDE. J Mater Sci. 2004;39:5547–50. doi: 10.1023/B:JMSC.0000039284.70028.fa.
Teng S, Shi J, Peng B, Chen L. The effect of alginate addition on the structure and morphology of hydroxyapatite/gelatin nanocomposites. Compos Sci Technol. 2006;66:1532–8. doi: 10.1016/j.compscitech.2005.11.021.
Chang MC, Ko CC, Douglas WH. Preparation of hydroxyapatite-gelatin nanocomposite. Biomaterials. 2003;24:2853–62. doi: 10.1016/S0142-9612(03)00115-7.
Mobini S, Javadpour J, Hosseinalipour M, Ghazi-Khansari M, Khavandi A, Rezaie HR. Synthesis and characterisation of gelatin-nano hydroxyapatite composite scaffolds for bone tissue engineering. Adv Appl Ceramics. 2008;107:4–8. doi: 10.1179/174367608X246817.
Lewandrowski KU, Bondre SP, Wise DL, Trantolo DJ. Enhanced bioactivity of a poly(propylene fumarate) bone graft substitute by augmentation with nano-hydroxyapatite. Biomed Mater Eng. 2003;13:115–24.
Jayabalan M, Shalumon KT, Mitha MK, Ganesan K, Epple M. Effect of hydroxyapatite on the biodegradation and biomechanical stability of polyester nanocomposites for orthopaedic applications. Acta Biomater. 2010;6:763–75. doi: 10.1016/j.actbio.2009.09.015.
Jayabalan M, Shalumon KT, Mitha MK, Ganesan K, Epple M. The effect of radiation processing and filler morphology on the biomechanical stability of a thermoset polyester composite. Biomed Mater. 2010;5:25009. doi: 10.1088/1748-6041/5/2/025009.
Jie W, Li Y, He Y. Processing properties of nano apatite-polyamide biocomposites. J Mater Sci. 2005;40:793–6. doi: 10.1007/s10853-005-6326-5.
Wei J, Li Y, Chen W, Zuo Y. A study on nano-composite of hydroxyapatite and polyamide. J Mater Sci. 2003;38:4033–9. doi: 10.1023/A:1026127018920.
Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338–48. doi: 10.1016/j.biomaterials.2007.04.014.
Sender C, Dantras E, Dantras-Laffont L, Lacoste MH, Dandurand J, Mauzac M, et al. Dynamic mechanical properties of a biomimetic hydroxyapatite/polyamide 6,9 nanocomposite. J Biomed Mater Res B Appl Biomater. 2007;83:628–35. doi: 10.1002/jbm.b.30930.
Yang K, Wei J, Wang CY, Li Y. A study on in vitro and in vivo bioactivity of nano hydroxyapatite/polymer biocomposite. Chin Sci Bull. 2007;52:267–71. doi: 10.1007/s11434-007-0035-1.
Zhang X, Li Y, Lv GY, Zuo Y, Mu YH. Thermal and crystallization studies of nano-hydroxyapatite reinforced polyamide 66 biocomposites. Polym Degrad Stabil. 2006;91:1202–7. doi: 10.1016/j.polymdegradstab.2005.02.006.
Huang M, Feng J, Wang J, Zhang X, Li Y, Yan Y. Synthesis and characterization of nano-HA/PA66 composites. J Mater Sci Mater Med. 2003;14:655–60. doi: 10.1023/A:1024087410890.
Zhang X, Li Y, Zuo Y, Lv GY, Mu YH, Li H. Morphology, hydrogen-bonding and crystallinity of nano-hydroxyapatite/polyamide 66 biocomposites. Composites A. 2007;38:843–8. doi: 10.1016/j.compositesa.2006.08.002.
Lan W, Li Y, Yi Z, Li Z, Mu YH, Jimei H. Study on the biomimetic properties of bone substitute material: nano-hydroxyapatite/polyamide 66 composite. Mater Sci Forum. 2006;511:938–41.
Li Z, Li Y, Wang X, Wei J, Peng X. Studies on the porous scaffold made of the nano-HA/PA66 composite. J Mater Sci. 2005;40:107–10. doi: 10.1007/s10853-005-5693-2.
Zhang X, Li Y, Lv GY, Zuo Y, Mu YH, Lan W. The study on interaction mechanism between n-HA and PA66 in n-HA/PA66 biocomposites. Funct Mater. 2005;36:896–9.
Menon D, Anoop Anand K, Anitha VC, Nair S. Hydroxyapatite-reinforced polyamide 6,6 nanocomposites through melt compounding. Int J Polym Mater. 2010;59:498–509. doi: 10.1080/00914031003627262.
Yusong P, Dangsheng X, Xiaolin C. Mechanical properties of nanohydroxyapatite reinforced poly(vinyl alcohol) gel composites as biomaterial. J Mater Sci. 2007;42:5129–34. doi: 10.1007/s10853-006-1264-4.
Fengland X, Li Y, Wang X, Wei J, Yang A. Preparation and characterization of nano-hydroxyapatite/poly(vinyl alcohol) hydrogel biocomposite. J Mater Sci. 2004;39:5669–72. doi: 10.1023/B:JMSC.0000040074.64787.b3.
Wang HS, Wang GX, Pan QX. Electrochemical study of the interactions of DNA with redox-active molecules based on the immobilization of dsDNA on the sol-gel derived nano porous hydroxyapatite-polyvinyl alcohol hybrid material coating. Electroanalysis. 2005;17:1854–60. doi: 10.1002/elan.200503314.
Pramanik N, Mohapatra S, Pramanik P, Bhargava P. Processing and properties of nano-hydroxyapatite (n-HAp)/poly(ethylene-co-acrylic acid) (EAA) composite using a phosphonic acid coupling agent for orthopedic applications. J Am Ceram Soc. 2007;90:369–75. doi: 10.1111/j.1551-2916.2006.01388.x.
Pramanik N, Bhargava P, Alam S, Pramanik P. Processing and properties of nano- and macro-hydroxyapatite/poly(ethylene-co-acrylic acid) composites. Polym Compos. 2006;27:633–41. doi: 10.1002/pc.20246.
Li Z, Yubao L, Aiping Y, Xuelin P, Xuejiang W, Xiang Z. Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J Mater Sci Mater Med. 2005;16:213–9. doi: 10.1007/s10856-005-6682-3.
Zhang YF, Cheng XR, Chen Y, Shi B, Chen XH, Xu DX, et al. Three-dimensional nanohydroxyapatite/chitosan scaffolds as potential tissue engineered periodontal tissue. J Biomater Appl. 2007;21:333–49. doi: 10.1177/0885328206063853.
Kong L, Gao Y, Lu G, Gong Y, Zhao N, Zhang X. A study on the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur Polym J. 2006;42:3171–9. doi: 10.1016/j.eurpolymj.2006.08.009.
Lu XY, Wang XH, Qu SX, Weng J. Preparation of nano-hydroxyapatite/chitosan hybrids. J Inorg Mater. 2008;23:332–6. doi: 10.3724/SP.J.1077.2008.00332.
Chen JD, Wang Y, Chen X. In situ fabrication of nano-hydroxyapatite in a macroporous chitosan scaffold for tissue engineering. J Biomater Sci Polym Ed. 2009;20:1555–65. doi: 10.1163/092050609X12464345036768.
Thein-Han WW, Misra RDK. Biomimetic chitosan-nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009;5:1182–97. doi: 10.1016/j.actbio.2008.11.025.
Chen J, Nan K, Yin S, Wang Y, Wu T, Zhang Q. Characterization and biocompatibility of nanohybrid scaffold prepared via in situ crystallization of hydroxyapatite in chitosan matrix. Colloids Surf B Biointerfaces. 2010;81:640–7. doi: 10.1016/j.colsurfb.2010.08.017.
Lu Y, Zhu A, Wang W, Shi H. New bioactive hybrid material of nano-hydroxyapatite based on N-carboxyethylchitosan for bone tissue engineering. Appl Surf Sci. 2010;256:7228–33. doi: 10.1016/j.apsusc.2010.05.056.
Zhou G, Li Y, Zhang L, Li H, Wang M, Cheng L, et al. The study of tri-phasic interactions in nano-hydroxyapatite/konjac glucomannan/chitosan composite. J Mater Sci. 2007;42:2591–7. doi: 10.1007/s10853-006-1337-4.
Huang J, Lin YW, Fu XW, Best SM, Brooks RA, Rushton N, et al. Development of nano-sized hydroxyapatite reinforced composites for tissue engineering scaffolds. J Mater Sci Mater Med. 2007;18:2151–7. doi: 10.1007/s10856-007-3201-8.
Lee HJ, Choi HW, Kim KJ, Lee SC. Modification of hydroxyapatite nanosurfaces for enhanced colloidal stability and improved interfacial adhesion in nanocomposites. Chem Mater. 2006;18:5111–8. doi: 10.1021/cm061139x.
Lee HJ, Kim SE, Choi HW, Kim CW, Kim KJ, Lee SC. The effect of surface-modified nano-hydroxyapatite on biocompatibility of poly(∑-caprolactone)/hydroxyapatite nanocomposites. Eur Polym J. 2007;43:1602–8. doi: 10.1016/j.eurpolymj.2007.02.030.
Grande CJ, Torres FG, Gomez CM, Bañó MC. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009;5:1605–15. doi: 10.1016/j.actbio.2009.01.022.
Zadegan S, Hossainalipour M, Ghassai H, Rezaie HR, Naimi-Jamal MR. Synthesis of cellulose-nanohydroxyapatite composite in 1-n-butyl-3-methylimidazolium chloride. Ceram Int. 2010;36:2375–81. doi: 10.1016/j.ceramint.2010.07.019.
Jia N, Li SM, Zhu JF, Ma MG, Xu F, Wang B, et al. Microwave-assisted synthesis and characterization of cellulose-carbonated hydroxyapatite nanocomposites in NaOH-urea aqueous solution. Mater Lett. 2010;64:2223–5. doi: 10.1016/j.matlet.2010.07.029.
Pang P, Li W, Liu Y. Effect of ball milling process on the microstructure of titanium-nanohydroxyapatite composite powder. Rare Met. 2007;26:118–23. doi: 10.1016/S1001-0521(07)60170-3.
Li W, Pang P, Liu Y. Microstructure and phase composition of Ti-based biocomposites with different contents of nano-hydroxyapatite. Trans. Nonferrous Metals Soc. China. 2007;17:1148–51.
Niespodziana K, Jurczyk K, Jakubowicz J, Jurczyk M. Fabrication and properties of titanium-hydroxyapatite nanocomposites. Mater Chem Phys. 2010;123:160–5. doi: 10.1016/j.matchemphys.2010.03.076.
Hao JY, Liu Y, Zhou S, Li Z, Deng X. Investigation of nanocomposites based on semi-interpenetrating network of [L-poly (epsilon-caprolactone)]/[net-poly (epsilon-caprolactone)] and hydroxyapatite nanocrystals. Biomaterials. 2003;24:1531–9. doi: 10.1016/S0142-9612(02)00516-1.
Yan Y, Li Y, Zheng Y, Yi Z, Wei J, Xia C, et al. Synthesis and properties of a copolymer of poly(1,4-phenylene sulfide)-poly(2,4-phenylene sulfide acid) and its nano-apatite reinforced composite. Eur Polym J. 2003;39:411–6. doi: 10.1016/S0014-3057(02)00109-X.
Bhattacharyya S, Nair LS, Singh A, Krogman NR, Bender J, Greish YE, et al. Development of biodegradable polyphosphazene-nanohydroxyapatite composite nanofibers via electrospinning. MRS Symp Proc 2005; 845:91-6.
Sinha A, Nayar S, Agrawal A, Bhattacharyya D, Ramachandrarao P. Synthesis of nanosized and microporous precipitated hydroxyapatite in synthetic polymers and biopolymers. J Am Ceram Soc. 2003;86:357–9. doi: 10.1111/j.1151-2916.2003.tb00024.x.
Zuo Y, Li Y, Wei J, Han J, Xu F. The preparation and characterization of n-HA/PA series biomedical composite. Funct Mater. 2004;35:513–6.
Zhou G, Li Y, Zhang L, Zuo Y, Jansen JA. Preparation and characterization of nano-hydroxyapatite/chitosan/konjac glucomannan composite. J Biomed Mater Res A. 2007;83:931–9. doi: 10.1002/jbm.a.31427.
Daniel-da-Silva AL, Lopes AB, Gil AM, Correia RN. Synthesis and characterization of porous |-carrageenan/calcium phosphate nanocomposite scaffolds. J Mater Sci. 2007;42:8581–91. doi: 10.1007/s10853-007-1851-z.
Furuzono T, Kishida A, Tanaka J. Nano-scaled hydroxyapatite/polymer composite I. Coating of sintered hydroxyapatite particles on poly(gamma-methacryloxypropyl trimethoxysilane)grafted silk fibroin fibers through chemical bonding. J Mater Sci Mater Med. 2004;15:19–23. doi: 10.1023/B:JMSM.0000010093.39298.5a.
Korematsu A, Furuzono T, Yasuda S, Tanaka J, Kishida A. Nano-scaled hydroxyapatite/polymer composite. II. Coating of sintered hydroxyapatite particles on poly(2-(o-[1'-methylpropylideneamino] carboxyamino) ethyl methacrylate)-grafted silk fibroin fibers through covalent linkage. J Mater Sci. 2004;39:3221–5. doi: 10.1023/B:JMSC.0000025865.44900.74.
Korematsu A, Furuzono T, Yasuda S, Tanaka J, Kishida A. Nano-scaled hydroxyapatite/polymer composite III. Coating of sintered hydroxyapatite particles on poly(4-methacryloyloxyethyl trimellitate anhydride)-grafted silk fibroin fibers. J Mater Sci Mater Med. 2005;16:67–71. doi: 10.1007/s10856-005-6448-y.
Yang K, Wang C, Wei J. A study on biocomposite of nano apatite/poly (1,4-phenylene-sulfide)-poly (2,4-phenylene sulfide acid) Composites B. 2007;38:306–10. doi: 10.1016/j.compositesb.2006.06.008.
Jiang L, Li Y, Zhang L, Wang XJ. Study on nano-hydroxyapatite/chitosan-carboxymethyl cellulose composite scaffold. J Inorg Mater. 2008;23:135–40. doi: 10.3724/SP.J.1077.2008.00135.
Liuyun J, Yubao L, Li Z, Jianguo L. Preparation and properties of a novel bone repair composite: nano-hydroxyapatite/chitosan/carboxymethyl cellulose. J Mater Sci Mater Med. 2008;19:981–7. doi: 10.1007/s10856-007-3208-1.
Liou SC, Chen SY, Liu DM. Phase development and structural characterization of calcium phosphate ceramics-polyacrylic acid nanocomposites at room temperature in water-methanol mixtures. J Mater Sci Mater Med. 2004;15:1261–6. doi: 10.1007/s10856-004-5733-5.
Liu L, Liu J, Wang M, Min S, Cai Y, Zhu L, et al. Preparation and characterization of nano-hydroxyapatite/silk fibroin porous scaffolds. J Biomater Sci Polym Ed. 2008;19:325–38. doi: 10.1163/156856208783721010.
Ren YJ, Sun XD, Cui FZ, Wei YT, Cheng ZJ, Kong XD. Preparation and characterization of antheraea pernyi silk fibroin based nanohydroxyapatite composites. J Bioact Compat Polym. 2007;22:465–74. doi: 10.1177/0883911507082407.
Mikołajczyk T, Rabiej S, Bogun M. Analysis of the structural parameters of polyacrylonitrile fibers containing nanohydroxyapatite. J Appl Polym Sci. 2006;101:760–5. doi: 10.1002/app.23978.
Wei J, Li Y, Lau KT. Preparation and characterization of a nano apatite/polyamide6 bioactive composite. Composites B. 2007;38:301–5. doi: 10.1016/j.compositesb.2006.05.006.
Sundaraseelan J, Sastry TP. Fabrication of a biomimetic compound containing nano hydroxyapatite—demineralised bone matrix. J Biomed Nanotechnol. 2007;3:401–5. doi: 10.1166/jbn.2007.042.
Leeuwenburgh SCG, Jansen JA, Mikos AG. Functionalization of oligo(poly(ethylene glycol)fumarate) hydrogels with finely dispersed calcium phosphate nanocrystals for bone-substituting purposes. J Biomater Sci Polym Ed. 2007;18:1547–64.
Hesaraki S, Ebadzadeh T, Ahmadzadeh-Asl S. Nanosilicon carbide/hydroxyapatite nanocomposites: structural, mechanical and in vitro cellular properties. J Mater Sci Mater Med. 2010;21:2141–9. doi: 10.1007/s10856-010-4068-7.
Zandi M, Mirzadeh H, Mayer C, Urch H, Eslaminejad MB, Bagheri F, et al. Biocompatibility evaluation of nano-rod hydroxyapatite/gelatin coated with nano-HAp as a novel scaffold using mesenchymal stem cells. J Biomed Mater Res A. 2010;92:1244–55. doi: 10.1002/jbm.a.32452.
Sun TS, Guan K, Shi SS, Zhu B, Zheng YJ, Cui FZ, et al. Effect of nano-hydroxyapatite/collagen composite and bone morphogenetic protein-2 on lumbar intertransverse fusion in rabbits. Chin J Traumatol. 2004;7:18–24.
Itoh S, Kikuchi M, Koyama Y, Takakuda K, Shinomiya K, Tanaka J. Development of a hydroxyapatite/collagen nanocomposite as a medical device. Cell Transplant. 2004;13:451–61. doi: 10.3727/000000004783983774.
Kester M, Heakal Y, Fox T, Sharma A, Robertson GP, Morgan TT, et al. Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells. Nano Lett. 2008;8:4116–21. doi: 10.1021/nl802098g.
Wang KW, Zhou LZ, Sun Y, Wu GJ, Gu HC, Duan YR, et al. Calcium phosphate/PLGA-mPEG hybrid porous nanospheres: a promising vector with ultrahigh gene loading and transfection efficiency. J Mater Chem. 2010;20:1161–6. doi: 10.1039/b917441a.
Gelinsky M, Welzel PB, Simon P, Bernhardt A, König U. Porous three-dimensional scaffolds made of mineralized collagen: preparation and properties of a biomimetic nanocomposite material for tissue engineering of bone. Chem Eng J. 2008;137:84–96. doi: 10.1016/j.cej.2007.09.029.
Hu Q, Li BQ, Wang M, Shen JC. Preparation and characterization of biodegradable chitosan/hydroxyapatite nanocomposite rods via in situ hybridization: a potential material as internal fixation of bone fracture. Biomaterials. 2004;25:779–85. doi: 10.1016/S0142-9612(03)00582-9.
Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–57. doi: 10.1016/j.biomaterials.2003.12.005.
Liou SC, Chen SY, Liu DM. Synthesis and characterization of needlelike apatitic nanocomposite with controlled aspect ratios. Biomaterials. 2003;24:3981–8. doi: 10.1016/S0142-9612(03)00303-X.
Liou SC, Chen SY, Liu DM. Manipulation of nanoneedle and nanosphere apatite/poly(acrylic acid) nanocomposites. J Biomed Mater Res B Appl Biomater. 2005;73:117–22. doi: 10.1002/jbm.b.30193.
Huang J, Best SM, Bonfield W, Brooks RA, Rushton N, Jayasinghe SN, et al. In vitro assessment of the biological response to nano-size hydroxyapatite. J Mater Sci Mater Med. 2004;15:415–44. doi: 10.1023/B:JMSM.0000021117.67205.cf.
Kong L, Gao Y, Cao W, Gong Y, Zhao N, Zhang X. Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J Biomed Mater Res A. 2005;75:275–82. doi: 10.1002/jbm.a.30414.
Christenson EM, Anseth KS, van den Beucken JJJP, Chan CK, Ercan B, Jansen JA, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25:11–22. doi: 10.1002/jor.20305.
Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155.
Fratzl P, ed. Collagen: structure and mechanics, Springer: New York NY, USA 2010; 510.
Olmo N, Turnay J, Herrera JI, Gavilanes JG, Lizarbe MA. Kinetics of in vivo degradation of sepiolite-collagen complexes: effect of glutaraldehyde treatment. J Biomed Mater Res. 1996;30:77–84. doi: 10.1002/(SICI)1097-4636(199601)30:1<77::AID-JBM10>3.0.CO;2-N.
Xie J, Baumann MJ, McCabe LR. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J Biomed Mater Res A. 2004;71:108–17. doi: 10.1002/jbm.a.30140.
Tcacencu I, Wendel M. Collagen-hydroxyapatite composite enhances regeneration of calvaria bone defects in young rats but postpones the regeneration of calvaria bone in aged rats. J Mater Sci Mater Med. 2008;19:2015–21. doi: 10.1007/s10856-007-3284-2.
Yamauchi K, Goda T, Takeuchi N, Einaga H, Tanabe T. Preparation of collagen/calcium phosphate multilayer sheet using enzymatic mineralization. Biomaterials. 2004;25:5481–9. doi: 10.1016/j.biomaterials.2003.12.057.
Du C, Cui FZ, Zhang W, Feng QL, Zhu XD, de Groot K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res. 2000;50:518–27. doi: 10.1002/(SICI)1097-4636(20000615)50:4<518::AID-JBM7>3.0.CO;2-W.
Hellmich Ch, Ulm FJ. Are mineralized tissues open crystal foams reinforced by crosslinked collagen? Some energy arguments. J Biomech. 2002;35:1199–212. doi: 10.1016/S0021-9290(02)00080-5.
Boskey AL. Hydroxyapatite formation in a dynamic collagen gel system—effects of type I collagen, lipids and proteoglycans. J Phys Chem. 1989;93:1628–33. doi: 10.1021/j100341a086.
Mathers NJ, Czernuszka JT. Growth of hydroxyapatite on type I collagen. J Mater Sci Lett. 1991;10:992–3. doi: 10.1007/BF00721823.
Sukhodub LF, Moseke C, Sukhodub LB, Sulkio-Cleff B, Maleev VY, Semenov MA, et al. Collagen-hydroxyapatite-water interactions investigated by XRD, piezogravimetry, infrared and Raman spectroscopy. J Mol Struct. 2004;704:53–8. doi: 10.1016/j.molstruc.2003.12.061.
Roveri N, Falini G, Sidoti MC, Tampieri A, Landi E, Sandri M, et al. Biologically inspired growth of hydroxyapatite nanocrystals inside self-assembled collagen fibers. Mater Sci Eng C. 2003;23:441–6. doi: 10.1016/S0928-4931(02)00318-1.
Tampieri A, Celotti G, Landi E. From biomimetic apatites to biologically inspired composites. Anal Bioanal Chem. 2005;381:568–76. doi: 10.1007/s00216-004-2943-0.
Tampieri A, Celotti G, Landi E, Sandri M, Roveri N, Falini G. Biologically inspired synthesis of bone-like composite: self-assembled collagen fibers/hydroxyapatite nanocrystals. J Biomed Mater Res A. 2003;67:618–25. doi: 10.1002/jbm.a.10039.
Mehlisch DR, Taylor TD, Leibold DG, Hiatt R, Waite DE, Waite PD, et al. Evaluation of collagen/hydroxylapatite for augmenting deficient alveolar ridges: a preliminary report. J Oral Maxillofac Surg. 1987;45:408–13. doi: 10.1016/0278-2391(87)90008-5.
Okazaki M, Ohmae H, Takahashi J, Kimura H, Sakuda M. Insolubilized properties of UV-irradiated CO3 apatite-collagen composites. Biomaterials. 1990;11:568–72. doi: 10.1016/0142-9612(90)90080-A.
TenHuisen KS, Martin RI, Klimkiewicz M, Brown PW. Formation and properties of a synthetic bone composite: hydroxyapatite-collagen. J Biomed Mater Res. 1995;29:803–10. doi: 10.1002/jbm.820290704.
Marouf HA, Quayle AA, Sloan P. In vitro and in vivo studies with collagen/hydroxyapatite implants. Int J Oral Maxillofac Implants. 1990;5:148–54.
Zerwekh JE, Kourosh S, Scheinberg R, Kitano T, Edwards ML, Shin D, et al. Fibrillar collagen-biphasic calcium phosphate composite as a bone graft substitute for spinal fusion. J Orthop Res. 1992;10:562–72. doi: 10.1002/jor.1100100411.
Clarke KI, Graves SE, Wong ATC, Triffit JT, Francis MJO, Czernuszka JT. Investigation into the formation and mechanical properties of a bioactive material based on collagen and calcium phosphate. J Mater Sci Mater Med. 1993;4:107–10. doi: 10.1007/BF00120378.
Rovira A, Bareille R, Lopez L, Rouasis F, Bordenave L, Rey C, et al. Preliminary report on a new composite material made of calcium phosphate, elastin peptides and collagens. J Mater Sci Mater Med. 1993;4:372–80. doi: 10.1007/BF00122195.
Zhang QQ, Ren L, Wang C, Liu LR, Wen XJ, Liu YH, et al. Porous hydroxyapatite reinforced with collagen protein. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:693–702. doi: 10.3109/10731199609118892.
Bakoš D, Soldán M, Hernández-Fuentes I. Hydroxyapatite-collagen-hyaluronic acid composite. Biomaterials. 1999;20:191–5. doi: 10.1016/S0142-9612(98)00163-X.
John A, Hong L, Ikada Y, Tabata Y. A trial to prepare biodegradable collagen-hydroxyapatite composites for bone repair. J Biomater Sci Polym Ed. 2001;12:689–705. doi: 10.1163/156856201316883485.
ltoh S, Kikuchi M, Takakuda K, Koyama Y, Matsumoto HN, Ichinose S, et al. The biocompatibility and osteoconductive activity of a novel hydroxyapatite/collagen composite biomaterial and its function as a carrier of rhBMP-2. J Biomed Mater Res 2001; 54:445-53; PMID: 11189053.
Shinomiya K, Itoh S, Kawauchi T, Kikuchi M, Tanaka J. Development of a novel hydroxyapatite/collagen composite biomaterial. Tissue Eng Therap Use. 2001;5:165–77.
Uskoković V, Ignjatovic N, Petranovic N.Synthesis and characterization of hydroxyapatite-collagen biocomposite materials. Mater Sci Forum 2002; 413:269-74; .
Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06.
Ishikawa H, Koshino T, Takeuchi R, Saito T. Effects of collagen gel mixed with hydroxyapatite powder on interface between newly formed bone and grafted achilles tendon in rabbit femoral bone tunnel. Biomaterials. 2001;22:1689–94. doi: 10.1016/S0142-9612(00)00331-8.
Sachlos E, Gotora D, Czernuszka JT. Collagen scaffolds reinforced with biomimetic composite nano-sized carbonate-substituted hydroxyapatite crystals and shaped by rapid prototyping to contain internal microchannels. Tissue Eng. 2006;12:2479–87. doi: 10.1089/ten.2006.12.2479.
Venugopal J, Low S, Choon AT, Sampath Kumar TS, Ramakrishna S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J Mater Sci Mater Med. 2008;19:2039–46. doi: 10.1007/s10856-007-3289-x.
Teng SH, Lee EJ, Park CS, Choi WY, Shin DS, Kim HE. Bioactive nanocomposite coatings of collagen/hydroxyapatite on titanium substrates. J Mater Sci Mater Med. 2008;19:2453–61. doi: 10.1007/s10856-008-3370-0.
Song JH, Kim HE, Kim HW. Collagen-apatite nanocomposite membranes for guided bone regeneration. J Biomed Mater Res B Appl Biomater. 2007;83:248–57. doi: 10.1002/jbm.b.30790.
Pek YS, Gao S, Arshad MSM, Leck KJ, Ying JY. Porous collagen-apatite nanocomposite foams as bone regeneration scaffolds. Biomaterials. 2008;29:4300–5. doi: 10.1016/j.biomaterials.2008.07.030.
Mittelmeier H, Nizzard M. Knochenregeneration mit industriell gefertigtem Collagen Apatit Implantat (“Collapat”). In: Osteogenese und Knochenwachstum, Hackenbroch MH, Refior HJ, Jäger MG, (Eds.); Thieme: Stuttgart, Germany 1982; 194-7.
Serre CM, Papillard M, Chavassieux P, Boivin G. In vitro induction of a calcifying matrix by biomaterials constituted of collagen and/or hydroxyapatite: an ultrastructural comparison of three types of biomaterials. Biomaterials. 1993;14:97–106. doi: 10.1016/0142-9612(93)90217-P.
Scabbia A, Trombelli L. A comparative study on the use of a HA/collagen/chondroitin sulphate biomaterial (Biostite) and a bovine-derived HA xenograft (Bio-Oss) in the treatment of deep intra-osseous defects. J Clin Periodontol. 2004;31:348–55. doi: 10.1111/j.1600-051X.2004.00483.x.
Yamasaki Y, Yoshida Y, Okazaki M, Shimazu A, Kubo T, Akagawa Y, et al. Action of FGMgCO3Ap-collagen composite in promoting bone formation. Biomaterials. 2003;24:4913–20. doi: 10.1016/S0142-9612(03)00414-9.
Wang X, Grogan SP, Rieser F, Winkelmann V, Maquet V, Berge ML, et al. Tissue engineering of biphasic cartilage constructs using various biodegradable scaffolds: an in vitro study. Biomaterials. 2004;25:3681–8. doi: 10.1016/j.biomaterials.2003.10.102.
Chang MC, Ikoma T, Kikuchi M, Tanaka J. The cross-linkage effect of hydroxyapatite/collagen nanocomposites on a self-organization phenomenon. J Mater Sci Mater Med. 2002;13:993–7. doi: 10.1023/A:1019825132610.
Iijima M, Moriwaki Y, Kuboki Y. Oriented growth of octacalcium phosphate on and inside the collagenous matrix in vitro. Connect Tissue Res. 1996;32:519–24. doi: 10.3109/03008209509017002.
Miyamoto Y, Ishikawa K, Takechi M, Toh T, Yuasa T, Nagayama M, et al. Basic properties of calcium phosphate cement containing atelocollagen in its liquid or powder phases. Biomaterials. 1998;19:707–15. doi: 10.1016/S0142-9612(97)00186-5.
Iijima M, Moriwaki Y, Kuboki Y. In vitro crystal growth of octacalcium phosphate on type I collagen fiber. J Cryst Growth. 1994;137:553–60. doi: 10.1016/0022-0248(94)90998-9.
Iijima M, Iijima K, Moriwaki Y, Kuboki Y. Oriented growth of octacalcium phosphate crystals on type I collagen fibrils under physiological conditions. J Cryst Growth. 1994;140:91–9. doi: 10.1016/0022-0248(94)90501-0.
Lawson AC, Czernuszka JT. Collagen--calcium phosphate composites. Proc Inst Mech Eng H. 1998;212:413–25. doi: 10.1243/0954411981534187.
Kamakura S, Sasaki K, Honda Y, Anada T, Suzuki O. Octacalcium phosphate combined with collagen orthotopically enhances bone regeneration. J Biomed Mater Res B Appl Biomater. 2006;79:210–7. doi: 10.1002/jbm.b.30531.
Kawai T, Anada T, Honda Y, Kamakura S, Matsui K, Matsui A, et al. Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Eng Part A. 2009;15:23–32. doi: 10.1089/ten.tea.2008.0141.
Masuda T, Kawai T, Anada T, Kamakura S, Suzuki O. Quality of regenerated bone enhanced by implantation of octacalcium phosphate-collagen composite. Tissue Eng Part C Methods. 2010;16:471–8. doi: 10.1089/ten.tec.2009.0212.
Rhee SH, Tanaka J. Hydroxyapatite coating on a collagen membrane by a biomimetic method. J Am Ceram Soc. 1998;81:3029–31. doi: 10.1111/j.1151-2916.1998.tb02734.x.
Itoh S, Kikuchi M, Takakuda K, Nagaoka K, Koyama Y, Tanaka J, et al. Implantation study of a novel hydroxyapatite/collagen (HAp/col) composite into weight-bearing sites of dogs. J Biomed Mater Res. 2002;63:507–15. doi: 10.1002/jbm.10305.
Kikuchi M, Ikoma T, Itoh S, Matsumoto HN, Koyama Y, Takakuda K, et al. Biomimetic synthesis of bone-like nanocomposites using the self-organization mechanism of hydroxyapatite and collagen. Compos Sci Technol. 2004;64:819–25. doi: 10.1016/j.compscitech.2003.09.002.
Yang XB, Bhatnagar RS, Li S, Oreffo RO. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10:1148–59. doi: 10.1089/ten.2004.10.1148.
Doi Y, Horiguchi T, Moriwaki Y, Kitago H, Kajimoto T, Iwayama Y. Formation of apatite-collagen complexes. J Biomed Mater Res. 1996;31:43–9. doi: 10.1002/(SICI)1097-4636(199605)31:1<43::AID-JBM6>3.0.CO;2-Q.
Bradt JH, Mertig M, Teresiak A, Pompe W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem Mater. 1999;11:2694–701. doi: 10.1021/cm991002p.
Scharnweber D, Born R, Flade K, Roessler S, Stoelzel M, Worch H. Mineralization behaviour of collagen type I immobilized on different substrates. Biomaterials. 2004;25:2371–80. doi: 10.1016/j.biomaterials.2003.09.025.
Yunoki S, Ikoma T, Monkawa A, Ohta K, Tanaka J. Preparation and characterization of hydroxyapatite/collagen nanocomposite gel. J Nanosci Nanotechnol. 2007;7:818–21. doi: 10.1166/jnn.2007.504.
Li X, Chang J. Preparation of bone-like apatite-collagen nanocomposites by a biomimetic process with phosphorylated collagen. J Biomed Mater Res A. 2008;85:293–300. doi: 10.1002/jbm.a.31397.
Sun T, Wang M. Electrochemical deposition of apatite/collagen composite coating on NiTi shape memory alloy and coating properties. Mater Res Soc Symp Proc 2010; 1239:141-6.
Zhao H, Huang C, Jin H, Cai J. A novel route for collagen/hydroxyapatite preparation by enzymatic decomposition of urea. J Compos Mater. 2010;44:2127–33. doi: 10.1177/0021998310364264.
Ficai A, Andronescu E, Voicu G, Ghitulica C, Vasile BS, Ficai D, et al. Self-assembled collagen/hydroxyapatite composite materials. Chem Eng J. 2010;160:794–800. doi: 10.1016/j.cej.2010.03.088.
Jee SS, Thula TT, Gower LB. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: influence of polymer molecular weight. Acta Biomater. 2010;6:3676–86. doi: 10.1016/j.actbio.2010.03.036.
Andronescu E, Ficai M, Voicu G, Ficai D, Maganu M, Ficai A. Synthesis and characterization of collagen/hydroxyapatite: magnetite composite material for bone cancer treatment. J Mater Sci Mater Med. 2010;21:2237–42. doi: 10.1007/s10856-010-4076-7.
Ficai M, Andronescu E, Ficai D, Voicu G, Ficai A. Synthesis and characterization of COLL-PVA/HA hybrid materials with stratified morphology. Colloids Surf B Biointerfaces. 2010;81:614–9. doi: 10.1016/j.colsurfb.2010.08.009.
Tamimi F, Kumarasami B, Doillon C, Gbureck U, Le Nihouannen D, Cabarcos EL, et al. Brushite-collagen composites for bone regeneration. Acta Biomater. 2008;4:1315–21. doi: 10.1016/j.actbio.2008.04.003.
Mai R, Reinstorf A, Pilling E, Hlawitschka M, Jung R, Gelinsky M, et al. Histologic study of incorporation and resorption of a bone cement-collagen composite: an in vivo study in the minipig. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e9–14. doi: 10.1016/j.tripleo.2007.09.016.
Moreau JL, Weir MD, Xu HHK. Self-setting collagen-calcium phosphate bone cement: mechanical and cellular properties. J Biomed Mater Res A. 2009;91:605–13. doi: 10.1002/jbm.a.32248.
Liu X, Wang XM, Chen Z, Cui FZ, Liu HY, Mao K, et al. Injectable bone cement based on mineralized collagen. J Biomed Mater Res B Appl Biomater. 2010;94:72–9. doi: 10.1002/jbm.b.31625.
Otsuka M, Nakagawa H, Ito A, Higuchi WI. Effect of geometrical structure on drug release rate of a three-dimensionally perforated porous apatite/collagen composite cement. J Pharm Sci. 2010;99:286–92. doi: 10.1002/jps.21835.
Young SW, Andrews WA, Muller H, Constantz B. Induction of fracture healing using fibrous calcium phosphate composite spherulites. Invest Radiol. 1991;26:470–3. doi: 10.1097/00004424-199105000-00016.
Rovira A, Amedee J, Bareille R, Rabaud M. Colonization of a calcium phosphate/elastin-solubilized peptide-collagen composite material by human osteoblasts. Biomaterials. 1996;17:1535–40. doi: 10.1016/0142-9612(96)89779-1.
Kazim M, Katowitz JA, Fallon M, Piest KL. Evaluation of a collagen/hydroxylapatite implant for orbital reconstructive surgery. Ophthal Plast Reconstr Surg. 1992;8:94–108. doi: 10.1097/00002341-199206000-00003.
Hirota K, Nishihara K, Tanaka H. Pressure sintering of apatite-collagen composite. Biomed Mater Eng. 1993;3:147–51.
Zahn D, Hochrein O, Kawska A, Brickmann J, Kniep R. Towards an atomistic understanding of apatite-collagen biomaterials: linking molecular simulation studies of complex, crystal- and composite-formation to experimental findings. J Mater Sci. 2007;42:8966–73. doi: 10.1007/s10853-007-1586-x.
Silva CC, Pinheiro AG, Figueiro SD, Goes JC, Sasaki JM, Miranda MAR, et al. Piezoelectric properties of collagen-nanocrystalline hydroxyapatite composites. J Mater Sci. 2002;37:2061–70. doi: 10.1023/A:1015219800490.
Yunoki S, Ikoma T, Tsuchiya A, Monkawa A, Ohta K, Sotome S, et al. Fabrication and mechanical and tissue ingrowth properties of unidirectionally porous hydroxyapatite/collagen composite. J Biomed Mater Res B Appl Biomater. 2007;80:166–73. doi: 10.1002/jbm.b.30581.
Keeney M, Collin E, Pandit A. Multi-channelled collagen-calcium phosphate scaffolds: their physical properties and human cell response. Tissue Eng Part C Methods. 2009;15:265–73. doi: 10.1089/ten.tec.2008.0365.
Chapman MW, Bucholz R, Cornell C. Treatment of acute fractures with a collagen-calcium phosphate graft material. A randomized clinical trial. J Bone Joint Surg Am. 1997;79:495–502. doi: 10.2106/00004623-199704000-00004.
Rodrigues CVM, Serricella P, Linhares ABR, Guerdes RM, Borojevic R, Rossi MA, et al. Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials. 2003;24:4987–97. doi: 10.1016/S0142-9612(03)00410-1.
Lickorish D, Ramshaw JAM, Werkmeister JA, Glattauer V, Howlett CR. Development of a collagen-hydroxyapatite composite biomaterial via biomimetic process. J Biomed Mater Res A. 2004;68:19–27. doi: 10.1002/jbm.a.20031.
Sionkowska A, Kozłowska J. Characterization of collagen/hydroxyapatite composite sponges as a potential bone substitute. Int J Biol Macromol. 2010;47:483–7. doi: 10.1016/j.ijbiomac.2010.07.002.
Hsu FY, Chueh SC, Wang YJ. Microspheres of hydroxyapatite/reconstituted collagen as supports for osteoblast cell growth. Biomaterials. 1999;20:1931–6. doi: 10.1016/S0142-9612(99)00095-2.
Wu TJ, Huang HH, Lan CW, Lin CH, Hsu FY, Wang YJ. Studies on the microspheres comprised of reconstituted collagen and hydroxyapatite. Biomaterials. 2004;25:651–8. doi: 10.1016/S0142-9612(03)00576-3.
Liao SS, Watari F, Uo M, Ohkawa S, Tamura K, Wang W, et al. The preparation and characteristics of a carbonated hydroxyapatite/collagen composite at room temperature. J Biomed Mater Res B Appl Biomater. 2005;74:817–21. doi: 10.1002/jbm.b.30315.
Yokoyama A, Gelinsky M, Kawasaki T, Kohgo T, König U, Pompe W, et al. Biomimetic porous scaffolds with high elasticity made from mineralized collagen--an animal study. J Biomed Mater Res B Appl Biomater. 2005;75:464–72. doi: 10.1002/jbm.b.30331.
Zou C, Weng W, Deng XJ, Cheng K, Liu X, Du P, et al. Preparation and characterization of porous beta-tricalcium phosphate/collagen composites with an integrated structure. Biomaterials. 2005;26:5276–84. doi: 10.1016/j.biomaterials.2005.01.064.
Amaro Martins VC, Goissis G. Nonstoichiometric hydroxyapatite-anionic collagen composite as support for the double sustained release of gentamicin and norfloxacin/ciprofloxacin. Artif Organs. 2000;24:224–30. doi: 10.1046/j.1525-1594.2000.06517.x.
Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, et al. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006;27:3387–95. doi: 10.1016/j.biomaterials.2006.01.041.
Martins VC, Goissis G, Ribeiro AC, Marcantônio E, Jr., Bet MR. The controlled release of antibiotic by hydroxyapatite: anionic collagen composites. Artif Organs. 1998;22:215–21. doi: 10.1046/j.1525-1594.1998.06004.x.
Jayaraman M, Subramanian MV. Preparation and characterization of two new composites: collagen-brushite and collagen-octacalcium phosphate. Med Sci Monit. 2002;8:481–7.
Matsui K, Matsui A, Handa T, Kawai T, Suzuki O, Kamakura S, Echigo S. Bone regeneration by octacalcium phosphate collagen composites in a dog alveolar cleft model 2010; 39:1218-25.
Masuda T, Kawai T, Anada T, Kamakura S, Suzuki O. Quality of regenerated bone enhanced by implantation of octacalcium phosphate-collagen composite. Tissue Eng Part C Methods. 2010;16:471–8. doi: 10.1089/ten.tec.2009.0212.
Iibuchi S, Matsui K, Kawai T, Sasaki K, Suzuki O, Kamakura S, et al. Octacalcium phosphate (OCP) collagen composites enhance bone healing in a dog tooth extraction socket model. Int J Oral Maxillofac Surg. 2010;39:161–8. doi: 10.1016/j.ijom.2009.12.006.
Ikeda H, Yamaza T, Yoshinari M, Ohsaki Y, Ayukawa Y, Kido MA, et al. Ultrastructural and immunoelectron microscopic studies of the peri-implant epithelium-implant (Ti-6Al-4V) interface of rat maxilla. J Periodontol. 2000;71:961–73. doi: 10.1902/jop.2000.71.6.961.
Uchida M, Oyane A, Kim HM, Kokubo T, Ito A. Biomimetic coating of laminin-apatite composite on titanium metal and its excellent cell-adhesive properties. Adv Mater (Deerfield Beach Fla) 2004;16:1071–4. doi: 10.1002/adma.200400152.
Oyane A, Uchida M, Ito A. Laminin-apatite composite coating to enhance cell adhesion to ethylene-vinyl alcohol copolymer. J Biomed Mater Res A. 2005;72:168–74. doi: 10.1002/jbm.a.30205.
Oyane A, Uchida M, Onuma K, Ito A. Spontaneous growth of a laminin-apatite nano-composite in a metastable calcium phosphate solution. Biomaterials. 2006;27:167–75. doi: 10.1016/j.biomaterials.2005.06.001.
Oyane A, Tsurushima H, Ito A. Highly efficient gene transfer system using a laminin-DNA-apatite composite layer. J Gene Med. 2010;12:194–206. doi: 10.1002/jgm.1425.
Liu WB, Qu SX, Shen R, Jiang CX, Li XH, Feng B, et al. Influence of pH values on preparation of hydroxyapatite/gelatin composites. J Mater Sci. 2006;41:1851–3. doi: 10.1007/s10853-005-3184-0.
Yaylaoğlu MB, Korkusuz P, Ors U, Korkusuz F, Hasirci V. Development of a calcium phosphate-gelatin composite as a bone substitute and its use in drug release. Biomaterials. 1999;20:711–9. doi: 10.1016/S0142-9612(98)00199-9.
Kim HW, Song JH, Kim HE. Nanofiber generation of gelatin-hydroxyapatite biomimetics for guided tissue regeneration. Adv Funct Mater. 2005;15:1988–94. doi: 10.1002/adfm.200500116.
Sivakumar M, Panduranga Rao K. Preparation, characterization and in vitro release of gentamicin from coralline hydroxyapatite-gelatin composite microspheres. Biomaterials. 2002;23:3175–81. doi: 10.1016/S0142-9612(02)00066-2.
Kim HW, Knowles JC, Kim HE. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: characterization and antibiotic drug release. J Biomed Mater Res B Appl Biomater. 2005;74:686–98. doi: 10.1002/jbm.b.30236.
Yin YJ, Zhao F, Song XF, Yao KD, Lu WW, Leong JC. Preparation and characterization of hydroxyapatite/chitosan-gelatin network composite. J Appl Polym Sci. 2000;77:2929–38. doi: 10.1002/1097-4628(20000923)77:13<2929::AID-APP16>3.0.CO;2-Q.
Kim HW, Yoon BH, Kim HE. Microsphere of apatite-gelatin nanocomposite as bone regenerative filler. J Mater Sci Mater Med. 2005;16:1105–9. doi: 10.1007/s10856-005-4714-7.
Hillig WB, Choi Y, Murthy S, Natravali N, Ajayan P. An open-pored gelatin/hydroxyapatite composite as a potential bone substitute. J Mater Sci Mater Med. 2008;19:11–7. doi: 10.1007/s10856-007-0154-x.
Chang MC, Douglas WH, Tanaka J. Organic-inorganic interaction and the growth mechanism of hydroxyapatite crystals in gelatin matrices between 37 and 80 ° C. J Mater Sci Mater Med. 2006;17:387–96. doi: 10.1007/s10856-006-8243-9.
Chang MC, Douglas WH. Cross-linkage of hydroxyapatite/gelatin nanocomposite using imide-based zero-length cross-linker. J Mater Sci Mater Med. 2007;18:2045–51. doi: 10.1007/s10856-007-3152-0.
Teng S, Chen L, Guo Y, Shi J. Formation of nano-hydroxyapatite in gelatin droplets and the resulting porous composite microspheres. J Inorg Biochem. 2007;101:686–91. doi: 10.1016/j.jinorgbio.2006.11.018.
Liu X, Smith LA, Hu J, Ma PX. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009;30:2252–8. doi: 10.1016/j.biomaterials.2008.12.068.
Zhou J, Tang J, Quan-Li L, Zhang JX, Dou XC. Rabbit tibial defects repaired by nano-gelatin-apatite-minocycline bionic composite. J Clin Rehabil Tissue Eng Res. 2010;14:4563–7.
Zhao F, Grayson WL, Ma T, Bunnell B, Lu WW. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials. 2006;27:1859–67. doi: 10.1016/j.biomaterials.2005.09.031.
Lin HR, Yeh YJ. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J Biomed Mater Res B Appl Biomater. 2004;71:52–65. doi: 10.1002/jbm.b.30065.
Turco G, Marsich E, Bellomo F, Semeraro S, Donati I, Brun F, et al. Alginate/Hydroxyapatite biocomposite for bone ingrowth: a trabecular structure with high and isotropic connectivity. Biomacromolecules. 2009;10:1575–83. doi: 10.1021/bm900154b.
Gelinsky M, Eckert M, Despang F. Biphasic, but mololithic scaffolds for the therapy of osteochondral defects. Int J Mater Res formerly Metallk Z 2007; 98:749-55.
Yamaguchi I, Tokuchi K, Fukuzaki H, Koyama Y, Takakuda K, Monma H, et al. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J Biomed Mater Res. 2001;55:20–7. doi: 10.1002/1097-4636(200104)55:1<20::AID-JBM30>3.0.CO;2-F.
Zhao F, Yin YJ, Lu WW, Leong JC, Zhang WJ, Zhang JY, et al. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials. 2002;23:3227–34. doi: 10.1016/S0142-9612(02)00077-7.
Shen X, Tong H, Jiang T, Zhu Z, Wan P, Hu J. Homogeneous chitosan/carbonate apatite/citric acid nanocomposites prepared through a novel in situ precipitation method. Compos Sci Technol. 2007;67:2238–45. doi: 10.1016/j.compscitech.2007.01.034.
Murugan R, Ramakrishna S. Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials. 2004;25:3829–35. doi: 10.1016/j.biomaterials.2003.10.016.
Yoshida A, Miyazaki T, Ishida E, Ashizuka M. Preparation of bioactive chitosan-hydroxyapatite nanocomposites for bone repair through mechanochemical reaction. Mater Trans. 2004;45:994–8. doi: 10.2320/matertrans.45.994.
Zhang Y, Ni M, Zhang MQ, Ratner B. Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003;9:337–45. doi: 10.1089/107632703764664800.
Zhang Y, Zhang MQ. Microstructural and mechanical characterization of chitosan scaffolds reinforced by calcium phosphates. J Non-Cryst Solids. 2001;282:159–64. doi: 10.1016/S0022-3093(01)00345-3.
Zhang Y, Zhang MQ. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J Biomed Mater Res. 2002;62:378–86. doi: 10.1002/jbm.10312.
Tachaboonyakiat W, Ogomi D, Serizawa T, Akashi M. Evaluation of cell adhesion and proliferation on a novel tissue engineering scaffold containing chitosan and hydroxyapatite. J Bioact Compat Polym. 2006;21:579–89. doi: 10.1177/0883911506070441.
Sreedhar B, Aparna Y, Sairam M, Hebalkar N. Preparation and characterization of HAP/carboxymethyl chitosan nanocomposites. J Appl Polym Sci. 2007;105:928–34. doi: 10.1002/app.26140.
Rusu VM, Ng CH, Wilke M, Tiersch B, Fratzl P, Peter MG. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomaterials. 2005;26:5414–26. doi: 10.1016/j.biomaterials.2005.01.051.
Pinheiro AG, Pereira FFM, Santos MRP, Freire FNA, Góes JC, Sombra ASB. Chitosan-hydroxyapatite-BIT composite films: preparation and characterization. Polym Compos. 2007;28:582–7. doi: 10.1002/pc.20267.
Tang XJ, Gui L, Lü XY. Hard tissue compatibility of natural hydroxyapatite/chitosan composite. Biomed Mater. 2008;3:044115. doi: 10.1088/1748-6041/3/4/044115.
Zhang Y, Venugopal JR, El-Turki A, Ramakrishna S, Su B, Lim CT. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials. 2008;29:4314–22. doi: 10.1016/j.biomaterials.2008.07.038.
Madhumathi K, Shalumon KT, Rani VVD, Tamura H, Furuike T, Selvamurugan N, et al. Wet chemical synthesis of chitosan hydrogel-hydroxyapatite composite membranes for tissue engineering applications. Int J Biol Macromol. 2009;45:12–5. doi: 10.1016/j.ijbiomac.2009.03.011.
Cai X, Tong H, Shen X, Chen W, Yan J, Hu J. Preparation and characterization of homogeneous chitosan-polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009;5:2693–703. doi: 10.1016/j.actbio.2009.03.005.
Ge H, Zhao B, Lai Y, Hu X, Zhang D, Hu K. From crabshell to chitosan-hydroxyapatite composite material via a biomorphic mineralization synthesis method. J Mater Sci Mater Med. 2010;21:1781–7. doi: 10.1007/s10856-010-4045-1.
Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol. 2010;47:1–4. doi: 10.1016/j.ijbiomac.2010.03.015.
Wan ACA, Khor E, Hastings GW. Preparation of a chitin-apatite composite by in situ precipitation onto porous chitin scaffolds. J Biomed Mater Res. 1998;41:541–8. doi: 10.1002/(SICI)1097-4636(19980915)41:4<541::AID-JBM5>3.0.CO;2-C.
Wan ACA, Khor E, Hastings GW. Hydroxyapatite modified chitin as potential hard tissue substitute material. J Biomed Mater Res. 1997;38:235–41. doi: 10.1002/(SICI)1097-4636(199723)38:3<235::AID-JBM8>3.0.CO;2-Q.
Geçer A, Yldz N, Erol M, Çalml A. Synthesis of chitin calcium phosphate composite in different growth media. Polym Compos. 2008;29:84–91. doi: 10.1002/pc.20385.
Dong H, Ye JD, Wang XP, Yang JJ. Preparation of calcium phosphate cement tissue engineering scaffold reinforced with chitin fiber. J Inorg Mater. 2007;22:1007–10.
Wang X, Ma J, Feng Q, Cui FZ. Skeletal repair in rabbits with calcium phosphate cements incorporated phosphorylated chitin. Biomaterials. 2002;23:4591–600. doi: 10.1016/S0142-9612(02)00205-3.
Wang J, Sun QZ, Gao J, Liu DM, Meng XC, Li MQ. Preparation and properties on silk fibers reinforced hydroxyapatite/chitosan composites. Adv Mater Res 2010; 106:557-60; .
Zhang Y, Reddy VJ, Wong SY, Li X, Su B, Ramakrishna S, et al. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng Part A. 2010;16:1949–60. doi: 10.1089/ten.tea.2009.0221.
Kousalya GN, Gandhi RM, Sundaram SC, Meenakshi S. Synthesis of nano-hydroxyapatite chitin/chitosan hybrid biocomposites for the removal of Fe(III) Carbohydr Polym. 2010;82:594–9. doi: 10.1016/j.carbpol.2010.05.013.
Sairam Sundaram C, Viswanathan N, Meenakshi S. Uptake of fluoride by nano-hydroxyapatite/chitosan, a bioinorganic composite. Bioresour Technol. 2008;99:8226–30. doi: 10.1016/j.biortech.2008.03.012.
Sairam Sundaram C, Viswanathan N, Meenakshi S. Fluoride sorption by nano-hydroxyapatite/chitin composite. J Hazard Mater. 2009;172:147–51. doi: 10.1016/j.jhazmat.2009.06.152.
Wen HB, de Wijn JR, van Blitterswijk CA, de Groot K. Incorporation of bovine serum albumin in calcium phosphate coating on titanium. J Biomed Mater Res. 1999;46:245–52. doi: 10.1002/(SICI)1097-4636(199908)46:2<245::AID-JBM14>3.0.CO;2-A.
Liu TY, Chen SY, Liu DM, Liou SC. On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J Control Release. 2005;107:112–21. doi: 10.1016/j.jconrel.2005.05.025.
Liu Y, Hunziker EB, Randall NX, de Groot K, Layrolle P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials. 2003;24:65–70. doi: 10.1016/S0142-9612(02)00252-1.
Dorozhkin SV, Dorozhkina EI. The influence of bovine serum albumin on the crystallization of calcium phosphates from a revised simulated body fluid. Colloids Surf A Physicochem Eng Asp. 2003;215:191–9. doi: 10.1016/S0927-7757(02)00438-7.
Fu HH, Hu YH, McNelis T, Hollinger JO. A calcium phosphate-based gene delivery system. J Biomed Mater Res A. 2005;74:40–8. doi: 10.1002/jbm.a.30267.
Bisht S, Bhakta G, Mitra S, Maitra A. pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int J Pharm. 2005;288:157–68. doi: 10.1016/j.ijpharm.2004.07.035.
Kakizawa Y, Miyata K, Furukawa S, Kataoka K. Size-controlled formation of a calcium phosphate-based organic-inorganic hybrid vector for gene delivery using poly(ethylene glycol)-block-poly(aspartic acid) Adv Mater (Deerfield Beach Fla) 2004;16:699–702. doi: 10.1002/adma.200305782.
Singh R, Saxena A, Mozumdar S. Calcium phosphate—DNA nanocomposites: morphological studies and their bile duct infusion for liver-directed gene therapy. Int J Appl Ceram Technol. 2008;5:1–10. doi: 10.1111/j.1744-7402.2008.02183.x.
Sporysh I, Shynkaruk E, Lysko O, Shynkaruk A, Dubok V, Buzaneva E, et al. Biomimetic hydroxyapatite nanocrystals in composites with C60 and Au-DNA nanoparticles: IR-spectral study. Mater Sci Eng B. 2010;169:128–33. doi: 10.1016/j.mseb.2009.10.039.
Taguchi T, Kishida A, Akashi M. Hydroxyapatite formation on/in poly(vinyl alcohol) hydrogel matrices using a novel alternate soaking process. Chem Lett. 1998;8:711–2. doi: 10.1246/cl.1998.711.
Tachaboonyakiat W, Serizawa T, Akashi M. Hydroxyapatite formation on/in iodegradable chitosan hydrogels by an alternate soaking process. Polym J. 2001;33:177–81. doi: 10.1295/polymj.33.177.
Schnepp ZAC, Gonzalez-McQuire R, Mann S. Hybrid biocomposites based on calcium phosphate mineralization of self-assembled supramolecular hydrogels. Adv Mater (Deerfield Beach Fla) 2006;18:1869–72. doi: 10.1002/adma.200502545.
Patel M, Patel KJ, Caccamese JF, Coletti DP, Sauk JJ, Fisher JP. Characterization of cyclic acetal hydroxyapatite nanocomposites for craniofacial tissue engineering. J Biomed Mater Res A. 2010;94:408–18. doi: 10.1002/jbm.a.32683.
Bigi A, Boanini E, Gazzano M, Kojdecki MA, Rubini K. Microstructural investigation of hydroxyapatite-polyelectrolyte composites. J Mater Chem. 2004;14:274–9. doi: 10.1039/b308687a.
Bigi A, Boanini E, Gazzano M, Rubini K, Torricelli P. Nanocrystalline hydroxyapatite-polyaspartate composites. Biomed Mater Eng. 2004;14:573–9.
Boanini E, Fini M, Gazzano M, Bigi A. Hydroxyapatite nanocrystals modified with acidic amino acids. Eur J Inorg Chem. 2006:4821–6. doi: 10.1002/ejic.200600423.
Boanini E, Torricelli P, Gazzano M, Giardino R, Bigi A. Nanocomposites of hydroxyapatite with aspartic acid and glutamic acid and their interaction with osteoblast-like cells. Biomaterials. 2006;27:4428–33. doi: 10.1016/j.biomaterials.2006.04.019.
Ikawa N, Kimura T, Oumi Y, Sano T. Amino acid containing amorphous calcium phosphates and the rapid transformation into apatite. J Mater Chem. 2009;19:4906–13. doi: 10.1039/b815154g.
Sánchez-Salcedo S, Nieto A, Vallet-Regi M. Hydroxyapatite/β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem Eng J. 2005;137:62–71. doi: 10.1016/j.cej.2007.09.011.
Román J, Cabañas MV, Peña J, Doadrio JC, Vallet-Regí M. An optimized beta-tricalcium phosphate and agarose scaffold fabrication technique. J Biomed Mater Res A. 2008;84:99–107. doi: 10.1002/jbm.a.31394.
Alcaide M, Serrano MC, Pagani R, Sánchez-Salcedo S, Nieto A, Vallet-Regí M, et al. L929 fibroblast and Saos-2 osteoblast response to hydroxyapatite-betaTCP/agarose biomaterial. J Biomed Mater Res A. 2009;89:539–49. doi: 10.1002/jbm.a.31985.
Abiraman S, Varma HK, Umashankar PR, John A. Fibrin glue as an osteoinductive protein in a mouse model. Biomaterials. 2002;23:3023–31. doi: 10.1016/S0142-9612(02)00064-9.
Bagot D’Arc M, Daculsi G. Micro macroporous biphasic ceramics and fibrin sealant as a moldable material for bone reconstruction in chronic otitis media surgery. A 15 years experience. J Mater Sci Mater Med. 2003;14:229–33. doi: 10.1023/A:1022828606312.
Bonucci E, Marini E, Valdinucci F, Fortunato G. Osteogenic response to hydroxyapatite-fibrin implants in maxillofacial bone defects. Eur J Oral Sci. 1997;105:557–61. doi: 10.1111/j.1600-0722.1997.tb00217.x.
Fortunato G, Marini E, Valdinucci F, Bonucci E. Long-term results of hydroxyapatite-fibrin glue implantation in plastic and reconstructive craniofacial surgery. J Craniomaxillofac Surg. 1997;25:124–35. doi: 10.1016/S1010-5182(97)80003-0.
Jegoux F, Goyenvalle E, Bagot D’arc M, Aguado E, Daculsi G. In vivo biological performance of composites combining micro-macroporous biphasic calcium phosphate granules and fibrin sealant. Arch Orthop Trauma Surg. 2005;125:153–9. doi: 10.1007/s00402-004-0748-4.
Le Guehennec L, Goyenvalle E, Aguado E, Pilet P, Bagot D’Arc M, Bilban M, et al. MBCP biphasic calcium phosphate granules and tissucol fibrin sealant in rabbit femoral defects: the effect of fibrin on bone ingrowth. J Mater Sci Mater Med. 2005;16:29–35. doi: 10.1007/s10856-005-6443-3.
Wittkampf A. Fibrin sealant as sealant for hydroxyapatite granules. J Craniomaxillofac Surg. 1989;17:179–81. doi: 10.1016/S1010-5182(89)80019-8.
Le Nihouannen D, Guehennec LL, Rouillon T, Pilet P, Bilban M, Layrolle P, et al. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006;27:2716–22. doi: 10.1016/j.biomaterials.2005.11.038.
Le Nihouannen D, Saffarzadeh A, Aguado E, Goyenvalle E, Gauthier O, Moreau F, et al. Osteogenic properties of calcium phosphate ceramics and fibrin glue based composites. J Mater Sci Mater Med. 2007;18:225–35. doi: 10.1007/s10856-006-0684-7.
Le Guehennec L, Goyenvalle E, Aguado E, Pilet P, Spaethe R, Daculsi G. Influence of calcium chloride and aprotinin in the in vivo biological performance of a composite combining biphasic calcium phosphate granules and fibrin sealant. J Mater Sci Mater Med. 2007;18:1489–95. doi: 10.1007/s10856-006-0086-x.
Le Nihouannen D, Goyenvalle E, Aguado E, Pilet P, Bilban M, Daculsi G, et al. Hybrid composites of calcium phosphate granules, fibrin glue, and bone marrow for skeletal repair. J Biomed Mater Res A. 2007;81:399–408. doi: 10.1002/jbm.a.31058.
Le Nihouannen D, Saffarzadeh A, Gauthier O, Moreau F, Pilet P, Spaethe R, et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008;19:667–75. doi: 10.1007/s10856-007-3206-3.
Yoh R, Matsumoto T, Sasaki JI, Sohmura T. Biomimetic fabrication of fibrin/apatite composite material. J Biomed Mater Res A. 2008;87:222–8. doi: 10.1002/jbm.a.31777.
Cui G, Li J, Lei W, Bi L, Tang P, Liang Y, et al. The mechanical and biological properties of an injectable calcium phosphate cement-fibrin glue composite for bone regeneration. J Biomed Mater Res B Appl Biomater. 2010;92:377–85. doi: 10.1002/jbm.b.31525.
Boanini E, Torricelli P, Gazzano M, Giardino R, Bigi A. Alendronate-hydroxyapatite nanocomposites and their interaction with osteoclasts and osteoblast-like cells. Biomaterials. 2008;29:790–6. doi: 10.1016/j.biomaterials.2007.10.040.
Li L, Wei KM, Lin F, Kong XD, Yao JM. Effect of silicon on the formation of silk fibroin/calcium phosphate composite. J Mater Sci Mater Med. 2008;19:577–82. doi: 10.1007/s10856-007-3004-y.
Wang L, Nemoto R, Senna M. Effects of alkali pretreatment of silk fibroin on microstructure and properties of hydroxyapatite-silk fibroin nanocomposite. J Mater Sci Mater Med. 2004;15:261–5. doi: 10.1023/B:JMSM.0000015486.02633.ce.
Wang L, Nemoto R, Senna M. Microstructure and chemical states of hydroxyapatite/silk fibroin nanocomposites synthesized via a wet-mechanochemical route. J Nanopart Res. 2002;4:535–40. doi: 10.1023/A:1022841507932.
Wang L, Nemoto R, Senna M. Three-dimensional porous network structure developed in hydroxyapatite-based nanocomposites containing enzyme pretreated silk fibroin. J Nanopart Res. 2004;6:91–8. doi: 10.1023/B:NANO.0000023228.49670.86.
Nemoto R, Wang L, Ikoma T, Tanaka J, Senna M. Preferential alignment of hydroxyapatite crystallites in nanocomposites with chemically disintegrated silk fibroin. J Nanopart Res. 2004;6:259–65. doi: 10.1023/B:NANO.0000034656.19386.99.
Wang L, Li CZ, Senna M. High-affinity integration of hydroxyapatite nanoparticles with chemically modified silk fibroin. J Nanopart Res. 2007;9:919–29. doi: 10.1007/s11051-006-9167-5.
Fan C, Li J, Xu G, He H, Ye X, Chen Y, et al. Facile fabrication of nano-hydroxyapatite/silk fibroin composite via a simplified coprecipitation route. J Mater Sci. 2010;45:5814–9. doi: 10.1007/s10853-010-4656-4.
Wang L, Li CZ. Preparation and physicochemical properties of a novel hydroxyapatite/chitosan-silk fibroin composite. Carbohydr Polym. 2007;68:740–5. doi: 10.1016/j.carbpol.2006.08.010.
Sogo Y, Ito A, Matsuno T, Oyane A, Tamazawa G, Satoh T, et al. Fibronectin-calcium phosphate composite layer on hydroxyapatite to enhance adhesion, cell spread and osteogenic differentiation of human mesenchymal stem cells in vitro. Biomed Mater. 2007;2:116–23. doi: 10.1088/1748-6041/2/2/009.
Rhee SH, Suetsugu Y, Tanaka J. Biomimetic configurational arrays of hydroxyapatite nanocrystals on bio-organics. Biomaterials. 2001;22:2843–7. doi: 10.1016/S0142-9612(01)00028-X.
Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC. Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J Biol Chem. 2005;280:15362–9. doi: 10.1074/jbc.M413504200.
Jung JY, Hong YJ, Choi YS, Jeong S, Lee WK. A new method for the preparation of bioactive calcium phosphate films hybridized with 1alpha,25-dihydroxyvitamin D3. J Mater Sci Mater Med. 2009;20:2441–53. doi: 10.1007/s10856-009-3817-y.
Shchukin DG, Sukhorukov GB, Möhwald H. Biomimetic fabrication of nanoengineered hydroxyapatite/polyelectrolyte composite shell. Chem Mater. 2003;15:3947–50. doi: 10.1021/cm0341585.
Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging. 2008;3:161–74. doi: 10.2147/cia.s2065.
Lizzul PF, Narurkar VA. The role of calcium hydroxylapatite (Radiesse) in nonsurgical aesthetic rejuvenation. J Drugs Dermatol. 2010;9:446–50.
Klesing J, Chernousova S, Kovtun A, Neumann S, Ruiz L, Gonzalez-Calbet JM, et al. An injectable paste of calcium phosphate nanorods, functionalized with nucleic acids, for cell transfection and gene silencing. J Mater Chem. 2010;20:6144–8. doi: 10.1039/c0jm01130d.
Thai VV, Lee BT. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J Mater Sci Mater Med. 2010;21:1867–74. doi: 10.1007/s10856-010-4058-9.
Low KL, Tan SH, Zein SHS, Roether JA, Mouriño V, Boccaccini AR. Calcium phosphate-based composites as injectable bone substitute materials. J Biomed Mater Res B Appl Biomater. 2010;94:273–86. doi: 10.1002/jbm.b.31619.
Weiss P, Gauthier O, Bouler JM, Grimandi G, Daculsi G. Injectable bone substitute using a hydrophilic polymer. Bone. 1999;25(Suppl):67S–70S. doi: 10.1016/S8756-3282(99)00146-5.
Daculsi G, Weiss P, Bouler JM, Gauthier O, Millot F, Aguado E. Biphasic calcium phosphate/hydrosoluble polymer composites: a new concept for bone and dental substitution biomaterials. Bone. 1999;25(Suppl):59S–61S. doi: 10.1016/S8756-3282(99)00135-0.
Turczyn R, Weiss P, Lapkowski M, Daculsi G. In situ self hardening bioactive composite for bone and dental surgery. J Biomater Sci Polym Ed. 2000;11:217–23. doi: 10.1163/156856200743661.
Bennett S, Connolly K, Lee DR, Jiang Y, Buck D, Hollinger JO, et al. Initial biocompatibility studies of a novel degradable polymeric bone substitute that hardens in situ. Bone. 1996;19:101–7. doi: 10.1016/S8756-3282(96)00130-5.
Liu X, Okada M, Maeda H, Fujii S, Furuzono T. Hydroxyapatite/biodegradable poly(L-lactide-co-ε-caprolactone) composite microparticles as injectable scaffolds by a Pickering emulsion route. Acta Biomater. 2011;7:821–8. doi: 10.1016/j.actbio.2010.08.023.
Daculsi G, Rohanizadeh R, Weiss P, Bouler JM. Crystal polymer interaction with new injectable bone substitute; SEM and Hr TEM study. J Biomed Mater Res. 2000;50:1–7. doi: 10.1002/(SICI)1097-4636(200004)50:1<1::AID-JBM1>3.0.CO;2-W.
Grimandi G, Weiss P, Millot F, Daculsi G. In vitro evaluation of a new injectable calcium phosphate material. J Biomed Mater Res. 1998;39:660–6. doi: 10.1002/(SICI)1097-4636(19980315)39:4<660::AID-JBM22>3.0.CO;2-9.
Weiss P, Lapkowski M, LeGeros RZ, Bouler JM, Jean A, Daculsi G. FTIR spectroscopic study of an organic/mineral composite for bone and dental substitute materials. J Mater Sci Mater Med. 1997;8:621–9. doi: 10.1023/A:1018519419539.
Weiss P, Bohic S, Lapkowski M, Daculsi G. Application of FT-IR microspectroscopy to the study of an injectable composite for bone and dental surgery. J Biomed Mater Res. 1998;41:167–70. doi: 10.1002/(SICI)1097-4636(199807)41:1<167::AID-JBM20>3.0.CO;2-J.
Schmitt M, Weiss P, Bourges X, Amador del Valle G, Daculsi G. Crystallization at the polymer/calcium-phosphate interface in a sterilized injectable bone substitute IBS. Biomaterials. 2002;23:2789–94. doi: 10.1016/S0142-9612(02)00015-7.
Gauthier O, Müller R, von Stechow D, Lamy B, Weiss P, Bouler JM, et al. In vivo bone regeneration with injectable calcium phosphate biomaterial: a three-dimensional micro-computed tomographic, biomechanical and SEM study. Biomaterials. 2005;26:5444–53. doi: 10.1016/j.biomaterials.2005.01.072.
Weiss P, Layrolle P, Clergeau LP, Enckel B, Pilet P, Amouriq Y, et al. The safety and efficacy of an injectable bone substitute in dental sockets demonstrated in a human clinical trial. Biomaterials. 2007;28:3295–305. doi: 10.1016/j.biomaterials.2007.04.006.
Fatimi A, Tassin JF, Axelos MAV, Weiss P. The stability mechanisms of an injectable calcium phosphate ceramic suspension. J Mater Sci Mater Med. 2010;21:1799–809. doi: 10.1007/s10856-010-4047-z.
Trojani C, Boukhechba F, Scimeca JC, Vandenbos F, Michiels JF, Daculsi G, et al. Ectopic bone formation using an injectable biphasic calcium phosphate/Si-HPMC hydrogel composite loaded with undifferentiated bone marrow stromal cells. Biomaterials. 2006;27:3256–64. doi: 10.1016/j.biomaterials.2006.01.057.
Zhang SM, Lü G. Clinical application of compound injectable bone substitutes in bone injury repair. J Clin Rehabil Tissue Eng Res. 2009;13:10117–20.
Daculsi G, Uzel PA, Bourgeois N, le François T, Rouvillain JL, Bourges X, et al. New injectable bone substitute using reversible thermosensitive hydrogel and BCP granules: in vivo rabbit experiments. Key Eng Mater 2009; 396-398:457-60; .
Iooss P, Le Ray AM, Grimandi G, Daculsi G, Merle C. A new injectable bone substitute combining poly(epsilon-caprolactone) microparticles with biphasic calcium phosphate granules. Biomaterials. 2001;22:2785–94. doi: 10.1016/S0142-9612(01)00022-9.
Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater. 2010;20:1–12. doi: 10.22203/ecm.v020a01.
Evis Z, Ergun C, Doremus RH. Hydroxylapatite-zirconia composites: thermal stability of phases and sinterability as related to the CaO-ZrO2 phase diagram. J Mater Sci. 2005;40:1127–34. doi: 10.1007/s10853-005-6928-y.
Rao RR, Kannan TS. Synthesis and sintering of hydroxyapatite-zirconia composites. Mater Sci Eng C. 2002;20:187–93. doi: 10.1016/S0928-4931(02)00031-0.
Mansur C, Pope M, Pascucci MR, Shivkumar S. Zirconia-calcium phosphate composites for bone replacement. Ceram Int. 1998;24:77–9. doi: 10.1016/S0272-8842(96)00078-8.
Kim HW, Kim HE, Salih V, Knowles JC. Dissolution control and cellular responses of calcium phosphate coatings on zirconia porous scaffold. J Biomed Mater Res A. 2004;68:522–30. doi: 10.1002/jbm.a.20094.
Milella E, Cosentino F, Licciulli A, Massaro C. Preparation and characterisation of titania/hydroxyapatite composite coatings obtained by sol-gel process. Biomaterials. 2001;22:1425–31. doi: 10.1016/S0142-9612(00)00300-8.
Goller G, Demirkiran H, Oktar FN, Demirkesen E. Processing and characterization of Bioglass reinforced hydroxyapatite composites. Ceram Int. 2003;29:721–4. doi: 10.1016/S0272-8842(02)00223-7.
Tancred DC, Carr AJ, McCormack BA. The sintering and mechanical behavior of hydroxyapatite with bioglass additions. J Mater Sci Mater Med. 2001;12:81–93. doi: 10.1023/A:1026773522934.
Lopes MA, Silva RF, Monteiro FJ, Santos JD. Microstructural dependence of Young’s and shear moduli of P2O5 glass reinforced hydroxyapatite for biomedical applications. Biomaterials. 2000;21:749–54. doi: 10.1016/S0142-9612(99)00248-3.
Juang HY, Hon MH. Fabrication and mechanical properties of hydroxyapatite-alumina composites. Mater Sci Eng C. 1994;2:77–81. doi: 10.1016/0928-4931(94)90033-7.
Li J, Forberg S, Hermansson L. Evaluation of the mechanical properties of hot isostatically pressed titania and titania-calcium phosphate composites. Biomaterials. 1991;12:438–40. doi: 10.1016/0142-9612(91)90015-3.
Noma T, Shoji N, Wada S, Suzuki T. Preparation of spherical Al2O3 particle dispersed hydroxyapatite ceramics. J Ceram Soc Jpn. 1993;101:923–7. doi: 10.2109/jcersj.101.923.
Gautier S, Champion E, Bernache-Assollant D. Toughening characterization in alumina platelet-hydroxyapatite matrix composites. J Mater Sci Mater Med. 1999;10:533–40. doi: 10.1023/A:1008964230258.
Fang Y, Roy DM, Cheng J, Roy R, Agrawal DK. Microwave sintering of hydroxyapatite-based composites. Ceram Trans. 1993;36:397–407.
Park K, Vasilosa T. Microstructure and mechanical properties of silicon carbide whisker/calcium phosphate composites produced by hot pressing. Mater Lett. 1997;32:229–33. doi: 10.1016/S0167-577X(97)00041-4.
Jin HB, Oktar FN, Dorozhkin S, Agathopoulos S. Sintering behavior and properties of reinforced hydroxyapatite/TCP biphasic bioceramics with ZnO-whiskers. J Compos Mater. 2011;45:1435–45. doi: 10.1177/0021998310383728.
de With G, Corbijn AT. Metal fibre reinforced hydroxyapatite ceramics. J Mater Sci. 1989;24:3411–5. doi: 10.1007/BF01139073.
Ruys AJ, Simpson SA, Sorrell CC. Thixotropic casting of fibre-reinforced ceramic matrix composites. J Mater Sci Lett. 1994;13:1323–5. doi: 10.1007/BF00624484.
Miao X, Ruys AJ, Milthorpe BK. Hydroxyapatite-316L fibre composites prepared by vibration assisted slip casting. J Mater Sci. 2001;36:3323–32. doi: 10.1023/A:1017915226015.
Kim HM, Chae WP, Chang KW, Chun S, Kim S, Jeong Y, et al. Composite nanofiber mats consisting of hydroxyapatite and titania for biomedical applications. J Biomed Mater Res B Appl Biomater. 2010;94:380–7. doi: 10.1002/jbm.b.31664.
Hirakura S, Kobayashi T, Ono S, Oaki Y, Imai H. Fibrous nanocrystals of hydroxyapatite loaded with TiO(2) nanoparticles for the capture and photocatalytic decomposition of specific proteins. Colloids Surf B Biointerfaces. 2010;79:131–5. doi: 10.1016/j.colsurfb.2010.03.041.
Wu JM, Yeh TS. Sintering of hydroxylapatite-zirconia composite materials. J Mater Sci. 1988;23:3771–7. doi: 10.1007/BF00540526.
Li J, Liao H, Hermansson L. Sintering of partially-stabilized zirconia and partially-stabilized zirconia-hydroxyapatite composites by hot isostatic pressing and pressureless sintering. Biomaterials. 1996;17:1787–90. doi: 10.1016/0142-9612(95)00356-8.
Takagi M, Mochida M, Uchida N, Saito K, Uematsu K. Filter cake forming and hot isostatic pressing for TZP-dispersed hydroxyapatite composite. J Mater Sci Mater Med. 1992;3:199–203. doi: 10.1007/BF00713450.
Silva VV, Domingues RZ. Hydroxyapatite-zirconia composites prepared by precipitation method. J Mater Sci Mater Med. 1997;8:907–10. doi: 10.1023/A:1018566124507.
Silva VV, Lameiras FS, Domingues RZ. Synthesis and characterization of calcia partially stabilized zirconia-hydroxyapatite powders prtepared by coprecipitation method. Ceram Int. 2001;27:615–20. doi: 10.1016/S0272-8842(00)00122-X.
Rapacz-Kmita A, Slosarczyk A, Paszkiewicz Z, Paluch D. Evaluation of HAp-ZrO2 composites and monophase HAp bioceramics. In vitro study. J Mater Sci. 2004;39:5865–7. doi: 10.1023/B:JMSC.0000040104.64482.4a.
Rapacz-Kmita A, Slosarczyk A, Paszkiewicz Z. HAp ZrO2 composite coatings prepared by plasma spraying for biomedical applications. Ceram Int. 2005;31:567–71. doi: 10.1016/j.ceramint.2004.06.022.
Sung YM, Kim DH. Crystallization characteristics of yttria-stabilized zirconia/hydroxyapatite composite nanopowder. J Cryst Growth. 2003;254:411–7. doi: 10.1016/S0022-0248(03)01191-6.
Silva VV, Lameiras FS. Synthesis and characterization of composite powders of partially stabilized zirconia and hydroxyapatite. Mater Charact. 2000;45:51–9. doi: 10.1016/S1044-5803(00)00048-6.
Shen Z, Adolfsson E, Nygren M, Gao L, Kawaoka H, Niihara K. Dense hydroxyapatite-zirconia ceramic composites with high strength for biological applications. Adv Mater (Deerfield Beach Fla) 2001;13:214–6. doi: 10.1002/1521-4095(200102)13:3<214::AID-ADMA214>3.0.CO;2-5.
Adolfsson E, Alberiushenning P, Hermansson L. Phase-analysis and thermal-stability of hot isostatically pressed zirconia-hydroxyapatite composites. J Am Ceram Soc. 2000;83:2798–802. doi: 10.1111/j.1151-2916.2000.tb01634.x.
Kim HW, Noh YJ, Koh YH, Kim HE, Kim HM. Effect of CaF2 on densification and properties of hydroxyapatite-zirconia composites for biomedical applications. Biomaterials. 2002;23:4113–21. doi: 10.1016/S0142-9612(02)00150-3.
Li W, Gao L. Fabrication of HAp-ZrO2 (3Y) nano-composite by SPS. Biomaterials. 2003;24:937–40. doi: 10.1016/S0142-9612(02)00428-3.
Kim HW, Knowles JC, Li LH, Kim HE. Mechanical performance and osteoblast-like cell responses of fluorine-substituted hydroxyapatite and zirconia dense composite. J Biomed Mater Res A. 2005;72:258–68. doi: 10.1002/jbm.a.30219.
Xiao XF, Liu RF, Zheng YZ. Hydrothermal-electrochemical codeposited hydoxyapatite/yttria-stabilized zirconia composite coating. J Mater Sci. 2006;41:3417–24. doi: 10.1007/s10853-005-5340-y.
Kumar BR, Prakash KH, Cheang P, Khor KA. Microstructure and mechanical properties of spark plasma sintered zirconia-hydroxyapatite nano-composite powders. Acta Mater. 2005;53:2327–35. doi: 10.1016/j.actamat.2005.01.039.
Ahn ES, Gleason NJ, Nakahira A, Ying JY. Nanostructure processing of hydroxyapatite-based bioceramics. Nano Lett. 2001;1:149–53. doi: 10.1021/nl0055299.
Silva VV, Lameiras FS, Domingues RZ. Microstructural and mechanical study of zirconia-hydroxyapatite (ZH) composite ceramics for biomedical applications. Compos Sci Technol. 2001;61:301–10. doi: 10.1016/S0266-3538(00)00222-0.
Khor KA, Fu L, Lim JP, Cheang P. The effects of ZrO2 on the phase compositions of plasma sprayed HA/YSZ composite coatings. Mater Sci Eng A. 2001;316:160–6.
Fu L, Khor KA, Lim JP. Effects of yttria-stabilized zirconia on plasma-sprayed hydroxyapatite/yttria-stabilized zirconia composite coatings. J Am Ceram Soc. 2002;85:800–6. doi: 10.1111/j.1151-2916.2002.tb00175.x.
Chou BY, Chang E, Yao SY, Chen JM. Phase transformation during plasma spraying of hydroxyapatite-10-wt%-zirconia composite coating. J Am Ceram Soc. 2002;85:661–9. doi: 10.1111/j.1151-2916.2002.tb00147.x.
Wang Q, Ge S, Zhang D. Nano-mechanical properties and biotribological behaviors of nanosized HA/partially-stabilized zirconia composites. Wear. 2005;259:952–7. doi: 10.1016/j.wear.2005.02.064.
Murugan R, Ramakrishna S. Effect of zirconia on the formation of calcium phosphate bioceramics under microwave irradiation. Mater Lett. 2003;58:230–4. doi: 10.1016/S0167-577X(03)00451-8.
Fu L, Khor KA, Lim JP. Processing, microstructure and mechanical properties of yttria stabilized zirconia reinforced hydroxyapatite coatings. Mater Sci Eng A. 2000;276:46–51.
Nagarajan VS, Rao KJ. Structural, mechanical and biocompatibility studies of hydroxyapatite-derived composites toughened by zirconia addition. J Mater Chem. 1993;3:43–51. doi: 10.1039/jm9930300043.
Tamari N, Kondo I, Mouri M, Kinoshita M. Effect of calcium fluoride addition on densification and mechanical properties of hydroxyapatite-zirconia composite ceramics. J Ceram Soc Jpn. 1988;96:1200–2. doi: 10.2109/jcersj.96.1200.
Evis Z, Doremus RH. Hot-pressed hydroxylapatite/monoclinic zirconia composites with improved mechanical properties. J Mater Sci. 2007;42:2426–31. doi: 10.1007/s10853-006-1299-6.
Evis Z, Doremus RH. Effect of MgF2 on hot pressed hydroxylapatite and monoclinic zirconia composites. J Mater Sci. 2007;42:3739–44. doi: 10.1007/s10853-006-0485-x.
Ahn ES, Gleason NJ, Ying JY. The effect of zirconia reinforcing agents on the microstructure and mechanical properties of hydroxyapatite-based nanocomposites. J Am Ceram Soc. 2005;88:3374–9. doi: 10.1111/j.1551-2916.2005.00636.x.
Erkmen ZE, Genç Y, Oktar FN. Microstructural and mechanical properties of hydroxyapatite-zirconia composites. J Am Ceram Soc. 2007;90:2885–92. doi: 10.1111/j.1551-2916.2007.01849.x.
Rapacz-Kmita A, Slosarczyk A, Paszkiewicz Z. Mechanical properties of HAp-ZrO2 composites. J Eur Ceram Soc. 2006;26:1481–8. doi: 10.1016/j.jeurceramsoc.2005.01.059.
Sung YM, Shin YK, Ryu JJ. Preparation of hydroxyapatite/zirconia bioceramic nanocomposites for orthopaedic and dental prosthesis applications. Nanotechnology. 2007;18:65602–8. doi: 10.1088/0957-4484/18/6/065602.
Quan R, Yang D, Wu X, Wang H, Miao X, Li W. In vitro and in vivo biocompatibility of graded hydroxyapatite-zirconia composite bioceramic. J Mater Sci Mater Med. 2008;19:183–7. doi: 10.1007/s10856-006-0025-x.
Khalil KA, Kim SW, Kim HY. Consolidation and mechanical properties of nanostructured hydroxyapatite-(ZrO2 + 3 mol% Y2O3) bioceramics by high-frequency induction heat sintering. Mater Sci Eng A. 2007;456:368–72. doi: 10.1016/j.msea.2006.12.005.
Kong YM, Kim S, Kim HE, Lee IS. Reinforcement of hydroxyapatite bioceramic by addition of ZrO2 coated with Al2O3. J Am Ceram Soc. 1999;82:2963–8. doi: 10.1111/j.1151-2916.1999.tb02189.x.
Wang LL, Wang XF, Jiang HT, Yu CL. Preparation of porous hydroxyapatite-zirconia composite scaffolds by combination of gel-casting and polymer sponge methods. Adv Mater Res 2010; 106:616-9; .
Choi JW, Kong YM, Kim HE, Lee IS. Reinforcement of hydroxyapatite bioceramic by addition of Ni3Al and Al2O3. J Am Ceram Soc. 1998;81:1743–8. doi: 10.1111/j.1151-2916.1998.tb02543.x.
Adolfsson E, Hermansson L. Phase stability aspects of various apatite-aluminium oxide composites. J Mater Sci. 2000;35:5719–23. doi: 10.1023/A:1004814726021.
Li J, Fartash B, Hermansson L. Hydroxyapatite-alumina composites and bone-bonding. Biomaterials. 1995;16:417–22. doi: 10.1016/0142-9612(95)98860-G.
Kim S, Kong YM, Lee IS, Kim HE. Effect of calcinations of starting powder on mechanical properties of hydroxyapatite-alumina bioceramic composite. J Mater Sci Mater Med. 2002;13:307–10. doi: 10.1023/A:1014019103240.
Chiba A, Kimura S, Raghukandan K, Morizono Y. Effect of alumina addition on hydroxyapatite biocomposites fabricated by underwater-shock compaction. Mater Sci Eng A. 2003;350:179–83. doi: 10.1016/S0921-5093(02)00718-9.
Pang YX, Bao X, Weng L. Preparation of tricalcium phosphate/alumina composite nanoparticles and self-reinforcing composites by simultaneous precipitation. J Mater Sci. 2004;39:6311–23. doi: 10.1023/B:JMSC.0000043601.46284.a0.
Jun YK, Kim WH, Kweon OK, Hong SH. The fabrication and biochemical evaluation of alumina reinforced calcium phosphate porous implants. Biomaterials. 2003;24:3731–9. doi: 10.1016/S0142-9612(03)00248-5.
Epure LM, Dimitrievska S, Merhi Y, Yahia LH. The effect of varying Al2O3 percentage in hydroxyapatite/Al2O3 composite materials: morphological, chemical and cytotoxic evaluation. J Biomed Mater Res A. 2007;83:1009–23. doi: 10.1002/jbm.a.31377.
Evis Z, Doremus RH. A study of phase stability and mechanical properties of hydroxylapatite-nanosize α -alumina composites. Mater Sci Eng C. 2007;27:421–5. doi: 10.1016/j.msec.2006.05.028.
Viswanath B, Ravishankar N. Interfacial reactions in hydroxyapatite/alumina nanocomposites. Scr Mater. 2006;55:863–6. doi: 10.1016/j.scriptamat.2006.07.049.
Lu YP, Li MS, Li ST, Wang ZG, Zhu RF. Plasma-sprayed hydroxyapatite+titania composite bond coat for hydroxyapatite coating on titanium substrate. Biomaterials. 2004;25:4393–403. doi: 10.1016/j.biomaterials.2003.10.092.
Li H, Khor KA, Cheang P. Impact formation and microstructure characterization of thermal sprayed hydroxyapatite/titania composite coatings. Biomaterials. 2003;24:949–57. doi: 10.1016/S0142-9612(02)00431-3.
Zheng XB, Ding CX. Characterization of plasma-sprayed hydroxyapatite/TiO2 composite coatings. J Therm Spray Technol. 2000;9:520–5.
Lee SH, Kim HW, Lee EJ, Li LH, Kim HE. Hydroxyapatite-TiO2 hybrid coating on Ti implants. J Biomater Appl. 2006;20:195–208. doi: 10.1177/0885328206050518.
Lin CM, Yen SK. Characterization and bond strength of electrolytic HA/TiO2 double layers for orthopedic applications. J Mater Sci Mater Med. 2004;15:1237–46. doi: 10.1007/s10856-004-5678-8.
Balamurugan A, Balossier G, Kannan S, Michel J, Rajeswari S. In vitro biological, chemical and electrochemical evaluation of titania reinforced hydroxyapatite sol-gel coatings on surgical grade 316L SS. Mater Sci Eng C. 2007;27:162–71. doi: 10.1016/j.msec.2006.04.006.
Gaona M, Limab RS, Marple BR. Nanostructured titania/hydroxyapatite composite coatings deposited by high velocity oxy-fuel (HVOF) spraying. Mater Sci Eng A. 2007;458:141–9. doi: 10.1016/j.msea.2006.12.090.
Boyd AR, Duffy H, McCann R, Meenan BJ. Sputter deposition of calcium phosphate/titanium dioxide hybrid thin films. Mater Sci Eng C. 2008;28:228–36. doi: 10.1016/j.msec.2006.12.004.
Fidancevska E, Ruseska G, Bossert J, Linc YM, Boccaccini AR. Fabrication and characterization of porous bioceramic composites based on hydroxyapatite and titania. Mater Chem Phys. 2007;103:95–100. doi: 10.1016/j.matchemphys.2007.01.015.
Harle J, Kim HW, Mordan N, Knowles JC, Salih V. Initial responses of human osteoblasts to sol-gel modified titanium with hydroxyapatite and titania composition. Acta Biomater. 2006;2:547–56. doi: 10.1016/j.actbio.2006.05.005.
Kim HW, Kim HE, Salih V, Knowles JC. Hydroxyapatite and titania sol-gel composite coatings on titanium for hard tissue implants; mechanical and in vitro biological performance. J Biomed Mater Res B Appl Biomater. 2005;72:1–8. doi: 10.1002/jbm.b.30073.
Pushpakanth S, Srinivasan B, Sreedhar B, Sastry TP. An in situ approach to prepare nanorods of titania-hydroxyapatite (TiO2-HAp) nanocomposite by microwave hydrothermal technique. Mater Chem Phys. 2008;107:492–8. doi: 10.1016/j.matchemphys.2007.08.029.
Sato M, Aslani A, Sambito MA, Kalkhoran NM, Slamovich EB, Webster TJ. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J Biomed Mater Res A. 2008;84:265–72. doi: 10.1002/jbm.a.31469.
Ramires PA, Romito A, Cosentino F, Milella E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials. 2001;22:1467–74. doi: 10.1016/S0142-9612(00)00269-6.
Que W, Khor KA, Xu JL, Yu LG. Hydroxyapatite/titania nanocomposites derived by combining high-energy ball milling with spark plasma sintering processes. J Eur Ceram Soc. 2008;28:3083–90. doi: 10.1016/j.jeurceramsoc.2008.05.016.
Nath S, Tripathi R, Basu B. Understanding phase stability, microstructure development and biocompatibility in calcium phosphate-titania composites, synthesized from hydroxyapatite and titanium powder mix. Mater Sci Eng C. 2009;29:97–107. doi: 10.1016/j.msec.2008.05.019.
Lee JH, Kim HE, Koh YH. Highly porous titanium (Ti) scaffolds with bioactive microporous hydroxyapatite/TiO2 hybrid coating layer. Mater Lett. 2009;63:1995–8. doi: 10.1016/j.matlet.2009.06.023.
Ün S, Durucan C. Preparation of hydroxyapatite-titania hybrid coatings on titanium alloy. J Biomed Mater Res B Appl Biomater. 2009;90:574–83. doi: 10.1002/jbm.b.31319.
Nathanael AJ, Mangalaraj D, Ponpandian N. Controlled growth and investigations on the morphology and mechanical properties of hydroxyapatite/titania nanocomposite thin films. Compos Sci Technol. 2010;70:1645–51. doi: 10.1016/j.compscitech.2010.06.010.
Ebrahimi-Kahrizsangi R, Nasiri-Tabrizi B, Chami A. Synthesis and characterization of fluorapatite-titania (FAp-TiO2) nanocomposite via mechanochemical process. Solid State Sci. 2010;12:1645–51. doi: 10.1016/j.solidstatesciences.2010.07.017.
Sun R, Li M, Lu Y, An X. Effect of titanium and titania on chemical characteristics of hydroxyapatite plasma-sprayed into water. Mater Sci Eng C. 2006;26:28–33. doi: 10.1016/j.msec.2005.05.003.
Lee BT, Lee CW, Gain AK, Song HY. Microstructures and material properties of fibrous Ap/Al2O3-ZrO2 composites fabricated by multi-pass extrusion process. J Eur Ceram Soc. 2007;27:157–63. doi: 10.1016/j.jeurceramsoc.2006.02.038.
Kong YM, Bae CJ, Lee SH, Kim HW, Kim HE. Improvement in biocompatibility of ZrO2-Al2O3 nano-composite by addition of HA. Biomaterials. 2005;26:509–17. doi: 10.1016/j.biomaterials.2004.02.061.
Oktar FN, Agathopoulos S, Ozyegin LS, Gunduz O, Demirkol N, Bozkurt Y, et al. Mechanical properties of bovine hydroxyapatite (BHA) composites doped with SiO2, MgO, Al2O3, and ZrO2. J Mater Sci Mater Med. 2007;18:2137–43. doi: 10.1007/s10856-007-3200-9.
Gunduz O, Erkan EM, Daglilar S, Salman S, Agathopoulos S, Oktar FN. Composites of bovine hydroxyapatite (BHA) and ZnO. J Mater Sci. 2008;43:2536–40. doi: 10.1007/s10853-008-2497-1.
Ajeesh M, Francis BF, Annie J, Harikrishna Varma PR. Nano iron oxide-hydroxyapatite composite ceramics with enhanced radiopacity. J Mater Sci Mater Med. 2010;21:1427–34. doi: 10.1007/s10856-010-4005-9.
Yang ZP, Gong XY, Zhang CJ. Recyclable Fe3O4/hydroxyapatite composite nanoparticles for photocatalytic applications. Chem Eng J. 2010;165:117–21. doi: 10.1016/j.cej.2010.09.001.
Liu Y, Zhong H, Li L, Zhang C. Temperature dependence of magnetic property and photocatalytic activity of Fe3O4/hydroxyapatite nanoparticles. Mater Res Bull. 2010;45:2036–9. doi: 10.1016/j.materresbull.2010.09.010.
Li XW, Yasuda HY, Umakoshi Y. Bioactive ceramic composites sintered from hydroxyapatite and silica at 1,200 ° C: preparation, microstructures and in vitro bone-like layer growth. J Mater Sci Mater Med. 2006;17:573–81. doi: 10.1007/s10856-006-8942-2.
Ragel CV, Vallet-Regí M, Rodríguez-Lorenzo LM. Preparation and in vitro bioactivity of hydroxyapatite/solgel glass biphasic material. Biomaterials. 2002;23:1865–72. doi: 10.1016/S0142-9612(01)00313-1.
Padilla S, Sánchez-Salcedo S, Vallet-Regí M. Bioactive and biocompatible pieces of HA/sol-gel glass mixtures obtained by the gel-casting method. J Biomed Mater Res A. 2005;75:63–72. doi: 10.1002/jbm.a.30405.
Lopes MA, Monteiro FJ, Santos JD. Glass-reinforced hydroxyapatite composites: fracture toughness and hardness dependence on microstructural characteristics. Biomaterials. 1999;20:2085–90. doi: 10.1016/S0142-9612(99)00112-X.
Fu Q, Zhou N, Huang W, Wang D, Zhang L, Li H. Preparation and characterization of a novel bioactive bone cement: glass based nanoscale hydroxyapatite bone cement. J Mater Sci Mater Med. 2004;15:1333–8. doi: 10.1007/s10856-004-5742-4.
Fu Q, Zhou N, Huang W, Wang D, Zhang L, Li H. Effects of nano HAP on biological and structural properties of glass bone cement. J Biomed Mater Res A. 2005;74:156–63. doi: 10.1002/jbm.a.30322.
Oktar FN, Goller G. Sintering effects on mechanical properties of glass-reinforced hydroxyapatite composites. Ceram Int. 2002;28:617–21. doi: 10.1016/S0272-8842(02)00017-2.
Padilla S, Román J, Sánchez-Salcedo S, Vallet-Regí M. Hydroxyapatite/SiO(2)-CaO-P(2)O(5) glass materials: in vitro bioactivity and biocompatibility. Acta Biomater. 2006;2:331–42. doi: 10.1016/j.actbio.2006.01.006.
Sych EE, Pinchuk ND, Ivanchenko LA. Effect of sintering temperature on the properties of biogenic hydroxyapatite-glass composites. Powder Metallurgy Metal Ceram. 2010;49:153–8. doi: 10.1007/s11106-010-9215-7.
Liu X, Ei-Ghannam A. Effect of processing parameters on the microstructure and mechanical behavior of silica-calcium phosphate nanocomposite. J Mater Sci Mater Med. 2010;21:2087–94. doi: 10.1007/s10856-010-4062-0.
Kokubo T, Shigematsu M, Nagashima Y, Tashiro M, Nakamura T, Yamamuro T, et al. Apatite- and wollastonite-containing glass ceramics for prosthetic applications. Bull Inst Chem Res Kyoto Univ. 1982;60:260–8.
Kitsugi T, Yamamuro T, Nakamura T, Higashi S, Kakutani Y, Hyakuna K, et al. Bone bonding behavior of three kinds of apatite containing glass ceramics. J Biomed Mater Res. 1986;20:1295–307. doi: 10.1002/jbm.820200906.
Kokubo T, Ito S, Shigematsu M, Sakka S, Yamamuro T. Fatigue and life-time of bioactive glass-ceramic A-W containing apatite and wollastonite. J Mater Sci. 1987;22:4067–70. doi: 10.1007/BF01133359.
Kokubo T, Ito S, Shigematsu M, Sakka S, Yamamuro T. Mechanical properties of a new type of apatite-containing glass-ceramic for prosthetic application. J Mater Sci. 1985;20:2001–4. doi: 10.1007/BF01112282.
Kokubo T. Bioactive glass ceramics: properties and applications. Biomaterials. 1991;12:155–63. doi: 10.1016/0142-9612(91)90194-F.
Kokubo T, Ito S, Huang ZT, Hayashi T, Sakka S, Kitsugi T, et al. Ca,P-rich layer formed on high-strength bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:331–43. doi: 10.1002/jbm.820240306.
Nishio K, Neo M, Akiyama H, Okada Y, Kokubo T, Nakamura T. Effects of apatite and wollastonite containing glass-ceramic powder and two types of alumina powder in composites on osteoblastic differentiation of bone marrow cells. J Biomed Mater Res. 2001;55:164–76. doi: 10.1002/1097-4636(200105)55:2<164::AID-JBM1003>3.0.CO;2-1.
Zhang D, Chang J, Zeng Y. Fabrication of fibrous poly(butylene succinate)/wollastonite/apatite composite scaffolds by electrospinning and biomimetic process. J Mater Sci Mater Med. 2008;19:443–9. doi: 10.1007/s10856-006-0043-8.
Acharya NK, Mahajan CV, Kumar RJ, Varma HK, Menon VK. Can iliac crest reconstruction reduce donor site morbidity?: a study using degradable hydroxyapatite-bioactive glass ceramic composite. J Spinal Disord Tech. 2010;23:266–71. doi: 10.1097/BSD.0b013e3181a990fc.
Encinas-Romero MA, Aguayo-Salinas S, Valenzuela-García JL, Payán SR, Castillón-Barraza FF. Mechanical and bioactive behavior of hydroxyapatite-wollastonite sintered composites. Int J Appl Ceram Technol. 2010;7:164–77. doi: 10.1111/j.1744-7402.2009.02377.x.
Ha NR, Yang ZX, Hwang KH, Kim TS, Lee JK. Improvement of the stability of hydroxyapatite through glass ceramic reinforcement. J Nanosci Nanotechnol. 2010;10:3459–62. doi: 10.1166/jnn.2010.2342.
Nath S, Biswas K, Wang K, Bordia RK, Basu B. Sintering, phase stability and properties of calcium phosphate-mullite composites. J Am Ceram Soc. 2010;93:1639–49.
Nath S, Ummethala R, Basu B. Fretting wear behavior of calcium phosphate-mullite composites in dry and albumin-containing simulated body fluid conditions. J Mater Sci Mater Med. 2010;21:1151–61. doi: 10.1007/s10856-009-3983-y.
Chaki TK, Wang PE. Densification and strengthening of silver-reinforced hydroxyapatite-matrix composite prepared by sintering. J Mater Sci Mater Med. 1994;5:533–42. doi: 10.1007/BF00124886.
Zhang X, Gubbels GHM, Terpstra RA, Metselaar R. Toughening of calcium hydroxyapatite with silver particles. J Mater Sci. 1997;32:235–43. doi: 10.1023/A:1018568308926.
Chu C, Lin P, Dong Y, Xue X, Zhu J, Yin Z. Fabrication and characterization of hydroxyapatite reinforced with 20 vol % Ti particles for use as hard tissue replacement. J Mater Sci Mater Med. 2002;13:985–92. doi: 10.1023/A:1019873015772.
Shi W, Kamiya A, Zhu J, Watazu A. Properties of titanium biomaterial fabricated by sinter-bonding of titanium/hydroxyapatite composite surface-coated layer to pure bulk titanium. Mater Sci Eng A. 2002;337:104–9. doi: 10.1016/S0921-5093(02)00003-5.
Ning CQ, Zhou Y. In vitro bioactivity of a biocomposite fabricated from HA and Ti powders by powder metallurgy method. Biomaterials. 2002;23:2909–15. doi: 10.1016/S0142-9612(01)00419-7.
Karanjai M, Sundaresan R, Rao GVN, Mohan RTR, Kashyap BP. Development of titanium based biocomposite by powder metallurgy processing with in situ forming of Ca-P phases. Mater Sci Eng A. 2007;447:19–26. doi: 10.1016/j.msea.2006.10.154.
Karanjai M, Kumarb MBV, Sundaresan R, Basu B, Mohan RTR, Kashyap BP. Fretting wear study on Ti-Ca-P biocomposite in dry and simulated body fluid. Mater Sci Eng A. 2008;475:299–307. doi: 10.1016/j.msea.2007.05.020.
Ning CQ, Zhou Y. On the microstructure of biocomposites sintered from Ti, HA and bioactive glass. Biomaterials. 2004;25:3379–87. doi: 10.1016/j.biomaterials.2003.10.017.
Chu C, Xue X, Zhu J, Yin Z. Mechanical and biological properties of hydroxyapatite reinforced with 40 vol. % titanium particles for use as hard tissue replacement. J Mater Sci Mater Med. 2004;15:665–70. doi: 10.1023/B:JMSM.0000030207.16887.f2.
Smirnov VV, Egorov AA, Barinov SM, Shvorneva LI. Composite calcium phosphate bone cements reinforced by particulate titanium. Dokl Chem. 2007;413:82–5. doi: 10.1134/S0012500807040027.
Chu C, Xue X, Zhu J, Yin Z. Fabrication and characterization of titanium-matrix composite with 20 vol% hydroxyapatite for use as heavy load-bearing hard tissue replacement. J Mater Sci Mater Med. 2006;17:245–51. doi: 10.1007/s10856-006-7310-6.
Li J, Habibovic P, Yuan H, van den Doel M, Wilson CE, de Wijn JR, et al. Biological performance in goats of a porous titanium alloy-biphasic calcium phosphate composite. Biomaterials. 2007;28:4209–18. doi: 10.1016/j.biomaterials.2007.05.042.
Pattanayak DK, Mathur V, Rao BT, Mohan TRR. Synthesis and characterization of titanium—calcium phosphate composites for bio applications. Trends Biomater Artif Organs. 2003;17:8–12.
Ding Y, Liu J, Wang H, Shen G, Yu R. A piezoelectric immunosensor for the detection of alpha-fetoprotein using an interface of gold/hydroxyapatite hybrid nanomaterial. Biomaterials. 2007;28:2147–54. doi: 10.1016/j.biomaterials.2006.12.025.
Miranda M, Fernández A, Díaz M, Esteban-Tejeda L, López-Esteban S, Malpartida F, et al. Silver-hydroxyapatite nanocomposites as bactericidal and fungicidal materials. Int J Mater Res. 2010;101:122–7. doi: 10.3139/146.110256.
Younesi M, Bahrololoom ME. Optimizations of wear resistance and toughness of hydroxyapatite nickel free stainless steel new bio-composites for using in total joint replacement. Mater Des. 2010;31:234–43. doi: 10.1016/j.matdes.2009.06.037.
Murakoshi Y, Kikuchi K, Katoh M, Matsuzaki K. Fabrication and property of degradable magnesium-calcium alloy composites with hydroxyapatite. IFMBE Proc. 2010;31:1226–9. doi: 10.1007/978-3-642-14515-5_311.
Razavi M, Fathi MH, Meratian M. Fabrication and characterization of magnesium-fluorapatite nanocomposite for biomedical applications. Mater Charact. 2010;61:1363–70. doi: 10.1016/j.matchar.2010.09.008.
Damien CJ, Parsons JR, Benedict JJ, Weisman DS. Investigation of a hydroxyapatite and calcium sulfate composite supplemented with an osteoinductive factor. J Biomed Mater Res. 1990;24:639–54. doi: 10.1002/jbm.820240602.
Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, et al. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26:2677–84. doi: 10.1016/j.biomaterials.2004.06.045.
Urban RM, Turner TM, Hall DJ, Inoue N, Gitelis S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin Orthop Relat Res. 2007;459:110–7. doi: 10.1097/BLO.0b013e318059b902.
Rauschmann M, Vogl T, Verheyden A, Pflugmacher R, Werba T, Schmidt S, et al. Bioceramic vertebral augmentation with a calcium sulphate/hydroxyapatite composite (Cerament SpineSupport): in vertebral compression fractures due to osteoporosis. Eur Spine J. 2010;19:887–92. doi: 10.1007/s00586-010-1279-z.
Kumar GS, Girija EK, Thamizhavel A, Yokogawa Y, Kalkura SN. Synthesis and characterization of bioactive hydroxyapatite-calcite nanocomposite for biomedical applications. J Colloid Interface Sci. 2010;349:56–62. doi: 10.1016/j.jcis.2010.05.038.
Smirnov VV, Goldberg MA, Shvorneva LI, Fadeeva IV, Shibaeva TV, Barinov SM. Synthesis of composite biomaterials in the hydroxyapatite-calcite system. Dokl Chem. 2010;432:151–4. doi: 10.1134/S0012500810050095.
Gittings JP, Bowena CR, Turner IG, Baxter F, Chaudhuri J. Characterisation of ferroelectric-calcium phosphate composites and ceramics. J Eur Ceram Soc. 2007;27:4187–90. doi: 10.1016/j.jeurceramsoc.2007.02.120.
Watanabe Y, Ikoma T, Suetsugu Y, Yamada H, Tamura K, Komatsu Y, et al. The densification of zeolite/apatite composites using a pulse electric current sintering method: a long-term assurance material for the disposal of radioactive waste. J Eur Ceram Soc. 2006;26:481–6. doi: 10.1016/j.jeurceramsoc.2005.07.032.
Lahiri D, Singh V, Benaduce AP, Seal S, Kos L, Agarwal A. Boron nitride nanotube reinforced hydroxyapatite composite: mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J Mech Behav Biomed Mater. 2011;4:44–56. doi: 10.1016/j.jmbbm.2010.09.005.
Agathopoulos S, Tulyaganov DU, Marques PAAP, Ferro MC, Fernandes MHV, Correia RN. The fluorapatite-anorthite system in biomedicine. Biomaterials. 2003;24:1317–31. doi: 10.1016/S0142-9612(02)00468-4.
Khor KA, Gu YW, Pan D, Cheang P. Microstructure and mechanical properties of plasma sprayed HA/YSZ/Ti-6Al-4V composite coatings. Biomaterials. 2004;25:4009–17. doi: 10.1016/j.biomaterials.2003.10.089.
Gu YW, Khor KA, Pan D, Cheang P. Activity of plasma sprayed yttria stabilized zirconia reinforced hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid. Biomaterials. 2004;25:3177–85. doi: 10.1016/j.biomaterials.2003.09.101.
Kalmodia S, Goenka S, Laha T, Lahiri D, Basu B, Balani K. Microstructure, mechanical properties, and in vitro biocompatibility of spark plasma sintered hydroxyapatite-aluminum oxide-carbon nanotube composite. Mater Sci Eng C. 2010;30:1162–9. doi: 10.1016/j.msec.2010.06.009.
Best SM, Porter AE, Thian ES, Huang J. Bioceramics: past, present and for the future. J Eur Ceram Soc. 2008;28:1319–27. doi: 10.1016/j.jeurceramsoc.2007.12.001.
De Aza PN, Guitián F, De Aza S. Bioeutectic: a new ceramic material for human bone replacement. Biomaterials. 1997;18:1285–91. doi: 10.1016/S0142-9612(97)00063-X.
Huang X, Jiang D, Tan S. Apatite formation on the surface of wollastonite/tricalcium phosphate composite immersed in simulated body fluid. J Biomed Mater Res B Appl Biomater. 2004;69:70–2. doi: 10.1002/jbm.b.20025.
Zhang F, Chang J, Lin K, Lu J. Preparation, mechanical properties and in vitro degradability of wollastonite/tricalcium phosphate macroporous scaffolds from nanocomposite powders. J Mater Sci Mater Med. 2008;19:167–73. doi: 10.1007/s10856-006-0056-3.
Juhasz JA, Best SM, Kawashita M, Miyata N, Kokubo T, Nakamura T, et al. Bonding strength of the apatite layer formed on glass-ceramic apatite-wollastonite-polyethylene composites. J Biomed Mater Res A. 2003;67:952–9. doi: 10.1002/jbm.a.10131.
Juhasz JA, Best SM, Bonfield W, Kawashita M, Miyata N, Kokubo T, et al. Apatite-forming ability of glass-ceramic apatite-wollastonite - polyethylene composites: effect of filler content. J Mater Sci Mater Med. 2003;14:489–95. doi: 10.1023/A:1023499728588.
Juhasz JA, Best SM, Brooks R, Kawashita M, Miyata N, Kokubo T, et al. Mechanical properties of glass-ceramic A-W-polyethylene composites: effect of filler content and particle size. Biomaterials. 2004;25:949–55. doi: 10.1016/j.biomaterials.2003.07.005.
Rea SM, Brooks RA, Best SM, Kokubo T, Bonfield W. Proliferation and differentiation of osteoblast-like cells on apatite-wollastonite/polyethylene composites. Biomaterials. 2004;25:4503–12. doi: 10.1016/j.biomaterials.2003.11.016.
Greish YE, Brown PW. Characterization of wollastonite-reinforced HAp--Ca polycarboxylate composites. J Biomed Mater Res. 2001;55:618–28. doi: 10.1002/1097-4636(20010615)55:4<618::AID-JBM1056>3.0.CO;2-9. [Erratum in: J Biomed Mater Res 2001; 56:459]
Greish YE, Brown PW. Characterization of bioactive glass-reinforced HAP-polymer composites. J Biomed Mater Res. 2000;52:687–94. doi: 10.1002/1097-4636(20001215)52:4<687::AID-JBM13>3.0.CO;2-K.
Radev L, Hristov V, Samuneva B, Ivanova D. Organic/inorganic bioactive materials. Part II: in vitro bioactivity of collagen-calcium phosphate silicate/wollastonite hybrids. Cent Eur J Chem. 2009;7:711–20. doi: 10.2478/s11532-009-0076-1.
Radev L, Hristov V, Fernandes MHV, Salvado IMM. Organic/inorganic bioactive materials part IV: in vitro assessment of bioactivity of gelatin-calcium phosphate silicate/wollastonite hybrids. Cent Eur J Chem. 2010;8:278–84. doi: 10.2478/s11532-009-0142-8.
Kangasniemi I, de Groot K, Wolke J, Andersson O, Luklinska Z, Becht JGM, et al. The stability of hydroxyapatite in an optimized bioactive glass matrix at sintering temperatures. J Mater Sci Mater Med. 1991;2:133–7. doi: 10.1007/BF00692970.
Kangasniemi IMO, de Groot K, Becht JGM, Yli-Urpo A. Preparation of dense hydroxylapatite or rhenanite containing bioactive glass composites. J Biomed Mater Res. 1992;26:663–74. doi: 10.1002/jbm.820260508.
Kangasniemi IMO, Vedel E, de Blick-Hogerworst J, Yli-Urpo AU, de Groot K. Dissolution and scanning electron microscopic studies of Ca,P particle-containing bioactive glasses. J Biomed Mater Res. 1993;27:1225–33. doi: 10.1002/jbm.820271003.
Maruno S, Ban S, Wang YF, Iwata H, Itoh H. Properties of functionally gradient composite consisting of hydroxyapatite containing glass coated titanium and characters for bioactive implant. J Ceram Soc Jpn. 1992;100:362–7. doi: 10.2109/jcersj.100.362.
Kobayashi S, Kawai W. Development of carbon nanofiber reinforced hydroxyapatite with enhanced mechanical properties. Composites A. 2007;38:114–23. doi: 10.1016/j.compositesa.2006.01.006.
Balani K, Anderson R, Laha T, Andara M, Tercero J, Crumpler E, et al. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials. 2007;28:618–24. doi: 10.1016/j.biomaterials.2006.09.013.
Chen Y, Gan CH, Zhang TH, Yu G, Bai P, Kaplan A. Laser-surface-alloyed carbon nanotubes reinforced hydroxyapatite composite coatings. Appl Phys Lett. 2005;86:251905–8. doi: 10.1063/1.1951054.
Chen Y, Zhang TH, Gan CH, Yu G. Wear studies of hydroxyapatite composite coating reinforced by carbon nanotubes. Carbon. 2007;45:998–1004. doi: 10.1016/j.carbon.2006.12.021.
Chen Y, Zhang YQ, Zhang TH, Gan CH, Zheng CY, Yu G. Carbon nanotube reinforced hydroxyapatite composite coatings produced through laser surface alloying. Carbon. 2006;44:37–45. doi: 10.1016/j.carbon.2005.07.011.
Xu JL, Khor KA, Sui JJ, Chen WN. Preparation and characterization of a novel hydroxyapatite/carbon nanotubes composite and its interaction with osteoblast-like cells. Mater Sci Eng C. 2009;29:44–9. doi: 10.1016/j.msec.2008.05.009.
Lee HH, Shin US, Won JE, Kim HW. Preparation of hydroxyapatite-carbon nanotube composite nanopowders. Mater Lett. 2010;65:208–11. doi: 10.1016/j.matlet.2010.10.012.
White AA, Kinloch IA, Windle AH, Best SM. Optimization of the sintering atmosphere for high-density hydroxyapatite-carbon nanotube composites. J R Soc Interface. 2010;7:529–39. doi: 10.1098/rsif.2010.0117.focus.
Ding Y, Liu J, Jin X, Lu H, Shen G, Yu R. Poly-L-lysine/hydroxyapatite/carbon nanotube hybrid nanocomposite applied for piezoelectric immunoassay of carbohydrate antigen 19-9. Analyst. 2008;133:184–90. doi: 10.1039/b713824e.
Zhao HY, Xu XX, Zhang JX, Zheng W, Zheng YF. Carbon nanotube-hydroxyapatite-hemoglobin nanocomposites with high bioelectrocatalytic activity. Bioelectrochemistry. 2010;78:124–9. doi: 10.1016/j.bioelechem.2009.08.009.
Slosarcyk A, Klisch M, Blazewicz M, Piekarczyk J, Stobierski L, Rapacz-Kmita A. Hot pressed hydroxyapatite-carbon fibre composites. J Eur Ceram Soc. 2000;20:1397–402. doi: 10.1016/S0955-2219(00)00014-5.
Dorner-Reisel A, Berroth K, Neubauer R, Nestler K, Marx G, Scislo M, et al. Unreinforced and carbon fibre reinforced hydroxyapatite: resistance against microabrasion. J Eur Ceram Soc. 2004;24:2131–9. doi: 10.1016/S0955-2219(03)00373-X.
Fu T, Zhao JL, Wei JH, Han Y, Xu KW. Preparation of carbon fiber fabric reinforced hydroxyapatite/epoxy composite by RTM processing. J Mater Sci. 2004;39:1411–3. doi: 10.1023/B:JMSC.0000013906.60034.e8.
Pecheva E, Pramatarova L, Hikov T, Fingarova D, Tanaka Y, Sakamoto H, et al. Apatite-nanodiamond composite as a functional coating of stainless steel. Surf Interface Anal. 2010;42:475–80. doi: 10.1002/sia.3213.
Yoshimura M. Phase stability of zirconia. Am Ceram Soc Bull. 1988;67:1950–5.
Thompson I, Rawlings RD. Mechanical behaviour of zirconia and zirconia-toughened alumina in a simulated body environment. Biomaterials. 1990;11:505–8. doi: 10.1016/0142-9612(90)90066-Y.
Monma H. Tricalcium phosphate ceramics complexed with hydroxyapatite. J Ceram Soc Jpn. 1987;96:60–4.
Farzadi A, Solati-Hashjin M, Bakhshi F, Aminian A. Synthesis and characterization of hydroxyapatite/β-tricalcium phosphate nanocomposites using microwave irradiation. Ceram Int. 2011;37:65–71. doi: 10.1016/j.ceramint.2010.08.021.
Wu CC, Huang ST, Tseng TW, Rao QL, Lin HC. FT-IR and XRD investigations on sintered fluoridated hydroxyapatite composites. J Mol Struct. 2010;979:72–6. doi: 10.1016/j.molstruc.2010.06.003.
Suchanek W, Yashima M, Kakihana M, Yoshimura M. Processing and mechanical properties of hydroxyapatite reinforced with hydroxyapatite whiskers. Biomaterials. 1996;17:1715–23. doi: 10.1016/0142-9612(96)87652-6.
Suchanek W, Yashima M, Kakihana M, Yoshimura M. Hydroxyapatite/hydroxyapatite-whisker composites without sintering additives: mechanical properties and microstructural evolution. J Am Ceram Soc. 1997;80:2805–13. doi: 10.1111/j.1151-2916.1997.tb03197.x.
Kaito T, Mukai Y, Nishikawa M, Ando W, Yoshikawa H, Myoui A. Dual hydroxyapatite composite with porous and solid parts: experimental study using canine lumbar interbody fusion model. J Biomed Mater Res B Appl Biomater. 2006;78:378–84. doi: 10.1002/jbm.b.30498.
Ramay HR, Zhang M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials. 2004;25:5171–80. doi: 10.1016/j.biomaterials.2003.12.023.
Matsumoto K, Tsuru K, Kawachi G, Maruta M, Matsuya S, Takahashi I, et al. Reinforcement of carbonate apatite bone substitutes with carbonate apatite by Ca salt introduction. J Ceram Soc Jpn. 2010;118:521–4. doi: 10.2109/jcersj2.118.521.
Tampieri A, Celotti G, Sprio S, Delcogliano A, Franzese S. Porosity-graded hydroxyapatite ceramics to replace natural bone. Biomaterials. 2001;22:1365–70. doi: 10.1016/S0142-9612(00)00290-8.
Werner J, Linner-Krcmar B, Friess W, Greil P. Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials. 2002;23:4285–94. doi: 10.1016/S0142-9612(02)00191-6.
Hsu YH, Turner IG, Miles AW. Fabrication of porous bioceramics with porosity gradients similar to the bimodal structure of cortical and cancellous bone. J Mater Sci Mater Med. 2007;18:2251–6. doi: 10.1007/s10856-007-3126-2.
Macchetta A, Turner IG, Bowen CR. Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-based freeze-casting method. Acta Biomater. 2009;5:1319–27. doi: 10.1016/j.actbio.2008.11.009.
Watari F, Yokoyama A, Saso F, Uo M, Kawasaki T. Fabrication and properties of functionally graded dental implant. Composites B. 1997;28:5–11. doi: 10.1016/S1359-8368(96)00021-2.
Watari F, Yokoyama A, Omori M, Hirai T, Kondo H, Uo M, et al. Biocompatibility of materials and development to functionally graded implant for bio-medical application. Compos Sci Technol. 2004;64:893–908. doi: 10.1016/j.compscitech.2003.09.005.
Chu C, Zhu J, Yin Z, Wang S. Hydroxyapatite-Ti functionally graded biomaterial fabricated by powder metallurgy. Mater Sci Eng A. 1999;271:95–100. doi: 10.1016/S0921-5093(99)00152-5.
Chu C, Zhu J, Yin Z, Lin P. Structure optimization and properties of hydroxyapatite-Ti symmetrical functionally graded biomaterial. Mater Sci Eng A. 2001;316:205–10. doi: 10.1016/S0921-5093(01)01239-4.
Chu C, Zhu J, Yin Z, Lin P. Optimal design and fabrication of hydroxyapatite-Ti asymmetrical functionally graded biomaterial. Mater Sci Eng A. 2003;348:244–50. doi: 10.1016/S0921-5093(02)00738-4.
Inagaki M, Yokogawa Y, Kameyama T. Effects of plasma gas composition on bond strength of hydroxyapatite/titanium composite coatings prepared by rf-plasma spraying. J Eur Ceram Soc. 2006;26:495–9. doi: 10.1016/j.jeurceramsoc.2005.07.030.
Nonami T, Kamiya A, Naganuma K, Kameyana T. Implantation of hydroxyapatite granules into superplastic titanium alloy for biomaterials. Mater Sci Eng C. 1998;6:281–4. doi: 10.1016/S0928-4931(98)00063-0.
Bai X, More K, Rouleau CM, Rabiei A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010;6:2264–73. doi: 10.1016/j.actbio.2009.12.002.
Liu C, Han Z, Czernuszka JT. Gradient collagen/nanohydroxyapatite composite scaffold: development and characterization. Acta Biomater. 2009;5:661–9. doi: 10.1016/j.actbio.2008.09.022.
Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and beta-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065–73. doi: 10.1016/j.biomaterials.2008.06.022.
Nindhia TGT, Koyoshi Y, Kaneko A, Sawada H, Ohta M, Hirai S, et al. Hydroxyapatite-silk functionally graded material by pulse electric current sintering. Trends Biomater Artif Organs. 2008;22:25–9.
Ban S, Hasegawa J, Maruno S.Fabrication and properties of functionally gradient bioactive composites comprising hydroxyapatite containing glass coated titanium. Mater Sci Forum 1999; 311:350-5; .
Stojanovic D, Jokic B, Veljovic Dj, Petrovic R, Uskokovic PS, Janackovic Dj. Bioactive glass-apatite composite coating for titanium implant synthesized by electrophoretic deposition. J Eur Ceram Soc. 2007;27:1595–9. doi: 10.1016/j.jeurceramsoc.2006.04.111.
Wong LH, Tio B, Miao X. Functionally graded tricalcium phosphate/fluoroapatite composites. Mater Sci Eng C. 2002;20:111–5. doi: 10.1016/S0928-4931(02)00020-6.
Okazaki M, Miake Y, Tohda H, Yanagisawa T, Matsumoto T, Takahashi J. Functionally graded fluoridated apatites. Biomaterials. 1999;20:1421–6. doi: 10.1016/S0142-9612(99)00049-6.
Okazaki M, Takahashi J. Synthesis of functionally graded CO3 apatite as surface biodegradable crystals. Biomaterials. 1999;20:1073–8. doi: 10.1016/S0142-9612(98)00244-0.
Peltola T, Pätsi M, Rahiala H, Kangasniemi I, Yli-Urpo A. Calcium phosphate induction by sol-gel-derived titania coatings on titanium substrates in vitro. J Biomed Mater Res. 1998;41:504–10. doi: 10.1002/(SICI)1097-4636(19980905)41:3<504::AID-JBM22>3.0.CO;2-G.
Heilmann F, Standard OC, Müller FA, Hoffman M. Development of graded hydroxyapatite/CaCO(3) composite structures for bone ingrowth. J Mater Sci Mater Med. 2007;18:1817–24. doi: 10.1007/s10856-007-3028-3.
Cavalcanti A, Shirinzadeh B, Zhang M, Kretly LC. Nanorobot hardware architecture for medical defense. Sensors (Basel Switzerland) 2008;8:2932–58. doi: 10.3390/s8052932.
Ding Y, Liu J, Jin X, Shen G, Yu R. A novel piezoelectric immunosensor for CA125 using a hydroxyapatite/chitosan nanocomposite-based biomolecular immobilization method. Aust J Chem. 2008;61:500–5. doi: 10.1071/CH07441.
Sánchez-Paniagua López M, Tamimi F, López-Cabarcos E, López-Ruiz B. Highly sensitive amperometric biosensor based on a biocompatible calcium phosphate cement. Biosens Bioelectron. 2009;24:2574–9. doi: 10.1016/j.bios.2009.01.002.
Wang B, Zhang JJ, Pan ZY, Tao XQ, Wang HS. A novel hydrogen peroxide sensor based on the direct electron transfer of horseradish peroxidase immobilized on silica-hydroxyapatite hybrid film. Biosens Bioelectron. 2009;24:1141–5. doi: 10.1016/j.bios.2008.06.053.
Lu L, Zhang L, Zhang X, Huan S, Shen G, Yu R. A novel tyrosinase biosensor based on hydroxyapatite-chitosan nanocomposite for the detection of phenolic compounds. Anal Chim Acta. 2010;665:146–51. doi: 10.1016/j.aca.2010.03.033.
Wang S, Lei Y, Zhang Y, Tang J, Shen G, Yu R. Hydroxyapatite nanoarray-based cyanide biosensor. Anal Biochem. 2010;398:191–7. doi: 10.1016/j.ab.2009.11.029.
Tagaya M, Ikoma T, Migita S, Okuda M, Takemura T, Hanagata N, et al. Fetal bovine serum adsorption onto hydroxyapatite sensor monitoring by quartz crystal microbalance with dissipation technique. Mater Sci Eng B. 2010;173:176–81. doi: 10.1016/j.mseb.2010.01.034.
Wypych G. Handbook of fillers. ChemTec Publishing: New York USA 2009; 3:800.
Almora-Barrios N, de Leeuw NH. A density functional theory study of the interaction of collagen peptides with hydroxyapatite surfaces. Langmuir. 2010;26:14535, 42. doi: 10.1021/la101151e.
Rhee SH, Lee JD, Tanaka J. Nucleation of hydroxyapatite crystal through chemical interaction with collagen. J Am Ceram Soc. 2000;83:2890–2. doi: 10.1111/j.1151-2916.2000.tb01656.x.
Lin X, Li X, Fan H, Wen X, Lu J, Zhang X. In situ synthesis of bone-like apatite/collagen nano-composite at low temperature. Mater Lett. 2004;58:3569–72. doi: 10.1016/j.matlet.2004.06.044.
Zhang W, Liao SS, Cui FZ. Hierarchical self-assembly of nanofibrils in mineralized collagen. Chem Mater. 2003;15:3221–6. doi: 10.1021/cm030080g.
Liu Q, de Wijn JR, van Blitterswijk CA. Covalent bonding of PMMA, PBMA, and poly(HEMA)to hydroxyapatite particles. J Biomed Mater Res. 1998;40:257–63. doi: 10.1002/(SICI)1097-4636(199805)40:2<257::AID-JBM10>3.0.CO;2-J.
Li J, Chen YP, Yin Y, Yao F, Yao K. Modulation of nano-hydroxyapatite size via formation on chitosan-gelatin network film in situ. Biomaterials. 2007;28:781–90. doi: 10.1016/j.biomaterials.2006.09.042.
Ficai A, Andronescu E, Ghitulica C, Voicu G, Trandafir V, Manzu D, et al. Colagen/hydroxyapatite interactions in composite biomaterials. Materiale Plastice. 2009;46:11–5.
Danilchenko SN, Kalinkevich OV, Kuznetsov VN, Kalinkevich AN, Kalinichenko TG, Poddubny IN, et al. Thermal transformations of the mineral component of composite biomaterials based on chitosan and apatite. Cryst Res Technol. 2010;45:685–91. doi: 10.1002/crat.201000163.
Boanini E, Gazzano M, Rubini K, Bigi A. Composite nanocrystals provide new insight on alendronate interaction with hydroxyapatite structure. Adv Mater (Deerfield Beach Fla) 2007;19:2499–502. doi: 10.1002/adma.200602497.
Tjandra W, Yao J, Ravi P, Tam KC, Alamsjah A. Nanotemplating of calcium phosphate using a double-hydrophilic block copolymer. Chem Mater. 2005;17:4865–72. doi: 10.1021/cm050679b.
Tsuchiya K, Yoshioka T, Ikoma T, Tanaka J. Chemical interaction between hydroxyapatite and organic molecules in biomaterials. Ceram Trans. 2010;210:531–5.
Grossman RF. Coupling agents. In: Polymer modifiers and additives, Lutz JT Jr, Grossman RF, (Eds.); CRC Press: Boca Raton FL, USA 2000; 95-106.
Chang MC, Ikoma T, Kikuchi M, Tanaka J. Preparation of a porous hydroxyapatite/collagen nanocomposite using glutataldehyde as a cross-linkage agent. J Mater Sci Lett. 2001;20:1199–201. doi: 10.1023/A:1010914621089.
Sousa RA, Reis RL, Cunha AM, Bevis MJ. Coupling of HDPE/hydroxyapatite composites by silane-based methodologies. J Mater Sci Mater Med. 2003;14:475–87. doi: 10.1023/A:1023471011749.
Dupraz AMP, de Wijn JR, van der Meer SAT, Goedemoed JH. Biocompatibility screening of silane-treated hydroxyapatite powders for use as filler in resorbable composites. J Mater Sci Mater Med. 1996;7:731–8. doi: 10.1007/BF00121408.
Dupraz AMP, de Wijn JR, v d Meer SA, de Groot K. Characterization of silane-treated hydroxyapatite powders for use as filler in biodegradable composites. J Biomed Mater Res. 1996;30:231–8. doi: 10.1002/(SICI)1097-4636(199602)30:2<231::AID-JBM13>3.0.CO;2-P.
Liao JG, Wang XJ, Zuo Y, Zhang L, Wen JQ, Li Y. Surface modification of nano-hydroxyapatite with silane agent. J Inorg Mater. 2008;23:145–9. doi: 10.3724/SP.J.1077.2008.00145.
Sousa RA, Reis RL, Cunha AM, Bevis MJ. Structure development and interfacial interactions in HDPE/HA composites moulded with preferred orientation. J Appl Polym Sci. 2002;73:2886–72.
Misra DN. Adsorption of zirconyl salts and their acids on hydroxyapatite: use of the salts as coupling agents to dental polymer composites. J Dent Res. 1985;64:1405–8. doi: 10.1177/00220345850640121701.
Liu Q, de Wijn JR, van Blitterswijk CA. A study on the grafting reaction of isocyanates with hydroxyapatite particles. J Biomed Mater Res. 1998;40:358–64. doi: 10.1002/(SICI)1097-4636(19980605)40:3<358::AID-JBM3>3.0.CO;2-E.
Tanaka H, Yasukawa A, Kandori K, Ishikawa T. Surface modification of calcium hydroxyapatite with hexyl and decyl phosphates. Colloids Surf A Physicochem Eng Asp. 1997;125:53–62. doi: 10.1016/S0927-7757(96)03876-9.
Tanaka H, Watanabe T, Chikazawa M, Kandori K, Ishikawa T. TPD, FTIR and molecular adsorption studies of calcium hydroxyapatite surface modified with hexanoic and decanoic acids J Coll Interf Sci 1999; 214:31-7; DOI: 10.1006/jcis.1999.6154.
Borum-Nicholas L, Wilson OC., Jr. Surface modification of hydroxyapatite. Part I. Dodecyl alcohol. Biomaterials. 2003;24:3671–9. doi: 10.1016/S0142-9612(03)00239-4.
Borum L, Wilson OC., Jr. Surface modification of hydroxyapatite. Part II. Silica. Biomaterials. 2003;24:3681–8. doi: 10.1016/S0142-9612(03)00240-0.
Li Y, Weng W. Surface modification of hydroxyapatite by stearic acid: characterization and in vitro behaviors. J Mater Sci Mater Med. 2008;19:19–25. doi: 10.1007/s10856-007-3123-5.
Lee SC, Choi HW, Lee HJ, Kim KJ, Chang JH, Kim SY, et al. In-situ synthesis of reactive hydroxyapatite nano-crystals for a novel approach of surface grafting polymerization. J Mater Chem. 2007;17:174–80. doi: 10.1039/b611401f.
Morita S, Furuya K, Ishihara K, Nakabayashi N. Performance of adhesive bone cement containing hydroxyapatite particles. Biomaterials. 1998;19:1601–6. doi: 10.1016/S0142-9612(97)00120-8.
Shinzato S, Nakamura T, Tamura J, Kokubo T, Kitamura Y. Bioactive bone cement: effects of phosphoric ester monomer on mechanical properties and osteoconductivity. J Biomed Mater Res. 2001;56:571–7. doi: 10.1002/1097-4636(20010915)56:4<571::AID-JBM1129>3.0.CO;2-H.
Dorozhkin SV. Is there a chemical interaction between calcium phosphates and hydroxypropylmethylcellulose (HPMC) in organic/inorganic composites? J Biomed Mater Res. 2001;54:247–55. doi: 10.1002/1097-4636(200102)54:2<247::AID-JBM12>3.0.CO;2-G.
Omori M, Okubo A, Otsubo M, Hashida T, Tohji K.Consolidation of multi-walled carbon nanotube and hydroxyapatite coating by the spark plasma system (SPS). Key Eng Mater 2004; 256:395-8; .
Zhao B, Hu H, Mandal SK, Haddon RC. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem Mater. 2005;17:3235–41. doi: 10.1021/cm0500399.
Kasuga T, Yoshida M, Ikushima AJ, Tuchiya M, Kusakari H. Bioactivity of zirconia-toughened glass-ceramics. J Am Ceram Soc. 1992;75:1884–8. doi: 10.1111/j.1151-2916.1992.tb07212.x.
Dorozhkin SV. Inorganic chemistry of the dissolution phenomenon: the dissolution mechanism of calcium apatites at the atomic (ionic) level. Comments Inorg Chem. 1999;20:285–99. doi: 10.1080/02603599908021447.
Dorozhkin SV. A review on the dissolution models of calcium apatites. Prog Crystal Growth Charact. 2002;44:45–61. doi: 10.1016/S0960-8974(02)00004-9.
Furukawa T, Matsusue Y, Yasunaga T, Shikinami Y, Okuno M, Nakamura T. Biodegradation behavior of ultra-high-strength hydroxyapatite/poly (L-lactide) composite rods for internal fixation of bone fractures. Biomaterials. 2000;21:889–98. doi: 10.1016/S0142-9612(99)00232-X.
Furukawa T, Matsusue Y, Yasunaga T, Nakagawa Y, Okada Y, Shikinami Y, et al. Histomorphometric study on high-strength hydroxyapatite/poly(L-lactide) composite rods for internal fixation of bone fractures. J Biomed Mater Res. 2000;50:410–9. doi: 10.1002/(SICI)1097-4636(20000605)50:3<410::AID-JBM16>3.0.CO;2-Y.
Yasunaga T, Matsusue Y, Furukawa T, Shikinami Y, Okuno M, Nakamura T. Bonding behavior of ultrahigh strength unsintered hydroxyapatite particles/poly(L-lactide) composites to surface of tibial cortex in rabbits. J Biomed Mater Res. 1999;47:412–9. doi: 10.1002/(SICI)1097-4636(19991205)47:3<412::AID-JBM17>3.0.CO;2-B.
Marques AP, Reis RL, Hunt JA. In vitro evaluation of the biocompatibility of novel starch based polymeric and composite material. Biomaterials. 2002;23:1471–8. doi: 10.1016/S0142-9612(01)00272-1.
Mendes SC, Reis RL, Bovell YP, Cunha AM, van Blitterswijk CA, de Bruijn JD. Biocompatibility testing of novel starch-based materials with potential application in orthopaedic surgery: a preliminary study. Biomaterials. 2001;22:2057–64. doi: 10.1016/S0142-9612(00)00395-1.
Habraken WJEM, Liao HB, Zhang Z, Wolke JGC, Grijpma DW, Mikos AG, et al. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater. 2010;6:2200–11. doi: 10.1016/j.actbio.2009.12.028.
Ngiam M, Liao S, Patil AJ, Cheng Z, Chan CK, Ramakrishna S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone. 2009;45:4–16. doi: 10.1016/j.bone.2009.03.674.
Hasegawa S, Ishii S, Tamura J, Furukawa T, Neo M, Matsusue Y, et al. A 5-7 year in vivo study of high-strength hydroxyapatite/poly(L-lactide) composite rods for the internal fixation of bone fractures. Biomaterials. 2006;27:1327–32. doi: 10.1016/j.biomaterials.2005.09.003.
Bongio M, van den Beucken JJJP, Leeuwenburgh SCG, Jansen JA. Development of bone substitute materials: from ‘biocompatible’ to ‘instructive’. J Mater Chem. 2010;20:8747–59. doi: 10.1039/c0jm00795a.
Glossary
Abbreviations:
- A-W
apatite-wollastonite
- BMP
bone morphogenetic protein
- BSA
bovine serum albumin
- EVOH
a copolymer of ethylene and vinyl alcohol
- HDPE
high-density polyethylene
- HIPS
high impact polystyrene
- HPMC
hydroxypropylmethylcellulose
- IBS
injectable bone substitute
- PAA
polyacrylic acid
- PBT
polybutyleneterephthalate
- PCL
poly(∑-caprolactone)
- PDLLA
poly(D,L-lactic acid)
- PE
polyethylene
- PEEK
polyetheretherketone
- PEG
polyethylene glycol
- PGA
polyglycolic acid
- PHB
polyhydroxybutyrate
- PHBHV
poly(hydroxybutyrate-co-hydroxyvalerate)
- PHEMA
polyhydroxyethyl methacrylate PHV, polyhydroxyvalerate
- PLA
polylactic acid
- PLGA
poly(lactic-co-glycolic) acid
- PLGC
co-polyester lactide-co-glycolide-co-∑-caprolactone
- PLLA
poly(L-lactic acid)
- PMMA
polymethylmethacrylate
- PP
polypropylene
- PPF
poly(propylene-co-fumarate)
- PS
polysulfone
- PSZ
partially stabilized zirconia
- PTFE
polytetrafluoroethylene
- PVA
polyvinyl alcohol
- PVAP
polyvinyl alcohol phosphate
- SEVA-C
a blend of EVOH with starch
- UHMWPE
ultrahigh molecular weight polyethylene
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/16782


