Abstract
Tissue engineering holds enormous challenges for materials science, wherein the ideal scaffold to be used is expected to be biocompatible, biodegradable and possess mechanical and physical properties that are suitable for target application. In this context, we have prepared degradable polyesters in different ratios by a simple polycondensation technique with citric acid and polycaprolactone triol. Differential scanning calorimetry indicated that the materials were amorphous based the absence of a crystalline melting peak and the presence of a glass transition temperature below 37°C. These polyesters were found to be hydrophilic and could be tailor-made into tubes and films. Porosity could also be introduced by addition of porogens. All the materials were non-cytotoxic in an in vitro cytotoxicity assay and may degrade via hydrolysis to non-toxic degradation products. These polyesters have potential implications in the field of soft tissue engineering on account of their similarity of properties.
Keywords: biodegradable, citric acid, elastomeric, polycaprolactone triol, polyester, soft tissue engineering
Introduction
Many of the diseases of the human population in recent years often lead to the impairment or loss of organs or tissues. Although allogenic/autologous transplantation is a commonly accepted therapy to cure patients, a critical shortage of organs/tissues of interest has restricted their use.1 While Xenogenic transplants are possible, immunological rejection upon transplantation requires accompanying immunosuppression therapy.1,2
The emerging field of tissue engineering may thus help to solve many of these problems. It mainly involves the recreation of a tissue or organ from a source of cells that may be derived from an endogenous source in the patient or from a donor. Tissue engineering often makes use of polymeric scaffolds to guide and promote controlled cellular growth and differentiation in order to generate new tissue.3-5 The minimum requirements of a material that can be used as a scaffold in tissue engineering is that it must be biocompatible, biodegradable with non-toxic by products and should be readily processed into complicated shapes with appropriate porosity. They should have appropriate mechanical properties as well as maintain mechanical strength during most of the tissue regeneration process.
Many biodegradable polymers, like poly(glycolide), poly(lactide) and PLA-PGA copolymers, have been used as tissue engineering scaffolds but have limited use in soft tissue engineering, as they are more stiff because of their elastic deformation characteristics.6-8 Acidic degradation products and lack of specific cell recognition signals are their other limitations. Serrano et al. and Bettinger et al. in their recent reviews have highlighted the uses and the limitations of various polyesters that have been developed for use in regenerative therapy or tissue engineering.9,10 Modification of the biomaterial surfaces with bioactive ligands, such as peptides, gelatin and other polysaccharides, by either adsorbing or covalently grafting to the surface has also been reported.11-14 Among the synthetic polymers polycaprolactone is being used extensively as scaffolds, both in its native form and also as blends with other materials, owing to their physicochemical and mechanical characteristics and easy processability.8 Polycaprolactone is also an FDA approved material that is also non-toxic and cell friendly, although it lacks the appropriate cell recognition sites. Their degradation and resorption kinetics are very slow; however, the products generated are either metabolized via the tricarboxylic acid cycle (TCA) or eliminated by direct renal secretion. Poly(caprolactone-triol) (PCLT) is a semi-crystalline polymer with a melting point around 30°C and glass transition temperature around -68°C and is potentially interesting for use as a biomaterial.15
In this context, to improve upon the biodegradation rates and to introduce sites for attachment of cell recognition ligands, we have synthesized biodegradable polyesters from polycaprolactone triol and citric acid in varying ratios through a polycondensation reaction. Citric acid is a polyfunctional monomer that is non-toxic and a metabolic product of the body (Krebs cycle). Citric acid has been used for the polyesterification reaction to introduce more active carboxyl sites that are feasible for conjugation with peptides and also to improve upon biodegradability, owing to a higher number of active scission sites.16 Due to the multifunctional groups, cross-linking can be induced to get a more stable structure. Moreover, the sodium salt of citric acid, sodium citrate, is a commonly known anticoagulant, and hence, it possesses the hemocompatibility required for blood-contacting applications, like soft tissue engineering. Ameer et al. have earlier reported on the synthesis of polyesters from citric acid and diols with several applications and surface affinitites to many cell types.17,18 In this paper, we report our studies on the polyester of citric acid and polycaprolactone triol. Three different formulations, i.e., 1:1, 3:2 and 2:1 weight ratios of Citric acid/Polycaprolactone triol (C-PCLT), were used in order to investigate the effect of polymer composition on the different physicochemical and mechanical properties of the scaffold. The results of our studies indicate that the polyester has an application potential for use as a scaffold for vascular tissue engineering and other soft tissue engineering, where the “holy grail” of developing a small diameter vascular graft still needs to be addressed.19,20
Results and Discussion
Polyesters have been widely considered in recent years as tissue engineering scaffolds. Most of the polyesters that have been synthesized and fabricated, such as PLLA, PLGA, etc., are rigid.7,8 Hence, the search is on for a biodegradable, biocompatible and elastomeric scaffold for soft tissue engineering. The polyester that we report here is shown to meet these demands. (Citric acid-co-polycaprolactone triol) polyester with intermolecular hydrogen bonding has been effectively synthesized via a simple reaction shown in Scheme 1. The citric acid and the polycaprolactone triol act as polyfunctional cross-linking monomers. Polycaprolactone triol is a sticky paste at room temperature and has a Tg at -68°C and a Tm at 23°C. After the post polymerization at 70°C, stable films are obtained. The large increase in Tg after polyesterification is caused by decreased mobility due to the reaction with citric acid and the cross-linking that is induced.
Scheme 1.
Synthetic scheme of (citric acid – co – polycaprolactone triol) polyester [C-PCLT ]
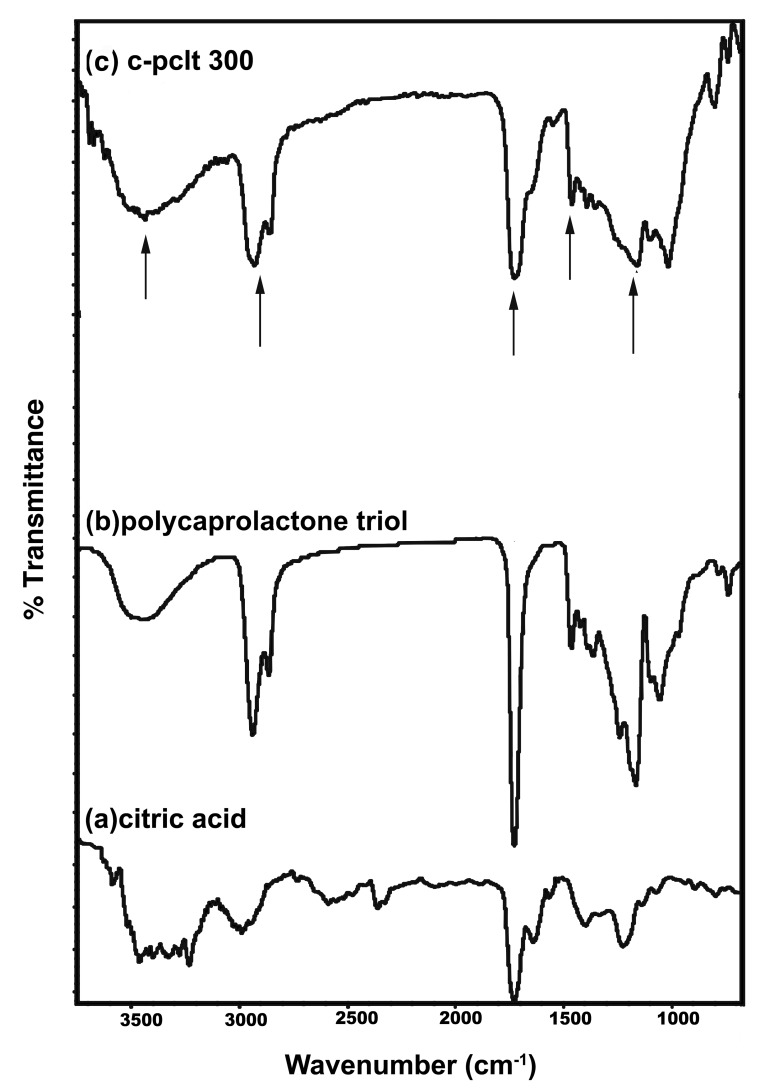
The reaction procedure was verified by FTIR. The typical FTIR spectra for the polyester preparation is shown in Figure 1. The plots of citric acid and Polycaprolactone triol are shown in Figure 1A and B. The intense C = O stretch at 1,730 cm−1 and the CO stretch at 1,157 cm−1 in the spectrum confirmed the formation of ester bonds in Figure 1C. The intense CH2 stretch at 2,942 and 2,864 cm−1 and the bend at 1,460 cm−1 also confirmed that the esterification reaction occurred. The broad OH stretch at 3,450 cm−1 indicates intermolecular hydrogen bonding.
Figure 1.
FTIR Spectra of (A) citric acid (B) polycaprolactone triol (C) C-PCLT 300.
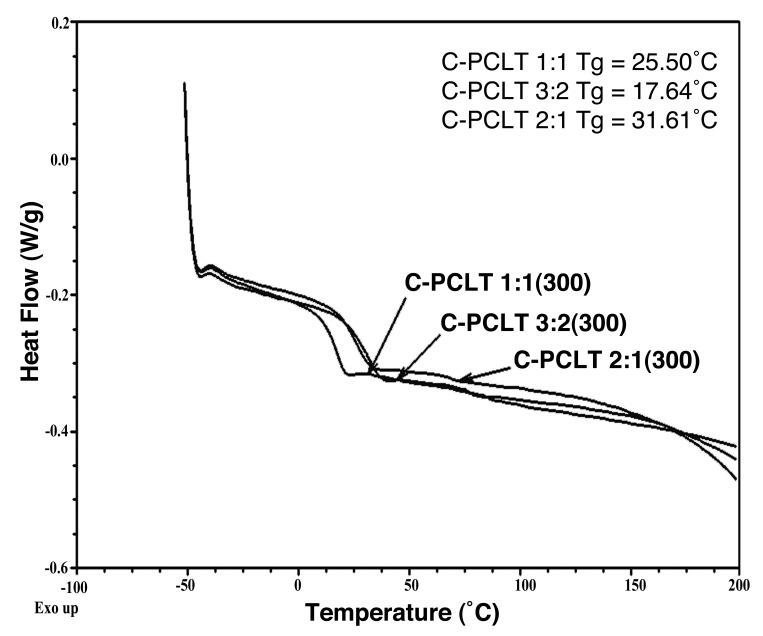
Thermal properties of the synthesized polyesters were evaluated by DSC and TGA. The DSC curves in the thermogram showed that there are no melting and crystallization peaks. Only low glass transition was observed. The polyester with composition 2:1 showed the highest Tg of 31.61°C, followed by 25.50°C and 17.64°C for 1:1 and 3:2 C-PCLT samples (Fig. 2). The samples are hence amorphous at 37°C, their use temperature, making the material more elastomeric.
Figure 2.
DSC Thermogram of C-PCLT 300 samples.
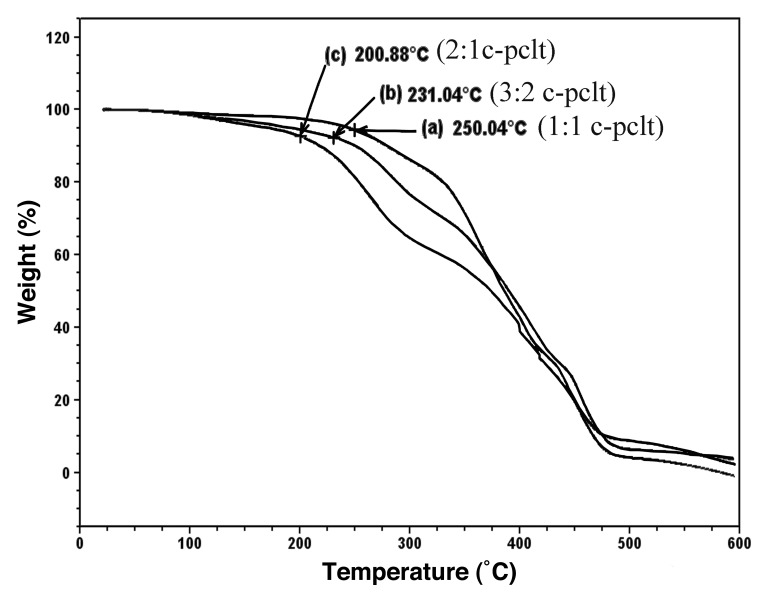
The thermal stability data in the TGA thermograms suggested that all the samples were thermally stable up to 200°C (Fig. 3), and it was observed that the rates of decomposition were found to increase as the concentration of citric acid was decreased.
Figure 3.
TGA plot for the C-PCLT (300) samples.
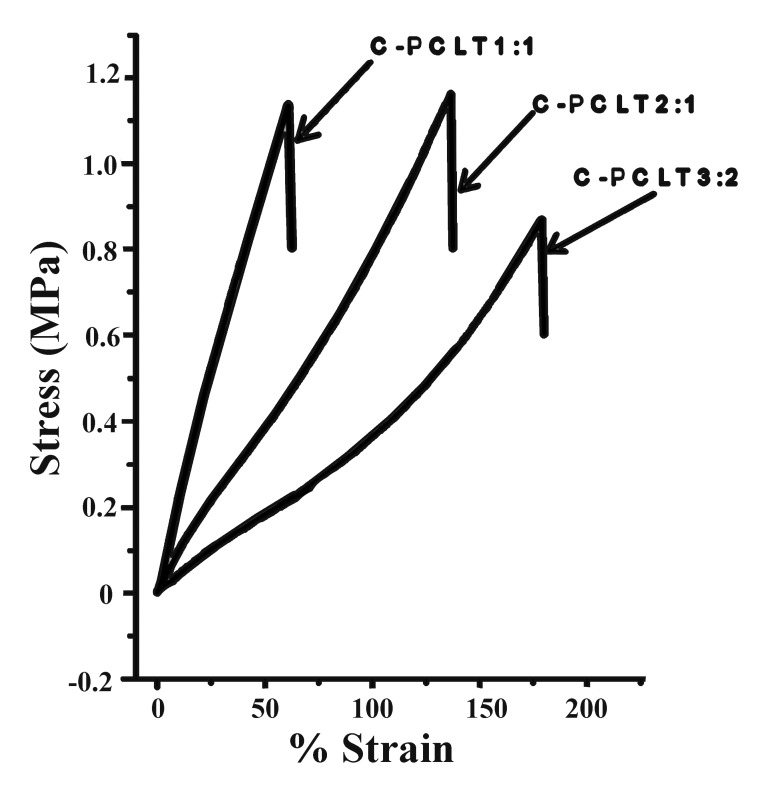
The stress strain curves for all the polyester samples were obtained, as shown in Figure 4. No permanent deformation was observed after the test. The samples prepared from 3:2 composition of C-PCLT were shown to have higher % elongation but lower tensile strength and toughness values (Table 1). This is also evident from the thermal studies, which show the lowest Tg value for the 3:2 composition, which may be attributed to the ester linkage formations and greater number of cross-links in this composition. The Young’s modulus values ranged from 0.56 ± 0.12 MPa to 2.44 ± 0.26 MPa for the investigated polyesters. From the data shown in the Table, these polyesters were found to have better mechanical properties than collagen, fibrin and blends of collagen and fibrin.21 The results were comparable to the % elongation at break requirements of arteries and veins as well as elastin.21,22 Also similar results to that of the arterial tissue were observed, which has a tensile strength of 0.1 MPa in the longitudinal direction and a tensile strength of 1.1 MPa in the transverse direction.23,24
Figure 4.
Stress-%strain plot of the C-PCLT polyester samples.
Table 1. Mechanical properties of the c-pclt polyester samples.
| SAMPLE | TENSILE STRENGTH (MPa) |
% ELONGATION AT BREAK (%) |
YOUNGS MODULUS (MPa) |
MODULUS OF TOUGHNESS (KPa) |
|---|---|---|---|---|
| 1:1 C-PCLT |
1.46 ± 0.27 |
61 ± 07 |
2.44 ± 0.26 |
7.61 ± 1.13 |
| 3:2 C-PCLT |
1.12 ± 0.19 |
177 ± 15 |
0.56 ± 0.12 |
13.20 ± 0.87 |
| 2:1 C-PCLT | 1.73 ± 0.22 | 146 ± 16 | 1.08 ± 0.16 | 15.08 ± 1.16 |
The compositions used for the study were based on different mole ratios of citric acid and polycaprolactone triol. The reaction is a polyesterification reaction between the hydroxyl groups of polycaprolactone triol and the carboxyl groups of citric acid. Since multifunctional groups are present, cross-linked structures are formed. The usual expectation from cross-linking is an increase in Tg (glass transition temperature) due to the restriction imposed by the newly formed chemical bonds (cross-links) on the segmental mobility of the polymer chains. However, these newly formed bonds can also impede the chain packing because of an increase in the chain length or formation of new bulky side chains, hence reducing the density. In the 3:2 composition, more bulky polycaprolactone triol groups are introduced, and the chemical bonds (increased chain length or bulky side chains) formed would interfere with crystal formation; therefore, the crystallinity of such polymers decreases with cross-linking. This leads to a low Tg value of 17.64°C for the 3:2 composition. Hence, the 3:2 c-pclt sample was found to be more amorphous at 37°C, their use temperature, making the material more elastomeric. This result could also be related to the mechanical strength results, which showed higher % elongation for the 3:2 compositions, showing the elastomeric nature of the scaffold.
Cell responses are highly dependant on the hydrophilicity or hydrophobicity of the surfaces they come in contact with.25 Here, the initial contact angles and equilibrium contact angle of the polyesters are as shown in Table 2. The graphical representation is shown in Figure 5. The polyesters were found to show a higher contact angle. This is due to the smaller chain length and greater amount of cross-linking. Also, the 3:2 C-PCLT compositions showed a higher contact angle when compared with the 1:1 and 2:1 samples, which may be attributed to maximum cross-linking in 3:2 C-PCLT compositions. Even though all the samples showed a high contact angle, it was seen that the water drop spreads out with time. The contact angles after spreading were in the range of 44° to 19°. From the mechanical and thermal properties it was seen that the polyester is an elastomeric and rubber-like material with more amorphous domains. Hence, the polymer chains were highly mobile at room temperature, and the polar water groups attracted the polar groups on the polymer, thereby inducing a surface rearrangement. This explains the spreading of the water drop with time. Hence, these polyesters are hydrophilic in nature. Also, the good wettability characteristics prove that these materials have surfaces suitable for a good cell-material interaction.
Table 2. Contact angle values for the c-pclt polyester samples.
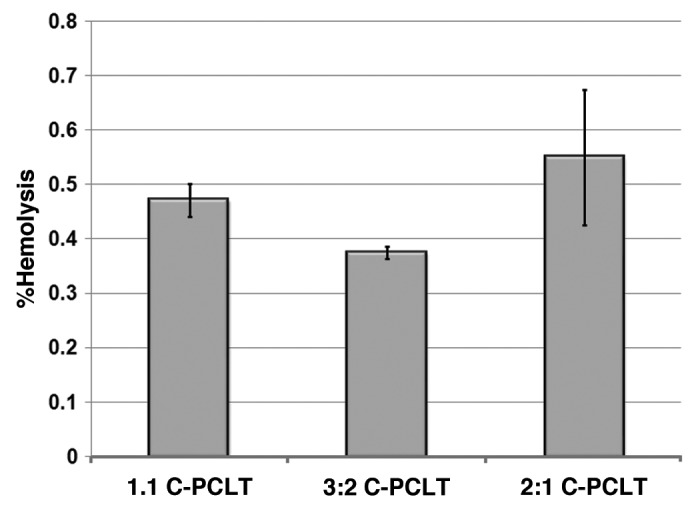
| SlNo | Sample name | Initial Contact angle(°) | Equilibrium Contact angle(°) | % Hemolysis |
|---|---|---|---|---|
| 1 |
1:1 C-PCLT |
74.55 ± 0.50 |
44.00 ± 0.24 |
0.47 ± 0.03 |
| 2 |
3:2 C-PCLT |
78.05 ± 0.83 |
28.00 ± 0.32 |
0.36 ± 0.01 |
| 3 | 2:1 C-PCLT | 67.75 ± 0.55 | 19.00 ± 0.56 | 0.55 ± 0.12 |
Figure 5.
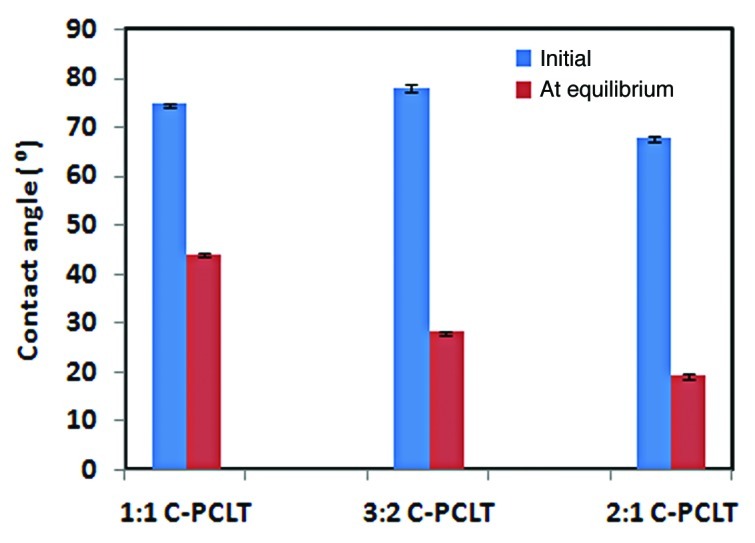
Contact angle plot of the polyester samples.
Figure 6 shows the degradation of C-PCLT polyester samples after incubation in PBS at 37°C for 4 weeks. The 2:1 C-PCLT sample showed the highest degradation rate, reaching 100%. The 3:2 C-PCLT sample showed the lowest degradation rate of 16.1% and the 1:1 C-PCLT sample a degradation rate of 31.5%, which is due to maximum cross-linking in the 3:2 composition, which is also confirmed by the contact angle studies. Hence, the degradation rates could be controlled in the required way by varying the ratio of the monomer feed. The major function of a biodegradable polymer-based scaffold is to remain at the implantation site while maintaining its physical characteristics and ordinary mechanical properties as well as supporting the attachment, proliferation and differentiation of cells until the complete regeneration of tissues on an injured site occurs. Ideally, the degradation rate of the scaffold should be matched with the rate of neo-tissue formation so as to provide a smooth transition of the load transfer from the scaffold to the tissue. Faster degradation rates, like that reported for polyglycolic acid (1.5 mo), may lead to sudden outbursts of byproducts that may be acidic in nature and create a low pH microenvironment, which stimulates chronic inflammation and induces fibrocollagenous tissue formation. It may also lead to sudden loss of mechanical strength. Most of the other reported polyesters show complete degradation from 10 mo onwards. Hibino et al. used a copolymer of lactic acid and caprolactone undergoing hydrolytic degradation of ~24 weeks in their in vivo clinical studies, where a long-term patency was observed at a follow up of 5.8 y.26 The 2:1 C-PCLT polyester shows a complete degradation at 1 mo, itself showing that this composition is not stable. The sudden degradation effects are also evident from the direct contact cytotoxicity assay and the MTT assay. The 3:2 compositions show a degradation rate of 16.1%. Comparing biodegradation pattern in the present study, the 3:2 composition is also expected to show a similar profile to the polylactides and, hence, stands as an ideal material for use as a scaffold in tissue engineering.
Figure 6.
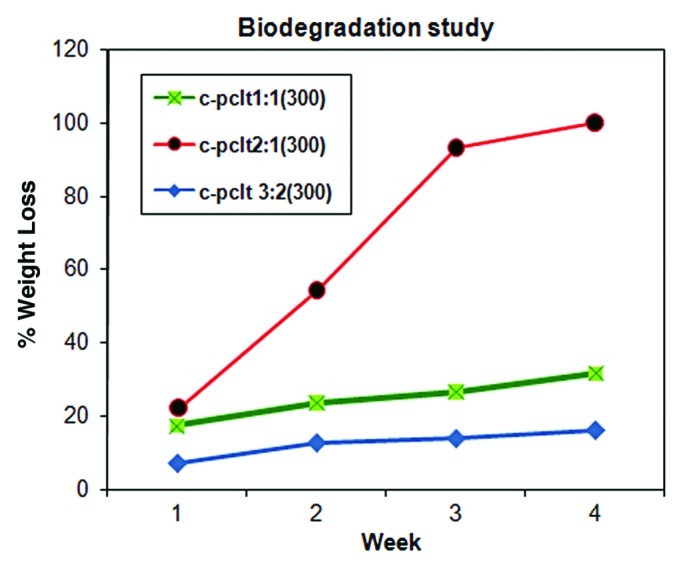
Biodegradation plot for C-PCLT (300) samples.
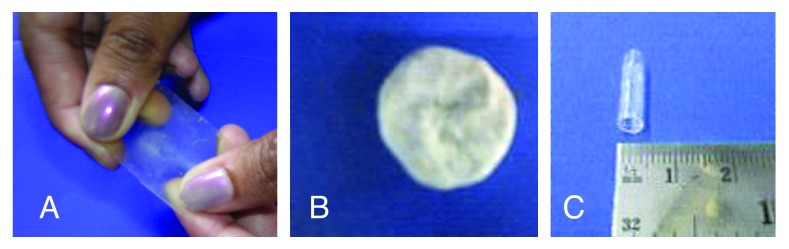
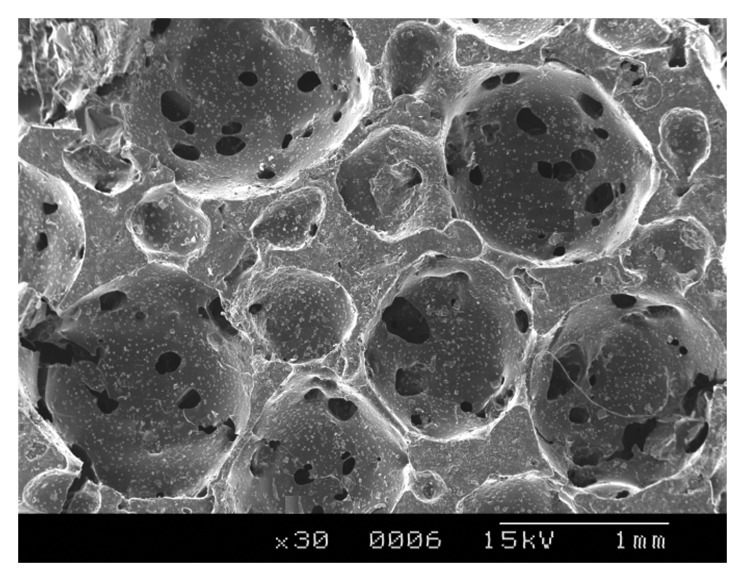
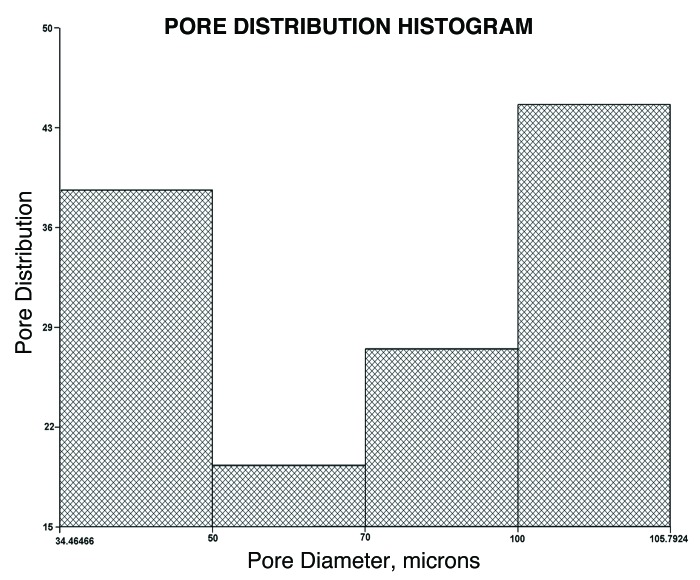
The fabrication of polymer scaffolds for tissue engineering applications has long been a subject of interest.27 The polyester with the composition 3:2 was taken for the rest of the studies owing to its lower degradation rates and good elastomeric characteristics. These could be fabricated in the form of thin transparent films, tubes and porous scaffolds, as shown in Figure 7. The pore sizes were determined by conducting SEM analysis (Fig. 8), which showed that the pore sizes varied from 125 μm to 1 mm and interconnected pores were obtained. The porosity measurements showed that the % porosity of interconnected pores was 46.837%, with the pore sizes ranging from 34 microns to 105 microns (Fig. 9). These materials have shown that they have excellent tailorable properties, and the pore size can be controlled by just varying the size of the porogen added.
Figure 7.
Polyester samples fabricated in the form of film, tuba and porous film.
Figure 8.
Scanning Electron microscopy image of 3:2 C-PCLT porous sample showing interconnected pores.
Figure 9.
Histogram showing pore distribution vs. pore diameter of the 3:2 polyester composition.
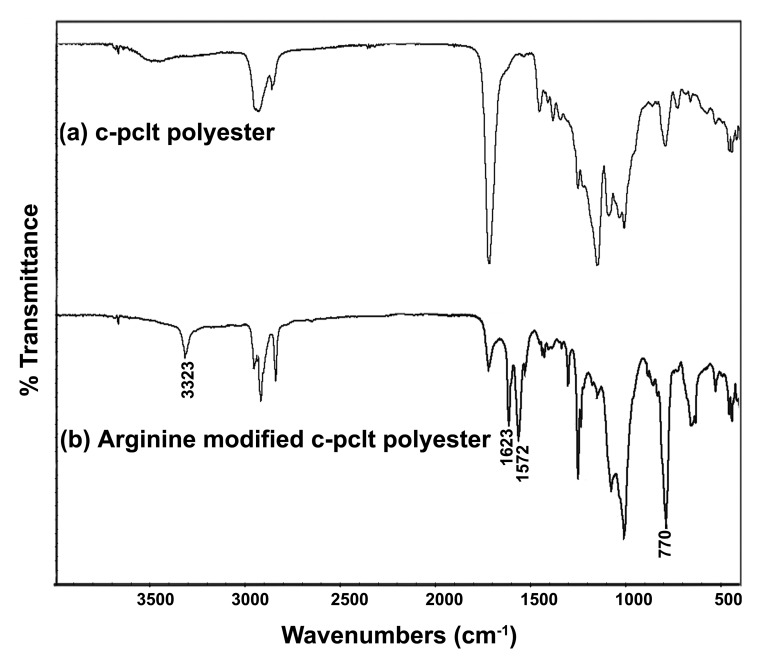
Bioconjugating a model amino acid like l-arginine onto the surface was found to be successful, and the reaction was confirmed by FTIR (Fig. 10). The peak at 3,323 cm−1 confirmed the N-H stretch, and the peak at 1,623 cm−1 showed the presence of guanido groups.28 The peak at 1,572 cm−1 showed the N-H bending, and the peak at 770 cm−1 showed the COO- scissoring. This was indicative of the fact that peptides like RGD or YIGSR could potentially be incorporated into the polymer surface through the free -NH2 groups to trigger a cell response. Thus these materials could be easily functionalized to meet specific requirements for cell interaction, unlike other materials, which require complex treatments to functionalize groups for further conjugation.
Figure 10.
FTIR plot of (A) unconjugated C-PCLT polyester and (B) L-Arginine Bioconjugated C-PCLT.
The percentage hemolysis for the samples was evaluated, and the results were formulated as shown in Figure 11. This test was done to give an idea of the hemolytic properties of the material intended for use in contact with blood. A material such as polyethylene, which is usually used as a negative control, produces little or no hemolysis (< 2%), and a material used as a positive control is one that will produce a hemolysis of at least 8%.29 So it is desirable that, for materials to be non-hemolytic, the % hemolysis should be less than 2%. All our materials showed a % hemolysis of less than 2% (Table 2). Hence, they are non-hemolytic and can be used in blood contacting surfaces.
Figure 11.
Graphical representation of % haemolysis of the polyester samples.
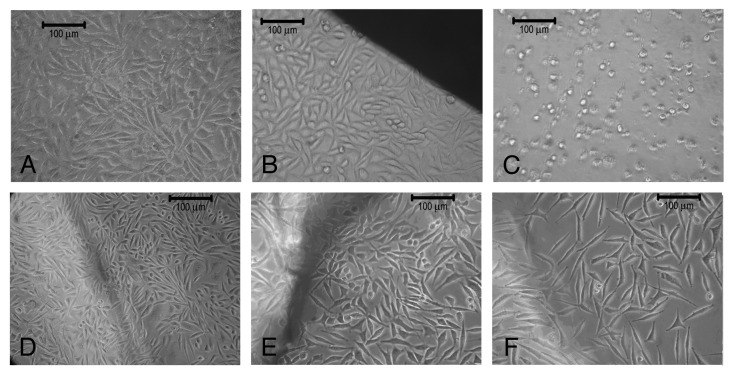
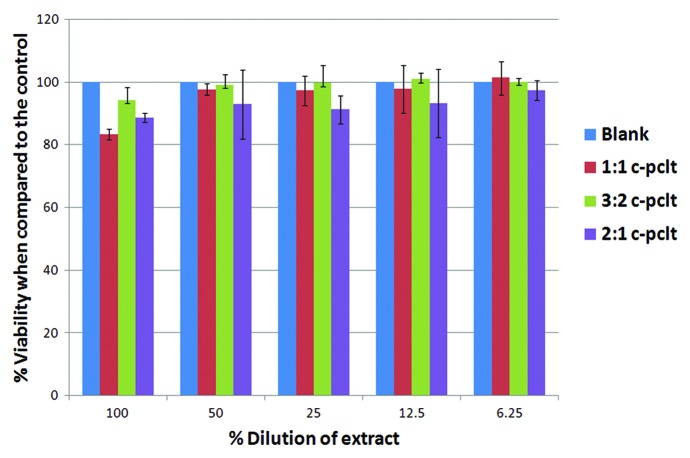
The cytotoxicity test by the direct contact method shows the zone of diffusion (a concentration gradient of toxic chemical) and eliminates extraction preparation. Hence this method was adapted to access the safety of the material. The results of direct contact between cells and samples are summarized in Table 3. In general, all the three samples were shown to be non-cytotoxic when compared with the negative reference material (high-density polyethylene) (Fig. 12B) and were observed to retain their spindle shape morphology except for the C-PCLT sample of ratio 2:1, showing only mild toxicity. This may be attributed to the increase in the citric acid concentration in the sample and the higher degradation rates of the samples. The cells under and around positive control were detached on the culture dish. Cells in contact with the polyester network did not undergo any deleterious effects, such as detachment, degeneration or lysis. The cytotoxicity or cell viability on the extracts of the samples was assessed using MTT assay. The results were in compliance with the cytotoxicity test on direct contact (Fig. 13). All the samples showed above 70% viability when compared with the control (taken as 100%).
Table 3. Scoring card for the direct contact cytotoxicity test with the polyester samples.
| Sample Composition | Scoring |
|---|---|
| HDPE (High density polyethylene) negative control |
0 |
| PVC (Poly vinyl chloride) positive control |
3 |
| 1:1 c-pclt |
0 |
| 3:2 c-pclt |
0 |
| 2:1 c-pclt | 1 |
Figure 12.
(A) L929 Fibroblast cells. (B) L929 Fibroblast cells in contact with negative control (high density polyethylene), (C) positive control (PVC), (D) 1:1 C-PCLT, (E) 3:2 C-PCLT, (F) 2:1 C-PCLT.
Figure 13.
MTT Assay done on the polyester samples and comparing the % viability with respect to the control.
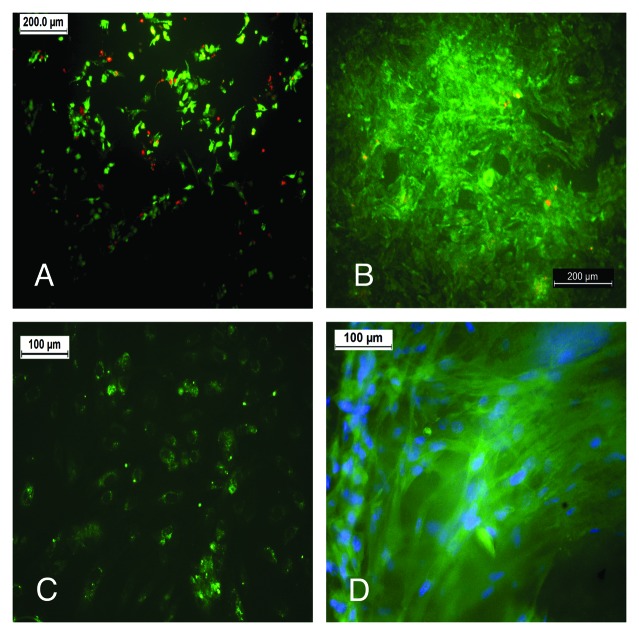
Preliminary in vitro culture studies with primary endothelial and smooth muscle cells confirms their potential as a cell friendly scaffold, especially in the area of soft tissue engineering (Fig. 14). The cells are seen to be proliferating and viable, expressing specific markers. Future work includes the differentiation of stem cells, like the umbilical cord mesenchymal stem cells, to cells of vascular origin on these novel scaffolds as well as their phenotypic and matrix remodeling studies, which we propose in our next paper.
Figure 14.
(A) HUVEC cells, cultured on the 3:2 composition on day 7 are viable in a Live Dead assay (live cells, green; dead cells, red). (B) RASMC cultured on the 3:2 C-PCLT sample on day 7 from a dense layer of viable cells (Live Dead assay). (C) vWF immunostaining for the Endothelial cell seeded constructs. (D) RASMC stained for Smooth muscle actin on the 3:2 samples.
Materials and Methods
Materials
Hydroxyl end functionalized polycaprolactone triols with a molecular weight of 300, citric acid, p-toluene sulphonic acid and L-Arginine supplied by Aldrich Chemical Corporation were used. 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) and N,N’-Dicyclohexyl carbodiimide (DCC) were purchased from Sigma Chemical Co. All other solvents and chemicals used were of reagent/analytical grade and were procured locally and utilized without further purification. L929 mouse fibroblast cells for cytotoxicity evaluation were subcultured from stock culture obtained from the National Centre for Cell Sciences, Pune, India.
Synthesis of citric acid-co-polycaprolactone triol polyester
The procedure of the polyester synthesis is illustrated in Scheme 1. A typical polycondensation reaction was performed. The monomers citric acid and polycaprolactone triol, taken in ratios of 1:1, 3:2 and 2:1, were charged into a rigorously dried round-bottom flask. The flask was then immersed into a preheated oil bath at 160°C with a flow of nitrogen gas provided continuously through inlet and outlet nozzles and with continuous stirring for about 15 min to obtain a clear homogenous melt system. Then the reaction temperature was lowered to 140°C, and 1% w/w of p-toluene sulphonic acid catalyst was added. The reaction was allowed to proceed for 1 h at that temperature to form the prepolymer. The prepolymer was then dissolved in acetone and the solution cast into Teflon molds and post-polymerized at 70°C for 2 d to obtain the polyester films, which were cleaned by continuous washings with distilled water in a sonicator and dried under vacuum.
FTIR analysis
The FTIR-ATR spectra of film samples were recorded at room temperature in the range of 400 to 6,000 cm−1 region using a NICOLET Inc., Model Impact 410 FTIR spectrometer with HATR accessory.
Thermal analysis
The glass transition temperature of the polyester was determined by Differential Scanning Calorimetry as per ASTM E1356–03. The thermograms were recorded in the range of -50°C to 200°C on a TA Instrument, DSC 2920 with TA 4000 controller at a heating rate of 10°C/min in a N2 atmosphere. The thermal stability of the samples was determined by Thermogravimetric Analysis as per ASTM E1131–03. The thermograms were recorded using a simultaneous TGA-DTA instrument (model SDT 2920 TA Instruments Inc.). Approximately 10 mg of the samples were heated from room temperature to 600°C at a heating rate of 10°C/min in a N2 atmosphere.
Mechanical properties testing
Tensile tests were conducted according to ISO 527–2:1993(E) on an Instron Mechanical tester (Series IX Automated Materials Testing System, INSTRON Corp.) equipped with 100 N load cell. Dumb bell specimens were cut using type 5B die and pulled at a rate of 100 mm/min. Tensile strength (MPa) and elongation at break (%), Youngs modulus (MPa), % strain at break and modulus of toughness (KPa) were calculated, and the stress vs. % strain plotted.
Contact angle measurement
The hydrophobicity or hydrophilicity is usually characterized by the water contact angle technique.30,31 The samples used for this analysis was thoroughly washed and cleaned. Deionized water was used for the study. The water in air contact angles of the polyester films were measured at room temperature (approx. 23°C) using the sessile drop method by a video-based contact angle measuring device (DataPhysics OCA15 plus) and imaging software (SCA20 software) within 10 sec after introduction of water droplet. The contact angle formed between a sessile drop and the film is directly related to the forces at the liquid-solid interface, indicating the hydrophilic or hydrophobic characteristics of the surface. Four independent measurements at different sites were averaged. The contact angle changes over time were also monitored. Five individual experiments were performed for the contact angle study. The results were presented as means ± standard deviation.
In vitro degradation
Phosphate Buffered Saline (PBS, pH 7.4, 0.1 M) was prepared by dissolving 17.97 g of disodium hydrogen phosphate, 5.73 g of monosodium hydrogen phosphate and 9 g of sodium chloride in 1 L of distilled water. The Polyester sample (10 x 10 x 1.5 mm) was placed in a container containing 10 ml phosphate buffer saline and incubated at 37°C for one week. The samples were removed and dried under vacuum. The mass loss was calculated by comparing the initial mass (Wo) with that at the given point (Wt), as shown in Equation (1). Six individual experiments for a period of four weeks were performed for the degradation test. The results were presented as means ± standard deviation.
Mass loss (%) = 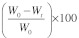 |
Preparation of porous films
Preparation of porous scaffolds was done by salt leaching method.32,33 The prepolymer formed was dissolved in acetone, followed by addition of sieved salt (70%), which serves as the porogen. The resulting slurry was put in an oven for post polymerization (70°C for 5 d). The salt in the resultant slurry was leached out by successive washing with double distilled water for 3 d. The film was then freeze-dried for 24 h and stored in a dessicator. The range of pore sizes was determined by gold coating the sample and observing by scanning electron microscopy (HITACHI model, S-2400). Specimens were mounted on aluminum stubs and coated with an ultra thin (300 Å) layer of gold in a coating apparatus and observed under microscope. SEM images were analyzed using image analysis software (Optima’sTM 6.1). The % porosity of the total interconnected pores, mean pore diameter and pore distribution was determined using liquid extrusion porosimetry (Model LEP-1100A, PMI, Porous Materials, Inc.). A porous sample of specific mass and density was taken and placed on a 5 mm Millipore membrane. Galwick (Propene-1,1,2,3,3,3-hexafluoro, oxidized, polymerized) was used as the wetting liquid, which spontaneously fills the pores of the sample and the membrane. Pressure of a non-reacting gas, nitrogen, was slowly increased over the sample so as to displace the liquid from the pores of the sample. From the displaced liquid, the porosity measurements are taken.
Surface modification of polyesters with L-Arginine
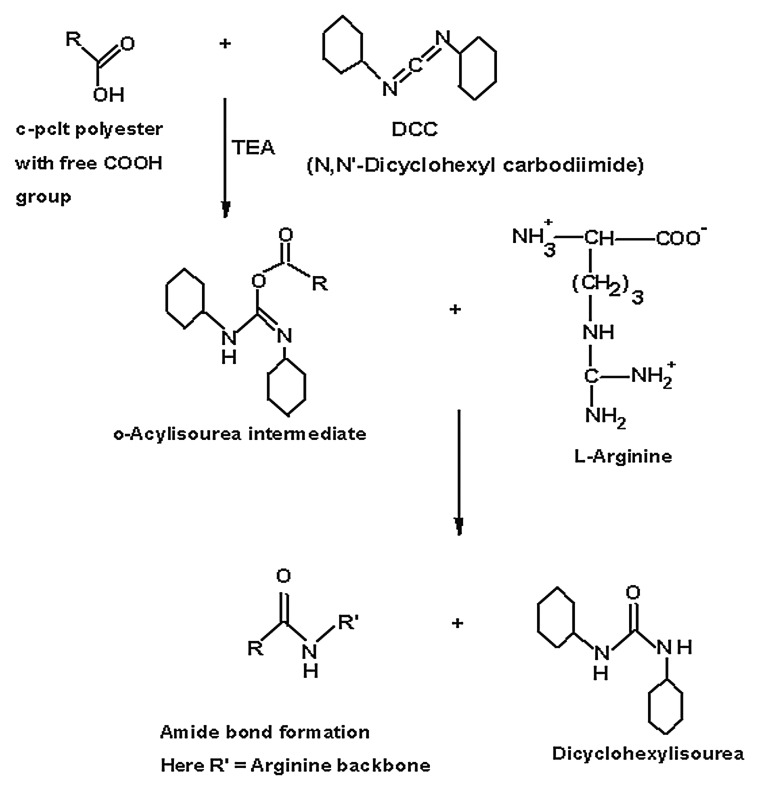
Surface modification was performed on the 3:2 C-PCLT sample, having good elastomeric properties, by conjugation reaction with N,N’-Dicyclohexylcarbodiimide (DCC). L-Arginine was taken as the model for the binding of RGD (Scheme 2).34 N,N’-Dicyclohexyl carbodiimide was first dissolved in chloroform. Ten mg/ml of L-Arginine to be conjugated was dissolved in water by using a vortex shaker. A film of polyester of known weight was then placed in 5 ml DCC solution. Two drops of Triethyl amine were added and kept for 2 h, then the polyester was removed and placed in 2 ml of the prepared L-Arginine solution for 24 h. The sample was then washed thoroughly with water and dried, and surface modification was confirmed by FTIR (NICOLET Inc., Madison, Model Impact 410 FTIR spectrometer with HATR accessory).
Scheme 2.
Synthetic scheme of bioconjugation of L-Arginine onto the Polyester using N, N’Dicyclohexyl carbodiimide (DCC)
Percentage hemolysis test
The percentage hemolysis test for examining the effect of material contact with blood was performed.35 Blood from human volunteer was collected into the anticoagulant, sodium citrate. The test materials were placed in polystyrene culture plates and agitated with phosphate buffered saline before they were exposed to blood. To each plate 1.5 ml blood was added and a 0.5 ml sample was collected immediately for analysis and the remaining 1.0 ml was exposed to the materials for 30 min under agitation at 75 ± 5 rpm using an Environ shaker thermostated at 35 ± 2°C. Two empty polystyrene culture dishes were exposed with blood as reference. The total hemoglobin in the initial samples was measured using automatic hematology analyzer (cobas minos vet). The platelet-poor plasma from blood exposed for 30 min was prepared. The free hemoglobin liberated in the plasma after exposure was measured in each sample using diode array spectrophotometer and the percentage hemolysis was calculated using the formula: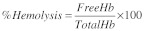
In vitro cytotoxicity studies
In vitro cytotoxicity test by direct contact
The in vitro cytotoxicity test was done as per ISO standard procedure.36 The ETO sterilized samples, negative control high-density polyethylene (HDPE) and positive control polyvinyl chloride (PVC disc) in triplicate were placed gently on top of a confluent monolayer of L929 mouse fibroblast cells. After incubation of cell with test samples at 37 ± 2°C for 24 ± 1 h, cell culture was examined microscopically for cellular response around test samples. Morphology of the cells was assessed in comparison to negative and positive control materials. The cellular responses of the test samples were scored as 0, 1, 2 and 3 according to non-cytotoxic, mildly cytotoxic, moderately cytotoxic and severely cytotoxic.
Viability test on extract by MTT assay
MTT assay measures colorimetrically only in in vitro living cells, and the results are directly related to the number of viable cultured cells.37 This principle is based on the cleavage of a yellow tetrazolium salt (3-{4,5-dimethylthiazol-2-yl}-2,5-diphenyl tetrazolium bromide) to purple formozan crystals by mitochondrial enzymes of metabolically active cells. L929 cells [6,000 cells in 100 ∝l Dulbecco’s modified Eagle’s medium (Sigma) supplemented with 10% (v/v) fetal bovine serum] were plated in 96-well plates. When monolayer was obtained, culture medium was removed, rinsed with PBS, and 100 ∝l of the extracts were added to each plate and again kept overnight. Cells with medium alone served as a control. After the plates were incubated for 24 h at 37°C in 5% carbon dioxide atmosphere, the medium was removed, and 100 ∝l of MTT working solution (5 mg/ml in PBS was the stock solution, and a 1:10 dilution was taken as the working solution) was introduced into each well and incubated at 95% humidified atmosphere at 37°C for 3 h. After the reagent was removed, and the material was rinsed with PBS, 100 ∝l of DMSO was added to each well and incubated for 5 min at 37°C in a shaker incubator until the converted dye dissolved completely. The absorbance of the resulting solution in each well was recorded at 540 nm after subtracting background at 670 nm using an automated microplate reader (ASYS UVM-340 Reader with DigiRead/ScanPlus Software, Austria).
In vitro cell culture studies
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured via a protocol similar to Jaffe et al. 0.5 cm2 porous samples (3:2 C-PCLT), after sterilizing with Ethyelene oxide gas and incubation in media for 24 h, were placed in cell culture dishes (6 cm in diameter). HUVEC at a density of 1.5 x 104 cells/ml was added to the porous samples by pippetting in tissue culture dishes and incubated for a period of 1.5 h for cell attachment. Further, the unattached cells were removed by gentle washing of the films with medium. Rat aortic SMC (approximately 1.5 x 104 cells) was also seeded on separate scaffolds by the same methodology. The seeded constructs were then placed in an incubator operating at 37°C and 5% CO2 for a period of 7 d, during which the culture medium was changed every day due to the cell density. For ascertaining the viability of cells and their ingrowth into the scaffolds, LIVE/DEAD® Viability/Cytotoxicity Kit (Invitrogen, Molecular probes, Eugene, OR) was used after a culture period of one week. Live cells are distinguished by the presence of intracellular esterase activity, as determined by the enzymatic conversion of the non-fluorescent, cell-permeant calcein AM to the intensely green fluorescent calcein. Ethidium homodimer-1 (EthD-1) enters cells with damaged membranes and produces a bright red fluoresence upon binding to nucleic acids in dead cells. After staining the films were imaged with argon 488 laser of confocal laser scanning microscopy (cLSM; Carl Zeiss LSM). Specific staining for cell markers, like von Willibrand factor for endothelial cells and smooth muscle actin, was performed to confirm the viability of the cells on the construct.
Statistical methods
Data are expressed as means ± standard deviation. The statistical significance between two sets of data was calculated using a two-tailed Student t-test. Data were taken to be significant when a p-value of 0.05 or less was obtained.
Conclusion
In summary, we have demonstrated the synthesis of polyesters from citric acid and polycaprolactone triol that proves to be a promising material with tunable properties in terms of their physical and degradative properties, which will play an active role in tissue replacement technology in the near future. These materials have good thermal and mechanical properties. Further, the polymers did not show any evidence of cytotoxicity and could be fabricated into porous scaffolds with interconnected porous structures. Experiments also showed that these polyesters could also be surface modified by bioconjugation with peptides so as to enhance cell adhesion and growth. Further investigations still remain to be done with these polymers, including culturing various cell types, like endothelial and smooth muscle cells, etc., into the porous scaffolds and evaluating the in vivo degradation and inflammatory responses. Hence, these materials show implications of potential application as soft tissue engineering and vascular tissue engineering scaffolds, which will help in tissue growth and regeneration.
Acknowledgments
The authors thank the Department of Biotechnology, the Government of India and the Director and Head, BMT Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, India for their immense support.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/17301
References
- 1.DiBardino DJ. The history and development of cardiac transplantation. Tex Heart Inst J. 1999;26:198–205. [PMC free article] [PubMed] [Google Scholar]
- 2.Calne R. Clinical transplantation: current problems, possible solutions. Philos Trans R Soc Lond B Biol Sci. 2005;360:1797–801. doi: 10.1098/rstb.2005.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vacanti CA, Vacanti JP. The science of tissue engineering. Orthop Clin North Am. 2000;31:351–6. doi: 10.1016/S0030-5898(05)70155-3. [DOI] [PubMed] [Google Scholar]
- 6.Ma PX, Langer R. Degradation, structure and properties of fibrous non-woven poly(glycolic acid) scaffolds for tissue engineering. Mater Res Soc Symp Proc 1995; 394:99-104. [Google Scholar]
- 7.Walton M, Cotton NJ. Long-term in vivo degradation of poly-L-lactide (PLLA) in bone. J Biomater Appl. 2007;21:395–411. doi: 10.1177/0885328206065125. [DOI] [PubMed] [Google Scholar]
- 8.Pitt CG, Greatzl MM, Kimmel GL. Aliphatic polyester II. The degradation of PLA, PCL and their copolymers in vivo. Biomaterials. 1981;2:215–20. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- 9.Serrano MC, Chung EJ, Ameer GA. Advances and applications of biodegradable elastomers in regenerative medicine. Adv Funct Mater. 2010;20:192–208. doi: 10.1002/adfm.200901040. [DOI] [Google Scholar]
- 10.Bettinger CJ. Biodegradable elastomers for tissue engineering and cell-biomaterial interactions. Macromol Biosci. 2011;11:467–82. doi: 10.1002/mabi.201000397. [DOI] [PubMed] [Google Scholar]
- 11.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–415. doi: 10.1016/S0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 12.Porjazoska, Yilmaz OK, Baysal K, Cvettovska M, Sirvanci S, Ercan F, et al. Synthesis and characterization of Poly(ethylene glycol)-poly(D,L-lactide-co-glycolide)poly(ethylene glycol) tri-block copolymers modified with collagen: a model surface suitable for cell interaction. J Biomater Sci Polymer Edn 2006; 17:323-40. [DOI] [PubMed]
- 13.Gabriel M, Amerongen GPVN, Victor WM, Hinsbergh V, Amerongen AVVN, Zentner A. Direct Grafting of RGD-Motif-containing peptide on the surface of polycaprolactone films. J Biomater Sci Polymer Edn 2006; 17:567-77. [DOI] [PubMed] [Google Scholar]
- 14.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–64. doi: 10.1016/S0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 15.Lin H, Kuo C, Lin Y. Synthesis and characterization of biodegradable Polyhydroxy butyrate-based polyurethane foams. J Cell Plast. 2003;39:101–16. doi: 10.1177/0021955X03039002002. [DOI] [Google Scholar]
- 16.Barrett DG, Yousaf MN. Design and applications of biodegradable polyester tissue scaffolds based on endogenous monomers found in human metabolism. Molecules. 2009;14:4022–50. doi: 10.3390/molecules14104022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Webb AR, Ameer GA. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv Mater (Deerfield Beach Fla) 2004;16:511–6. doi: 10.1002/adma.200306264. [DOI] [Google Scholar]
- 18.Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27:1889–98. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 19.Schmedlen RHBS, Elbjeirami WM, Gobin AS, West JL. Tissue engineered small-diameter vascular grafts. Clin Plast Surg. 2003;30:507–17. doi: 10.1016/S0094-1298(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 20.Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12:43–5. doi: 10.1096/fasebj.12.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elastic proteins: biological roles and mechanical properties. Philos Trans R Soc Lond B Biol Sci. 2002;357:121–32. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MC, Haut RC. Strain rate effects on tensile failure properties of the common carotid artery and jugular veins of ferrets. J Biomech. 1992;25:925–7. doi: 10.1016/0021-9290(92)90232-P. [DOI] [PubMed] [Google Scholar]
- 23.Calvert P. Biomimetic ceramics and composites. MRS Bull. 1992;17:37–40. [Google Scholar]
- 24.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: A review. Comp Sci Technol 2001; 1189-224. [Google Scholar]
- 25.Lydon MJ, Minett TW, Tighe BJ. Cellular interactions with synthetic polymer surfaces in culture. Biomaterials. 1985;6:396–402. doi: 10.1016/0142-9612(85)90100-0. [DOI] [PubMed] [Google Scholar]
- 26.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–6, 436, e1-2. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 27.Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003;5:29–39, discussion 39-40. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- 28.Liu WG, Zhang JR, Cao ZQ, Xu FY, Yao KD. A chitosan-arginine conjugate as a novel anticoagulation biomaterial. J Mater Sci Mater Med. 2004;15:1199–203. doi: 10.1007/s10856-004-5672-1. [DOI] [PubMed] [Google Scholar]
- 29.Document ASTM. Standard practice for assessment of hemolytic properties of materials designation: F 756-00. Annu Book ASTM Stand. 2005;•••:309–13. [Google Scholar]
- 30.Ratner BD. Characterisation of biomaterial surfaces. Cardiovasc Pathol. 1993;2:87–100. doi: 10.1016/1054-8807(93)90049-8. [DOI] [Google Scholar]
- 31.Andrade JD, King RM, Gregonis DE, Coleman DL. J Polymer Sci Poly Symbosium. 1979;66:313. [Google Scholar]
- 32.Kuo YC, Leou SN. Effects of composition, solvent, and salt particles on the physicochemical properties of polyglycolide/poly(lactide-co-glycolide) scaffolds. Biotechnol Prog. 2006;22:1664–70. doi: 10.1021/bp0602303. [DOI] [PubMed] [Google Scholar]
- 33.Mikos AG, Sarakinos G, Vacanti JP, Langer RS, Cima LG. Polymer membranes and methods of preparation of three dimensional membrane structures. US Patent No. 5, May 7, 1996; 514:378.
- 34.Greg TH. Zero Length Cross-linkers. Bioconjugate Techniques. Academic Press 1995; 177. [Google Scholar]
- 35.Document ISO. 10993-4, Biological evaluation of medical devices. Part 4. Selection of tests for interaction with blood. International Organization of Standardisation, Geneva 2002. [Google Scholar]
- 36.Document ISO. 10993-5, Biological compatibility of medical devices. Part 5. Tests for in vitro cytotoxicity. International Organization of Standardisation, Geneva 1999. [Google Scholar]
- 37.Ciapetti G, Cenni E, Pratelli L, Pizzoferrato A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials. 1993;14:359–64. doi: 10.1016/0142-9612(93)90055-7. [DOI] [PubMed] [Google Scholar]
- 38.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]