Abstract
Neutrophils are an important cellular component of the innate immune system that provides immediate protection to the host from infection. Neutrophil infiltration into inflamed peripheral tissues during infection is beneficial for immunity through phagocytosis of microbes, the release of antimicrobial factors, and secretion of proinflammatory cytokines. Recent reports further suggest that spleen-infiltrating neutrophils play a role in the adaptive immune response by providing survival signals to B cells. However, neutrophils may have detrimental effects on immunity in inflammatory diseases where their recruitment to lymphoid tissues and activation occur abnormally. To determine the contribution of neutrophils that reside in secondary lymphoid tissues to adaptive immunity, direct evaluation of the functional properties of tissue-resident neutrophils is required. We have developed a modified magnetic bead isolation approach for purifying neutrophils from inflamed spleens of autoimmune-prone mice by negative selection. Using this approach, we yielded neutrophils with greater than 90% purity without compromising cell viability. Equally important, the isolation procedure had little effect on the activation of neutrophils and did not impair phagocytic function. Thus, isolation of spleen-resident neutrophils by this optimized approach could be useful for interrogating the functional role of murine neutrophils in normal and abnormal immune responses.
Keywords: magnetic bead isolation, murine neutrophil, spleen, inflammation, autoimmunity, flow cytometry
Neutrophils have been considered mainly as effector cells of the innate immune response that control bacterial and fungal infections (1). Recently, however, neutrophils have been recognized as important regulators of adaptive immunity (1, 2). Neutrophils can interact directly with T cells and, through the production of IFNγ by neutrophils, promote T cell proliferation (3, 4). In addition, a subset of spleen-resident neutrophils expressing CD15 and CD16 has been shown to induce antibody secretion from marginal-zone B cells in healthy individuals through the production of the cytokines B cell activator of the TNF family (BAFF), a proliferation inducing ligand (APRIL), and IL-21 (2). Therefore, neutrophils may not only serve to recruit immune cells to sites of inflammation but may also activate them directly through the production of cytokines (5, 6). In addition to their beneficial role during infections, neutrophils are thought to contribute to the pathogenesis of diseases that involve inflammation. Activated neutrophils release a large number of inflammatory cytokines including IL-1α, IL-1β, IL-6, IL-4, and IL-17 that are linked to allergy, certain cancers, and autoimmune disorders such as systemic lupus erythematosus (1, 6). Therefore, a greater understanding of neutrophil function in both innate and adaptive immune responses has clinical implications for multiple diseases.
Neutrophils are produced in the bone marrow, are released into the bloodstream and, under normal conditions, survey the body for invading pathogens. In response to pathogens, circulating neutrophils migrate into peripheral tissues to the site of infection where they exert their effector activity (7). Neutrophils are phenotypically defined as Ly6G+ CD11b+ , regardless of their localization (8). Tissue-infiltrating neutrophils can be phenotypically differentiated further based on expression levels of the integrin family of cell adhesion molecules [(9–12); C.M.C. and L.D.E., personal observations]. Peripheral tissue- resident neutrophils also produce greater amounts of inflammatory cytokines and chemokines that further distinguish them from circulating neutrophils (13). Therefore, the tissue microenvironment is important for driving the activation of neutrophils that may impact the response of other immune cell types.
Methods have been previously designed for isolating murine neutrophils from blood (14, 15), bone marrow (16), peritoneum (15), and liver (17). In general, these methods use two different platforms for enrichment of neutrophils: density gradient centrifugation and magnetic beads that label specific cell populations using antibodies against cell surface antigens and then used for isolation either by positive or negative selection. Isolation of neutrophils by positive selection has also been achieved by flow cytometric cell sorting. These methods, tailored for purifying murine neutrophils from the sources described above, are simple and yield excellent cell purities; however, are less effective for isolating neutrophils within secondary lymphoid organs as we present here. Such a method to reliably isolate murine neutrophils from spleen and, in particular, from inflamed spleen where the composition of hematopoietic cells is altered would greatly facilitate the in vitro and in vivo analysis of these innate cells in their effector state during normal and abnormal immune responses. We developed an efficient method to isolate murine neutrophils in inflamed spleen by negative selection for subsequent analysis. We analyzed the steady-state frequencies of neutrophils in the blood, bone marrow, and spleens of the autoimmune-prone B6.Faslpr/J mouse model. Neutrophils were significantly elevated in the spleens of B6.Faslpr/J mice compared to healthy control animals. Based on the analysis of other major immune cell types in the spleen, we generated an optimized antibody cocktail (OAC) targeting these immune cell types to isolate splenic neutrophils by magnetic bead negative selection. The purity and viability of neutrophils using this method were highly reproducible and this technique could be used to not only isolate neutrophils from the spleen but also from the bone marrow and blood. Splenic neutrophils isolated with the OAC protocol were not impaired in cell viability or phagocytic activity, indicating that neutrophils were not adversely affected by this isolation procedure. Therefore, the OAC protocol provides a rapid and effective process for isolating spleen-resident neutrophils for further investigation.
Materials and Methods
Mice
B6.Faslpr J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at the University of Virginia animal facility. Age-matched wild-type C57BL/6 (B6) mice were purchased from Charles River Laboratories (Wilmington, MA). All experiments were performed using 4- to 5-monthold female mice from June, 2011 through March, 2012. Mice were housed in a specific pathogen-free animal facility at the University of Virginia. All animal procedures were conducted in compliance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Neutrophil Isolation Protocols and Staining Procedure
Spleens were removed from mice and were homogenized into a single-cell suspension using Dulbecco’s phosphate buffered saline (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (HyClone, Rockford, IL), designated isolation buffer. Red blood cells were lysed by resuspending spleen cells in ammonium chloride-Tris (ACT) buffer and incubating on ice for 10 min. Splenocytes were washed and resuspended in isolation buffer at a concentration of 1 × 106 cells/mL. Four different isolation techniques were used to develop a protocol optimal for purifying spleen-resident neutrophils and maintaining neutrophil viability to enable subsequent functional analyses.
EasySep® isolation technique
First, negative selection by magnetic beads using the EasySep protocol (Stemcell Technologies, BC, Canada), designed for isolating murine neutrophils from peripheral blood and bone marrow, was performed as per the manufacturer’s instructions. The commercial EasySep antibody cocktail contains mAbs against mouse CD4, CD5, CD11c, B220, CD49b, CD117, Ter119, and F4/80 of undisclosed concentrations.
OAC isolation technique
The second technique used the EasySep protocol, but was supplemented with additional antibodies specific for contaminating cell populations present in inflamed murine spleen. This modified magnetic bead isolation technique is referred to as OAC. For the OAC protocol, the commercial antibody cocktail provided by EasySep (50 µL per 1 × 106 total cells/mL) was supplemented with the following biotin-conjugated mAbs: anti-CD3 (clone 145-2C11; used at 3.25 µg/mL), anti-Ter119 (clone TER119; used at 0.25 µg/mL), and anti-B220 (clone RA3-6B2; used at 2.25 µg/mL), all purchased from BD Biosciences (San Jose, CA); anti-F4/80 (clone BM8; used at 4.35 µg/mL) and anti-CD11c (clone N418; used at 4.35 µg/mL) from BioLegend (San Diego, CA); anti-CD19 (clone 1D3; used at 4.35 µg/mL) and anti-NK1.1 (clone PK136; used at 4.35 µg/mL) from eBioscience (San Diego, CA). Spleen cells were incubated with antibodies at 4°C for 15 min and were subsequently washed with isolation buffer. Cells were then resuspended in isolation buffer (100 × 106 cells/mL), streptavidin microbeads were added (270 µL/mL; MiltenyiBiotec, Auburn, CA), and the cell mixture was incubated for 15 min at 48C. Cells were washed and resuspended in isolation buffer at 2.5 3 106 cells/mL. Neutrophils were isolated using the EasySep magnet (Stemcell Technologies) by pouring off the nonadherent cell fraction.
Density gradient centrifugation isolation technique
The third method used a density gradient to isolate neutrophils by centrifugation of splenic single-cell suspensions on Ficoll (GE Healthcare, Pittsburgh, PA) as previously described for peripheral blood neutrophils (15, 18).
Electronic cell sorting
Finally, splenic neutrophils were purified using a cell sorter. For cell sorting, ACT-treated splenocytes were stained at a concentration of 1 × 107 cells/mL with anti-CD11b FITC or anti-CD11b PE (clone M1/70; eBioscience; used at 5 µg/mL for FITC, 1 µg/mL for PE) and anti-Ly6G PE-Cy7 (clone IA8; BioLegend; used at 0.25 µg/mL) for 30 min on ice. Immediately before sorting, cells were resuspended in Live/Dead Fixable Aqua (Invitrogen, Grand Island, NY) as per the manufacturer’s instructions. All steps were performed on ice and cells were spun at 175g for 8 min at 4°C.
Peripheral blood neutrophils were isolated via retro-or-bital puncture and treated as described above, except that 10 mL of ACT was added per 1 mL of blood and incubated for 15 min to lyse red blood cells. The total number of cells was counted from a 0.5-mL volume of blood and the overall numbers of neutrophils in 0.5 mL of blood were calculated by multiplying the percentage of neutrophils, determined by flow cytometric analysis, with the total white blood cell count. This value was multiplied by 3 to calculate the absolute numbers of blood neutrophils in a 20-g mouse as previously described (19). Bone marrow-resident neutrophils were isolated from single cell suspensions prepared from the femurs and tibias of individual animals, and treated as described for the spleen.
Viability Assay
Purified neutrophils were cultured at 5 × 104 cells per well using 96-well Costar round bottom tissue culture plates (Corning, Corning, NY) in 100 µL of complete media containing: low glucose DMEM (Gibco), 5% nonessential amino acids (Gibco), and 10% FBS (HyClone). Cells were incubated at 37°C for the duration of the experiment. Cells were harvested at the indicated time points (refer to Fig. 2) and analyzed for viability by staining cells with Live/Dead Fixable Aqua (Invitrogen) as per the manufacturer’s instructions prior to fixation in 1% formaldehyde/PBS. Data were collected and analyzed as described below in the Flow Cytometry section.
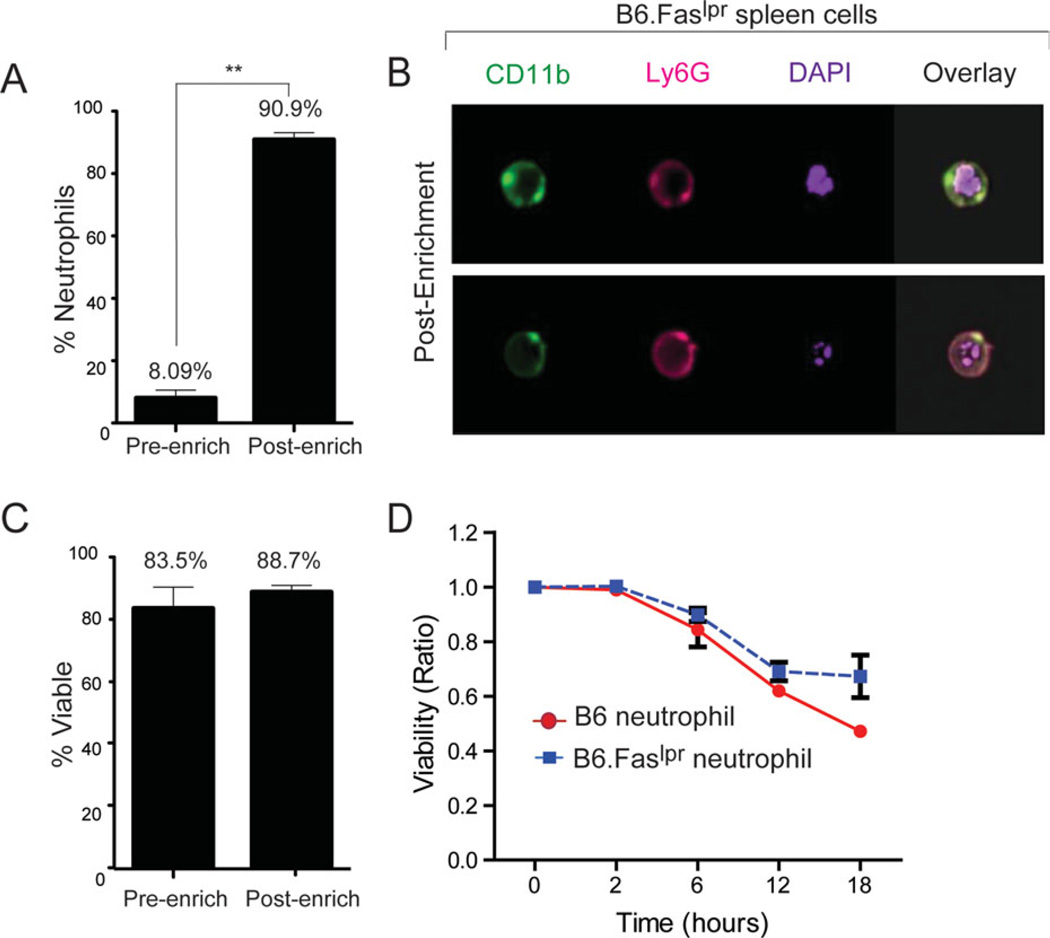
Figure 2.
Isolation of splenic neutrophils using the OAC protocol generates a pure neutrophil population with limited cell death. Spleens were isolated from 5-month-old B6.Faslpr/J and B6 mice. Single cell suspensions were analyzed before (pre-enrich) and after (post-enrich) isolation of neutrophils using the OAC method. (A) Data from four separate animals of the percentages of splenic neutrophils are shown. **P < 0.0005 compared to the pre-enriched group. (B) Enriched CD11b+Ly6G+ neutrophils were analyzed for multilobulated nuclei on the ImageStreamX cytometer. Samples were stained with CD11b-FITC, Ly6G-PECy7, and DAPI to stain nuclei. Original magnification 60×. Data shown are from 500 images and are representative of three independent experiments. (C) Neutrophils from samples shown in panel (A) were analyzed for cell viability by flow cytometry. Data from four separate experiments of the percentages of viable splenic neutrophils are shown. (D) Enriched neutrophils were analyzed for cell viability over a period of 18 h using the Live/Dead Aqua discriminator by flow cytometry. Data are presented as a viability ratio that was calculated by dividing the percentage of viable neutrophils at the outset of the experiment (0 h) by the percentage of viable neutrophils at each of the indicated time points. Data from four independent experiments are shown. *P < 0.05 compared to B6 neutrophils at 18 h.
S. aureus Uptake Assay
Purified neutrophils were added to 96-well U-bottom plates at 5 × 104 cells/well in 100 µL of complete media. AlexaFluor488-labeled S. aureus particles (Molecular Probes, Invitrogen; used at 5 µL per well) were added either with or without 1-h pretreatment with an opsonizing reagent, derived from S. aureus specific rabbit polyclonal IgG antibodies (Molecular Probes). Cells were incubated at 37°C for 2 h. Cells were washed twice to remove excess, nonadherent bacteria and were stained with Live/Dead Fixable Aqua (Invitrogen) and then fixed in 1% formaldehyde/PBS. Before data acquisition, the Alexafluor488 on the surface of the cells was quenched with trypan blue to eliminate detection of surface-bound bacteria that had not been internalized, as previously described (20). Data were collected and analyzed as described below.
FLOW CYTOMETRY
Total Spleen Cell Populations
Spleens from B6 and B6.Faslpr/J mice were homogenized into single-cell suspensions. Following ACT treatment, cells were stained with the following mAbs: anti-GR-1 FITC (clone RB6-8C5; BioLegend; used at 5 µg/mL), anti-CD11b PE (clone M1/70; eBioscience; used at 1 µg/mL), anti-CD11c APC (clone N418; BioLegend; used at 2 µg/mL), anti-B220 Pacific Blue (clone RM2628; Invitrogen; used at 1 µg/mL), anti-Ly-6G PECy7 (clone IA8; BioLegend; used at 0.25 µg/mL), anti-F4/80 FITC (clone BM8; eBioscience; used at 5 µg/mL), anti-CD3 PE (clone 145-2C11; BD Bioscience; used at 1 µg/mL), anti- CD19 PECy5.5 (clone eBiofD3; eBioscience; used at 0.5 µg/mL), anti-NK1.1 biotin (clone PK136; eBioscience; used at 5 lg/mL), and Live/Dead AQUA (described earlier).
Costimulatory Cell-Surface Markers
Isolated neutrophils were stained with the following mAbs: anti-CD18 biotin (cloneM18/2; eBioscience; used at 5 µg/mL), anti-CD11a FITC (clone M17/4; BioLegend; used at 5 µg/mL), and anti-CD62L PE (clone MEL-14; eBioscience; used at 1 µg/mL) for 30 min on ice. Cells isolated via EasySep, OAC, and Ficoll density gradient protocols were also stained with anti-CD11b and anti-Ly6G mAbs as described above. Sorted cells did not receive additional neutrophil marker staining.
Staining Protocol
Cells were incubated with primary Abs for 30 min on ice. After incubation with primary Abs, cells were stained with StrepAvidin-eFluor450 (Cat 48-4317-82; eBioscience; used at 0.5 µg/mL) for 20 min on ice, followed by staining with the viability marker Live/Dead Fixable Aqua (Invitrogen) for 20 min on ice according to the manufacturer’s instructions. Nonspecific staining was minimized by using normal rat serum or the addition of FcR blocking prior to mAb staining. Compensation was performed using single-stain controls from total spleen cells stained with the same concentration of mAbs as neutrophil samples at a concentration of 5 × 104 in 100 µL of PBS supplemented with 5% FBS. Cells were washed once between each staining condition. Samples were fixed in 200 µL of 1% formaldehyde/PBS prior to flow cytometric analysis.
Instrumentation, Data Analysis, and Gating Strategies
Neutrophils were sorted on a FACSVantage SE Turbo Sort DIVA version 5.0 (Becton Dickinson, Franklin Lakes, NJ) using an 80-µm nozzle. Data acquisition was performed on a CyAn ADP LX (Beckman Coulter, Brea, CA) using Summit software version 4.3.02 build 2451 (Beckman Coulter) and analyzed using FlowJo software version 9.3.3 (TreeStar, Ashland, OR). The CyAn ADP LX is equipped with three lasers (488/635/405). The 488 laser consists of five emitter filters: 488/10, 530/40, 575/25, 613/20, 680/30, 750LP. The 405 laser contains two emitter filer sets: 405/50, 530/40. The 635 laser contains two emitter filter sets: 665/20, 750LP. Compensation was calculated using the Compensation Wizard (FlowJo). Data displays were transformed biexponentially and are presented as 5% probability contours (depicting outliers) or overlay histograms. Gating was performed based on fluorescence-minus one controls. Neutrophils were identified based on the following gating strategy: Singlet+ Aqua− CD11b+Ly6G+ (Supporting Information Fig. 1). The instruments were calibrated as follows: Daily QC—Spherotec (Lake Forest, IL) Sphero Ultra Rainbow URFP-30-2 (3-µm fluorescent particles), running in linear mode. Monthly QC—Thermo Scientific (Waltham, MA) Cyto-Cal Multifluor plus Violet Fluorescence Intensity Calibrator FC3MV (mixture of 3.5-µm microspheres with five different fluorescent intensities and blank microspheres), running in logarithmic mode.
To confirm the morphology of neutrophils isolated using the OAC protocol, cells were stained with anti-CD11b-FITC and anti-Ly6G-PE-Cy7 as described above. Neutrophils were then fixed and permeabilized using the BD Cytofix/Cytoperm Kit (Becton Dickinson) as per the manufacturer’s instructions and stored at 4°C overnight. Cells were washed three times and then resuspended in PBS with DAPI (0.01 µg/mL; Sigma, St Louis, MO). Data were acquired on an ImageStreamX (Amnis, Seattle, WA) at a 60× magnification and analyzed using IDEAS software version 4.0 (Amnis). For uptake assays, fixed samples (described above) were acquired on an ImageStreamX at a 60× magnification and analyzed using IDEAS software version 4.0. After developing a mask that defines the inside of the cell, the ratio of the fluorescence intensity of the S. aureus inside the cell was compared to the ratio of the intensity of the entire cell to generate a score, with positive scores representing a greater ratio of internal fluorescence to external fluorescence and a higher score corresponding to greater fluorescence intensity (21). A detailed description of the multicolor staining panels used for all experiments can be found in Supporting Information Table 1.
Statistics
Statistics were determined using Prism Software (GraphPad Software, La Jolla, CA). All P-values were derived from the two-tailed Student’s t -test or two-way ANOVA as stated in the figure legend (*P < 0.05, **P < 0.0005).
RESULTS AND DISCUSSION
Frequencies of Circulating and Lymphoid Tissue-Resident Neutrophils in the B6.Faslpr/J Autoimmune-Prone Mouse Model
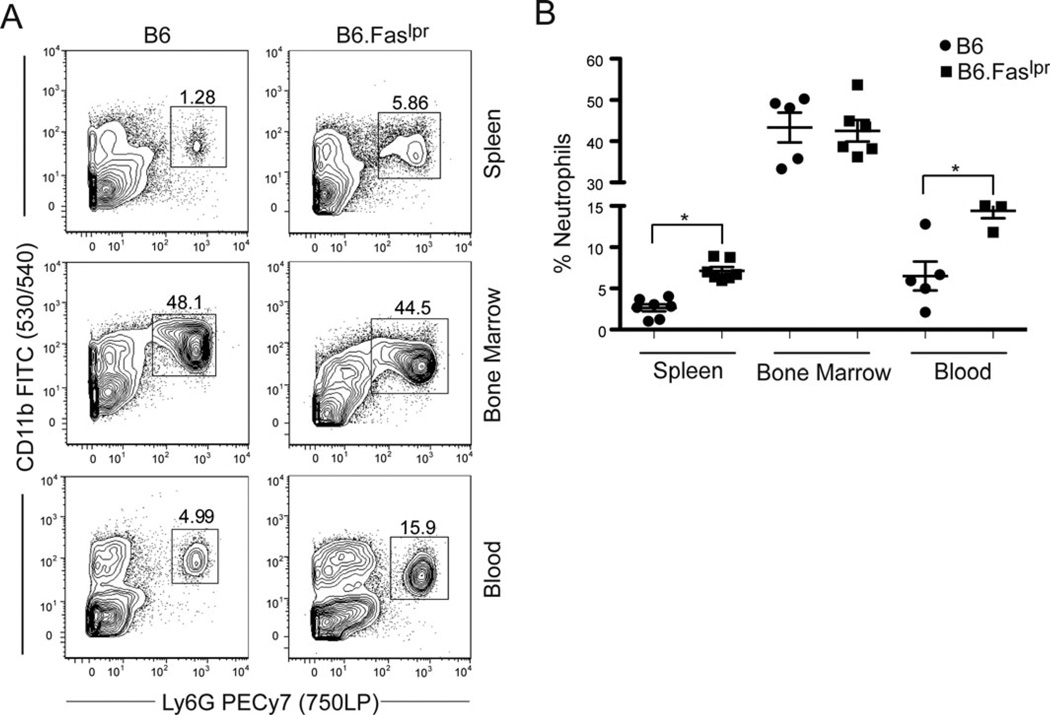
To analyze the role of spleen-resident neutrophils in autoimmune responses, we determined the frequency of CD11b+Ly6G+ neutrophils in the spleens, bone marrow, and blood of B6.Faslpr/J mice compared to age-matched control B6 mice. B6.Faslpr/J mice on the nonautoimmune C57BL/6 background carry a naturally occurring Faslpr mutation and develop a benign, slow-progressing lupus-like disease (22). Results demonstrated that B6.Faslpr/J mice had significantly increased percentages of neutrophils in the spleen and blood compared to B6 control mice (Fig. 1). In contrast, the percentages of neutrophils in the bone marrow were comparable between the two mouse strains. These data suggest that the lupus-like disease in B6.Faslpr/J mice alters the recruitment of circulating neutrophils into the spleen and/or enhances the viability of circulating and tissue-resident neutrophils, leading to increased frequencies of neutrophils in blood and spleen. The observation that there is no change in the percentages of neutrophils in the bone marrow suggests that neutrophil development itself is not altered in B6.Faslpr/J mice.
Figure 1.
B6.Faslpr/J mice have increased percentages of circulating and spleen-resident neutrophils. Single cell suspensions from spleens, blood, and bone marrow of age-matched B6 and B6.Faslpr/J mice were analyzed for the percentage of CD11b+Ly6G+ neutrophils by flow cytometry. (A) Representative contour plots demonstrating that the frequency of neutrophils is higher in spleen and blood samples from B6.Faslpr/J mice are shown. (B) Data from 5 to 7 individual mice are shown. Student’s t -test was performed on the indicated groups. *P < 0.05 compared to B6.
Development of an Optimized Antibody Cocktail for Splenic Neutrophil Isolation
A single-cell suspension of purified neutrophils is important for studying the biology of neutrophils. Magnetic bead isolation of neutrophils is an effective method that is frequently used for positive or negative selection of murine neutrophils (14). However, this method has at least two limitations for isolating murine spleen-resident neutrophils. First, a reliable protocol for magnetic bead separation of splenic neutrophils is lacking. In addition, the commercially available magnetic bead-based kits for isolating murine neutrophils are developed for purifying neutrophils from blood and bone marrow and not from peripheral tissue. Second, positive selection of neutrophils by either magnetic bead selection or flow cytometric cell sorting pose a risk for adversely affecting cell viability and function through direct labeling of the target cell population. We have employed a modified magnetic bead isolation method for the negative selection of neutrophils from spleen. To account for changes in the cellular composition of spleens compared to blood that would guide us in optimizing the isolation of splenic neutrophils, we measured the percentages of myeloid- and lymphoid-derived immune cell types. Flow cytometric analysis demonstrated that the percentages of cells staining positive for CD11b, Ly6G, GR-1 (Ly6G/Ly6C), F4/80, and NK1.1 were lower in spleens compared to blood of B6.Faslpr/J mice (Supporting Information Fig. 2). Although the percentages of cells staining positive for F4/80 and NK1.1 were lower in spleen compared to blood, the overall numbers of these cells were higher in spleen (data not shown). The percentages of cells staining positive for B220, CD19, CD11c, and CD3 were higher in spleen compared to blood (Supporting Information Fig. 2), indicating that these cell populations were over-represented in spleen compared to blood of B6.Faslpr mice. Based on these findings, we selected biotin-conjugated mAbs to the markers B220, CD19, CD11c, CD3, F4/80, and NK1.1 and, using increasing titers of these mAbs (0.1–10 µg/mL), performed magnetic bead isolation of splenic neutrophils by negative selection. As a result of these preliminary experiments (data not shown), we developed an OAC suited for isolating a pure population of splenic neutrophils. Because naïve healthy B6 animals consistently had low frequencies of splenic neutrophils, we developed the OAC magnetic bead isolation method of splenic neutrophils using B6.Faslpr/J mice. Enrichment using the OAC protocol consistently resulted in greater than 90% purity of splenic neutrophils compared to neutrophils before enrichment where they represented ~8% of total spleen cells (Fig. 2A and Supporting Information Fig. 3A). Because some dendritic cell subsets also express CD11b and Ly6G, we confirmed that the OAC-enriched CD11b+Ly6G+ spleen cells were neutrophils by morphology using Imagestream analysis. Results demonstrated that the CD11b+Ly6G+ cells isolated by the OAC method were neutrophils as defined by the classic polymorphonuclear granulocyte morphology (DAPI staining; Fig. 2B), which is not a feature of dendritic cells.
The half-life of circulating neutrophils in vivo is estimated to be 6–8 h (23), while in vitro, neutrophils have been reported to spontaneously undergo apoptosis in as few as 2–4 h (24). To determine whether enrichment of splenic neutrophils by the OAC protocol affected their viability, we measured the percentage of cells that excluded the viability dye, Aqua. Results demonstrated that purified splenic neutrophils maintained greater than 80% viability immediately following OAC isolation and was equivalent to the percent viability of total spleen cells before enrichment (Fig. 2C and Supporting Information Fig. 3B). Further analysis of cell viability over time demonstrated that the half-life of purified neutrophils from spleens of B6.Faslpr/J mice was between 12 and 18 h as measured by the viability ratio (Fig. 2D), calculated as the percent of viable neutrophils immediately following isolation relative to the percent of viable neutrophils cultured for the indicated times. To determine whether the half-life of OAC-purified splenic neutrophils was spontaneously affected by the B6.Faslpr/J genetic background, we measured the steady-state viability of splenic neutrophils from B6 control mice when cultured over the course of 18 h. Results demonstrated that neutrophils isolated from B6 mice (96% purity) have a kinetics of cell viability similar to B6.Faslpr/J-derived neutrophils (Fig. 2D), suggesting that the 12–18 h half-life of splenic neutrophils is independent of genetic background. These findings indicate that the OAC isolation protocol can be used for purifying murine splenic neutrophils, even when they are not over-represented in tissue, and does not adversely affect cell viability.
The OAC protocol was similarly effective at enriching neutrophils from the bone marrow (> 90% purity) and slightly less effective at isolating neutrophils from the blood (~73% purity) (Supporting Information Fig. 4A). Isolation of neutrophils from both blood and bone marrow yielded greater than 90% cell viability (Supporting Information Fig. 4B).
Comparison of the OAC Method to Previously Established Methods for Isolating Neutrophils
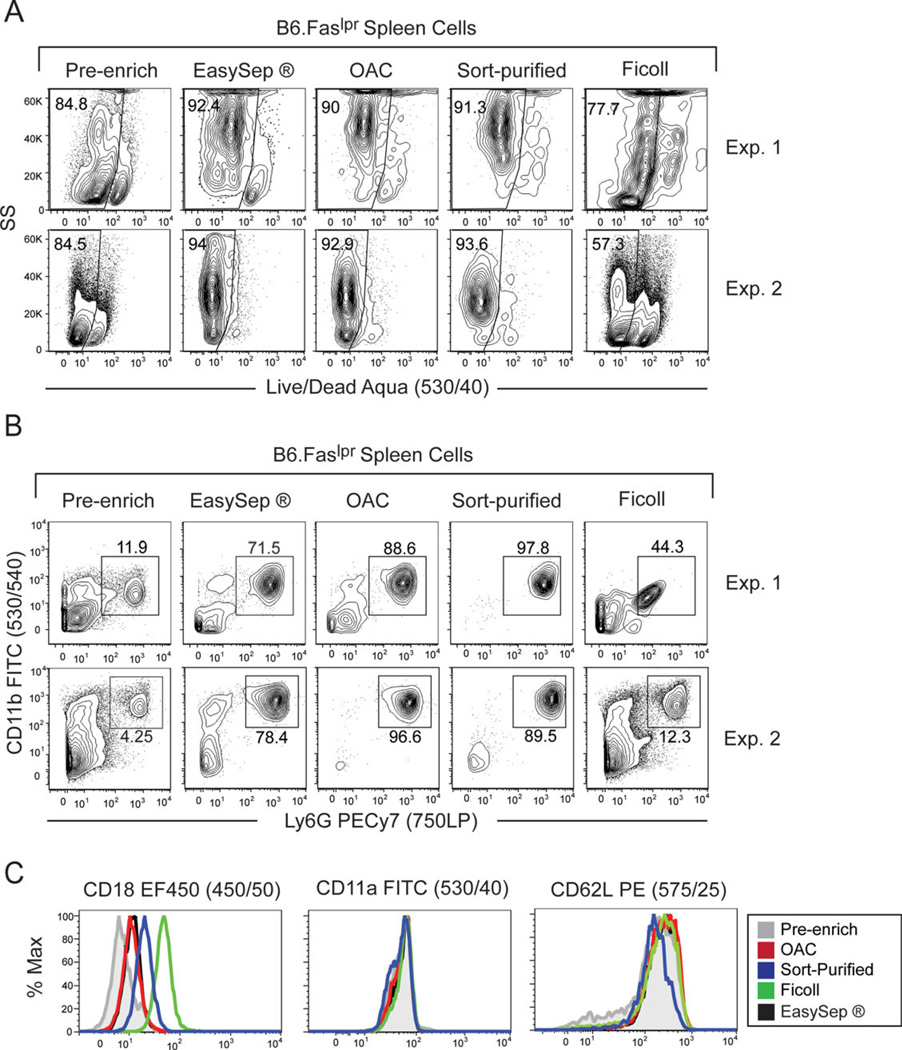
Multiple methods for isolating neutrophils have been reported in the literature (14, 15). We compared the efficacy of our OAC protocol to three different protocols for the purity of the resultant neutrophil population, cell viability, and the activation status of neutrophils following each protocol. These protocols included the EasySep magnetic bead isolation kit, fluorescence-activated cell sorting, and cell separation using the density gradient Ficoll. Using each of these protocols, we determined the viability of cells after enrichment of neutrophils from spleens of B6.Faslpr/J mice. Results from two representative experiments demonstrated that, like the OAC protocol, cell viability was greater than 90% using the EasySep and sort-purification protocols (Fig. 3A). In contrast, cell viability was consistently lower following isolation of neutrophils by the Ficoll method compared to the other protocols. Analysis of the purity of splenic neutrophils following each protocol demonstrated that the OAC and sort-purification methods resulted in greater than 89% purity (Fig. 3B). In contrast, the EasySep and Ficoll isolation protocols resulted in a much lower purity of neutrophils. Taken together, our results demonstrate that the OAC and sort-purification protocols are the most effective methods for isolating a pure population of neutrophils without compromising cell viability.
Figure 3.
Comparison of the OAC method to previously established neutrophil isolation procedures. Neutrophils were isolated from 5-month-old B6.Faslpr/J mice using the indicated method. Cell viability (A) and cell purity (B) were measured before and after each isolation protocol. Data presented from two independent experiments. (C) Expression of the activation markers CD18, CD11a, and CD62L on isolated neutrophils 4 h after purification. One representative experiment from two independent experiments is shown.
Following activation, neutrophils upregulate membrane expression of CD18 and CD11a (25, 26), lose expression of CD62L (27), and then rapidly undergo apoptosis (28). Therefore, limiting neutrophil activation brought about by the isolation procedure is crucial for the subsequent functional analysis of neutrophils. To determine if the OAC procedure activated neutrophils any differently than the other isolation methods, we measured the expression levels of CD18, CD11a, and CD62L on enriched neutrophils. Results demonstrated that neutrophils isolated by the OAC and EasySep methods displayed the least amount of activation compared to pre-enriched neutrophils, as measured by modest expression levels of CD18 and no detectable changes in CD11a and CD62L expression levels (Fig. 3C). In contrast, neutrophils that were sort-purified or enriched by Ficoll had higher expression levels of CD18 and lower expression levels of CD62L, suggesting that these isolation procedures activated neutrophils more than the OAC and EasySep procedures.
The isolation of splenic neutrophils using the OAC method can be performed within 2 h. Therefore, using equivalent numbers of single cell suspensions prepared from spleens of B6.Faslpr/J mice, we compared the OAC method to sort-purification for cell yields that can be achieved in a 2-h time period. Results from three independent experiments demonstrated that from 10 × 106 spleen cells, the OAC method yielded 3.5 × 105 ± 0.34 × 105 (mean ± SEM) neutrophils while sort-purification yielded 2.59 × 105 ± 0.27 × 105 neutrophils. Taken together, these findings suggest that the OAC method is the most effective of the four isolation procedures for isolating a high yield of pure neutrophils from spleen without significant adverse effects on cell viability and activation.
Neutrophils Isolated by the OAC Method Maintain Their Phagocytic Capacity
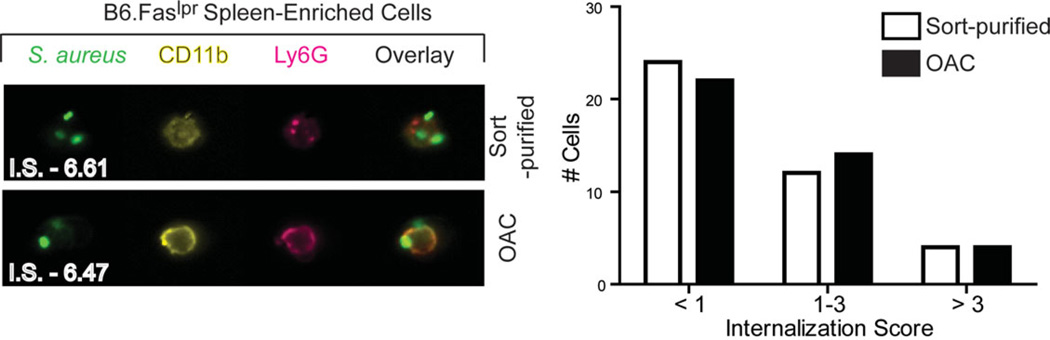
Neutrophils are professional phagocytes, capable of engulfing cell debris as well as microbial products (29). To address whether the OAC protocol altered the phagocytic function of neutrophils, we measured the capacity of neutrophils to engulf fluorescently labeled (AlexaFluor488) S. aureus particles compared to sort-purified neutrophils (30). Results demonstrated that, after 2 h of culture in the presence of S. aureus particles, ~22–23% of neutrophils engulfed S. aureus particles regardless of the isolation method (Supporting Information Fig. 5A). As expected, engulfment of S. aureus particles by neutrophils was significantly enhanced following opsonization of the particles (Ops. S. aureus). Results demonstrated that ~62–66% of neutrophils engulfed Ops. S. aureus regardless of the isolation method (Supporting Information Figs. 5A and 5B). Thus, neutrophils isolated by the OAC method maintain their phagocytic function as measured by flow cytometry. Multispectral image flow cytometric analysis of neutrophils using the ImageStreamX cytometer further confirmed the internalization of the AlexaFluor488-conjugated S. aureus particles. This was achieved by calculating the internalization score (I.S.) on individual neutrophils. The I.S. here compares the intensity of AlexaFluor488 inside the cell to the intensity of AlexaFluor488 throughout the entire cell. Thus, the greater the I.S. assigned to a cell, the greater the amount of engulfment. Results demonstrated that the ability of neutrophils to internalize opsonized S. aureus particles was not affected by the isolation procedure (Fig. 4). By measuring the I.S., nearly 50% of neutrophils had a score less than 1 (24 out of 50 cells for Sort; 22 out of 50 cells for OAC), suggesting minimal or no internalization. These results are consistent with what was observed by flow cytometry. Moderate uptake of particles, measured by an I.S. from 1 to 3, was similar between sort-purified and OAC-purified neutrophils (12 out of 50 cells for Sort; 14 out of 50 cells for OAC). High levels of uptake, measure by an I.S. greater than 3, were identical between the two isolation procedures (4 out of 50 cells; Fig. 4).
Figure 4.
Isolation of cells by the OAC and sort-purification methods result in similar phagocytic function. Neutrophils were isolated from 5-month-old B6.Faslpr/J mice using the OAC protocol or by sort-purification. Isolated neutrophils were incubated in the presence of fluorescently labeled S. aureus particles with prior opsonization for 2 h. Engulfment of opsonized S. aureus by neutrophils was confirmed by high-resolution microscopy and flow cytometry using ImageStreamX technology. One representative image from more than 250 images taken from two separate animals for each purification method is shown. Uptake of S. aureus for each neutrophil was calculated as an internalization score (I.S.). The I.S. from 50 randomly selected neutrophils for each isolation method is shown in the right panel, demonstrating that the uptake of particles by neutrophils is equivalent between the two isolation methods.
CONCLUSION
In this study, we describe a modified magnetic bead isolation protocol for enriching spleen-resident neutrophils by negative selection. EasySep and Ficoll isolation methods failed to effectively purify neutrophils and particularly, in the case of Ficoll, yielded a population of cells with low viability. Although sort-purification generates a highly pure and viable population of neutrophils, the process of sorting leads to a substantial activation of the cells. Our results demonstrate that the OAC protocol yields a pure population of neutrophils. Equally important, the OAC protocol does not adversely affect cell viability, activation, or phagocytic function. This method provides investigators with an efficient option for separating spleen-resident neutrophils, which can be used in a broad range of in vitro and in vivo experiments to study their biology.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Sebastien Coquery and Michael Solga for excellent technical assistance.
Grant sponsor: NIH (NIAID; Immunology Training Grant); Grant number: AI093722
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- 1.Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 4.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scapini P, Bazzoni F, Cassatella MA. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett. 2008;116:1–6. doi: 10.1016/j.imlet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 7.Appelberg R. Neutrophils and intracellular pathogens: Beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 9.Roussel E, Gingras MC. Transendothelial migration induces rapid expression on neutrophils of granule-release VLA6 used for tissue infiltration. J Leukoc Biol. 1997;62:356–362. doi: 10.1002/jlb.62.3.356. [DOI] [PubMed] [Google Scholar]
- 10.al-Mokdad M, Shibata F, Nakagawa H. Effects of cytokine-induced neutrophil chemoattractants (CINCs) on shape change, adhesiveness and phagocytosis of rat neutrophils. Biol Pharm Bull. 1997;20:920–923. doi: 10.1248/bpb.20.920. [DOI] [PubMed] [Google Scholar]
- 11.Kubes P, Niu XF, Smith CW, Kehrli ME, Jr, Reinhardt PH, Woodman RC. A novel b 1-dependent adhesion pathway on neutrophils: A mechanism invoked by dihydrocytochalasin B or endothelial transmigration. FASEB J. 1995;9:1103–1111. [PubMed] [Google Scholar]
- 12.Poon BY, Ward CA, Giles WR, Kubes P. Emigrated neutrophils regulate ventricular contractility via a4 integrin. Circ Res. 1999;84:1245–1251. doi: 10.1161/01.res.84.11.1245. [DOI] [PubMed] [Google Scholar]
- 13.Milot E, Filep JG. Regulation of neutrophil survival/apoptosis by Mcl-1. Scientific- WorldJournal. 2011;11::1948–1962. doi: 10.1100/2011/131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotter MJ, Norman KE, Hellewell PG, Ridger VC. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am J Pathol. 2001;159:473–481. doi: 10.1016/S0002-9440(10)61719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y, Dorf ME. Isolation of mouse neutrophils. Curr Protoc Immunol. 2001;Chapter 3, Unit 3.20:3.20.1–3.20.6. doi: 10.1002/0471142735.im0320s22. [DOI] [PubMed] [Google Scholar]
- 16.Hasenberg M, Kohler A, Bonifatius S, Borucki K, Riek-Burchardt M, Achilles J, Mann L, Baumgart K, Schraven B, Gunzer M. Rapid immunomagnetic negative enrichment of neutrophil granulocytes from murine bone marrow for functional studies in vitro and in vivo. PLoS One. 2011;6:e17314. doi: 10.1371/journal.pone.0017314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotter MJ, Muruve DA. Isolation of neutrophils from mouse liver: A novel method to study effector leukocytes during inflammation. J Immunol Methods. 2006;312:68–78. doi: 10.1016/j.jim.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Botran R, Větvička V. Advanced Methods in Cellular Immunology. Boca Raton: CRC Press; 2000. 173 pp. [Google Scholar]
- 19.Riches AC, Sharp JG, Thomas DB, Smith SV. Blood volume determination in the mouse. J Physiol. 1973;228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loike JD, Silverstein SC. A fluorescence quenching technique using trypan blue to differentiate between attached and ingested glutaraldehyde-fixed red blood cells in phagocytosing murine macrophages. J Immunol Methods. 1983;57:373–379. doi: 10.1016/0022-1759(83)90097-2. [DOI] [PubMed] [Google Scholar]
- 21.Megjugorac NJ, Jacobs ES, Izaguirre AG, George TC, Gupta G, Fitzgerald-Bocarsly P. Image-based study of interferongenic interactions between plasmacytoid dendritic cells and HSV-infectedmonocyte-derived dendritic cells. Immunol Invest. 2007;36:739–761. doi: 10.1080/08820130701715845. [DOI] [PubMed] [Google Scholar]
- 22.Cohen PL, Eisenberg RA. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 23.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 25.Anderson SI, Hotchin NA, Nash GB. Role of the cytoskeleton in rapid activation of CD11b/CD18 function and its subsequent downregulation in neutrophils. J Cell Sci. 2000;113 (Part 15)::2737–2745. doi: 10.1242/jcs.113.15.2737. [DOI] [PubMed] [Google Scholar]
- 26.Hentzen ER, Neelamegham S, Kansas GS, Benanti JA, McIntire LV, Smith CW, Simon SI. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 2000;95:911–920. [PubMed] [Google Scholar]
- 27.Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–2555. [PubMed] [Google Scholar]
- 28.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 29.Colucci-Guyon E, Tinevez JY, Renshaw SA, Herbomel P. Strategies of professional phagocytes in vivo: Unlike macrophages, neutrophils engulf only surface-associated microbes. J Cell Sci. 2011;124:3053–3059. doi: 10.1242/jcs.082792. [DOI] [PubMed] [Google Scholar]
- 30.Benati D, Ferro M, Savino MT, Ulivieri C, Schiavo E, Nuccitelli A, Pasini FL, Baldari CT. Opposite effects of simvastatin on the bactericidal and inflammatory response of macrophages to opsonized S aureus. J Leukoc Biol. 2010;87:433–442. doi: 10.1189/jlb.0409273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.