Abstract
Background
Acute coronary syndrome is a leading cause of death in developed countries. Follistatin-like 1 (FSTL1) is a myocyte-derived secreted protein that is upregulated in the heart in response to ischemic insult. Here, we investigated the therapeutic impact of FSTL1 on acute cardiac injury in small and large preclinical animal models of ischemia/reperfusion and dissected its molecular mechanism.
Methods and Results
Administration of human FSTL1 protein significantly attenuated myocardial infarct size in a mouse or pig model of ischemia/reperfusion, which was associated with a reduction of apoptosis and inflammatory responses in the ischemic heart. Administration of FSTL1 enhanced the phosphorylation of AMP-activated protein kinase in the ischemia/reperfusion–injured heart. In cultured cardiac myocytes, FSTL1 suppressed apoptosis in response to hypoxia/reoxygenation and lipopolysaccharide-stimulated expression of proinflammatory genes through its ability to activate AMP-activated protein kinase. Ischemia/reperfusion led to enhancement of bone morphogenetic protein-4 expression and Smad1/5/8 phosphorylation in the heart, and FSTL1 suppressed the increased phosphorylation of Smad1/5/8 in ischemic myocardium. Treating cardiac myocytes with FSTL1 abolished the bone morphogenetic protein-4 –stimulated increase in apoptosis, Smad1/5/8 phosphorylation, and proinflammatory gene expression. In cultured macrophages, FSTL1 diminished lipopolysaccharide-stimulated expression of proinflammatory genes via activation of AMP-activated protein kinase and abolished bone morphogenetic protein-4 – dependent induction of proinflammatory mediators.
Conclusions
Our data indicate that FSTL1 can prevent myocardial ischemia/reperfusion injury by inhibiting apoptosis and inflammatory response through modulation of AMP-activated protein kinase– and bone morphogenetic protein-4 – dependent mechanisms, suggesting that FSTL1 could represent a novel therapeutic target for post-myocardial infarction, acute coronary syndrome.
Keywords: apoptosis, inflammation, ischemia, myocytes, cardiac, reperfusion
Coronaryheart disease, including acute coronary syndrome, is one of the major causes of morbidity and mortality worldwide.1 Successful reperfusion therapy for acute coronary syndrome with percutaneous coronary intervention can minimize myocardial infarction and prevent heart failure, thus leading to reduced mortality.2–4 However, tissue damage and pathological remodeling can be a side effect of myocardial reperfusion.1,5 Thus, a strategy to protect against cardiac injury and dysfunction in response to tissue ischemia and reperfusion could be useful as an adjunct therapy for the treatment of post-myocardial infarction, acute coronary syndrome.
Accumulating evidence indicates that heart tissue secretes a variety of bioactive molecules, also known as cardiokines, that modulate the cellular processes in the heart, including cardiac remodeling, in an autocrine, paracrine, or endocrine manner.6 –9 Cardiokines include atrial natriuretic peptide, brain natriuretic peptide, adrenomedullin, and protease inhibitor 16.10 –13 Several cardiokines are upregulated in response to cardiac stress and play important roles in cardiac pathology, implying that cardiokines may serve as both biomarkers and targets for the management of the disease process. Follistatin-like 1 (FSTL1), also known as TSC-36, is a secreted glycoprotein that belongs to the follistatin family of proteins.14 Follistatin family members bind to transforming growth factor-β superfamily proteins and inhibit their functions.15 We have previously shown that FSTL1 is a cardiokine that is upregulated in ischemia-injured and hypertrophic hearts of mice.16 It has been reported that the FSTL1 transcript is increased in the myocardium in patients with end-stage heart failure.17 We have demonstrated that elevated levels of circulating FSTL1 are associated with chronic systolic heart failure and that FSTL1 protein expression is increased in the failing heart in humans.18 Furthermore, circulating FSTL1 levels are elevated in patients with acute coronary syndrome.19,20 Thus, FSTL1 can serve as a useful biomarker of cardiac disease.
The functional role of FSTL1 in the regulation of cardiovascular disease has also been investigated. We have shown that systemic delivery of adenoviral vectors encoding murine Fstl1 can prevent myocardial ischemia/reperfusion (I/R) injury in mice, which is associated with reduced myocyte apoptosis.16 We have also shown that overexpression of Fstl1 promotes revascularization in response to hind-limb ischemia in mice.21 Recently, we have demonstrated that Fstl1 attenuates cardiac hypertrophy in response to pressure overload in mice.22 From these genetic gain- and loss-of-function experiments, we conclude that Fstl1 has broad cardiovascular-protective activities. However, the therapeutic impact of acute FSTL1 administration on cardiac injury in preclinical models has not been previously investigated. Furthermore, the molecular mechanism by which FSTL1 exerts the protective actions on the heart is incompletely understood. Here, we investigated the therapeutic effects of human FSTL1 protein on cardiac injury and remodeling in preclinical models of myocardial I/R and assessed its molecular mechanism.
Methods
An expanded Methods section can be found in the online-only Data Supplement.
Statistical Analysis
Data are presented as mean±SE. The Student t test was performed for comparison between 2 independent groups. One-way ANOVA was performed for comparison of ≥3 independent groups. The Fisher protected least-significant-difference test was used only if the overall comparison by 1-way ANOVA was statistically significant. All continuous variables were assumed to be normally distributed. A value of P<0.05 denoted the presence of a statistically significant difference. Given the large number of statistical comparisons performed, all at the 0.05 level of significance may result in the possibility of a type I error.
Results
Administration of FSTL1 Protein Reduces Myocardial Infarct Size After I/R in Murine and Porcine Models
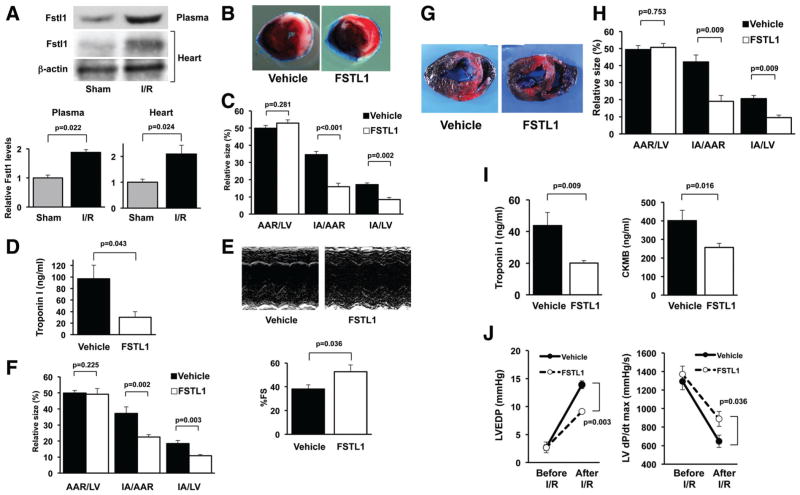
To examine the role of Fstl1 in the ischemic heart, Fstl1 protein expression was measured in the myocardium and plasma in male wild-type C57BL/6J mice after I/R injury or sham surgery. Myocardial I/R injury led to an increase in Fstl1 protein expression in the heart compared with sham operation (Figure 1A). Plasma Fstl1 levels were also increased after myocardial I/R. These data indicate that Fstl1 may be released from the heart during I/R.
Figure 1.
Administration of follistatin-like 1 (FSTL1) protein decreases myocardial infarct size in animal models of ischemia/reperfusion (I/R). A, Fstl1 protein levels in the heart and plasma in mice at 24 hours after myocardial I/R injury or sham operation. Fstl1 protein expression was analyzed by Western blotting and expressed relative to β-actin levels (n=4). B and C, Systemic delivery of human FSTL1 protein reduces myocardial infarct size in mouse I/R models. After intravenous injection of human FSTL1 protein (100 ng/g mouse) or vehicle, wild-type mice were subjected to 60 minutes of ischemia followed by 24 hours of reperfusion. Representative photographs of the mouse heart sections stained with Evans blue and subsequent 2,3,5-triphenyl tetrazolium chloride (TTC) are shown (B). Left ventricular (LV) area, the area at risk (AAR), and the infarct area (IA) were measured, and quantitative analysis of infarct size is shown (C; n=7– 8). D, FSTL1 reduces plasma troponin I levels of mice at 3 hours after I/R (n=4). E, Administration of FSTL1 protein improves LV function as assessed by echocardiography. Representative M-mode echocardiograms for vehicle- or FSTL1-treated mice at 24 hours after I/R are shown (top); quantitative analysis of LV fractional shortening (%FS) in mice treated with vehicle or FSTL1 protein is also shown (bottom; n=5). F, FSTL1 administration during reperfusion reduces infarct size in mice. Recombinant human FSTL1 protein (100 ng/g mouse) or vehicle was administered intravenously to mice at 5 minutes after reperfusion. Quantitative analysis of infarct size is shown (n=7). G and H, Intracoronary administration of human FSTL1 protein suppresses myocardial infarct size after I/R in pigs. Pigs were subjected to 45 minutes of ischemia followed by 24 hours of reperfusion, and recombinant human FSTL1 protein (3 μg/kg pig) or vehicle was injected through the wire lumen of the catheter during the first 10 minutes of ischemia. Representative photographs of the heart sections stained with Evans blue and TTC are shown (G). Quantitative analysis of the AAR/LV, IA/AAR, and IA/LV ratios is shown (H; n=5). I, FSTL1 treatment inhibits circulating levels of troponin I and creatine phosphokinase-MB (CKMB) in pigs at 24 hours after I/R (n=5). J, FSTL1 decreases LV end-diastolic pressure (LVEDP) and increases LV dP/dtmax in pigs at 24 hours after I/R (n=3–5).
To test whether systemic delivery of FSTL1 protein affects acute cardiac ischemic injury in mice, male C57BL/6J mice were intravenously treated with recombinant human FSTL1 protein or vehicle and subsequently subjected to 60 minutes of myocardial ischemia and 24 hours of reperfusion. Figure 1B shows the representative photographs of the heart sections stained with Evans blue dye to delineate the area at risk (AAR) and 2,3,5-triphenyl tetrazolium chloride (TTC) to delineate the infarct area (IA) at 24 hours after reperfusion. Administration of FSTL1 protein significantly reduced the ratios of IA to AAR and of IA to left ventricle (LV) by 54±3% and 51±3%, respectively (Figure 1C). There were no significant differences in the AAR/LV ratios between the 2 groups. Treating mice with FSTL1 protein also reduced the plasma levels of troponin I, a marker of heart damage, at 3 hours after I/R (Figure 1D). Furthermore, treatment with FSTL1 significantly increased LV fractional shortening in mice at 24 hours after myocardial I/R as measured by echocardiography (Figure 1E).
To further assess whether FSTL1 administration during reperfusion can affect myocardial injury, we intravenously administered recombinant human FSTL1 protein or vehicle to mice at 5 minutes after reperfusion. Administration of FSTL1 protein reduced the IA/AAR and IA/LV ratios by 40±4% and 41±4%, respectively, compared with vehicle (Figure 1F). Thus, FSTL1 administration, either before ischemia or after reperfusion, is effective at minimizing cardiac ischemic damage in mice.
To examine the effects of FSTL1 on cardiac injury in a large-animal model of I/R, female Yorkshire-Duroc pigs were subjected to 45 minutes of ischemia and 24 hours of reperfusion. Intracoronary injection of recombinant human FSTL1 protein or vehicle was performed via the wire lumen of the catheter during the first 10 minutes of cardiac ischemia. Figure 1G shows representative photographs of the pig heart sections stained with Evans blue dye and TTC. Quantitative analysis of infarct size demonstrated that the intracoronary administration of FSTL1 protein attenuated the IA/AAR and IA/LV ratios by 55±5% and 54±5%, respectively (Figure 1H). Treatment of pigs with FSTL1 protein also reduced the circulating levels of troponin I and creatine phosphokinase-MB, another marker of heart damage, at 24 hours after I/R (Figure 1I).
To examine the actions of FSTL1 on cardiac function in the porcine model, hemodynamic parameters were determined before the induction of ischemia and at 24 hours after I/R with a manometer-tipped catheter. All parameters before the I/R operation were not significantly different between FSTL1- and vehicle-treated pigs. FSTL1 administration to pigs led to a decrease in LV end-diastolic pressure and an increase in dP/dtmax at 24 hours after I/R (Figure 1J).
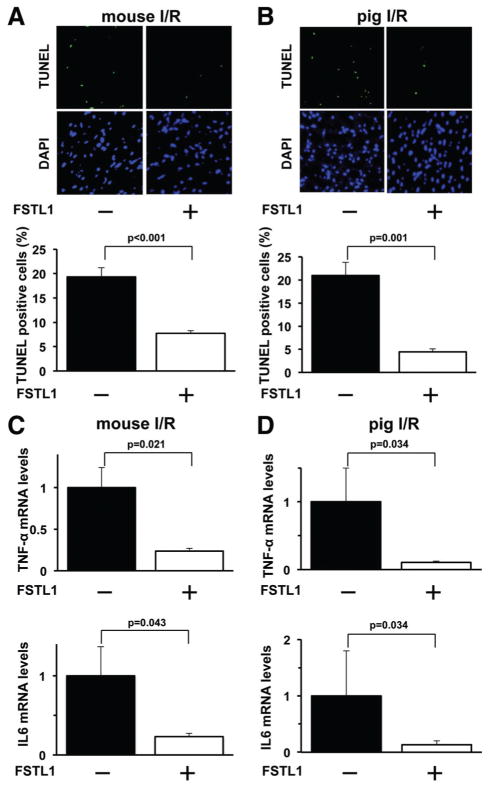
Delivery of FSTL1 Protein Suppresses Apoptosis and Inflammatory Responses in Ischemic Heart In Vivo
Apoptosis is the key feature of various pathological heart conditions.23,24 To investigate whether administration of FSTL1 protein modulates the apoptosis in the ischemic hearts, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining in the AAR regions was performed. Figure 2A shows representative photographs of TUNEL-positive nuclei in the heart in mice. Systemic delivery of FSTL1 protein significantly reduced the frequency of TUNEL-positive cells in the mouse hearts after I/R compared with vehicle treatment (Figure 2A). Similarly, intracoronary administration of FSTL1 to pigs led to a marked reduction of the percentage of TUNEL-positive cells in the myocardium after I/R (Figure 2B).
Figure 2.
Administration of follistatin-like 1 (FSTL1) protein attenuates apoptosis and inflammatory responses in the ischemic myocardium of mice and pigs. A, Systemic delivery of FSTL1 attenuates apoptosis in the ischemic heart of mice. Upper panels show representative photographs of mouse heart sections stained with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; green) and DAPI (blue). Lower graph shows quantitative analysis of TUNEL-positive nuclei (n=5). B, Intracoronary administration of FSTL1 to pigs inhibits apoptosis in the ischemic myocardium. Upper panels show representative pictures of heart sections stained with TUNEL (green) and DAPI (blue). Lower graph shows quantitative analysis of TUNEL-positive nuclei (n=4). C and D, Delivery of human FSTL1 suppresses expression of proinflammatory cytokines in the ischemic hearts of mice (C) and pigs (D). The mRNA expression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) was measured by reverse transcription–polymerase chain reaction method and expressed relative to β-actin levels (n=5). I/R indicates ischemia/reperfusion.
Because inflammation contributes to myocardial injury after I/R,25–27 the expression of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), was evaluated in the ischemic hearts on the different experimental groups by quantitative reverse transcription–polymerase chain reaction. Systemic administration of FSTL1 protein to mice attenuated the mRNA levels of TNF-α and IL-6 in the I/R-injured hearts (Figure 2C). Similarly, intracoronary injection of FSTL1 protein significantly reduced the transcript levels of TNF-α and IL-6 in the pig heart after I/R (Figure 2D).
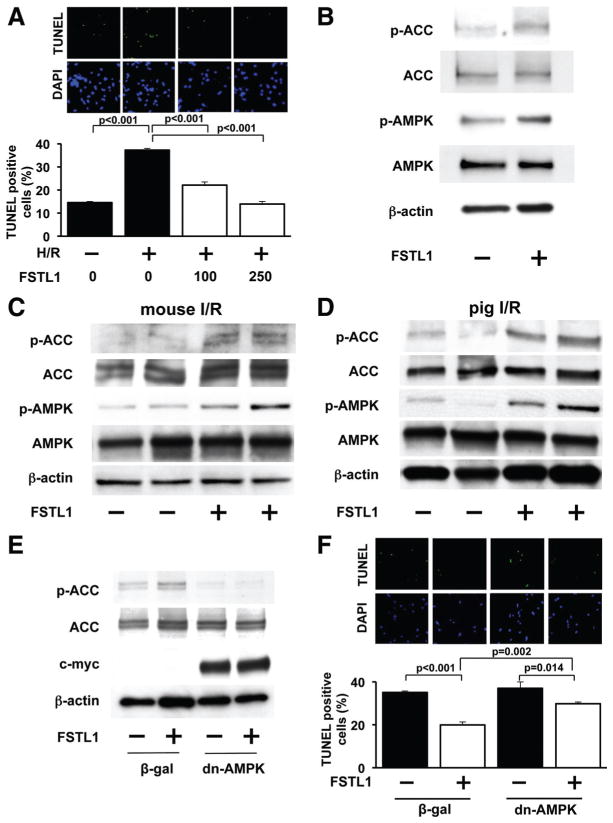
FSTL1 Attenuates Apoptosis of Cardiac Myocytes via Activation of AMP-Activated Protein Kinase and Inhibition of Bone Morphogenetic Protein-4 Signaling
To investigate whether FSTL1 directly influences cardiomyocyte apoptosis, neonatal rat ventricular myocytes (NRVMs) were cultured under conditions of normoxia or hypoxia/reoxygenation (H/R) in the presence of recombinant human FSTL1 protein or vehicle. H/R significantly increased the percentage of TUNEL-positive cells, whereas treatment with FSTL1 protein at concentrations of 100 and 250 ng/mL attenuated the frequency of TUNEL-positive cells (Figure 3A).
Figure 3.
Follistatin-like 1 (FSTL1) inhibits apoptosis via activation of AMP-regulated protein kinase (AMPK). A, FSTL1 suppresses hypoxia/reoxygenation (H/R)–induced apoptosis of neonatal rat ventricular myocytes (NRVMs). NRVMs were treated with FSTL1 protein (100 or 250 ng/mL) or vehicle under conditions of normoxia or H/R. NRVMs were stained with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; green) and DAPI (blue), and quantitative analysis of TUNEL-positive myocytes was performed (n=4). B, FSTL1 promotes AMPK phosphorylation in NRVMs. NRVMs were treated with FSTL1 protein (250 ng/mL) or vehicle for 30 minutes. The phosphorylation status of acetyl-coenzyme A carboxylase (p-ACC) and AMPK (p-AMPK) was analyzed by Western blotting. C and D, FSTL1 promotes AMPK signaling pathways in I/R-injured hearts of mice (C) and pigs (D). The phosphorylation of ACC and AMPK was assessed by Western blot analysis. Representative blots are shown from 4 independent experiments. E, AMPK inhibition abolishes FSTL1-induced ACC phosphorylation as assessed by Western blot analysis. After transduction with a dominant-negative form of AMPK tagged with c-myc (Ad-dn-AMPK) or β-galactosidase (Ad-β-gal) at a multiplicity of infection of 10 for 24 hours, NRVMs were treated with FSTL1 (250 ng/mL) or vehicle for 30 minutes. Representative blots are shown from 3 independent experiments. F, AMPK is involved in the suppressive action of FSTL1 on H/R-induced apoptosis of NRVMs. NRVMs were transduced with Ad-dn-AMPK or Ad-β-gal and cultured in the presence or absence of FSTL1 (250 ng/mL) under conditions of H/R. Apoptotic nuclei were identified by TUNEL staining (n=4).
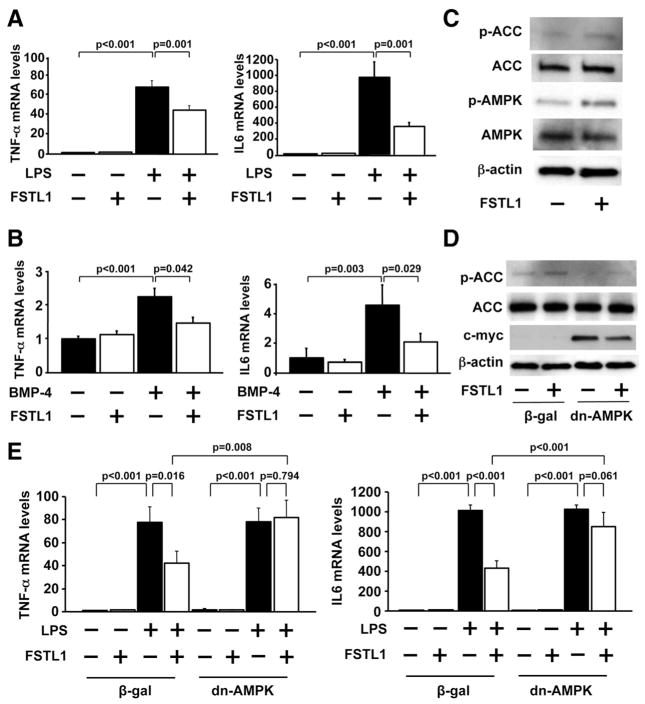
Because activation of AMP-activated protein kinase (AMPK) protects cardiomyocytes from apoptosis,28 –30 we investigated the effects of FSTL1 on AMPK signaling pathways. In a cell culture model, treatment of NRVMs with FSTL1 protein augmented the activating phosphorylation levels of AMPK at Thr-172, which was accompanied by increased phosphorylation of its downstream substrate acetyl-coenzyme A carboxylase (ACC) at Ser-79 (Figure 3B). Systemic injection of human FSTL1 protein also promoted the phosphorylation of AMPK and ACC in the ischemic heart of mice (Figure 3C), and the intracoronary delivery of FSTL1 protein enhanced the phosphorylation of AMPK and ACC in I/R-injured hearts of pigs (Figure 3D).
To investigate the involvement of AMPK signaling in antiapoptotic effects of FSTL1 in cardiac myocytes, NRVMs were transduced with adenoviral vectors expressing a dominant-negative form of AMPK tagged with c-myc (Ad-dn-AMPK) or β-galactosidase (Ad-β-gal) as a control, followed by treatment with human FSTL1 protein or vehicle. FSTL1-induced phosphorylation of ACC in NRVMs was abolished by transduction with Ad-dn-AMPK (Figure 3E). Transduction with Ad-dn-AMPK also inhibited the suppressive effects of FSTL1 on H/R-induced apoptosis of NRVMs (Figure 3F), indicating that the actions of FSTL1 on myocyte survival are partly dependent on its ability to activate AMPK.
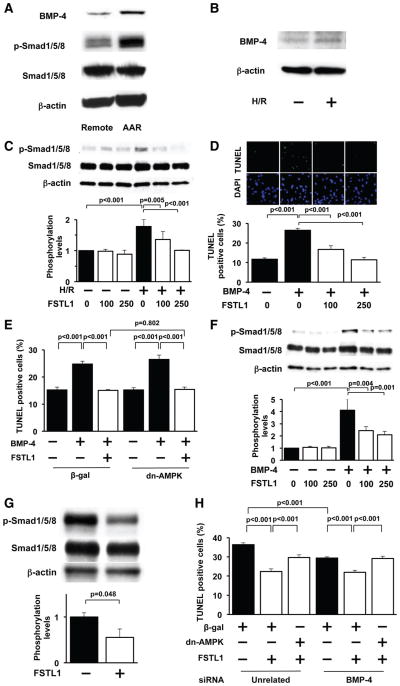
FSTL1 has been reported to bind to bone morphogenetic protein-4 (BMP-4) and to antagonize BMP-4, thereby regulating lung development.31 It has also been shown that BMP-4 promotes the apoptosis of cardiac myocytes.32 These findings led us to speculate that BMP-4 signaling contributes to the cardioprotective actions of FSTL1. Myocardial I/R in pigs led to an increase in BMP-4 protein expression in AAR regions, which was accompanied by an increase in phosphorylation levels of Smad1/5/8, a downstream target of BMP-4 (Figure 4A). Likewise, H/R stimulation significantly increased BMP-4 protein expression in cultured NRVMs (Figure 4B). H/R also stimulated the phosphorylation of Smad1/5/8 in NRVMs, which was abolished by treatment with FSTL1 protein at concentrations of 100 and 250 ng/mL (Figure 4C). Treatment of NRVMs with recombinant BMP-4 protein significantly increased the percentage of TUNEL-positive cells under normoxic conditions, and the simultaneous addition of FSTL1 protein to BMP-4 abolished the BMP-4 –induced increase in TUNEL-positive cells (Figure 4D). Transduction with Ad-dn-AMPK did not affect the inhibitory actions of FSTL1 on BMP-4 –stimulated myocyte apoptosis under conditions of normoxia (Figure 4E). BMP-4 treatment also increased the phosphorylation levels of Smad1/5/8, which were diminished by the addition of FSTL1 protein (Figure 4F). Moreover, intracoronary infusion of FSTL1 protein significantly reduced the phosphorylation levels of Smad1/5/8 in the ischemic myocardium of pigs (Figure 4G). These data indicate that FSTL1 promotes the survival of cardiomyocytes in part via inhibition of BMP-4 signaling that is independent of AMPK.
Figure 4.
Follistatin-like 1 (FSTL1) antagonizes bone morphogenetic protein-4 (BMP-4) signaling in the ischemic heart and cardiac myocytes. A, BMP-4 is upregulated in the heart in response to ischemia/reperfusion (I/R). Representative blots of BMP-4 and phosphorylated Smad1/5/8 (p-Smad1/5/8) at remote nonischemic area (remote) and ischemic area at risk (AAR) of pigs are shown from 4 independent experiments. B, Hypoxia/reoxygenation (H/R) increases the expression of BMP-4 in NRVMs. Representative blots of BMP-4 are shown from 4 independent experiments. C, FSTL1 attenuates the phosphorylation of Smad1/5/8 in neonatal rat ventricular myocytes (NRVMs) under conditions of H/R. NRVMs were treated with FSTL1 (100 or 250 ng/mL) or vehicle under conditions of normoxia or H/R. The phosphorylation levels of Smad1/5/8 (p-Smad1/5/8) were analyzed by Western blotting and expressed relative to β-actin levels (n=3). D, FSTL1 abolishes BMP-4 –induced apoptosis of NRVMs. NRVMs were treated with BMP-4 protein (100 ng/mL) or vehicle along with FSTL1 protein (100 or 250 ng/mL) or vehicle for 18 hours under normoxic conditions. Top, Representative pictures of NRVMs stained with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; green) and DAPI (blue). Bottom, Quantitative analysis of TUNEL-positive NRVMs (n=4). E, Effect of AMP-regulated protein kinase (AMPK) inactivation on FSTL1-mediated inhibition of BMP4-stimulated apoptosis of NRVMs. NRVMs were transduced with dominant-negative form of AMPK tagged with c-myc (Ad-dn-AMPK) or β-galactosidase (Ad-β-gal) at a multiplicity of infection of 10 for 24 hours and treated with BMP-4 protein (100 ng/mL) or vehicle along with FSTL1 protein (250 ng/mL) or vehicle for 18 hours under normoxic conditions (n=4). F, FSTL1 suppresses BMP-4 –stimulated phosphorylation of Smad1/5/8 in NRVMs. NRVMs were treated with BMP-4 protein (100 ng/mL) or vehicle along with FSTL1 protein (100 or 250 ng/mL) or vehicle for 18 hours under normoxic conditions. The phosphorylation levels of Smad1/5/8 (p-Smad1/5/8) were determined by Western blot analysis and expressed relative to β-actin levels (n=4). G, Intracoronary administration of FSTL1 attenuates the phosphorylation of Smad1/5/8 in the ischemic myocardium in pigs. Top, Representative blots of p-Smad1/5/8, Smad1/5/8, and β-actin. Bottom, Quantitative analysis of relative phosphorylation levels of Smad1/5/8 (n=4). H, Involvement of BMP-4 antagonization and AMPK activation in the inhibitory action of FSTL1 on H/R-induced myocyte apoptosis. NRVMs are transfected with siRNAs targeting BMP-4 or unrelated siRNAs (40 nmol/L) and transduced with an Ad-dn-AMPK or Ad-β-gal followed by treatment with FSTL1 (250 ng/mL) or vehicle (n=4).
To assess the relative contribution of BMP-4 antagonization and AMPK activation to the cardioprotection by FSTL1, NRVMs were transfected with siRNAs targeting BMP-4 or unrelated siRNAs and transduced with Ad-dn-AMPK or Ad-β-gal, followed by treatment with FSTL1 protein or vehicle under H/R conditions. Transfection of NRVMs with siRNAs against BMP-4 led to a reduction of BMP-4 mRNA expression by 69±3%. Knockdown of BMP-4 suppressed the apoptosis of NRVMs in the absence of FSTL1 under conditions of H/R (Figure 4H). Treatment of NRVMs with FSTL1 significantly attenuated the H/R-stimulated apoptosis under conditions of BMP-4 ablation, which was reversed by transduction with Ad-dn-AMPK. Furthermore, knockdown of BMP-4 did not affect the H/R-stimulated apoptosis of NRVMs in the presence of FSTL1. Collectively, these data indicate that FSTL1 reduces H/R-induced apoptosis through 2 independent pathways involving activation of AMPK and inhibition of BMP-4 signaling.
FSTL1 Reduces Inflammatory Responses in Cultured Cardiac Myocytes
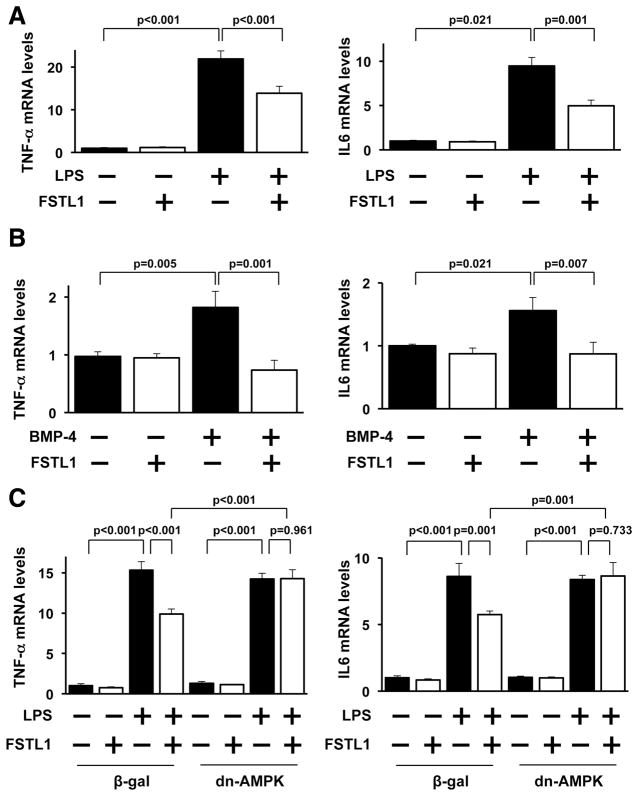
To analyze the antiinflammatory actions of FSTL1 at the cellular level, NRVMs were pretreated with human FSTL1 protein or vehicle, followed by stimulation with lipopolysac-charide (LPS). LPS exposure increased the mRNA levels of TNF-α and IL-6 in NRVMs, and FSTL1 treatment significantly suppressed the LPS-induced increases in TNF-α and IL-6 expression (Figure 5A). Stimulation with BMP-4 protein also increased the mRNA levels of TNF-α and IL-6 in NRVMs, and BMP-4 –induced increases in TNF-α and IL-6 expression were completely blocked by the addition of FSTL1 (Figure 5B).
Figure 5.
Follistatin-like 1 (FSTL1) suppresses inflammatory responses in cultured cardiac myocytes. A, FSTL1 diminishes lipopolysaccharide (LPS)-stimulated expression of proinflammatory genes in neonatal rat ventricular myocytes (NRVMs). NRVMs were pretreated with FSTL1 (250 ng/mL) or vehicle for 30 minutes, followed by stimulation with LPS (100 ng/mL) or vehicle for 6 hours. The mRNA expression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) was measured by reverse transcription–polymerase chain reaction (RT-PCR) and expressed relative to β-actin levels (n=4). B, Effect of FSTL1 on bone morphogenetic protein-4 (BMP-4)–stimulated expression of proinflammatory cytokines. NRVMs were treated with BMP-4 protein (100 ng/mL) or vehicle along with FSTL1 protein (100 or 250 ng/mL) or vehicle for 18 hours. The transcript levels of TNF-α and IL-6 were determined by RT-PCR and expressed relative to β -actin levels (n=4). C, AMPK participates in the effect of FSTL1 on the LPS-induced increase in proinflammatory gene expression. After transduction with a dominant-negative form of AMPK tagged with c-myc (Ad-dn-AMPK) or β -galactosidase (Ad-β -gal) at a multiplicity of infection of 10 for 24 hours, NRVMs were pre-treated with FSTL1 (250 ng/mL) or vehicle for 30 minutes, followed by treatment with LPS (100 ng/mL) or vehicle for 6 hours. The mRNA levels were analyzed by RT-PCR and expressed relative to β -actin levels (n=4).
To determine the potential contribution of AMPK signaling to the antiinflammatory actions of FSTL1 in cardiomyocytes, NRVMs were transduced with Ad-dn-AMPK or Ad-β -gal in the presence or absence of FSTL1 protein, followed by exposure to LPS. Transduction with Ad-dn-AMPK reversed the suppressive actions of FSTL1 on LPS-stimulated expression of TNF-β and IL-6 in NRVMs (Figure 5C).
FSTL1 Inhibits Expression of Inflammatory Mediators in Cultured Macrophages
Macrophages are one of the major types of cells that produce proinflammatory cytokines during myocardial I/R.33,34 To test the actions of FSTL1 on inflammatory responses in macrophages, RAW264.7 macrophage cells were pretreated with human FSTL1 or vehicle, followed by stimulation with LPS. LPS treatment dramatically stimulated the mRNA levels of TNF-α and IL-6 in cultured macrophages, which were significantly attenuated by FSTL1 protein (Figure 6A). BMP-4 stimulation also increased the expression of TNF-α and IL-6 in macrophages, and treatment with FSTL1 completely blocked the upregulation of TNF-α and IL-6 induced by BMP-4 (Figure 6B).
Figure 6.
Follistatin-like 1 (FSTL1) protein diminishes inflammatory responses in cultured macrophages. A, FSTL1 inhibits lipopolysaccharide (LPS)-stimulated expression of proinflammatory genes in macrophages. Macrophage RAW264.7 cells were cultured in the presence of FSTL1 (250 ng/mL) or vehicle for 30 minutes, followed by treatment with LPS (100 ng/mL) or vehicle for 6 hours. The mRNA expression of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) was quantified by reverse transcription–polymerase chain reaction (RT-PCR) analysis and expressed relative to β-actin levels (n=4). B, FSTL1 abolishes bone morphogenetic protein-4 (BMP-4)–stimulated expression of proinflammatory mediators in macrophages. Macrophages were treated with BMP-4 protein (100 ng/mL) or vehicle along with FSTL1 protein (250 ng/mL) or vehicle for 18 hours. The transcript levels of TNF-α and IL-6 were analyzed by RT-PCR and expressed relative to β-actin levels (n=4). C, FSTL1 promotes the AMP-regulated protein kinase (AMPK) signaling pathway in macrophages. Macrophages were treated with FSTL1 (250 ng/mL) or vehicle for 15 minutes. The phosphorylation levels of ACC (p-ACC) and AMPK (p-AMPK) were determined by Western blot analysis. D, AMPK inactivation cancels FSTL1-stimulated ACC phosphorylation in macrophages as determined by Western blot analysis. Macrophages were transduced with Ad- dominant-negative form of AMPK tagged with c-myc (Ad-dn-AMPK) or β-galactosidase (Ad-β-gal), and treated with FSTL1 (250 ng/mL) or vehicle for 15 minutes. E, AMPK signaling is involved in FSTL1-mediated inhibition of LPS-stimulated expression of proinflammatory cytokines. After transduction with Ad-dn-AMPK or Ad-β-gal, macrophages were treated with FSTL1 (250 ng/mL) or vehicle for 30 minutes followed by stimulation with LPS (100 ng/mL) or vehicle for 6 hours. Transcript levels were determined by RT-PCR analysis and expressed relative to β-actin levels (n=4).
To assess the participation of AMPK in the inhibitory actions of FSTL1 on inflammatory response in macrophages, AMPK signaling pathways were assessed in cultured macrophages by Western blot analysis. Treatment of macrophages with FSTL1 protein resulted in an increase in phosphoryla-tion of AMPK and ACC (Figure 6C). Transduction with Ad-dn-AMPK blocked the FSTL1-induced increase in ACC phosphorylation in cultured macrophages (Figure 6D). Furthermore, transduction with Ad-dn-AMPK cancelled the inhibitory effects of FSTL1 on LPS-stimulated expression of TNF-α and IL-6 in macrophages (Figure 6E). Thus, the antiinflammatory actions of FSTL1 may be partly dependent on activation of AMPK in macrophages.
AMPK Contributes to the Myocardial Infarct–Sparing Effect of FSTL1
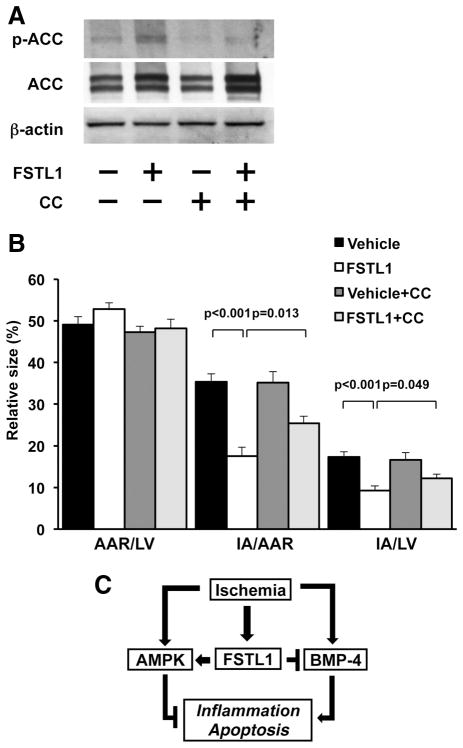
To further analyze the involvement of AMPK in FSTL1-mediated protection from acute cardiac injury in vivo, we intraperitoneally injected AMPK inhibitor compound C into mice. Treatment with compound C significantly diminished the FSTL1-induced increase in ACC phosphorylation in the ischemic heart (Figure 7A). Compound C had little effect on ACC phosphorylation of ischemic heart in vehicle-treated mice. Although compound C did not influence myocardial infarct size in vehicle-treated mice, it significantly reversed the suppressive actions of FSTL1 on myocardial infarct area of mice after I/R (Figure 7B). These data suggest that AMPK is involved in the beneficial actions of FSTL1 on acute ischemic injury in the heart.
Figure 7.
AMP-regulated protein kinase (AMPK) is involved in the myocardial infarct-sparing effect of follistatin-like 1 (FSTL1). A, Effect of AMPK inactivation on acetyl-coenzyme A carboxylase (ACC) phosphorylation in the ischemic heart. AMPK inhibitor compound C (CC; 20 mg/kg) dissolved in dimethyl sulfoxide (DMSO) or DMSO was injected intraperitoneally into mice. After intravenous injection of human FSTL1 protein (100 ng/g mouse) or vehicle, wild-type mice were subjected to ischemia/reperfusion (I/R). The phosphorylation of ACC (p-ACC) in the ischemic heart was assessed by Western blot analysis. Representative blots are shown from 4 independent experiments. B, Role of AMPK in FSTL1-mediated inhibition of infarct size. Quantitative analysis of the ratios of area at risk (AAR) to left ventricle (LV), infarct area (IA) to AAR, and the IA to LV is shown (n=6). C, Proposed scheme for the mechanism by which FSTL1 protects the heart from ischemic injury. FSTL1 is upregulated in the heart and plasma in response to ischemic insult. FSTL1 promotes AMPK signaling pathways in the ischemic heart, thereby leading to a reduction of inflammation and apoptosis. FSTL1 also antagonizes bone morphogenetic protein-4 (BMP-4)– dependent proinflammatory and proapoptotic signals in the myocardium. Therefore, FSTL1 confers beneficial actions on the ischemic hearts by reducing inflammatory response and apoptosis through modulation of AMPK- and BMP-4 – dependent mechanisms.
Discussion
Our study provides the first evidence that administration of FSTL1 protein improves acute myocardial injury and dysfunction after I/R in preclinical animal models. Systemic delivery of recombinant human FSTL1 protein to mice led to reductions of myocardial infarct size, systolic dysfunction, apoptosis, and inflammatory responses after I/R. Intracoronary injection of FSTL1 protein attenuated myocardial infarction and improved cardiac function in pigs after I/R, which was associated with suppression of apoptosis and inflammation in the ischemic heart. These data are consistent with our previous findings that adenovirus-mediated delivery of mouse Fstl1 is effective at attenuating myocardial I/R injury in mice.16 Our in vitro experiments showed that FSTL1 promotes the survival of cardiac myocytes in response to H/R and that FSTL1 attenuated agonist-stimulated expression of proinflammatory mediators in cardiac cells and macrophages. Thus, FSTL1 administration can protect the heart from ischemic damage through at least 2 mechanisms involving the reduction of cardiomyocyte death and suppression of inflammatory responses in myocardial cells. Because therapeutic approaches to minimize cell death and inflammation in the heart are believed to be logical strategies to treat acute cardiac injury, administration of FSTL1 may be a useful adjunctive therapy for post-myocardial infarction, acute coronary syndrome.
Increased apoptosis is a key feature of the pathological cardiac remodeling that occurs in response to ischemia.23,24 In the present study, FSTL1 attenuated apoptosis in the ischemic heart in animal models of I/R, which is associated with enhanced activation of AMPK. Our in vitro data showed that FSTL1 promoted AMPK phosphorylation in cardiac myocytes and that FSTL1 suppressed cardiomyocyte apoptosis in response to H/R partly via activation of AMPK, which is independent of inhibition of BMP-4 signaling. Our data also showed that I/R injury led to an increase in cardiac BMP-4 expression, which was associated with enhanced phosphorylation of Smad1/5/8. Administration of FSTL1 diminished the phosphorylation of Smad1/5/8 in the ischemic heart. In cultured myocytes, H/R stimulation increased BMP-4 expression and enhanced the phosphorylation of Smad1/5/8, which was abolished by FSTL1. Furthermore, FSTL1 abrogated BMP-4-induced apoptosis of myocytes via an AMPK-independent mechanism. It has been shown that BMP-4 heterozygous knockout mice exhibit reductions in myocardial infarct size and apoptosis after I/R.32 Therefore, the salutary effects of FSTL1 on cardiac injury under conditions of ischemia appear to be mediated, at least in part, through its abilities to both activate AMPK and inhibit BMP-4 signaling, leading to a suppression of cell death (Figure 7C).
Previous reports have demonstrated that BMP-4 acts as a proinflammatory mediator in endothelial cells.35,36 In line with these findings, treatment of cultured myocytes and macrophages with BMP-4 stimulated proinflammatory gene expression. The proinflammatory action of BMP-4 on myocytes and macrophages could be abolished by treatment with FSTL1. Similarly, FSTL1 abrogated the proapoptotic effects of BMP-4 on myocytes. Consistent with these observations, FSTL1 has been shown to directly bind to BMP-4 and to inhibit its signaling in cultured hepatoma cells.31 Taken together, the favorable effects of FSTL1 on acute ischemic damage in the myocardium appear to be mediated through its abilities to antagonize both the proinflammatory and proapoptotic functions of BMP-4 (Figure 7C).
Our data indicate that the suppressive actions of FSTL1 on inflammatory responses in ischemic heart are partly dependent on its ability to activate AMPK signaling in its target cells (Figure 7C). Administration of FSTL1 protein markedly reduced the expression of proinflammatory mediators in ischemic areas of myocardium in vivo. Treatment of cultured cardiomyocytes or macrophages with FSTL1 protein led to a reduction of proinflammatory gene expression in response to LPS. Moreover, FSTL1 enhanced the phosphorylation of AMPK in ischemic regions of the heart and in cultured cardiac myocytes and macrophages, and the antiinflammatory actions of FSTL1 in LPS-treated cells are reversed by inhibition of AMPK activation. These results are consistent with previous observations that activation of AMPK signaling negatively regulates the inflammatory responses in various types of cells, including macrophages.37
Myocardial AMPK is activated during various stresses, including ischemia and hypertrophy, and activation of AMPK signaling under these conditions is thought to confer a protective role.38 Thus, hormones or reagents that activate AMPK may protect the heart from ischemic injury and hypertrophy. In this regard, the cardioprotective effects of adiponectin and macrophage migration inhibitory factor are mediated in part through their ability to activate AMPK signaling.30,39,40 Recently, we have shown that FSTL1 functions to reduce myocardial hypertrophy in response to pressure overload by promoting AMPK activation.22 Here, we have extended these findings by showing that FSTL1-mediated activation of myocardial AMPK has salutary effects on ischemic heart disease in vivo and in vitro.
It has been shown that administration of FSTL1 protein improves joint inflammation in a model of antibody-induced arthritis.41 Overexpression of FSTL1 promotes heart allograft survival, which is associated with reduced expression of proinflammatory genes, including IL-6.42 In agreement with these findings, our data show that administration of FSTL1 attenuates the expression of TNF-α and IL-6 in ischemic heart in vivo and that FSTL1 treatment downregulates LPS-or BMP-4 –stimulated expression of TNF-α and IL-6 in cultured cardiac myocytes and macrophages. In contrast, Fstl1 overexpression is reported to exacerbate collagen-induced arthritis associated with enhanced expression of proinflammatory cytokines.43,44 The discrepancies among these studies may result from differences in experimental models or the context of other regulatory molecules that influence FSTL1 function. Thus, future research is required to clarify the role of FSTL1 in the regulation of inflammatory responses under various pathological conditions.
Conclusions
We show that FSTL1 can prevent apoptosis and inflammatory responses in the heart during I/R through its ability to promote AMPK signaling and to antagonize BMP-4 function, thereby contributing to protection against acute cardiac injury (Figure 7C). In particular, we demonstrated the effectiveness of FSTL1 treatment for myocardial damage and dysfunction in response to ischemia in a preclinical porcine I/R model that is applicable to human acute coronary syndrome,45 indicating the potential clinical utility of FSTL1. Collectively, these data suggest that FSTL1 represents a novel target molecule for the treatment of the pathological cardiac remodeling.
Supplementary Material
CLINICAL PERSPECTIVE.
Heart tissue secretes a variety of bioactive molecules, also known as cardiokines, that modulate the cellular processes in the heart. Follistatin-like 1 (FSTL1) is a cardiokine that is upregulated in the heart in response to ischemic insult. In the present study, we investigated the therapeutic impact of FSTL1 on acute cardiac injury in small and large preclinical animal models of ischemia/reperfusion. Systemic delivery of recombinant human FSTL1 protein to mice led to reductions of myocardial injury and dysfunction. Furthermore, intracoronary injection of human FSTL1 protein attenuated myocardial infarction and improved cardiac function in pigs after ischemia/reperfusion. The beneficial actions of FSTL1 were associated with suppression of apoptosis and inflammation in the ischemic heart. FSTL1 promoted AMP-activated protein kinase signaling pathways in the ischemic heart, thereby leading to a reduction of inflammation and apoptosis. FSTL1 also antagonized the proinflammatory and proapoptotic functions of bone morphogenetic protein-4. Therefore, FSTL1 can prevent myocardial ischemia/reperfusion injury by inhibiting apoptosis and inflammatory response through modulation of AMP-activated protein kinase– and bone morphogenetic protein-4 – dependent mechanisms. Our observations suggest that FSTL1 could represent a novel therapeutic target for the treatment of post-myocardial infarction, acute coronary syndrome.
Acknowledgments
We thank Boston Scientific Japan for allowing us to use their animal laboratory (Miyazaki T&E Center, Miyazaki, Japan). We gratefully acknowledge the technical assistance of Yoko Inoue, Miho Sakai, Naoki Komaki, Takao Kawaguchi, Hiromi Kanasugi, Shinobu Ando, Kazuko Ishikawa, Koichi Yamaguchi, Takahiro Uryu, Kazuyo Noda, Takuya Kan, and Yutaka Kose.
Sources of Funding
This work was supported by a Grant-in-Aid for Scientific Research and grants from the Takeda Science Foundation, Suzuken Memorial Foundation, Japan Research Foundation for Clinical Pharmacology, Senshin Medical Research Foundation, and Uehara Memorial Foundation to Dr Ouchi. Dr Shibata was supported by Grant-in-Aid for Young Scientists B and the Uehara Memorial Foundation. Dr Ohashi was supported with the Grant-in-Aid for Young Scientists B and the Cardiovascular Research Fund, Tokyo, Japan.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.112.115089/-/DC1.
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18– e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange RA, Hillis LD. Immediate angioplasty for acute myocardial infarction. N Engl J Med. 1993;328:726–728. doi: 10.1056/NEJM199303113281010. [DOI] [PubMed] [Google Scholar]
- 3.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM. Decline in rates of death and heart failure in acute coronary syndromes, 1999 –2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 4.Simoons ML, Windecker S. Controversies in cardiovascular medicine: chronic stable coronary artery disease: drugs vs. revascularization. Eur Heart J. 2010;31:530–541. doi: 10.1093/eurheartj/ehp605. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Fedak PW, Weisel RD, Butany J, Rao V, Maitland A, Li RK, Dhillon B, Yau TM. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation. 2002;105:2332–2336. doi: 10.1161/01.cir.0000016602.96363.36. [DOI] [PubMed] [Google Scholar]
- 6.Burley DS, Hamid SA, Baxter GF. Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev. 2007;12:279–291. doi: 10.1007/s10741-007-9029-y. [DOI] [PubMed] [Google Scholar]
- 7.Glembotski CC. Functions for the cardiomyokine, MANF, in cardioprotection, hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:512–517. doi: 10.1016/j.yjmcc.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, Sussman MA, Glembotski CC. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ Res. 2008;103:1249–1258. doi: 10.1161/CIRCRESAHA.108.180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doroudgar S, Glembotski CC. The cardiokine story unfolds: ischemic stress-induced protein secretion in the heart. Trends Mol Med. 2011;17:207–214. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–1763. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura R, Kato J, Kitamura K, Onitsuka H, Imamura T, Cao Y, Marutsuka K, Asada Y, Kangawa K, Eto T. Adrenomedullin administration immediately after myocardial infarction ameliorates progression of heart failure in rats. Circulation. 2004;110:426– 431. doi: 10.1161/01.CIR.0000136085.34185.83. [DOI] [PubMed] [Google Scholar]
- 12.Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K, Toyokuni S, Yutani C, Kangawa K. Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation. 2004;109:242–248. doi: 10.1161/01.CIR.0000109214.30211.7C. [DOI] [PubMed] [Google Scholar]
- 13.Frost RJ, Engelhardt S. A secretion trap screen in yeast identifies protease inhibitor 16 as a novel antihypertrophic protein secreted from the heart. Circulation. 2007;116:1768–1775. doi: 10.1161/CIRCULATIONAHA.107.696468. [DOI] [PubMed] [Google Scholar]
- 14.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 16.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–5827. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 18.El-Armouche A, Ouchi N, Tanaka K, Doros G, Wittkopper K, Schulze T, Eschenhagen T, Walsh K, Sam F. Follistatin-like 1 in chronic systolic heart failure: A marker of left ventricular remodeling. Circ Heart Fail. 2011;4:621– 627. doi: 10.1161/CIRCHEARTFAILURE.110.960625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widera C, Horn-Wichmann R, Kempf T, Bethmann K, Fiedler B, Sharma S, Lichtinghagen R, Leitolf H, Ivandic B, Katus HA, Giannitsis E, Wollert KC. Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin Chem. 2009;55:1794–1800. doi: 10.1373/clinchem.2009.129411. [DOI] [PubMed] [Google Scholar]
- 20.Widera C, Giannitsis E, Kempf T, Korf-Klingebiel M, Fiedler B, Sharma S, Katus HA, Asaumi Y, Shimano M, Walsh K, Wollert KC. Identification of follistatin-like 1 by expression cloning as an activator of the growth differentiation factor 15 gene and a prognostic biomarker in acute coronary syndrome. Clin Chem. 2012;58:1233–1241. doi: 10.1373/clinchem.2012.182816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJ, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011;108:E899–E906. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbate A, Bussani R, Amin MS, Vetrovec GW, Baldi A. Acute myocardial infarction and heart failure: role of apoptosis. Int J Biochem Cell Biol. 2006;38:1834–1840. doi: 10.1016/j.biocel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekhar Y. Role of apoptosis in ventricular remodeling. Curr Heart Fail Rep. 2005;2:18–22. doi: 10.1007/s11897-005-0003-5. [DOI] [PubMed] [Google Scholar]
- 25.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240–247. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 26.Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, LaRosa GJ, Hawkins HK, Smith CW, Michael LH, Entman ML, Rossen RD. Complement C5A, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684– 692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- 27.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, Ma XL. Amp-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119:835– 844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 30.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, Nakajima M, Furuichi T, Ikegawa S, Gao X, Chen YG, Jiang D, Ning W. Follistatin-like 1 (FSTL1) is a bone morphogenetic protein (bmp) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pachori AS, Custer L, Hansen D, Clapp S, Kemppa E, Klingensmith J. Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. J Mol Cell Cardiol. 2010;48:1255–1265. doi: 10.1016/j.yjmcc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Formigli L, Manneschi LI, Nediani C, Marcelli E, Fratini G, Orlandini SZ, Perna AM. Are macrophages involved in early myocardial reperfusion injury? Ann Thorac Surg. 2001;71:1596–1602. doi: 10.1016/s0003-4975(01)02400-6. [DOI] [PubMed] [Google Scholar]
- 34.Zuidema MY, Zhang C. Ischemia/reperfusion injury: the role of immune cells. World J Cardiol. 2010;2:325–332. doi: 10.4330/wjc.v2.i10.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 36.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 39.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 40.Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, Shintani S, Walsh K, Ouchi N, Murohara T. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166–173. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660– 668. doi: 10.1002/art.20023. [DOI] [PubMed] [Google Scholar]
- 42.Le Luduec JB, Condamine T, Louvet C, Thebault P, Heslan JM, Heslan M, Chiffoleau E, Cuturi MC. An immunomodulatory role for follistatin-like 1 in heart allograft transplantation. Am J Transplant. 2008;8:2297–2306. doi: 10.1111/j.1600-6143.2008.02398.x. [DOI] [PubMed] [Google Scholar]
- 43.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, Robbins P, Hirsch R. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758– 4762. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 44.Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–1088. doi: 10.1002/art.33422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.