Abstract
Background
Addictive behaviors such as cigarette smoking and coffee drinking have been associated with a reduced risk of Parkinson disease. Whether alcohol consumption is also associated with risk is less certain.
Methods
We prospectively followed 132,403 participants in the Cancer Prevention Study II Nutrition Cohort from 1992 to 2005. Alcohol intake was assessed at baseline. Incident cases of Parkinson Disease (n = 605; 389 male and 216 female) were confirmed by treating physicians and medical record review. Relative risks were estimated using proportional hazards models, adjusting for age, smoking and other risk factors.
Results
Alcohol consumption was not significantly associated with Parkinson Disease risk. After adjustment for age, smoking, and other risk factors, the Relative Risk comparing men consuming 30 or more grams of alcohol (highest category) to non-drinker men was 1.29 (95% CI: 0.90, 1.86, p-trend: 0.40) and the Relative Risk comparing women consuming 15 or more grams of alcohol (highest category) per day to non-drinker women was 0.77 (95% CI: 0.41, 1.45, p-trend: 0.87). Consumption of beer, wine or liquor was also not associated with Parkinson Disease risk.
Conclusions
The results of this large prospective study do not support an association between alcohol intake and risk of Parkinson disease.
INTRODUCTION
Smoking and coffee drinking, two common addictive behaviors, have been consistently associated with reduced risk of Parkinson disease (PD).1–4 A protective effect of alcohol intake on PD risk has also been suggested by some studies,5–7 but this relationship is less certain and requires further investigation.
One hypothesis to explain the observed association of smoking and caffeine intake with risk of PD is that of a pre-morbid personality characterized by temperance, conscientiousness and risk aversion. Such a personality may lead persons destined to develop PD to avoid novelty-seeking and addictive behaviors or more easily discontinue them, years before the diagnosis of PD.8 Alternatively, it is possible that persons who go on to develop PD have an underlying metabolism (genetic or as a result of a toxic insult early in life) that makes them less vulnerable to the use of addicting substances.8 Under either of these scenarios, it is likely that alcohol intake would also be linked to a lower risk of PD.
Alternatively, it is possible that caffeinated beverages and cigarette smoke contain biological substances that render them protective against the neurodegeneration underlying PD, independently of their addictive nature. Several biological mechanisms have been proposed for the neuroprotective effects of caffeine and tobacco constituents such as nicotine9–12 and monoamine oxidase B inhibitors.13, 14 It is also conceivable that some components of alcoholic beverages, such as the flavonoids in red wine could have neuroprotective properties.15 Also, it is possible that inverse associations observed in prior case-control studies on alcohol and PD were due to residual confounding by smoking and caffeine use.
To distinguish between these scenarios, and to gain a deeper understanding of the role that addictive behaviors play in predisposition to PD, it is important to clearly understand the extent of the association between alcohol intake and risk of PD. Therefore, we examined in the Cancer Prevention Study (CPS) II – Nutrition cohort, a large prospective study of men and women, whether total intake of alcohol as well as the intake of beer, wine and liquor at baseline was related to altered risk of PD.
METHODS
Study population
Established in 1992, the CPS-II Nutrition cohort is a subgroup of the approximately 1.2 million members of the American Cancer Society CPS II mortality cohort.16 It includes 184,190 participants (86,404 men and 97,786 women) from 21 U.S. states, who reported their medical histories, lifestyle characteristics, and dietary habits in response to a mailed baseline (1992–1993) questionnaire.16 Selection into the CPS-II Nutrition cohort was based on residence in states with cancer registries16, which is not likely to bias our study on the relationship between alcohol intake and Parkinson disease. In 2001, as described previously,17 as part of cohort follow-up, participants were asked to report if they had ever been diagnosed with PD. Every two years thereafter participants were asked whether they had a new diagnosis of PD. We included in this study 132,509 (58,388 men and 74,121 women) cohort participants who returned one or more of the 2001, 2003 or 2005 questionnaires and had neither the symptoms nor the diagnosis of PD at study baseline in 1992. We excluded subjects who returned none of the 2001, 2003 or 2005 questionnaires (36,839), who were missing 15% or more of the dietary questions on the 1992 questionnaire or had caloric intake outside the reasonable ranges (N = 12,973), who left all of the beverage section on the questionnaire (including the alcohol questions) blank (N = 466), who were missing alcohol (N = 24)or who had an unreadable response to any of the alcohol questions were also excluded from the analysis (N = 1239). We also made exclusions for confirmed PD cases that had onset before baseline (1992) (134) and unconfirmed cases prior to baseline (5), as discussed below. This study was approved by the Human Subjects Committee at the Harvard School of Public Health (HSPH) and the Institutional Review Board (IRB) at Emory University.
Assessment of alcohol intake
On the 1992 baseline questionnaire, alcoholic beverage consumption over the previous year was assessed using the 68-item modified Block food frequency questionnaire 18. Participants recorded their usual intake of beer, wine or wine coolers, and liquor, separately, according to serving size (i.e., small, medium, large) and to one of nine possible frequencies (from never or less than once per month, 1–3 drinks per month, one per week, 2–4 per week, 5–6 per week, 1 per day, 2–3 per day, 4–5 per day, or six more per day). Estimated gram weights for alcoholic beverages consumed per day were estimated based on age, gender, beverage, and serving size originally obtained from national survey data 19, and multiplying that number by frequency to obtain grams of beverage per day. Average total number of alcoholic beverages consumed per day was calculated by summing the contribution from each type. Total ethanol consumption (in grams/day) was calculated assuming an average alcohol content of 12.3 g for 4 ounces (118 g) glass of wine, 13.9g for a 33 cL (356 g) can of beer, and 14g for a 1.5oz (42 g) serving of liquor.20 In a validation study using four random 24-hour recalls over a one year period as the comparison measure, the energy adjusted, de-attenuated correlation coefficients for grams of alcoholic beverages reported was 0.80 for men and 0.79 for women 21.
Alcohol intake was assessed again in 1999, and participants were asked to assess their average total use of typical serving sizes of liquor (1 drink or shot), white and red wine (4 oz glass), and beer and light beer (1 glass, bottle or can) according to one of ten possible frequencies (never, less than once per month, 1–3 drinks per month, 1 drink per week, 2–4 drinks per week, 2–4 drinks per week, 5–6 drinks per week, 1 drink per day, 2–3 drinks per ay, 4–5 drinks per day or 6+ drinks per day). The calculation of total ethanol consumption and consumption from beer, wine and liquor from these values was analogous for that described above.
Parkinson Disease Case Ascertainment
The procedure for PD case ascertainment is described in detail in our prior publications in this cohort. 4, 22, 23 Briefly, all CPSII-N participants who reported a diagnosis of PD on the 2001, 2003 and 2005 follow-up questionnaires were asked for permission to contact their treating neurologists and obtain copies of their medical records. The treating neurologists (or internists, who were contacted if the neurologists did not respond) were asked to fill out a diagnostic questionnaire or to send us a copy of the participant’s medical record. Questions on the questionnaire included those on cardinal signs of PD (rest tremor, rigidity, bradykinesia, and postural instability), response to levodopa treatment, and the presence of other symptoms or features to support a diagnosis of PD or suggest an alternative diagnosis. For the purpose of this study, cases were deemed confirmed if the PD diagnosis was considered definite or probable by the treating neurologist or internist, or if the medical record indicated a final diagnosis of PD made by a neurologist or evidence at a neurological evaluation of at least two of the four cardinal signs (with one being rest tremor or bradykinesia), a progressive course, and the absence of unresponsiveness to levodopa or dopaminergic agonist or of other features suggesting an alternative diagnosis. To confirm PD cases reported in the 2003 and 2005 follow-up questionnaires, similar procedures were implemented except that copies of the medical records were requested for all cases and these were reviewed by a movement disorder specialist (M.A.S.).
Within the cohort, 1810 participants self-reported PD in 2001, 2003 or 2005 and returned a follow-up consent form. Of these, 1055 consented to have their medical records reviewed, 246 confirmed that they have PD but did not consent to medical record review, 328 denied having PD, 54 refused to participate and 127 had died. A diagnosis of PD was confirmed in 865 cases. Of the confirmed cases, we excluded 134 because their symptoms onset was before the baseline survey.. An additional 58 cases were excluded because they had either left the entire beverage section blank, had unreadable values for the alcohol variables or had caloric intakes outside the reasonable range and 68 cases were excluded because they were not considered to be definite or probable PD by the reviewing neurologist. Thus, 605 incident cases were included in this analysis, 76% confirmed by the treating neurologists or movement disorders specialists, 13% by the review of neurological medical records, and 11% by the treating internists or family physicians. Sensitivity analyses were performed including an additional 217 cases (148 men and 69 women) who reported a diagnosis of PD but did not provide permission to review their medical records.
Statistical Analyses
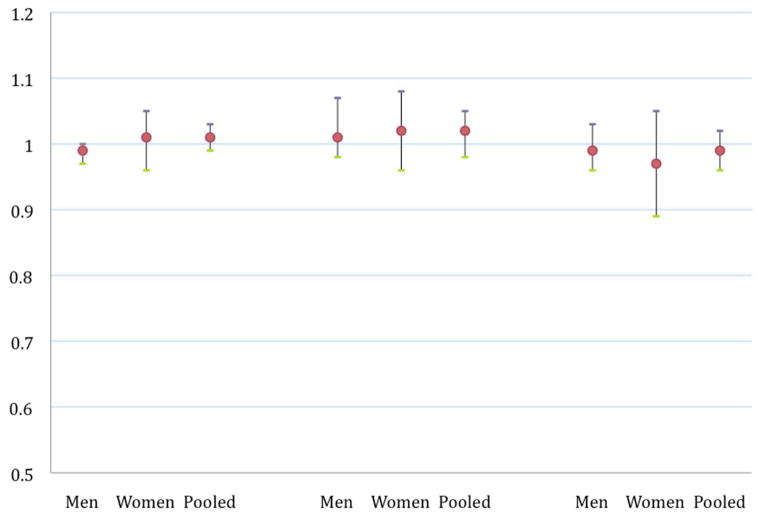
Follow-up in this study was defined from the date of return of the 1992 questionnaire to the earlier of date of return of the latest complete questionnaire (August 31, 2001, 2003 or 2005 respectively), date of onset of the first symptoms of PD or date of death. Total alcohol intake was analyzed as a categorical variable, with the median value in each category used to create a continuous variable for linear trend tests. Beer, wine and liquor were analyzed both as categorical variables (results not presented) and as continuous variables with relative risks (RR’s) calculated for 10g/day increments (Figure 1).
Figure 1.
Relative rates and 95% confidence intervals for Parkinson’s disease incidence by the g/day* of beer, wine or liquor at baseline (1992)a
*modeled as a linear variable in increments of 10g/day
We used Cox proportional hazards models to calculate multivariate relative risks (RR) with adjustment for model 1) age in months and model 2) age in months, smoking in quintiles of pack years, and coffee intake (<3 cups/week, 3–6 cups/week, 1 cup/day, 2–3 cups/day, 4–5 cups/day, 4–5 cups/day >=6 cups/day). We also considered adjustment for caloric intake, dairy intake, use of ibuprofen, physical activity and baseline body mass index from baseline, and pesticide exposure and education from 10 years prior to baseline in the parent CPS-II cohort, but estimates did not change significantly after these additional adjustments, and we thus report the age, smoking and caffeine adjusted estimates. We calculated 95 percent confidence intervals (95% CI) for all relative risks.
We performed several sensitivity analyses. Because smoking has been consistently associated with PD risk, we performed analyses stratified on smoking status (ever/never). In women, we performed analyses stratified by use of hormone replacement therapy (HRT). Also, because PD patients may change their alcohol consumption prior to diagnosis due to early symptoms, we performed further lag-time analyses excluding the first five years of follow up. Finally, 217 PD cases (148 men and 69 women) in this study reported that they had been diagnosed with PD but did not give consent for us to contact their treating neurologist or internist. To address possible bias from their exclusion, we repeated our analysis including these participants.
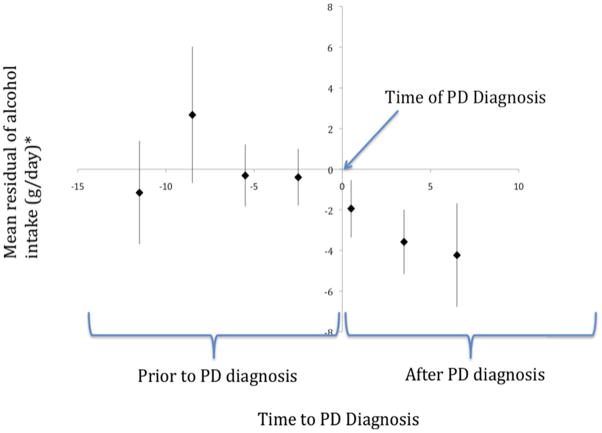
The protective association of alcohol with risk of PD reported in case-control studies could be due to patients reducing their alcohol consumption after the PD diagnosis. In our cohort, among individuals with PD the mean alcohol intake was higher before diagnosis than after diagnosis (mean difference = −1.28 g/day (SD 12.39 g/day). Because this change may be due to aging rather than to a specific effect of PD on alcohol consumption, we also examined whether alcohol consumption changed during the years leading up to and following a diagnosis of PD, as compared with changes in individuals without PD of the same age, smoking status and sex over the same period of time. For this purpose, we fitted two linear regression models among all cohort participants (with and without PD) with alcohol intake (in g/day, as reported in 1992 for one model and in 1999 for the other) as the dependent variable, and age, sex and smoking (never/former/current) as predictors. The regression residuals of these models reflect the difference between each individual reported alcohol intake and that expected at the same time for individuals of the same age, sex, and smoking status. The time to diagnosis (y-axis in Figure 2) was defined as the difference in time between when data on alcohol intake was collected for a each individual (1992 or 1999 questionnaire) and their date of first symptoms of PD; this time was negative if the symptoms onset before the questionnaire and positive if the symptoms of PD onset after; participants who reported their alcohol intake in both 1992 and 1999 contributed two points to the analysis. The times to diagnosis were combined into 3 year categories. Using the time of diagnosis as the reference, we then plotted the mean of the alcohol residuals in each e-year category of time to diagnosis, combining data from 1992 and 1999. We tested the statistical significance of changes in alcohol consumption in individuals with PD as compared to individual without PD using generalized estimating equations24, accounting for the date of the questionnaire (1992, 1999) used to measure alcohol intake. The observational period included all available data and spanned from 13 years prior to diagnosis to 6 years after to diagnosis. Separate tests were conducted for the whole observational period and then separately for the interval before the sharp decline in alcohol intake (13 to 4 years before the diagnosis) and for the period during which the decline occurred (4 years before diagnosis to 6 years after diagnosis).. All p-values reported are two sided (α = 0.05).
Figure 2.
Residual and 95% confidence intervals of alcohol intake (g/day) over time from diagnosis in PD cases adjusting for age, gender, smoking and time period (alcohol data from the 1992 and 1999 questionnaires). Zero represents date of PD diagnosis.
*Mean residual (difference between actual and expected based on a model that included age, smoking and gender) within each category of time to PD diagnosis. Based on the figure, alchol intake begins to decline, on average 4 years prior to PD diagnosis. The p values for trend were 0.002 for the whole observational period, 0.94 for the period 13 years prior to diagnosis to 4 years prior to diagnosis and 0.008 for the period starting 4 years prior to the date of diagnosis and thereafter.
RESULTS
During the follow up, we documented a total of 389 male and 216 female individuals who were diagnosed with incident PD. The mean age of the cohort participants at baseline was 63.5 years in men and 61.8 years in women. The mean age at diagnosis of the PD cases was 72.6 years in men and 72.2 years in women. Heavy alcohol users were more likely to smoke and drink coffee, and had lower dairy consumption compared to moderate and non-users. (Table 1)
Table 1.
Baseline characteristics of Participants in the Cancer Prevention Study II Nutrition Cohort by alcohol intake(g/day)
| Alcohol (g/day) in 1992 Category
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||
|
| ||||||||||
| 0 | 1–9.9 | 10–19.9 | 20–29.9 | >=30 | 0 | 1–4.9 | 5–9.9 | 10–14.9 | >=15 | |
|
| ||||||||||
| N | 113 | 145 | 71 | 18 | 42 | 108 | 56 | 17 | 24 | 11 |
| Age, y | 64.0 | 64.0 | 64.0 | 64.0 | 63.9 | 62.3 | 62.3 | 62.3 | 62.4 | 62.3 |
| BMI in 1992 (mean) | 26.6 | 26.4 | 26.0 | 26.2 | 26.3 | 26.3 | 25.4 | 24.5 | 24.0 | 24.2 |
| MET-h/week exercise | 12.4 | 13.8 | 14.8 | 14.8 | 13.5 | 11.43 | 12.5 | 13.6 | 13.2 | 13.9 |
| Current smokers, % | 7 | 6 | 7 | 8 | 13 | 6 | 7 | 9 | 11 | 15 |
| Former smokers, % | 48 | 58 | 65 | 66 | 69 | 26 | 39 | 47 | 53 | 58 |
| Total energy intake, kcal/d | 1742 | 1739 | 1796 | 1878 | 2131 | 1334 | 1351 | 1365 | 1405 | 1555 |
| Coffee intake (1982), cups/d | 2.6 | 2.8 | 2.9 | 3.1 | 3.2 | 2.2 | 2.6 | 2.8 | 2.8 | 2.8 |
| Dairy intake, g/d | 393.0 | 376.9 | 348.3 | 324.2 | 312.9 | 359.7 | 356.1 | 336.0 | 325.8 | 293.8 |
| Pesticide exposure (1982), % | 10 | 8 | 6 | 6 | 6 | 4 | 3 | 3 | 3 | 3 |
| Ibuprofen use, % | 5 | 4 | 4 | 4 | 5 | 7 | 7 | 7 | 7 | 8 |
| Highest education high school graduation, % | 22 | 18 | 14 | 11 | 16 | 36 | 29 | 25 | 22 | 21 |
| Highest education : some graduate school or above% | 23 | 28 | 31 | 33 | 27 | 11 | 14 | 16 | 17 | 16 |
Overall, total alcohol intake at baseline was not associated with risk of PD. In men, the RR comparing those in the highest category of alcohol intake (30 grams or more per day) to those reporting no alcohol consumption was 1.29 (95% CI: 0.90, 1.86, p-trend = 0.40). In women, the RR comparing those in the highest category of alcohol consumption (15 grams or more per day) to non-drinkers was 0.77 (95% CI: 0.41, 1.45; p-trend = 0.87) (Table 2). Smoking was the strongest confounder included in multivariate models.
Table 2.
Relative Risk (RR) and 95% Confidence Intervals (CI) for Parkinson disease incidence by total alcohol intake (g/day) at baseline
| Alcohol intake (g/day) | Cases | Person Years | Age-Adjusted RR | Mltva RR1 | p-trend |
|---|---|---|---|---|---|
| Men | |||||
| 0 | 113 | 227,337 | 1.00 (Ref) | 1.00 (Ref) | |
| <9.9 | 145 | 239,705 | 1.27 (0.99, 1.63) | 1.36 (1.06, 1.74) | |
| 10–19.9 | 71 | 110,309 | 1.32 (0.98, 1.79) | 1.48 (1.09, 2.01) | |
| 20–29.9 | 18 | 37,078 | 1.00 (0.61, 1.65) | 1.15 (0.69, 1.90) | |
| 30+ | 42 | 85,072 | 1.06 (0.74, 1.52) | 1.29 (0.90, 1.86) | 0.40 |
| Women | |||||
| 0 | 108 | 413,660 | 1.00 (Ref) | 1.00 (Ref) | |
| <4.9 | 56 | 252,206 | 0.90 (0.65, 1.25) | 0.95 (0.68, 1.31) | |
| 5–9.9 | 17 | 83,315 | 0.85 (0.51, 1.43) | 0.95 (0.57, 1.60) | |
| 10–14.9 | 24 | 65,984 | 1.41 (0.91, 2.20) | 1.67 (1.06, 2.64) | |
| 15+ | 11 | 77,163 | 0.59 (0.32, 1.10) | 0.77 (0.41, 1.45) | 0.87 |
Adjusted for age in months and pack-years of smoking (never-smokers, 1–9, 10–19, 20–29, 30–39, ≥ 40 pack-years) and coffee intake (<3 cups/week, 3–6 cups/week, 1 cup/day, 2–3 cups/day, 4–5 cups/day, 4–5 cups/day ≥ 6 cups/day).
RR: relative rate; CI: confidence interval.
Consumption of beer, wine, or liquor was not associated with altered risk of PD (Figure 1). In pooled analyses combining men and women, assuming a linear relationship, a 10g/day increase in the consumption of beer, wine and liquor was associated with, respectively a RR of 1.01 (95% CI: 0.99, 1.03) for beer, a RR of 1.02 (95% CI: 0.98, 1.05) for wine and a RR of 0.99 (95% CI : 0.96, 1.02) for liquor.
Because of the strong inverse relationship between smoking and PD risk, we performed analyses stratified by smoking at baseline (ever or ever-smokers). In these analyses estimates were adjusted for age and caffeine intake and in ever-smokers, additional adjustment for pack-years of smoking. We did not observe a significant interaction with smoking (Table 3). We also performed analyses of particular alcoholic beverages (beer, wine liquor) stratified by smoking. We did not observe significant associations with PD in these stratified analyses. In women, no significant interaction was observed between alcohol intake and use of HRT.
Table 3.
Relative rates and 95% confidence intervals for Parkinson disease by total alcohol intake (g/day) and smoking status at baseline (1992)
| Never Smokers | Ever Smokers | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Alcohol Intake (g/day) | Cases | Multiva RR (95% CI) | p- trend | Cases | Multiva RR (95% CI) | p-trend |
| Men | ||||||
| 0 | 60 | 1.00 (Ref) | 51 | 1.00 (Ref) | ||
| 1–9.9 | 62 | 1.33 (0.92, 1.91) | 81 | 1.34 (0.95, 1.91) | ||
| 10–19.9 | 25 | 1.49 (0.92, 2.41) | 46 | 1.49 (0.97, 2.23) | ||
| 20–29.9 | 4 | 0.79 (0.28, 2.20) | 14 | 1.35 (0.74, 2.44) | ||
| 30+ | 8 | 1.11 (0.52, 2.35) | 0.85 | 34 | 1.40 (0.90, 1.18) | 0.26 |
| Women | ||||||
| 0 | 77 | 1.00 (Ref) | 28 | 1.00 (Ref) | ||
| 1–4.9 | 41 | 1.13 (0.77, 1.66) | 14 | 0.60 (0.31, 1.15) | ||
| 5–9.9 | 8 | 0.84 (0.40, 1.76) | 9 | 1.05 (0.49, 2.26) | ||
| 10–14.9 | 11 | 1.71 (0.90, 3.24) | 11 | 1.27 (0.63, 2.59) | ||
| 15+ | 5 | 0.99 (0.37, 2.46) | 0.80 | 6 | 0.64 (0.26, 1.57) | 0.78 |
Adjusted for age in months, and coffee intake (<3 cups/week, 3–6 cups/week, 1 cup/day, 2–3 cups/day, 4–5 cups/day, 4–5 cups/day >=6 cups/day); in ever-smokers, additional adjustment for pack-years of smoking (never-smokers, 1–9, 10–19, 20–29, 30–39, ≥ 40 pack-years)
RR: relative rate; CI: confidence interval
To address the possible influence of undiagnosed PD on our results, we performed sensitivity analyses by leaving a lag of at least 5 years between the reported alcohol consumption and date of onset of PD. Our results were not materially altered by this exclusion ((p-trend: 0.09 (men) and 0.67 (women)). The results were also similar in analyses including 84 men and 53 women who reported that they had been diagnosed with PD but did not give consent for us to review their medical records (data not shown).
Alcohol intake among PD cases, as compared to that of individuals of the same age and sex without PD, started to decline 2–3 years prior to diagnosis of PD and continued to decline thereafter (Figure 2). This decline was significant over the entire observational period (p-trend = 0.002), and for the period starting 4 years prior to diagnosis and thereafter (p-trend 0.008). There was no significant change in alcohol residuals for the period up to 4 years prior to PD diagnosis (p-trend = 0.94).
DISCUSSION
In this large prospective study of men and women, we found no association between total alcohol consumption and the incidence of PD.
We previously reported strong inverse associations between both smoking and coffee drinking and risk of incident PD in this and in the larger American Cancer Society CPS II mortality cohort.4, 25 Under the hypothesis of a premorbid, non-addictive personality preceding the onset of Parkinson disease determining a general aversion to the use of addictive substances, one would expect future Parkinson patients to avoid the intake of alcohol as well as cigarettes and caffeine. Thus, the lack of an association with alcohol in this study provides evidence against a general aversion to addicting substances in persons ‘destined’ to develop PD later in life.
A number of case-control studies examined the association between alcohol intake and risk of PD.26–39 Most of these studies report either a moderately decreased risk, or no change in risk associated with alcohol intake. However, findings of these studies should be interpreted cautiously due to the known limitations of case control studies such as the potential for selection bias, retrospective assessment of alcohol intake, and the use of prevalent cases; these limitations may be compounded by the fact, shown in our study, that individuals with PD tend to decrease their alcohol consumption around the time of their PD diagnosis. Although participants in case-control studies were asked to recall their alcohol consumption before the diagnosis of PD, recall of past consumption is likely to be affected by current consumption.40 Confounding by smoking and caffeine intake could have also contributed to the protective association with alcohol reported by case-control studies.
The results of three early studies, including the Leisure World Study6, the Honolulu Heart Program5, and the Rotterdam cohort41, although not significant individually, when combined suggested a possible lower PD risk in alcohol drinkers as compared to non-drinkers7. This inverse association, however, was not confirmed among participants in the Nurses Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) in which alcohol consumption was repeatedly assessed using a comprehensive and well-validated questionnaire.7 Our results, which are based on a larger investigation, do not support a protective effect of alcohol consumption on PD risk. Of note, although alcohol consumption and particularly beer consumption, tend to increase blood urate levels 42 and higher blood urate concentrations are associated with lower PD risk PD risk43, this indirect effect of alcohol on PD risk would be too small to be reliably detected even in large epidemiological studies and therefore the lack of association between alcohol and PD risk does not contradict the hypothesis that higher blood urate reduces PD risk.
While we did not observe an association between alcohol intake and subsequent risk of PD, we found that PD patients start to decrease their alcohol intake around the time of diagnosis or shortly before, (Figure 2). Reasons for this decrease might include overall decline in physical, emotional and social functioning, and increased health consciousness. Also, this decrease in alcohol intake may explain some of the inverse associations between alcohol intake and risk of PD reported in case-control studies, where patients are asked to recall alcohol intake usually one to two years before diagnosis.
The strengths of our study include its large size, longitudinal design with a large number of confirmed incident PD cases, and the thoroughly collected prospective data on alcohol intake as well as on potential confounders. Also, the diagnosis of PD was based on medical records obtained from the patient’s neurologist and reports from treating physicians, which have been found to have over 90% accuracy.44 Thus, bias from misdiagnosis is likely to be modest.
A limitation that should be considered in interpreting the results of this study is that the information on alcohol consumption was based on self-report and thus may be subject to misclassification, as individuals are known to under-report alcohol consumption. However, the alcohol consumption in this cohort was shown to be highly correlated between two dietary assessment methods 21, and alcohol intake has been linked to a significantly increased risk of breast cancer in this cohort.45 Thus, an association with alcohol would be unlikely to be missed if it existed.
In summary, in this large prospective study of men and women, we did not observe an association between either total alcohol intake or the intake of beer, wine or liquor and risk of Parkinson disease. These results are consistent with most epidemiological studies that show either a weak or no effect of alcohol intake and risk of PD.
References
- 1.Hernán MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson’s disease in two prospective studies. Ann Neurol. 2001;50:780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- 2.Morozova N, O’Reilly EJ, Ascherio A. Variations in gender ratios support the connection between smoking and Parkinson’s disease. Mov Disord. 2008;23(10):1414–1419. doi: 10.1002/mds.22045. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly EJ, Chen H, Gardener H, Gao X, Schwarzschild MA, Ascherio A. Smoking and Parkinson’s disease: using parental smoking as a proxy to explore causality. Am J Epidemiol. 2009;169(6):678–682. doi: 10.1093/aje/kwn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thacker EL, O’Reilly EJ, Weisskopf MG, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68(10):764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandinetti A, Morens DM, Reed D, MacEachern D. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson’s disease. Am J Epidemiol. 1994;139:1129–1138. doi: 10.1093/oxfordjournals.aje.a116960. [DOI] [PubMed] [Google Scholar]
- 6.Paganini-Hill A. Risk factors for parkinson’s disease: the leisure world cohort study. Neuroepidemiology. 2001;20(2):118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 7.Hernán MA, Chen H, Schwarzschild MA, Ascherio A. Alcohol consumption and the incidence of Parkinson’s disease. Ann Neurol. 2003;54(2):170–175. doi: 10.1002/ana.10611. [DOI] [PubMed] [Google Scholar]
- 8.Menza M. The personality associated with Parkinson’s disease. Curr Psychiatry Rep. 2000;2(5):421–426. doi: 10.1007/s11920-000-0027-1. [DOI] [PubMed] [Google Scholar]
- 9.Brodie C, Blumberg P, Jacobson K. Activation of the A2A adenosine receptor inhibits nitric oxide production in glial cells. FEBS Letters. 1998;429:139–142. doi: 10.1016/s0014-5793(98)00556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J-F, Staal R, Xu K, Beilstein M, Sonsalla PK, Schwarzschild MA. Novel Neuroprotection by Adenosine A2A Receptor Inactivation in an MPTP Model of Parkinson’s Disease. Movement Disorders. 2000;15(Suppl 3):37, abstr. P304. [Google Scholar]
- 11.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 12.Morens DM, Grandinetti A, Reed D, White LR, Ross GW. Cigarette smoking and protection from Parkinson’s disease -- false association or etiologic clue (review) Neurology. 1995;45:1041–1051. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- 13.Khalil AA, Davies B, Castagnoli N., Jr Isolation and characterization of a monoamine oxidase B selective inhibitor from tobacco smoke. Bioorg Med Chem. 2006;14(10):3392–3398. doi: 10.1016/j.bmc.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 14.Castagnoli KP, Steyn SJ, Petzer JP, Van der Schyf CJ, Castagnoli N., Jr Neuroprotection in the MPTP Parkinsonian C57BL/6 mouse model by a compound isolated from tobacco. Chem Res Toxicol. 2001;14(5):523–527. doi: 10.1021/tx000224v. [DOI] [PubMed] [Google Scholar]
- 15.Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of Flavonoids and Risk of Dementia. European Journal of Epidemiology. 2000;16(4):357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol. 2005;58(6):963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 18.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Agriculture. ARS USDA National Nutrient Database for Standard Reference, Release 22. 2009. [Google Scholar]
- 21.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11(4):462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 23.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson’s disease. Mov Disord. 2008;23(1):69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol. 1997;26(6):1323–1333. doi: 10.1093/ije/26.6.1323. [DOI] [PubMed] [Google Scholar]
- 25.Ascherio A, Weisskopf MG, O’Reilly EJ, et al. Coffee consumption, gender, and Parkinson’s disease mortality in the Cancer Prevention Study-II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 26.Haack DG, Baumann RJ, McKean HE, Jameson HD, Turbek JA. Nicotine exposure and Parkinson disease. Am J Epidemiol. 1981;114:191–200. doi: 10.1093/oxfordjournals.aje.a113182. [DOI] [PubMed] [Google Scholar]
- 27.Behari M, Srivastava AK, Das RR, Pandey RM. Risk factors of Parkinson’s disease in Indian patients. J Neurol Sci. 2001;190(1–2):49–55. doi: 10.1016/s0022-510x(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 28.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson’s disease: a case-control study. Neurology. 2000;55(9):1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 29.Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Smoking and Parkinson’s disease: a dose-response relationship. Neurology. 1999;52:115–119. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Fall P, Frederikson M, Axelson O, Granérus A. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for parkinson’s disease in the Emilia-Romagna region of Italy. Neurotoxicology. 1998;19:709–712. [PubMed] [Google Scholar]
- 32.Liou HH, Tsai MC, Chen CJ, et al. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- 33.Hellenbrand W, Seidler A, Boeing H, et al. Diet and the Parkinson’s disease. I: a possible role for the past intake of specific foods and food groups. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology. 1996;47:636–643. doi: 10.1212/wnl.47.3.636. [DOI] [PubMed] [Google Scholar]
- 34.Morano A, Jiménez-Jiménez FJ, Molina JA, Antolín MA. Risk-Factors for Parkinson’s disease: case -control study in the province of Cáceres, Spain. Acta Neurol Scand. 1994;89:164–170. doi: 10.1111/j.1600-0404.1994.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 35.Mayeux R, Tang M, Marder K, Coté LJ, Stern Y. Smoking and Parkinson’s disease. Mov Disord. 1994;9:207–212. doi: 10.1002/mds.870090215. [DOI] [PubMed] [Google Scholar]
- 36.Jiménez-Jiménez FJ, Mateo D, Giménez-Roldan S. Premorbid smoking, alcohol consumption, and coffee drinking habits in Parkinson’s disease: a case-control study. Mov Disord. 1992;7:339–344. doi: 10.1002/mds.870070407. [DOI] [PubMed] [Google Scholar]
- 37.Ho SC, Woo J, Lee CM. Epidemiologic study of Parkinson’s disease in Hong Kong. Neurology. 1989;39:1314–1318. doi: 10.1212/wnl.39.10.1314. [DOI] [PubMed] [Google Scholar]
- 38.Ngim C, Devathasan G. Epidemiologic study on the association between body burden mercury level and idiopathic Parkinson’s disease. Neuroepidemiology. 1989;8:128–141. doi: 10.1159/000110175. [DOI] [PubMed] [Google Scholar]
- 39.Godwin-Austen RB, Lee PN, Marmot MG, Stern GM. Smoking and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1982;45:577–581. doi: 10.1136/jnnp.45.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willett WC. Nutritional Epidemiology. 2. New York: Oxford University Press, Inc; 1998. [Google Scholar]
- 41.Willems-Giesbergen PCLM, de Rijk MC, van Swieten JC, Hofman A, Breteler MM. Smoking, alcohol, and coffee consumption and the risk of pd: results from the rotterdan study. Neurology. 2000;54(Suppl 3):A347–A348. [Google Scholar]
- 42.Gibson T, Rodgers AV, Simmonds HA, Toseland P. Beer drinking and its effect on uric acid. Br J Rheumatol. 1984;23(3):203–209. doi: 10.1093/rheumatology/23.3.203. [DOI] [PubMed] [Google Scholar]
- 43.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166(5):561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57(8):1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 45.Feigelson HS, Jonas CR, Robertson AS, McCullough ML, Thun MJ, Calle EE. Alcohol, Folate, Methionine, and Risk of Incident Breast Cancer in the American Cancer Society Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiology Biomarkers & Prevention. 2003;12(2):161–164. [PubMed] [Google Scholar]