Abstract
Background
Bacterial vaginosis (BV) recurs frequently after metronidazole treatment. This randomized, single-blinded clinical trial (RCT) evaluated the efficacy of topical application of 62% ethyl alcohol in emollient gel (gel) to the penis by male partners of women diagnosed with BV for preventing post-treatment BV recurrence.
Methods
Among 587 Kenyan women presenting with vulvovaginal symptoms, 236 had BV (vaginal Gram stain Nugent score ≥ 7), of whom 223 (94.3%) agreed, along with their partners, to be randomized: 115 to the intervention and 108 to the control arm. In the intervention arm, male partners agreed to apply gel each morning, and before and after sexual intercourse. All couples received counseling, condoms, and syndromic treatment of STI symptoms. Follow-up visits were scheduled 1 week, 1 month, and 2 months post-enrollment, with vaginal Gram stains at every visit and culture for H2O2-producing lactobacilli at the 2 month visit. The primary outcome was time to diagnosis of BV during follow-up.
Results
In the primary intent-to-treat analysis, diagnosis of BV was significantly more frequent in the intervention arm (Hazard ratio 1.44, 95% CI 1.01-2.04). After adjustment for baseline covariates, the hazard ratio was 1.39 (95% CI 0.98-1.99). At the 2 month visit, prevalences of any vaginal lactobacilli or of H2O2-producing lactobacilli did not differ appreciably in the two study arms (p=0.81, and 0.32 respectively).
Conclusion
Daily use of the 62% ethyl alcohol gel by men before and after sex significantly increased persistence or early recurrence of BV in their partners through two months after metronidazole treatment. However, no difference was observed in prevalences of vaginal lactobacilli within this same period.
Keywords: Bacterial vaginosis, recurrence, male factor, Kenya, microbicide
INTRODUCTION
In bacterial vaginosis (BV), anaerobic and facultative bacteria replace the normal, lactobacillus-dominated vaginal flora [1]. Molecular techniques have demonstrated a variety of new BV-associated bacterial species [2]. BV represents the most common cause of vaginal discharge symptoms among women of reproductive age and may cause considerable gynecologic and obstetric morbidity [1,3-8], and increase risk of acquisition of human immunodeficiency virus type 1 (HIV-1) infection [9-12], especially in African women with high BV prevalence.
BV is associated with sexually transmitted infections (STI), multiple or new sexual partners and recent sexual contact [13-16]. The hypothesis that BV can be sexually acquired [17-19] is consistent with isolation of BV-associated facultative and anaerobic bacteria from the male perineum, urethra and subprepuce [20,21] and reduction of BV incidence and/ or recurrence by condom use [15,16]. Inoculation of vaginal fluid from women with BV into the vaginas of non-infected women reproduced the syndrome [22]. In lesbian couples, BV concordance is associated with shared use of sex toys [23].
Following successful treatment with oral or intravaginal medication [24], BV commonly recurs within 3-6 months [25, 26]. Alternative treatments. e.g., iodine washes [27], intravaginal acid gel [28,29] and suppressive intermittent intravaginal therapy [30] have had limited or no success. Although oral probiotic use reportedly improved BV treatment [31], efforts to recolonize the vagina with lactobacilli during or following treatment of BV have given mixed results [32]. Oral antimicrobial treatment of male partners of women with BV in studies reported from 1988 to 1993 did not reduce recurrence [33-35] and the effect of male circumcision on BV among female partners has not been consistent [36,37].
This randomized, single-blinded trial assessed the efficacy of topical application to the penis of 62% ethanol in emollient gel (Purell®) before and after sexual intercourse by male partners of monogamous women with BV to reduce recurrence of BV following metronidazole treatment. We also assessed the impact on prevalence of bacterial morphotypes resembling G. vaginalis, Prevotella sp., and Mobiluncus sp. on Gram stain of vaginal fluid, and on vaginal recolonization by Lactobacillus spp. To the best of our knowledge, data on the effect of Purell has not been reported other than hand sanitation and in vitro application to epithelial specimens.
MATERIALS AND METHODS
The Institutional Review Board of University of Washington, Kenya Medical Research Institute, and University of Nairobi approved the protocol. The trial was registered with clinicaltrials.gov, registration number NCT00542074.
Between June 2003-June 2005 at the Nairobi City Council STI Special Treatment Clinic (STC), four satellite City Council clinics, and a faith-based health facility, women with vaginal symptoms, or whose partner had urogenital symptoms, were referred toether with their male sex partners to the study, together or within 24 hours of each other. Study clinic visits took place at the STC.
On day 0, both partners independently gave written informed consent for participation, received HIV counseling and testing, and underwent face-to-face interviews regarding demographic, sexual and behavioral characteristics, and symptoms. Female participants underwent physical examination, including pelvic examination and collection of vaginal swabs for determination of vaginal pH and detection of volatile amines, using the QuickVue Advance pH & Amine test® (Quidel, San Diego, CA), diagnosis of BV using Nugent’s criteria [38], detection of Trichomonas vaginalis by wet mount examination and by culture (InPouch®, Biomed Diagnostics, Inc., White City, OR); and endocervical specimens for N. gonorrhoeae and C. trachomatis DNA detection by polymerase chain reaction amplification (PCR) (Amplicor, Roche Diagnostics Inc., NJ). Men underwent genital examination and urethral swab for T. vaginalis culture and N. gonorrhoeae and C. trachomatis PCR. Participants provided blood for HIV-1 serologic screening (Detect HIV-1, Biochem ImmunoSystems, Inc, Montreal, Canada), with positive tests confirmed by ELISA (Recombigen, Cambridge Biotech LTD, Ireland), and for syphilis serodiagnosis by Rapid Plasma Reagin (RPR, Beckton-Dickinson, Baltimore, MD), confirmed by Treponema pallidum hemaglutination assay (TPHA, Biotech Laboratories, UK).
BV diagnosis was based upon Nugent score ≥ 7 determined by a laboratory technologist; every fifth smear was reviewed, re-scored and discussed at a weekly meeting of four laboratory technologists, blinded to initial interpretations. Scores were compared (kappa = .90), discussed, and a final consensus score determined. These smears were photographed in at least 8 fields and sent to a blinded microbiologist (CS), who independently scored the slides. The laboratory technologists’ consensus diagnosis of BV had high concordance with that of the microbiologist (kappa = 0.87). All technicians were blinded to randomization assignment for all specimens.
Participants with BV received metronidazole 400 mg TID for 7 days and became eligible for randomization. All couples received STI prevention counseling, free condoms, and STD syndromic management per Kenya Ministry of Health guidelines as indicated. Using a computer-generated random sequence, the study statistician placed single sheets of paper labeled “Intervention” or “Control” into a series of sealed opaque envelopes, kept in a locked cabinet by the study coordinator. Once a couple was deemed willing and eligible, the study nurse consecutively obtained the next envelope from the study coordinator, and provided it to participants, who opened it themselves.
Randomized couples participated in a discussion on improved genital hygiene, and were advised to bathe daily. Men in the intervention arm were requested to use 62% ethanol gel (Purell®, GOJO Healthcare Inc., Akron, OH) at least once daily, and immediately prior to and following intercourse, squeezing a shilling-sized (size of a US quarter) amount of gel on their palm, rubbing it gently onto the palms of both hands, and applying a second similar-sized amount to the genitalia from the tip of the penis towards the base. Uncircumcised men were instructed to retract the foreskin before gel application. We provided gel in pocket-sized 4.25 fluid ounce plastic squeeze bottles and encouraged men to carry it with them at all times. Participants received a brochure concerning STI control and hygienic practices, and (for the intervention arm) information on use of the study product. Participants received diaries to record when (s)he had sex, bathed, and, in the intervention arm, when the male applied gel. Neither participants nor those administering the intervention were blinded to group assignment. The men began the trial immediately following the enrollment visit. Women were advised to abstain for the first week of treatment or to use condoms until they returned to clinic for the one week visit.
Upon completing enrollment visits, community health workers (CHW) escorted couples home to verify locator information, and visited each couple again on days 14 and 45, to encourage continued participation, monitor side effects, and review and replenish study diaries. Female articipants were asked to return one week, one month, and two months after enrollment. Male partners were encouraged to return for follow-up visits but were not required for female participation. Intervention and control participants were seen for follow-up visits on different days to prevent cross-arm communication. Each follow-up included interview and examination; women underwent syndromic management for STI-related symptoms, and vaginal swabbing for determining Nugent’s score. Men also underwent interview, examination, and syndromic management for STI-related symptoms. At the one-week visit, couples received post-test counseling, treatment for additional STI detected, and referral to support groups and/or care for those HIV-infected. After the one-week visit, only the woman was required to return, bringing diaries for herself and her partners. Because men were instructed to wait until the gel had evaporated before starting sex, womens’ motivation to douche to remove gel was expected to be minimal and questionnaires did not include information on douching or other intravaginal practices.
At the two-month follow-up, vaginal swabs were taken for isolation of lactobacilli and transported anaerobically (Anaerobe Systems, San Jose, Calif.), emulsified in pre-reduced 1% yeast extract broth for inoculation onto Brucella medium enriched with vitamin K and hemin, Columbia Agar containing colistin and nalidixic acid (CNA), and Rogosa agar. Cultures were incubated anaerobically for 5 days at 35°C. Growth of each organism type was recorded semi-quantitatively. Each colony type found on any of the three plates was Gram stained, those appearing consistent with lactobacillus underwent additional tests. Lactobacilli were alpha hemolytic, catalase-negative, produced acid in both slant and butt of triple sugar iron agar, and did not grow on bile esculin agar. Strict anaerobes were identified as nonsporeforming Gram-positive rods, negative for indole and nitrate production, and then identified to species level using simple enzymatic tests; (WeeTabs, Key Scientific, Round Rock, TX). For isolates confirmed as lactobacillus, H2O2 production was further determined (Protects, Key Scientific).
Before classification as lost-to-follow-up, participants not returning for study visits were sought three times at home by the CHW. When participants declined further study participation, reasons for discontinuation were elicited.
The estimated target sample size (364 couples), providing 80% power to detect a 33% reduction in recurrence (50% vs. 33.3%) with alpha=0.05 (2-sided), after 20% loss-to-follow-up. However, prolonged transportation strikes in Nairobi limited enrollment to 223.
The primary analysis was comparative time to first BV (Nugent score ≥ 7) by randomization arm, using Wilcoxon’s log rank test. Cox regressions were used to estimate hazard ratios (HRs) for the intervention effect, and to estimate the intervention effect, before and after adjusting for baseline characteristics differing between study arms. Time-varying covariates were used to examine a dose-response relationship between gel use and time to BV, and post-randomization explanatory variables (e.g., reported sexual practices during follow-up).
Because women received syndromic treatment for STI-related symptoms, including vaginal symptoms, at follow-up visits, and because Nugent scores were not concurrently available to clinicians during the visit, some symptomatic women with Nugent score < 7 received metronidazole. Since this could bias comparisons between arms, another analyses compared time to failure defined as either Nugent score ≥ 7 or receipt of treatment with metronidazole during follow-up, by randomization arm. Analyses were intent-to-treat; all couples returning for any follow-up were included, and analyzed according to randomization group. Analyses employed SAS version 9 (Cary, NC) and Stata version 9 (College Station, TX).
RESULTS
Of 7153 low-middle income women approached for participation, 587 (8.2%) and their male partners sought participation in the trial; in 236 (40%) of these couples, the female partner had a Nugent score ≥ 7. Of these, 223 (93%) the male partner agreed to participate; 115 couples (52%) were randomized to the intervention arm and 108 (48%) to the control arm (Figure 1). The number of women still in follow-up or already having the study outcome were 99 (86%) in the intervention arm and 93 (86%), control arm, at one month; and: 93 (81%), intervention and 89 (82%), control arm at two months. Six participants relocated during the study, 1 died, 1 spouse was murdered, 3 couples separated, 1 couple discontinued after learning they were HIV-discordant, 1 was too busy, and 37 withdrew or were lost-to-follow-up without providing reasons. None reported discontinuing due to adverse events.
Figure 1.
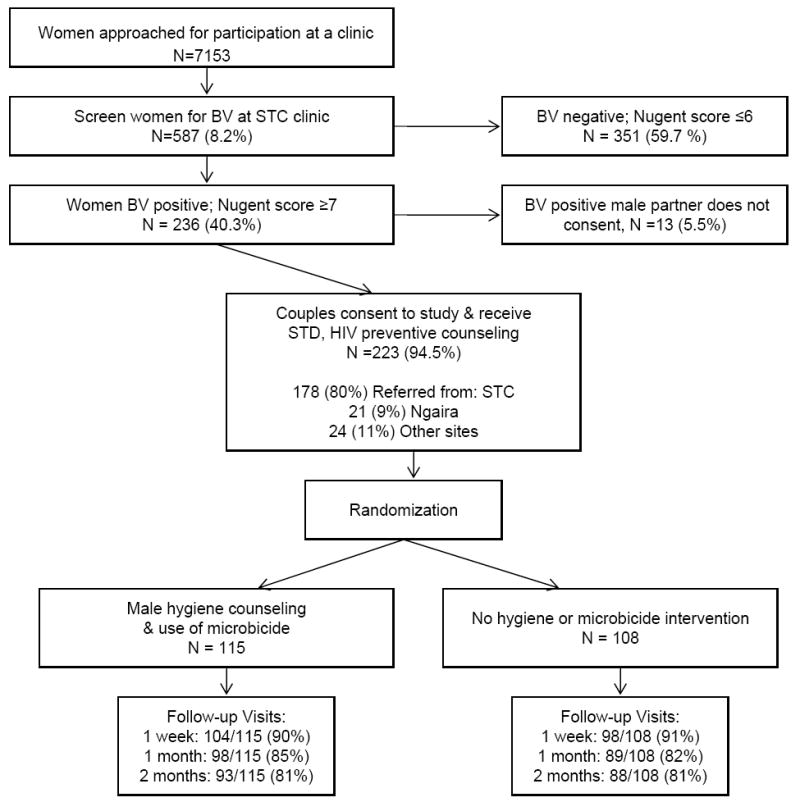
Study profile for the randomized controlled trial. Numerator for follow-up is those either still in follow-up and at risk, or who already had BV during follow-up.
Age, years of education, marital status, contraception use, circumcision status of the male partner, and sexual behavior data did not differ between intervention and control groups. Mean duration of the sexual relationship with the current partner was longer in the control group (5.8 ± 5.5 years) than in the intervention group (4.5 ± 3.9 years) (Table 1).
TABLE 1.
Demographic Features, Clinical History, and Laboratory Findings by Randomization Arm Among 223 Women and Their Male Partners
| Women | Male Partners | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Intervention | P* | Control | Intervention | P* | |
|
|
|
|||||
| 108 (48%) | 115 (52%) | 108 (48%) | 115 (52%) | |||
| Sociodemographic history | ||||||
| Mean age in yr ([plusmn] SD) | 27.5 [plusmn] 6.2 | 26.1 [plusmn] 5 | 0.06 | 33.7 [plusmn] 6.6 | 32.3 [plusmn] 7.2 | 0.15 |
| Mean yr of education ([plusmn] SD) | 9.0 [plusmn] 2.7 | 9.1 [plusmn] 2.8 | 0.69 | 9.9 [plusmn] 2.6 | 9.6 [plusmn] 2.7 | 0.37 |
| Marital status: | 0.86 | 0.92 | ||||
| Single | 3 (3%) | 5 (4%) | 4 (4%) | 4 (3%) | ||
| Married | 99 (92%) | 104 (90%) | 97 (90%) | 105 (91%) | ||
| Cohabiting | 6 (6%) | 6 (5%) | 7 (6%) | 6 (5%) | ||
| Contraceptive use | ||||||
| IUD | 7 (6%) | 2 (2%) | 0.09 | N/A | N/A | N/A |
| Depo-Provera | 20 (19%) | 18 (16%) | 0.60 | N/A | N/A | N/A |
| Circumcised | N/A | N/A | N/A | 84 (78%) | 92 (80%) | 0.74 |
| Mean age of circumcision | N/A | N/A | N/A | 14.5 [plusmn] 3.4 | 14.5 [plusmn] 4.7 | 0.99 |
| Mean age of sexual début ([plusmn] SD) | 17.5 [plusmn] 2.5 | 17.4 [plusmn] 2.6 | 0.65 | 17.3 [plusmn] 3.6 | 16.7 [plusmn] 3.7 | 0.21 |
| Mean duration of relationship with regular partner in yr ([plusmn] SD) | 5.8 [plusmn] 5.5 | 4.5 [plusmn] 3.9 | 0.03 | 5.7 [plusmn] 5.5 | 4.8 [plusmn] 3.9 | 0.15 |
| Median, mean (range) no. times had sex with partner last month | 8, 9.2 (0–31) | 8, 8.4 (0–40) | 0.34 | 8, 8.97 (0–31) | 8, 8.74 (0–60) | 0.56 |
| Median, mean, (range) no. sexual partners: | ||||||
| Last 30 d | 1, 0.96 (0–1) | 1, 0.97 (0–2) | 0.48 | 1, 1.06 (0–3) | 1, 1.01 (0–3) | 0.39 |
| Last 3 mo | 1. 0.99 (0–2) | 1, 1.04 (1–2) | 0.09 | 1, 1.15 (0–4) | 1, 1.15 (0–5) | 0.99 |
| Last 1 yr | 1, 1.08 (0–3) | 1, 1.13 (1–3) | 0.24 | 1, 1.44 (1–6) | 1, 1.57 (1–10) | 0.71 |
| Lifetime | 2, 4.74 (1–200) | 3, 3.04 (1–20) | 0.83 | 6, 9.60 (1–100) | 5, 9.48 (1–99) | 0.26 |
| Laboratory findings | ||||||
| Syphilis seroreactive | 5 (5%) | 2 (2%) | 0.27 | 3 (3%) | 1 (1%) | 0.36 |
| HIV-1 seropositive | 33 (31%) | 34 (29%) | 0.88 | 26 (24%) | 30 (26%) | 0.76 |
P values were obtained using Fisher exact test for binary variables, Pearson [chi]2 test for categorical variables, and t test for continuous variables, except Wilcoxon Rank Sum test was used for numbers of times having sex or number of sex partners.</.>
BV-free survival curves show women in the intervention arm had a significantly shorter time to BV detection (log rank test, p = 0.04) (Figure 2). The unadjusted HR for BV persistence or recurrence in the intervention arm vs. the control arm was 1.44 (95% CI 1.01-2.04), and did not differ significantly by circumcision status of the male partner (HR=1.37 (0.91-12.04) and 1.77 (0.84-3.71) for partners of circumcised and uncircumcised men, respectively, (p=0.22). Nor did it differ for condom use or HIV status measured at baseline. The association of the intervention with BV persistence or recurrence was similar after adjustment for baseline factors associated with persistent or recurrent BV at p<0.20 (duration of partnership, female HIV status, and male partner circumcision status) but was not statistically significant (adjusted HR=1.39 (0.98-1.99)), Table 2.
Figure 2.
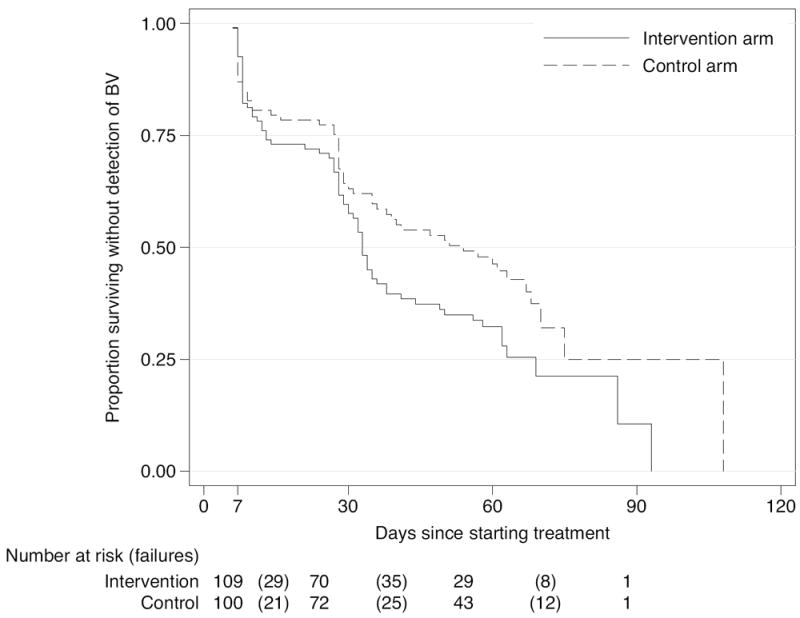
Survival until first detection of BV by Nugent Score ≥ 7 after the baseline visit (Kaplan-Meier estimates), by randomization arm (p=0.04, log rank test). Visits were scheduled at 1 week, 1 month, and 2 months following initiation of the treatment regimen. In the intervention arm there were 72 failures during 117 person-months at risk (61.5% incidence of BV per month in those at risk). In the control arm there were 58 failures during 132.5 person-months at risk (43.8% incidence of BV per month in those at risk). The number at risk for both study arms for a given follow-up visit is given on the first day a subject in either arm returned for that follow-up visit. For the 1 week visit, this was 5 days, for the 1 month visit, 24 days, for the 2 month visit, 55 days.
TABLE 2.
Unadjusted and Adjusted Associations With Potential Risk Factors (Including the Intervention per se) for Bacterial Vaginosis, With Nugent Score [mtequ]7, for N = 209 Women Who Were Randomized and With At Least 1 Follow-Up Visit
| Factor | N (%) | Hazard Ratio (95% CI)* | P | Adjusted Hazard Ratio (95% CI)† | Adjusted P |
|---|---|---|---|---|---|
| Intervention arm (vs. control) | 1.44 (1.01–2.04) | 0.04 | 1.39 (0.98–1.99) | 0.07 | |
| Age of woman [mt]30 | 44 (21%) | 0.73 (0.47–1.14) | 0.23 | ||
| Duration of partnership [mtequ]6 yr‡ | 61 (29%) | 0.63 (0.42–0.93) | 0.02 | 0.65 (0.43–0.97) | 0.03 |
| IUD use | 8 (4%) | 0.77 (0.28–2.09) | 0.61 | ||
| Female HIV status | 62 (30%) | 1.44 (1.00–1.07) | 0.05 | 1.41 (0.93–2.07) | 0.08 |
| Female report of unprotected sex, last 30 d | 184 (88%) | 1.21 (0.70–2.10) | 0.49 | ||
| Female reports sex last 7 d | 177 (85%) | 1.14 (0.71–1.87) | 0.59 | ||
| Male reports [mt]1 sex partner in last year | 69 (33%) | 0.97 (0.82–1.16) | 0.77 | ||
| Male circumcision status | 167 (80%) | 0.72 (0.48–1.08) | 0.11 | 0.82 (0.52–1.26) | 0.37 |
Hazard ratios and 95% confidence intervals estimated using Cox proportional hazards model.
Hazard ratios and 95% confidence intervals adjusted for factors associated with BV at P [lt] 0.20.
Mean duration of partnership reported by female partners was 5.8 years.</.>
Syndromic management resulted in metronidazole treatment of 8 women (8%) in each arm at one week, and 47 (51%) in the intervention arm and 38 (44%) in the control arm at one month. Of these women, 6 in the intervention arm and 7 in the control arm did not have Nugent score ≥ 7 at one week and 9 intervention and 15 control women, respectively, did not at one month. Using either Nugent score ≥ 7 or treatment with metronidazole during follow-up to define “failure,” we found HR=1.31 (95% CI 0.96-1.79) for the intervention arm vs. the control arm. Prevalence of this definition of “failure” at one month was 67% in the intervention arm and 59% in the control arm.
Vaginal swab specimens at one- and two-month visits showed fewer lactobacillus morphotypes (p= 0.08), and more mobiluncus morphotypes (p=0.03) in intervention than in control women at one month, but no differences in Gardnerella sp morphotypes at either visit (p=0.45 and p=0.14) (Table 3). At the two-month visit, rates of isolation of any lactobacilli or of H2O2-producing lactobacilli did not differ significantly in the two study arms (Table 4).
TABLE 3.
Examination of Bacterial Morphotypes on Gram Stain at 1- and 2- Month Follow-Up Visits by Randomization Arm, Number (%)
| Morphotypes Observed on Gram Stain* | 1-mo Visit | 2-mo Visit | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control N = 87 |
Intervention N = 94 |
P Value for Trend† | Control N = 84 |
Intervention N = 80 |
P Value for Trend† | |
| Lactobacillus | 0.08 | 0.14 | ||||
| None | 29 (33) | 42 (45) | 26 (31) | 25 (31) | ||
| 1+ | 15 (17) | 17 (18) | 10 (12) | 17 (21) | ||
| 2+ | 8 (9) | 8 (9) | 10 (12) | 11 (14) | ||
| 3+ | 14 (16) | 11 (12) | 13 (15) | 15 (19) | ||
| 4+ | 21 (24) | 16 (17) | 25 (30) | 12 (15) | ||
| Gardnerella | 0.45 | 0.14 | ||||
| None | 7 (8) | 9 (10) | 10 (12) | 7 (9) | ||
| 1+ | 8 (9) | 9 (10) | 9 (11) | 7 (9) | ||
| 2+ | 14 (16) | 4 (4) | 12 (14) | 9 (11) | ||
| 3+ | 13 (15) | 15 (16) | 15 (18) | 8 (10) | ||
| + | 45 (52) | 57 (61) | 38 (45) | 49 (61) | ||
| Mobiluncus | 0.03 | 0.31 | ||||
| None | 85 (98) | 86 (91) | 82 (98) | 76 (95) | ||
| 1+ to 2+ | 2 (2) | 2 (2) | 1 (1) | 1 (1) | ||
| 3+ to 4+ | 0 (0) | 6 (6) | 1 (1) | 3 (4) | ||
1+ [lt]1/1000[times] microscopic field; 2+ 1–5/1000[times]; 3+ 6–30/1000[times]; 4+ [mt]30/1000[times].
Score test for trend of odds.</.>
TABLE 4.
Lactobacillus Culture Results at 2-month Visit, by Treatment Arm: Number (%) Unless Otherwise Noted
| Lactobacillus Culture Results | Intervention | Control | P* |
|---|---|---|---|
|
| |||
| N = 61 | N = 64 | ||
| Any Lactobacillus | 48 (79) | 52 (81) | 0.82 |
| Any faculative Lactobacillus | 48 (79) | 51 (80) | [mt]0.99 |
| Any faculative H2O2-producing Lactobacillus | 20 (33) | 14 (22) | 0.23 |
| Any anaerobic Lactobacillus | 6 (9) | 3 (5) | 0.49 |
| Number of H2O2-producing Lactobacillus, mean CFU/mL | 1.1 [times] 104 | 7.8 [times] 103 | 0.35 |
P value is by Fisher exact test, for binary outcomes, and Wilcoxon Rank Sum test for number of lactobacilli.</.>
Women in the two study arms differed little for reported consistent male condom use (27% vs 31%, p=0.6) or having an outside sexual partner(0% vs 2%, p= 0.2), as did male partner report of having an outside sexual partner (5% vs 0%, p= 0.13) during the first month of study participation. Men in the intervention arm did report in their diaries more frequent sexual intercourse during the first month of follow-up (median 9 acts vs 6, p=0.03). Adjustment for number of episodes of sexual intercourse did not explain the effect of the intervention on BV.
Of 87 male partners in the intervention arm reporting sex in the first month only 39 (45%) reported 100% gel use compliance on days when sex was reported; the mean was 75%, and the lower 25th percentile was 56%. On days when sex without a condom was reported, 39 (53%) of 72 reported 100% compliance (mean 81%, lower 25th percentile 70%). Of 39 women whose male partners reported 100% compliance in the intervention arm, 25 (64%) had BV vs. 36 (41%) of 87 in the control arm. In the intervention arm, we saw no dose response relationship between the number of gel uses and BV, even when adjusted for the number of sexual acts and condom use(data not shown).
No serious adverse events were reported in either arm. Penile rash was found at follow-up visits in 8 of 107 in the intervention arm and 6 of 97 controls, p=0.72. Urethral discharge was seen in 4 men in the intervention arm and one control, p=0.21.
DISCUSSION
We had postulated that because BV-associated bacteria could be carried on the male genitalia [21,39], particularly in the sub-prepuce of uncircumcised men [40], that male partners of women with BV might subsequently re-inoculate the vagina after BV treatment, resulting in BV recurrence [22]. We had also hypothesized that men with other partners might transfer BV-associated microorganisms from those partners to their index partner. Therefore we evaluated whether self-application to the penile epidermis of a gel that rapidly kills most bacteria [41,42] might prevent vaginal reintroduction of BV-associated bacteria to partners after metronidazole treatment, thus reducing BV recurrence.
However, we actually observed by survival analysis through two months a significant increase in post-treatment recurrence or persistence of BV among women whose partners used the topical microbicide, although (1) no significant difference was observed in prevalence of BV or in isolation of lactobacillus or H2O2-producing lactobacillus from the vagina two months post-initiation of treatment; (2) when treatment failure was defined as either detection of BV or decision of the clinician to give syndromic treatment for BV during follow-up, the difference between intervention and control arms was not statistically significant; (3) at one month, although prevalence of BV was significantly higher in the 62% ethanol gel arm, we did not observe a dose-response relationship between gel use and BV; and (4) neither percent compliance with gel use, percent compliance with gel use in the absence of condom use, or the reported number of gel uses adjusted for the number of sex acts was associated with BV in women in the intervention arm. Thus, use of the alcohol gel by male partners clearly did not reduce risk of post-treatment persistence or recurrence of BV, and may have increased that risk. Although the sample size was smaller than planned, results were sufficient to indicate no need to proceed to a larger trial.
Incident or recurrent BV has been linked to frequent and recent sexual intercourse, unprotected sexual intercourse, and new sexual partners [12]. Vaginal recolonization by H202-producing lactobacilli reflects restoration of the normal vaginal ecosystem. Similar prevalences and concentrations by culture of lactobacillus or any H2O2-producing lactobacillus at the 2-month visit for both study arms suggests no effect of male gel use on vaginal recolonization by lactobacilli post-treatment, despite the trend toward fewer lactobacillus morphotypes and more mobiluncus morphotypes of the vaginal specimens obtained at the 1-month visit. Fredricks et al hypothesize that mobiluncus morphotype resembles BVAB on Gram stain, and the increase in Mobiluncus morphotypes may reflect increase in BVAB.
Several hypotheses might explain an increase in persistence or recurrence of BV in the intervention arm. Microbicide use daily and before and after sex might alter the penile flora or the penile epithelium. Use just before sex might have altered the vaginal flora of the female partner. Rapid evaporation of ethanol during application to the skin, and the lack of dose response relationship argue against these hypotheses. The effects of the study product on penile flora of a subset of the uncircumcised male partners are being analyzed in a separate report. Although topical microbicide use could potentially decrease condom use or cause increased sexual risk-taking because of sensation-seeking behavior, we saw no evidence of either in the diaries returned by female participants. If BV-associated organisms persist in the urethra instead of or in addition to persisting on the surface of the penis, then microbicide use on the penile epithelium might not influence reintroduction of such bacteria into the vagina; this would not explain any increase in persistent or recurrent BV with the intervention arm. We don’t believe the results of this trial rule out the “male factor” hypothesis for recurrence or persistence of BV.
This study had 14% loss-to-follow-up through 1-month for the primary outcome and 18-19% through 2 months. The first dose of metronidazole treatment was taken during the enrollment visit; we did not subsequently verify compliance of the women in completing the dose of metronidazole provided. Syndromic treatment of some women with metronidazole while still in follow-up is a concern. Although results of our secondary analysis, which defined “failure” as either BV or syndromic treatment with metronidazole during follow-up, did not give HRs that differed substantially from those of our primary analysis, the differences were no longer statistically significant. The small numbers of uncircumcised male participants did not allow for detailed subanalysis of the hypothesis that uncircumcised men are more likely to transfer BV-associated organisms to female partners. Women submitted their partner’s diaries, which may have influenced the self-reporting by the male participants. Our study population consisted of urban Kenyan women with BV and vaginal symptoms or a partner with urogenital symptoms, seen in STI or general health clinics and who were themselves willing and whose male partners were willing to participate. The modest rate uptake (8.2% of those presenting for screening) was likely partly attributable to male partners declining participation. Thus our results cannot necessarily be generalized to all women with BV.
Better understanding of the vaginal ecosystem, the pathogenesis and host factors responsible for BV, and mechanisms responsible for post-treatment persistence or recurrence, could help guide development of interventions to reduce frequent persistence or recurrence of this condition.
Acknowledgments
The authors would like to thank Carol Spiegel (CS), PhD, University of Wisconsin School of Medicine for quality control on slides, and GOJO Healthcare Inc. for initial consultation, and donation of Purell® for the project. The manuscript is submitted for publication with the permission of the Director KEMRI.
Supported by the University of Washington Fogarty International Center (#T22TW00001), FIRCA grant No: R03 TW05820, the University of Washington Center for AIDS Research (P30-AI-27757), and a WHIN supplementary grant (HD 40540-04).
Footnotes
Presented Abstract P-488,17th Biannual meeting of the ISSTDR, Seattle, July 29, 2007
References
- 1.Hillier S, Marrazzo J, Holmes KK. Bacterial vaginosis. In: Holmes KK, Cohen M, Piot P, Sparling PF, Stamm WE, Corey L, Wasserheit JN, Watts DH, editors. Sexually Transmitted Diseases. 4. New York: McGraw Hill; 2008. pp. 737–68. [Google Scholar]
- 2.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 3.Eschenbach DA. Bacterial vaginosis: emphasis on upper genital tract complications. Obstet Gynecol Clin North Am. 1989;16:593–610. [PubMed] [Google Scholar]
- 4.Eschenbach DA. Bacterial vaginosis and anaerobes in obstetric-gynecologic infection. Clin Infect Dis. 1993;16(Suppl 4):S282–S7. doi: 10.1093/clinids/16.supplement_4.s282. [DOI] [PubMed] [Google Scholar]
- 5.Eschenbach DA, Gravett MG, Chen KC, Hoyme UB, Holmes KK. Bacterial vaginosis during pregnancy. An association with prematurity and postpartum complications. Scand J Urol Nephrol Suppl. 1984;86:213–22. [PubMed] [Google Scholar]
- 6.Hillier SL, Krohn MA, Cassen E, Easterling TR, Rabe LK, et al. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clin Infect Dis. 1995;20(Suppl 2):S276–S8. doi: 10.1093/clinids/20.supplement_2.s276. [DOI] [PubMed] [Google Scholar]
- 7.Goepfert AR, Goldenberg RL, Andrews WW, Hauth JC, Mercer B, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The Preterm Prediction Study: association between cervical interleukin 6 concentration and spontaneous preterm birth. Am J Obstet Gynecol. 2001;184:483–8. doi: 10.1067/mob.2001.109653. [DOI] [PubMed] [Google Scholar]
- 8.Govender L, Hoosen AA, Moodley J, Moodley P, Sturm AW. Bacterial vaginosis and associated infections in pregnancy. Int J Gynaecol Obstet. 1996;55:23–8. doi: 10.1016/0020-7292(96)02744-0. [DOI] [PubMed] [Google Scholar]
- 9.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 11.Martin HL, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–9. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 12.Gray RH, Wawer MJ, Sewankambo N, Serwadda D. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:1780. doi: 10.1016/s0140-6736(05)63612-4. [DOI] [PubMed] [Google Scholar]
- 13.Schwebke JR, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis. 2005;32:654–8. doi: 10.1097/01.olq.0000175396.10304.62. [DOI] [PubMed] [Google Scholar]
- 14.Marrazzo JM, Coffey P, Elliott MN. Sexual practices, risk perception and knowledge of sexually transmitted disease risk among lesbian and bisexual women. Perspect Sex Reprod Health. 2005;37:6. doi: 10.1363/psrh.37.006.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008 Dec 1;47(11):1426–35. doi: 10.1086/592974. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006 Jun 1;193(11):1478–86. doi: 10.1086/503780. Epub 2006 Apr 26. [DOI] [PubMed] [Google Scholar]
- 17.Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–7. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson U, Hellberg D, Shoubnikova M, Nilsson S, Mardh PA. Sexual behavior risk factors associated with bacterial vaginosis and Chlamydia trachomatis infection. Sex Transm Dis. 1997;24:241–6. doi: 10.1097/00007435-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Fonck K, Kidula N, Kirui P, Ndinya-Achola J, Bwayo J, et al. Pattern of sexually transmitted diseases and risk factors among women attending an STD referral clinic in Nairobi, Kenya. Sex Transm Dis. 2000;27:417. doi: 10.1097/00007435-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lohr JA. The foreskin and urinary tract infections. J Pediatr. 1989;114:502–4. doi: 10.1016/s0022-3476(89)80583-9. [DOI] [PubMed] [Google Scholar]
- 21.Willen M, Holst E, Myhre EB, Olsson AM. The bacterial flora of the genitourinary tract in healthy fertile men. Scand J Urol Nephrol. 1996;30:387–93. doi: 10.3109/00365599609181315. [DOI] [PubMed] [Google Scholar]
- 22.Gardner HL, D C. Haemophilus vaginalis vaginitis. Am J Obstet Gynecol. 1955;69:962–72. [PubMed] [Google Scholar]
- 23.Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol. 2004;191:1898–906. doi: 10.1016/j.ajog.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 25.Thomas KK, Sanchez S, Garcia PJ, Holmes KK. Why do different criteria for ‘cure’ yield different conclusions in comparing two treatments for bacterial vaginosis? Sex Transm Dis. 2005;32:526–30. doi: 10.1097/01.olq.0000175293.46256.eb. [DOI] [PubMed] [Google Scholar]
- 26.Sobel JD, Schmitt C, Meriwether C. Long-term follow-up of patients with bacterial vaginosis treated with oral metronidazole and topical clindamycin. J Infect Dis. 1993;167:783–4. doi: 10.1093/infdis/167.3.783. [DOI] [PubMed] [Google Scholar]
- 27.Wewalka G, Stary A, Bosse B, Duerr HE, Reimer K. Efficacy of povidone-iodine vaginal suppositories in the treatment of bacterial vaginosis. Dermatology. 2002;204(Suppl 1):S79–S85. doi: 10.1159/000057731. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JD, Shann SM, Brady SK, Mammen-Tobin AG, Evans AL, et al. Recurrent bacterial vaginosis: the use of maintenance acidic vaginal gel following treatment. Int J STD AIDS. 2005;16:736–8. doi: 10.1258/095646205774763081. [DOI] [PubMed] [Google Scholar]
- 29.Andersch B, Lindell D, Dahlen I, Brandberg A. Bacterial vaginosis and the effect of intermittent prophylactic treatment with an acid lactate gel. Gynecol Obstet Invest. 1990;30:114–9. doi: 10.1159/000293230. [DOI] [PubMed] [Google Scholar]
- 30.Sobel JD, Ferris D, Schwebke J, Nyirjesy P, Wiesenfeld HC, Peipert J, Soper D, Ohmit SE. Hillier Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006 May;194(5):1283–9. doi: 10.1016/j.ajog.2005.11.041. Epub 2006 Apr 21. [DOI] [PubMed] [Google Scholar]
- 31.Falagas ME, Betsi GI, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clin Microbiol Infect. 2007;13(7):657–64. doi: 10.1111/j.1469-0691.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- 32.Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8(6):1450–4. doi: 10.1016/j.micinf.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Vutyavanich T, Pongsuthirak P, Vannareumol P, Ruangsri RA, Luangsook P. A randomized double-blind trial of tinidazole treatment of the sexual partners of females with bacterial vaginosis. Obstet Gynecol. 1993;82(4 Pt 1):550–4. [PubMed] [Google Scholar]
- 34.Vejtorp M, Bollerup AC, Vejtoryp L, Fanoe E, Nathan E, et al. Bacterial vaginosis: a double-blind randomized trial of the effect of treatment of the sexual partner. Br J Obstet Gynaecol. 1988;95:920–6. doi: 10.1111/j.1471-0528.1988.tb06581.x. [DOI] [PubMed] [Google Scholar]
- 35.Mengel MB, Berg AO, Weaver CH, Herman DJ, Herman SJ, et al. The effectiveness of single-dose metronidazole therapy for patients and their partners with bacterial vaginosis. J Fam Pract. 1989;28:163–71. [PubMed] [Google Scholar]
- 36.Zenilman JM, Fresia A, Berger B, McCormack WM. Bacterial vaginosis is not associated with circumcision status of the current male partner. Sex Transm Infect. 1999 Oct;75(5):347–8. doi: 10.1136/sti.75.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray RH, Kigozi G, Serwadda D, Makumbi F, Nalugoda F, Watya S, Moulton L, Chen MZ, Sewankambo NK, Kiwanuka N, Sempijja V, Lutalo T, Kagayii J, Wabwire-Mangen F, Ridzon R, Bacon M, Wawer MJ. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009 Jan;200(1):42.e1–7. doi: 10.1016/j.ajog.2008.07.069. Epub 2008 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdge DR, Bowie WR, Chow AW. Gardnerella vaginalis-associated balanoposthitis. Sex Transm Dis. 1986;13:159–62. doi: 10.1097/00007435-198607000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Wijesinha SS, Atkins BL, Dudley NE, Tam PK. Does circumcision alter the periurethral bacterial flora? Pediatr Surg Int. 1998;13:146–8. doi: 10.1007/s003830050270. [DOI] [PubMed] [Google Scholar]
- 41.Girou E, Loyeau S, Legrand P, Oppein F, Brun-Buisson C. Efficacy of handrubbing with alcohol-based solution versus standard handwashing with antiseptic soap: randomised clinical trial. Br Med J. 2002;325:362. doi: 10.1136/bmj.325.7360.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girou E, Oppein F. Handwashing compliance in a French university hospital: new perspective with the introduction of hand-rubbing with a waterless alcohol-based solution. J Hosp Infect. 2001;48(Suppl A):S55–S7. doi: 10.1016/s0195-6701(01)90015-5. [DOI] [PubMed] [Google Scholar]


