Abstract
Recently, the use of recombinant full-length amelogenin protein in combination with fluoride has shown promising results in the formation of densely packed enamel-like structures. In this study, amelogenin (rP172)-releasing hydrogels containing calcium, phosphate, and fluoride were investigated for remineralization efficacy using in vitro early enamel caries models. The hydrogels were applied to artificial caries lesions on extracted human third molars, and the remineralization efficacy was tested in different models: static gel remineralization in the presence of artificial saliva, pH cyclic treatment at pH 5.4 acetic buffer and pH 7.3 gel remineralization, and treatment with multispecies oral biofilms grown in a continuous flowing constant-depth film fermenter. The surface microhardness of remineralized enamel increased significantly when amelogenin was released from hydrogel. No cytotoxicity was observed when periodontal ligament cells were cultured with the mineralized hydrogels.
Keywords: Remineralization, biocompatibility, enamel-like crystals, amelogenin, oral bacterial biofilm
Introduction
Dental caries (tooth decay) is a result of chemical dissolution of tooth enamel minerals (demineralization) caused by acids of bacterial origin. Remineralization of enamel with materials containing calcium, phosphate, and fluoride has been extensively studied with limited success. Enamel, the hardest biosynthetic tissue in vertebrates, has an amazing ordered hierarchical structure from the nanoscopic to macroscopic scale. Biomimetic synthesis of enamel-like material is of great interest to dental clinicians because it may provide an alternative, noninvasive treatment for early carious lesions. Amelogenin, the most abundant enamel extracellular matrix protein, is believed to play a vital role in controlling the formation of hierarchically organized enamel crystals. Using recombinant amelogenin, the mechanism of amelogenin-assisted organized mineralization has been extensively studied with regards to self-assembly and mineral complex formation,1,2 crystal morphology control,3,4 and crystal nucleation kinetics.5,6 Although different models have been proposed for amelogenin–calcium phosphate interaction, it is accepted that amelogenin is a crucial promoter of enamel biomineralization and a modulator of calcium phosphate nanocrystal structure.7,8 Recent studies have shown that the self-assembly of amelogenin protein into nanospheres is the key factor in controlling elongated and oriented growth of carbonated apatite crystals, while the organized assembly of nanospheres into collinear arrays (chains and supramolecules) is critical at the initial stage of mineral deposition.6,9,10
The natural dynamic demineralization–remineralization process of enamel in the oral cavity maintains the integrity of tooth structure. The acids produced from fermentation of carbohydrates (sugars) by cariogenic bacteria such as Streptococcus mutans and Lactobacilli will enhance demineralization, leading to dissolution of enamel crystals11,12 and development of carious lesions.13 By proper enamel remineralization, the dominant demineralization process at early caries could be halted and even reversed.14,15 The most commonly used approaches for remineralization include supplementing mineral ions (i.e. calcium, phosphate, and fluoride) from dental varnishes; filling composites, glass ionomers, tooth pastes, dentifrice, or mouth rinse solutions; and depositing fluoroapatite (FAP)-like phases within the enamel.16 Enamel remineralization has been well accepted for controlling coronal caries since the 1980s. However, strategies to effectively manipulate remineralized crystals to achieve desired mechanical properties and to form enamel-like nanocrystal have not yet been realized.
Previous studies have evaluated the unique properties of recombinant full-length amelogenin (rP172) in enamel remineralization.17,18 The application of amelogenin combined with fluoride in remineralization solutions has proven to be effective in modulating the formation of densely packed enamel-like crystals in an in vitro etched enamel model. Within specific ranges of fluoride ion concentration and supersaturation degree of hydroxyapatite (HAP), enamel remineralization with densely packed nanorods is observed. Therefore, it is possible to formulate a biomaterial system that can locally deliver amelogenin and ions for enamel remineralization in a novel strategy to rebuild enamel-like crystals and treat early carious lesions.
Hydrogels have been reported as a mineralization template for synthesizing needle-shaped HAP nanoparticles. Self-assembled nanogels of cholesterol-bearing mannan act as templates for hierarchical hybrid nanostructures.19 Here, we developed a hydrogel system that can release amelogenin, calcium, phosphate, and fluoride in a controlled manner, and tested its efficacy in modulating enamel remineralization. In the oral cavity, hundreds of different bacterial species exist in highly complex communities, better known as plaque biofilms.20 S. mutans biofilms have often been used to study enamel erosion.21 To better mimic the oral environment, we also tested this system using an in vitro mixed-species biofilm model, termed “constant-depth film fermenter” (CDFF).22 The surface hardness of enamel caries before and after treatment was measured by a microhardness tester and evaluated statistically. The crystal morphology and mineral phase of the remineralized enamel surface were analyzed by field emission scanning electron microscopy (FE-SEM) and attenuated total reflectance–Fourier transform infrared (ATR-FTIR).
Materials and methods
All reagents and buffers were purchased from Sigma–Aldrich (Saint Louis, MO) or Thermo Fisher Scientific (Waltham, MA, USA). Supersaturated calcium phosphate solutions, Tris (tris(hydroxymethyl)-aminomethane), buffered at pH 7.3 were prepared by dissolving CaCl2·2H2O, KH2PO4, NaCl, and Tris-HCl in distilled deionized water (DDW) to achieve an ionic strength (IS) of 190 mM. The standard procedures of bacterial culture and biofilm formation were followed according to previous publications.23 Brain heart infusion (BHI) and a mucin-based medium (basal medium mucin (BMM)) were used for planktonic and biofilm cultures, respectively. Recombinant full-length porcine amelogenin (rP172) was prepared as described previously.24 The purity of rP172 (molecular weight (MW) = 19572.7 Da) was analyzed using the Agilent HP 1100 HPLC (Agilent Technologies Inc., Santa Clara, CA, USA) and Bruker Esquire 3000 ion trap mass spectrometer (Bruker Daltonics Inc., Billerica, MA, USA).
Preparation of remineralization hydrogel and assessing release of protein and ions
In the remineralization buffer solutions, the initial molar ratio of Ca2+ to PO43− ions was 1.67, the same as in HAP, with a supersaturation degree of σ(HAP) = 14.2, σ(FAP) = 51.6. Supersaturated hydrogel for the remineralization assay was prepared by adding agar powder (Cat# A1296; Sigma–Aldrich) to the remineralization solution, which was kept at 60°C after complete dissolution. The viscosity and gelation point of the agar gel were determined by ARES rheometer (TA Instruments-Waters LLC, New Castle, DE). The recombinant amelogenin was added to achieve a final concentration of 100 µg mL−1 in the hydrogel.
After the hydrogel (2 mL) was set at room temperature in a plastic vial, ultrapure water or buffer was added to the 15-mL plastic vial. The concentrations of rP172, calcium, and fluoride ions in the equilibrated aqueous solution were analyzed in triplicate samples. At the desired time interval, 2 mL of solution was removed, and the same amount of fresh solution was added. The amelogenin concentration was assayed using a QuantiPro BCA Assay Kit (Sigma–Aldrich). The mixture of 20 µL of sample solution with an equal-volume bicinchoninic acid (BCA) working solution was incubated at 65°C for 1 h, and a 3 µL droplet of the mixture was taken for measurement by a preset BCA method in the Nanodrop 2000 UV-Vis Spectrometer (Thermo Scientific, Madison, WI). Calcium ion concentration was measured by the Orion 960 Titrator System (Thermo Scientific) with a calcium ion–selective microelectrode (MI-600; Microelectrode Inc., Bedford, NH) in a 2.0 mL solution. The fluoride concentration was measured by a 720 pH/ISE meter (Thermo-Orion) with a fluoride ion–selective electrode (Thermo-Orion 96-09) in 2.0 mL of release solution with an additional 10% total ionic strength adjustment buffer III solution (TISAB III; Thermo Scientific). The release solution from hydrogel was also desalted using a centrifugal desalting column (exclusion MW = 7000 Da; Zeba Spin Column; Thermo Scientific) and lyophilized. Then the dry powder was dissolved and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and high-performance liquid chromatography–mass spectrometry (HPLC-MS) to determine the purity and integrity of the amelogenin.
Cytotoxicity evaluations
The cytotoxicity of the remineralization hydrogels was tested by coculturing with human periodontal ligament (PDL) fibroblasts. Human PDL fibroblasts were obtained from extracted molars from patients with healthy gingiva following informed written consent as prescribed in an approved Institutional Review Board (IRB) protocol (Louisiana State University Health Sciences Center (LSUHSC)-NO IRB #4826). PDL fibroblasts were maintained in minimum essential medium α(MEMα) containing 10% fetal calf serum and 200 units mL−1 penicillin and 200 µg mL−1 streptomycin. The cells were grown in 48-well plates for 24 h prior to exposure to hydrogel or hydrogel extracts. Remineralization hydrogels (8 g) were extracted with 40 mL growth medium for 24 h at 37°C. The hydrogel extractions (400 µL) were then added onto the cells. In direct contact tests, 200 µL remineralization hydrogel at 60°C was cast onto a monolayer of cells. After hydrogel gelation, 200 µL growth medium was added on top. Medium containing 10−4 M bisphenol-A-glycidyldimethacrylate (BisGMA) monomer (known to be cytotoxic to fibroblasts) served as a positive control. Untreated cells grown on tissue culture treated with polystyrene served as the negative control. After 24 h, cell survival was visualized using a fluorescent esterase substrate (acetomethoxy derivate of calcein (calcein-AM)) and a Nikon TE2000 (Nikon Inc., Melville, NY, USA) inverted fluorescent microscope. The live cells were stained with the fluorescent dye, whereas the dead cells were not. Cell survival was quantified using a BioTek Synergy 2 fluorescent multiwell plate reader. A midterm cytotoxicity test was performed on five plates of PDL fibroblast cultured with casting hydrogels. The survival rate for each plate was analyzed at 1, 3, 5, 7, and 9 days using analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test for post hoc tests. SAS version 9.2 (SAS Institute Inc., Cary, NC, 2008) was used for all statistical analyses.
Preparation of artificial enamel caries
Extracted human third molars with no detectable caries or fillings (from a tooth bank from unidentified donors at the School of Dentistry, LSUHSC) were randomly selected and cleaned. For remineralization tests, the teeth were embedded in epoxy and sectioned to expose the occlusal surfaces using a water-cooled diamond blade cutter (Accutom 50; Struers, Ballerup, Denmark). For testing in the CDFF biofilm model, teeth slabs were cut longitudinally to slices with 0.7–1.0 mm thickness. Tooth slabs were gently polished with 600 and 2000 grits SiC paper and 0.05 µm Al2O3 paste on a canvas cloth. The teeth were then demineralized in lactic acid solution (0.1 M, pH 4.5) containing 3 mM CaCl2, 3 mM KH2PO4, and 0.2% guar gum (Spectrum Chemical Co.) for 3 days at 37°C. The tooth slabs were stored at 4°C in Hank’s balanced buffer solution (NaCl: 8 g L−1, KCl: 0.4 g L−1, KH2PO4: 0.06 g L−1, CaCl2·2H2O: 0.18 g L−1, and MgSO4: 0.098 g L−1) before treatment.
Enamel remineralization evaluation
Static remineralization
The demineralized tooth slices were immersed in a 2 mL freshly prepared remineralization hydrogel containing 0.4% agar, 100 µg mL−1 amelogenin rP172, and 5 mg L−1 fluoride at 60°C in a 20-mL scintillation vial. After gelation, 3- to 5-mm-thick hydrogel formed on the enamel surface. The hydrogel surface was covered with 10 mL artificial saliva (NaCl: 0.8 g L−1, KCl: 1.2 g L−1, MgCl2·6H2O: 0.1 g L−1, K2HPO4: 0.3 g L−1, CaCl2·2H2O: 0.1 g L−1, and sodium carboxymethylcellulose: 1 g L−1)25 and was incubated at 37°C for 36 h. Hydrogels, without amelogenin as well as without either amelogenin or fluoride, were used as controls. The samples were removed, rinsed with deionized water, and then air-dried.
pH cycling
The tooth slab mounted on a 13 × ø25-mm epoxy cylinder block was soaked in 20 mL agar gel solution at 55°C–60°C in a 100-mL container and then 5 mg L−1 F−; and 100 µg mL−1 rP172 amelogenin were added. After fully setting at room temperature to form a 3- to 5-mm-thick hydrogel over the etched enamel, 20 mL artificial saliva (pH 7.0) was added on top of the hydrogel. The tooth in the hydrogel was then incubated at 37°C for 6 h. After rinsing the tooth surface in 70°C deionized water to remove hydrogel, the teeth were stored in artificial saliva overnight for 16 h. The teeth were then demineralized at pH 5.4 (50 mM acetic buffer containing 3 mM CaCl2 and 3 mM KH2PO4) for 1 h. The cyclic pH treatment was repeated for five cycles, and the hardness was measured after each cycle.
Multispecies oral biofilm model in CDFF
Tooth slabs were treated with one of three remineralization hydrogels in the presence of multispecies oral biofilms grown in a CDFF. Three groups were created by slabs with hydrogel containing amelogenin and fluoride (AM group), hydrogel containing no amelogenin but fluoride (F group), and blank hydrogel containing neither amelogenin nor fluoride (control group). Tooth slabs were sterilized by ultraviolet (UV) irradiation for 10 min on each side. The tooth was then transferred to the top of the plug in a CDFF flange, where the remineralization gel at 55°C was applied. After gelation at 37°C for 30 min, BMM of pH 7.2 was introduced continuously at a flow rate of 0.5 mL min−1 and the flange with 15 sample plugs was rotated at a constant 3 r min−1. A proportional mixture of different bacterial species was infused in the presence of an anaerobic gas phase containing 5% CO2 and 95% N2 for the first 48 h after inoculation to facilitate formation of mixed-species biofilms.
Ten species of bacteria were included in the biofilm26,27: S. mutans UA159, S. oralis 34, S. sanguinis SK150, Actinomyces naeslundii WVU45, Lactobacillus casei 4646, Neisseria subflava A1078, Veillonella dispar ATCC17745, Prevotella nigrescens T588, Porphyromonas gingvalis ATCC33277, and Fusobacterium nucleatum ATCC 10953. This group of bacteria is known to form stable biofilm communities with each individual species being well represented. These bacteria are all associated with dental plaque formation in both healthy individuals and patients with dental caries and periodontal disease. S. mutans and L. casei are known for their capacity to convert sugars to lactic acid, which causes demineralization of teeth. Porphyromonas gingvalis and P. nigrescens are known to cause periodontal disease. F. nucleatum is considered as a “bridge” organism capable of interacting with both primary and secondary colonizers of the tooth surface.
For samples undergoing multiple rounds of bacteria-induced caries formation, two types of hydrogels AM and F were tested (n = 6) and three holes on the flange were inserted with solid plugs as the reservoir for the multispecies of bacteria. After treatment for 2, 4, 6, and 8 days, the tooth slabs were taken out, cleaned, and the surface Knoop hardness number (KHN) was measured. Following UV sterilization, the tooth slabs were reinserted back into the rotary flange in CDFF, and freshly prepared hydrogel was applied atop. Multispecies biofilms that formed in the blank plugs were spread by the scraper to other plugs on the surface of the hydrogel embedded tooth samples.
Remineralized enamel characterization
Enamel microhardness is an essential indicator of tooth structural integrity and function. The KHN on the enamel surface before and after remineralization was measured using a Microhardness Tester (MicroMet 5400; Buhler, Lake Bluff, IL) under 25g force and 15 s dwell time. For each sample, 5–7 indentation measurements were randomly taken 100 µm away from dentino-enamel junction (DEJ) or enamel edge. ANOVA with post hoc analysis via Tukey’s HSD test was used to compare the mean in KHN after remineralization among different groups.
For data analysis of continuous remineralization measurements in the CDFF model, the MIXED procedure in SAS version 9.2 (SAS Institute Inc) was used to fit a mixed effects linear model to compare the change in KHN for the two treatments as the number of cycles increased. The MIXED procedure accommodated repeated KHN measurements made on the same tooth after each cycle. A compound symmetry covariance structure was assumed. Tests of simple effects28 were used to compare the two treatments’ mean KHN for each number of cycles.
The crystals on the remineralized enamel surfaces were analyzed by FE-SEM (LEO 1530 VP). The specimens were coated with carbon using a Carbon coater 208C (Cressington Scientific Instruments, Watford, UK). ATR-FTIR spectra were acquired with a Nexus 670 FT-IR spectrometer (Thermo-Nicolet, Thermo Scientific) equipped with a Vision Gladi diamond ATR (Pike Technology, Madison, WI). The enamel samples were scanned 128 times at 4 cm−1 resolution from 4000 to 400 cm−1.
Results
Controlled release of ions and amelogenin from the hydrogel
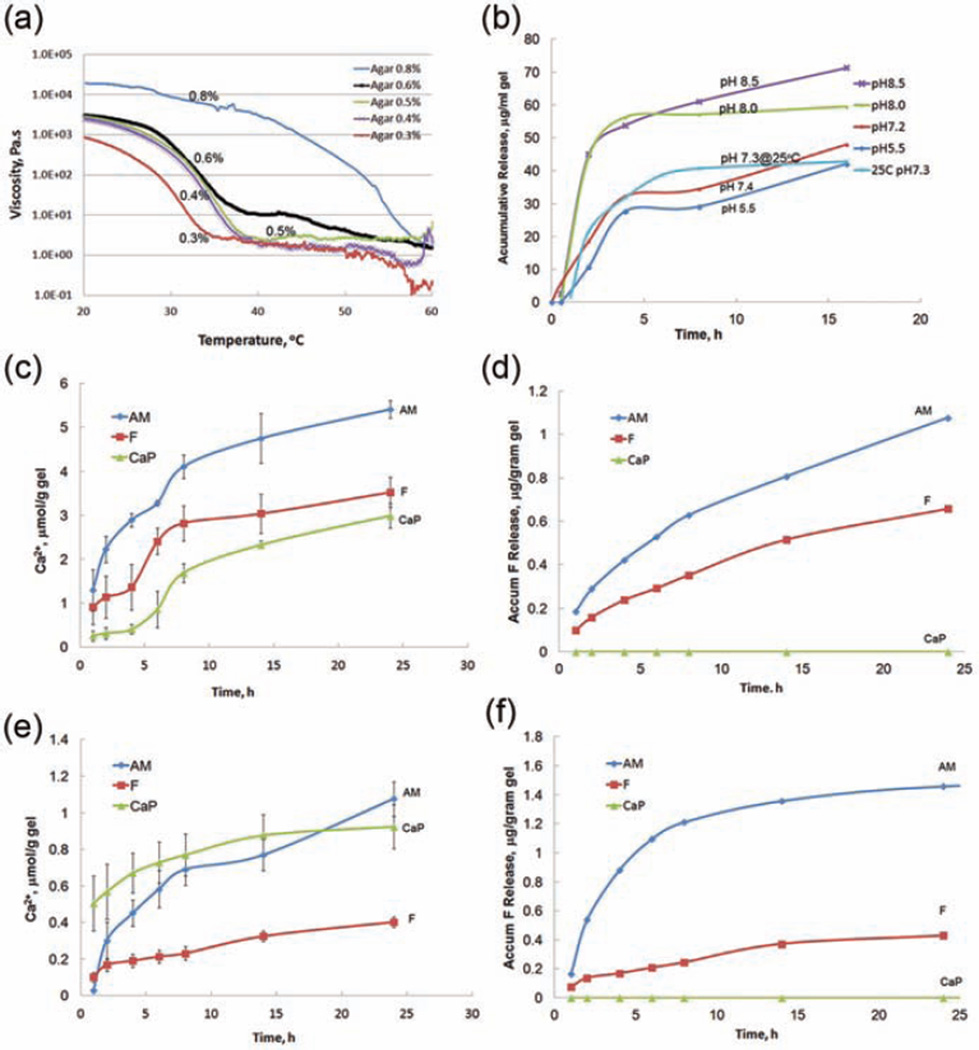
The viscosity–temperature curves of different concentrations of agar solutions are shown in Figure 1(a). It was found that the gelation temperature increased with agar concentration. The addition of buffer salts, phosphate, and calcium did not significantly change the gelation temperature. The gelation temperature and viscosity increased slightly from pH 7.3 to pH 8.9 in the Tris buffer. The agar gel with a concentration of 0.4–0.5 wt%, which was flowable above 55°C but solidified below 38°C–40°C, was chosen as the hydrogel for the release of remineralization agents and was the focus of all subsequent remineralization tests. As shown in Figure 1(b) to (c), the release profiles from a 0.5% agar hydrogel (pH 7.3) can reach 4.7 µmol of [Ca2+], 0.8 µg of [F−], and 60 µg of amelogenin from 1 g of hydrogel in 14 h. The release of rP172 from hydrogel lasted more than 24 h with a concentration between 40 and 100 µg mL−1. This shows that the release rate is dependent on the pH of the hydrogel within the pH range of 5.5–8.9. Amelogenin has an isoelectric point around pH 6.8. So its release rate was much higher at pH 8.0 than at pH 7.0–7.2 and was maintained at a similar level at pH 5.5. As for temperature effects, the release rate of [Ca2+] decreased dramatically (Figure 1(e)) at 25°C, but the release rate of [F−] from hydrogel remained the same (Figure 1(f)). Amelogenin has a relatively higher solubility at 25°C than at 37°C, therefore the release slightly increased at 25°C (Figure 1(b)).
Figure 1.
(a) Effect of temperature on the viscosity of mineralization agar gel at different concentrations (0.8%, 0.6%, 0.5%, 0.4%, and 0.3%) tested by a rheometer. Release profiles of (b) amelogenin protein, (c) calcium ions, and (d) fluoride ions from remineralization hydrogels at 37°C and release profiles of (e) calcium ions and (f) fluoride ions at 25°C.
AM: amelogenin and fluoride; CaP: group containing neither fluoride nor amelogenin; F: group containing fluoride but no amelogenin.
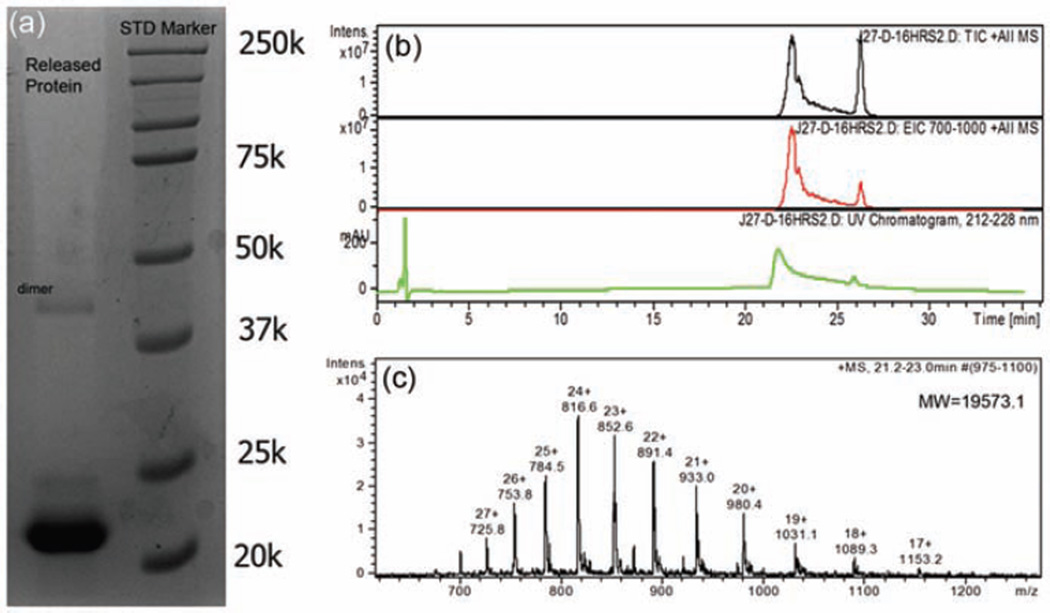
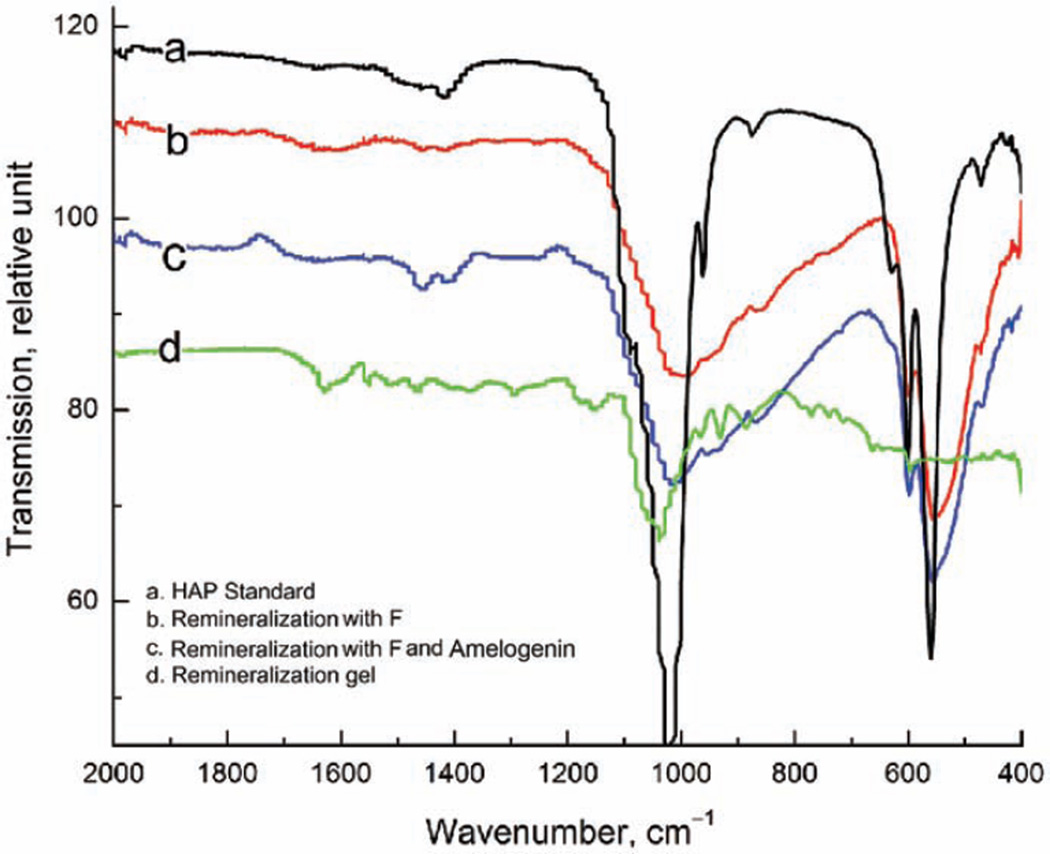
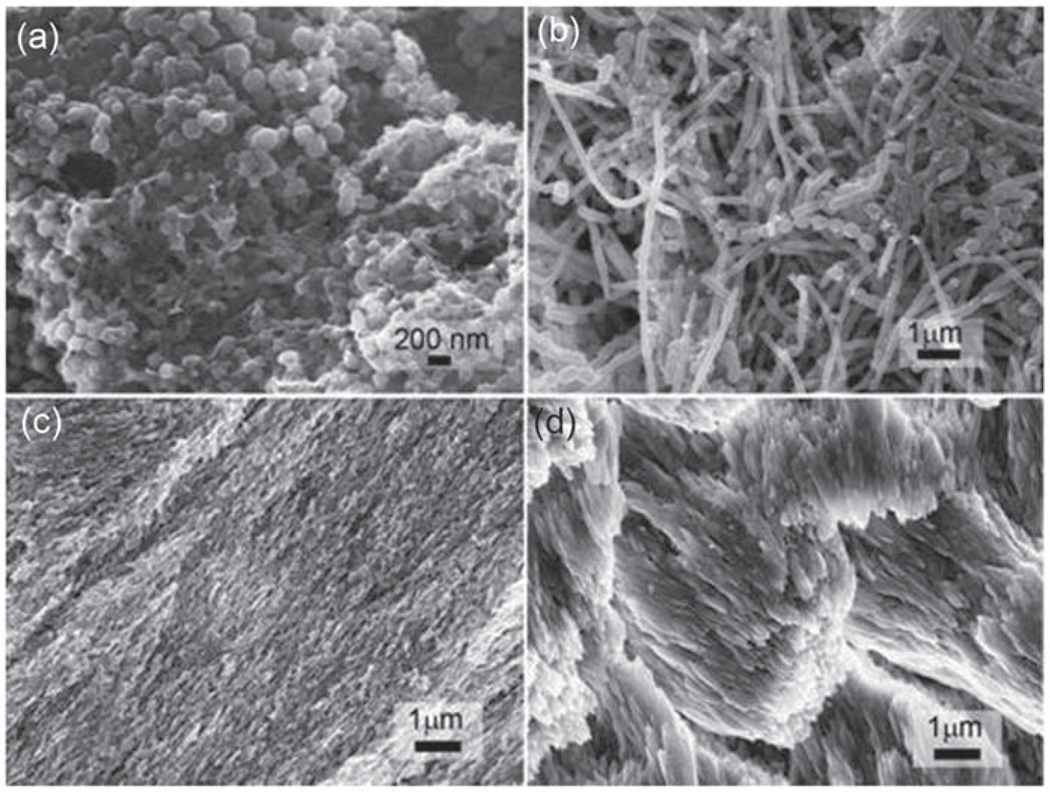
The stability of rP172 was analyzed by HPLC with a serial on-line UV detector and MS. When incubated at 37°C in a pH 2 trifluoroacetic acid (TFA) buffer and deionized water solutions, it remained >99% intact after 2 days and >96% after 7 days. The SDS-PAGE analysis of the released amelogenin reveals a single band with the same mass of intact rP172. The HPLC-MS indicates a single protein peak with the deconvolution MW of 19573.2 Da corresponding well to rP172 (Figure 2). The regenerated amelogenin promoted the formation of organized rod-like HAP bundles similar to those reported by Fan et al.18 FT-IR spectra on the freeze-dried hydrogels (Figure 3(d)) show only the broad peaks around 1020 cm−1 corresponding to amorphous calcium phosphate (ACP) but no dual peaks around 550 and 600 cm−1 corresponding to HAP or octacalcium phosphate (OCP), which indicates no substantial calcium phosphate crystallization inside the hydrogel. Observation of the hydrogel containing calcium, phosphate, and fluoride ions after 24-h incubation by FE-SEM, in Figure 4(a), reveals no large crystals, only ~180-nm spherical granules, suggesting no formation of crystalline HAP.
Figure 2.
(a) SDS-PAGE analysis of the equilibrated solution from mineralization hydrogel shows the band of 22k of full-length amelogenin rP172 and a big band of 43k of rP172 dimer. (b) HPLC-MS chromatograph peak of the equilibrated solution from the mineralization hydrogel and (c) corresponding MS spectrum of the protein at 21.2–23.0 min shows the intact molecular weight of rP172. The peak at 25 min impurity is from agar hydrogel.
SDS-PAGE: sodium dodecyl sulfate–polyacrylamide gel electrophoresis; HPLC-MS: high-performance liquid chromatography-mass spectrometry.
Figure 3.
ATR FT-IR spectra of (a) standard HAP powder, (b) enamel surface after treatment with remineralization hydrogel containing fluoride, (c) enamel surface after treatment with remineralization hydrogel containing fluoride and amelogenin, and (d) freeze-dried remineralization hydrogel containing fluoride and amelogenin after 24-h incubation at 37°C. Notably, the spectrum (d) has no absorption peak around 600 cm−1 corresponding to HAP or OCP.
ATR FT-IR: attenuated total reflectance–Fourier transform infrared; HAP: hydroxyapatite; OCP: octacalcium phosphate; F: fluoride.
Figure 4.
FE-SEM images of (a) the freeze-dried remineralization hydrogel containing fluoride and amelogenin after 24-h incubation at 37°C, (b) multispecies biofilms formed in a CDFF model after 6 days of culture, (c) enamel surface after acid etching, and (d) enamel surface after 3 days of remineralization gel treatment with a multispecies biofilm model in CDFF.
CDFF: constant-depth film fermenter; FE-SEM: field emission scanning electron microscopy.
Static enamel remineralization
The KHN of acid-etched enamel was determined before and after application of the remineralization hydrogel and immersion in artificial saliva buffer for 36 h (n = 4 for each group). The KHN increased significantly from 16.47 ± 3.73 to 28.55 ± 4.85 kg mm−2 (p = 0.008) after remineralization of the hydrogel with [F−] = 5 mg L−1, [Ca2+] = 4 mM, and [PO43−] = 2.4 mM. With the addition of 100 µg mL−1 rP172 to the above agar hydrogel, KHN increased from 15.67 ± 0.87 to 46.45 ± 3.91 kg mm−2 (p = 0.001).
Cyclic gel remineralization
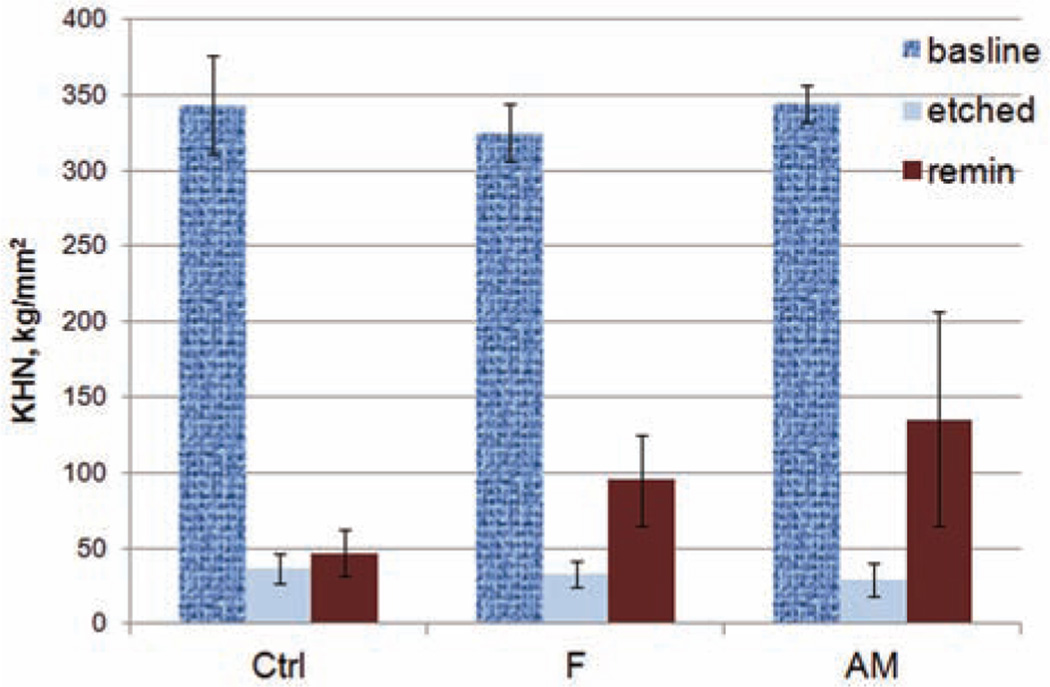
We tested the remineralization efficacy of demineralized tooth slabs (n = 10 per group) with 5-day cyclic treatment in a pH 7.3 remineralization hydrogel for 12 h, followed by pH 5.0, 0.1 M sodium acetate buffer for 2 h and artificial saliva for 10 h. As shown in Figure 5, the enamel treated with hydrogel containing 100 µg mL−1 rP172 and 5 mg L−1 fluoride showed a significant increase in the KHN from 28.78 ± 11.07 to 135.72 ± 70.25 kg mm−2 (p < 0.001). The mean hardness increase was significantly higher than the control groups without amelogenin (p = 0.043) and those without either amelogenin or fluoride (p < 0.001). These results indicate that a remineralization hydrogel containing full-length amelogenin can significantly increase the surface microhardness of remineralized enamel.
Figure 5.
Enamel KHN changes after demineralization (etched) and remineralization (remin) in hydrogel in cyclic treatment with artificial saliva (10 h), remineralization gel (12 h), and acetate buffer (2 h) after 5 days (remin).
Ctrl group: no fluoride and no amelogenin; F group: with fluoride but no amelogenin; AM group: with both fluoride and amelogenin; baseline: the KHN of intact enamel before demineralization; KHN: Knoop hardness number.
Remineralization in a multispecies oral biofilm model in CDFF
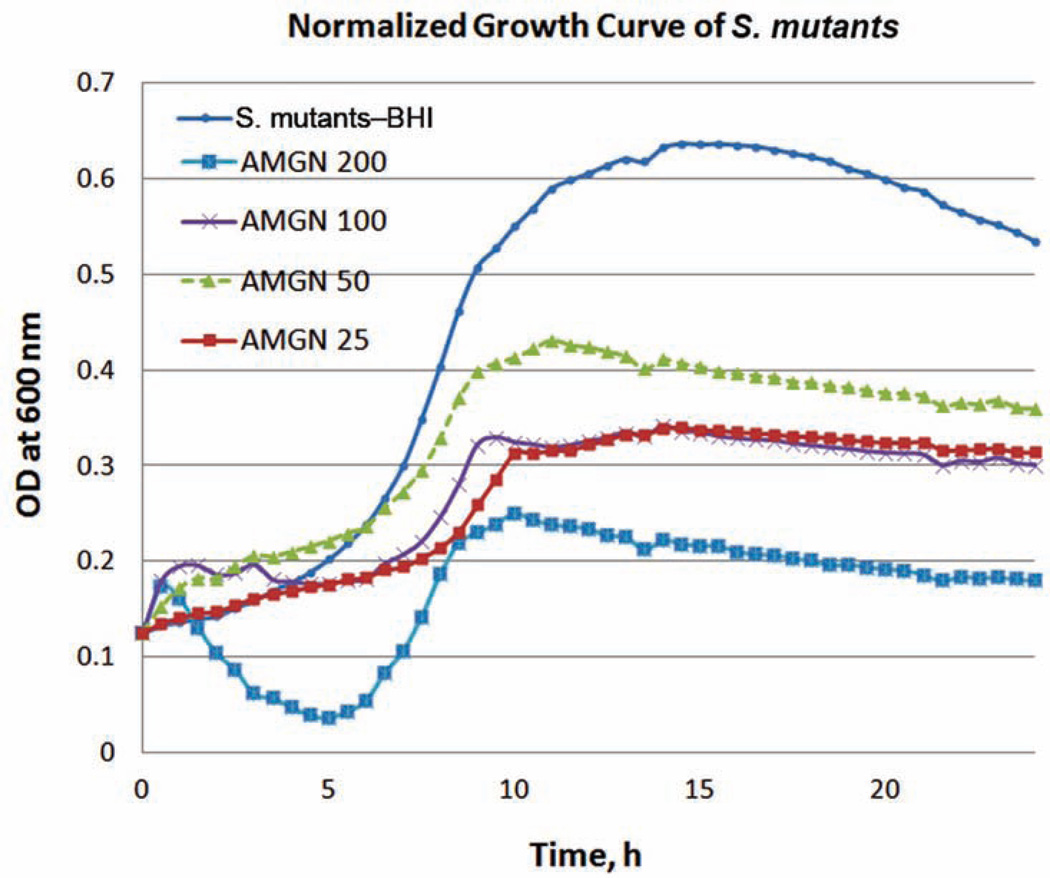
To mimic the oral environment, we used mixed-species biofilms grown in CDFF, a continuously flowing system commonly used in studying the ecology of oral biofilms.27 First, we analyzed interactions between amelogenin and S. mutans. As shown in Figure 6, inclusion of rP172 in the growth medium at a concentration of 200 µg mL−1 was found to decrease the optical density of S. mutans and S. sanguinis by more than 45% and 39%, respectively, when compared to culture medium controls. The number of colony-forming units (CFUs) of biofilms is significantly reduced at a concentration of 50 µg mL−1 rP172 compared to controls at 24 h (28% reduction, p = 0.015). While the underlying mechanism remains unclear, these results suggest that amelogenin is inhibitory to cariogenic biofilms, which may prevent biofilm invasion into the hydrogel. This is plausible since potential antimicrobial effects on ex vivo periodontal plaques by Emdogain® (extracted porcine enamel matrix, mainly amelogenin) have been reported.29
Figure 6.
Growth curve of S. mutans in the presence of different concentrations (25, 50, 100, and 200 µg mL−1) of amelogenin (AMGN) monitored by a Bioscreen C at 600 nm.
BHI: brain heart infusion; OD: optical density.
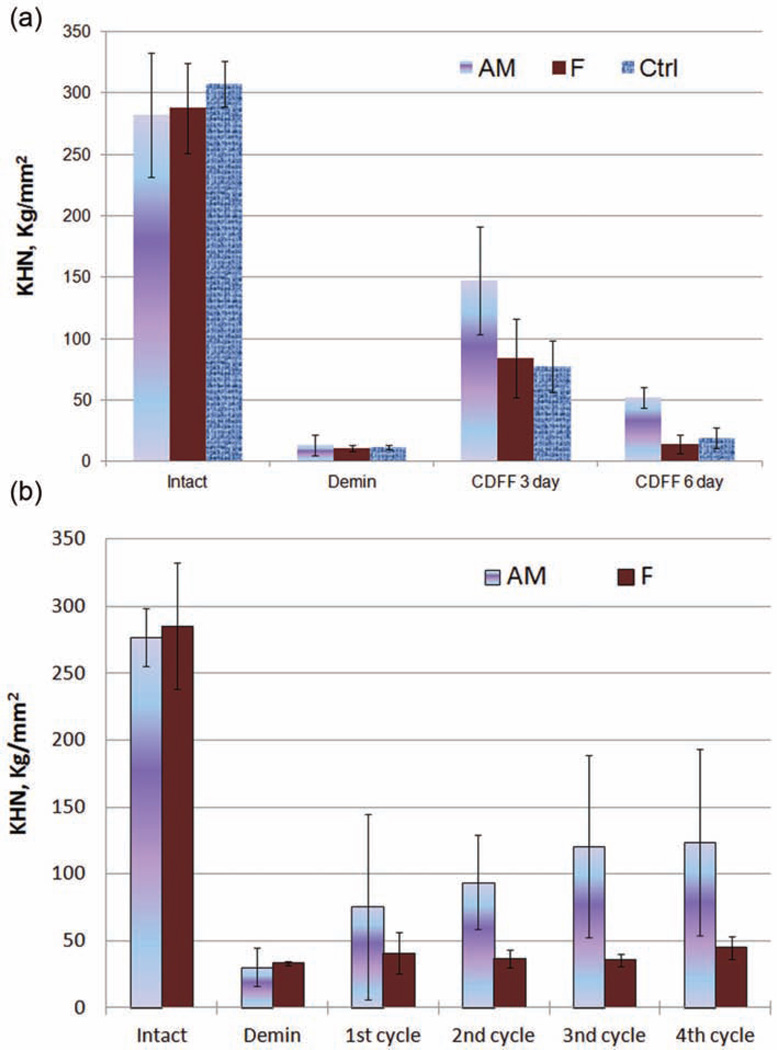
As seen in Figure 7, the tooth slabs covered with hydrogel were equilibrated with the biofilm culture medium (BMMG [i.e. glucose at the level of 10 mM was included in the BMM growth medium]26 supplemented with K2HPO4: 0.03% and CaCl2·2H2O: 0.01%) before mixed species of bacteria were infused into the CDFF system to allow biofilms to grow on the surface of the hydrogels. The SEM image (Figure 4(b)) shows significant amount of biofilm accumulated after 72 h, but the depth of biofilms in the system was kept constant (0.3 mm) using polytetrafluoroethylene (PTFE) scrapers. Enamel KHN changes after demineralization and remineralization in hydrogel treated with multispecies biofilms for 3 and 6 days (Figure 8(a)). The enamel KHN increase for the AM group is significantly greater than the F group without amelogenin (138. 56 ± 13.67 vs 53.17 ± 15.17, n = 10, p = 0.004), and the control group without either amelogenin or fluoride (138. 56 ± 13.67 vs 57.93 ± 9.23, n = 10, p = 0.019) after 3 days. But there was no significant difference in the mean increase for the F group compared to controls (53.17 ± 15.17 vs 57.93 ± 9.23, n = 10, p = 0.680). At the sixth day, however, the mineralization balance changed to more demineralization resulting in less surface hardness. The AM group had a significantly greater mean increase in enamel microhardness than the F group (38.67 ± 15.32 vs 3.33 ± 5.97, p < 0.001) and the control group (38.67 ± 15.32 vs 7.74 ± 7.18, p = 0.001), but there was no significant difference in the mean increase for the F group compared to controls (3.33 ± 5.97 vs 7.74 ± 7.18, p = 0.783).
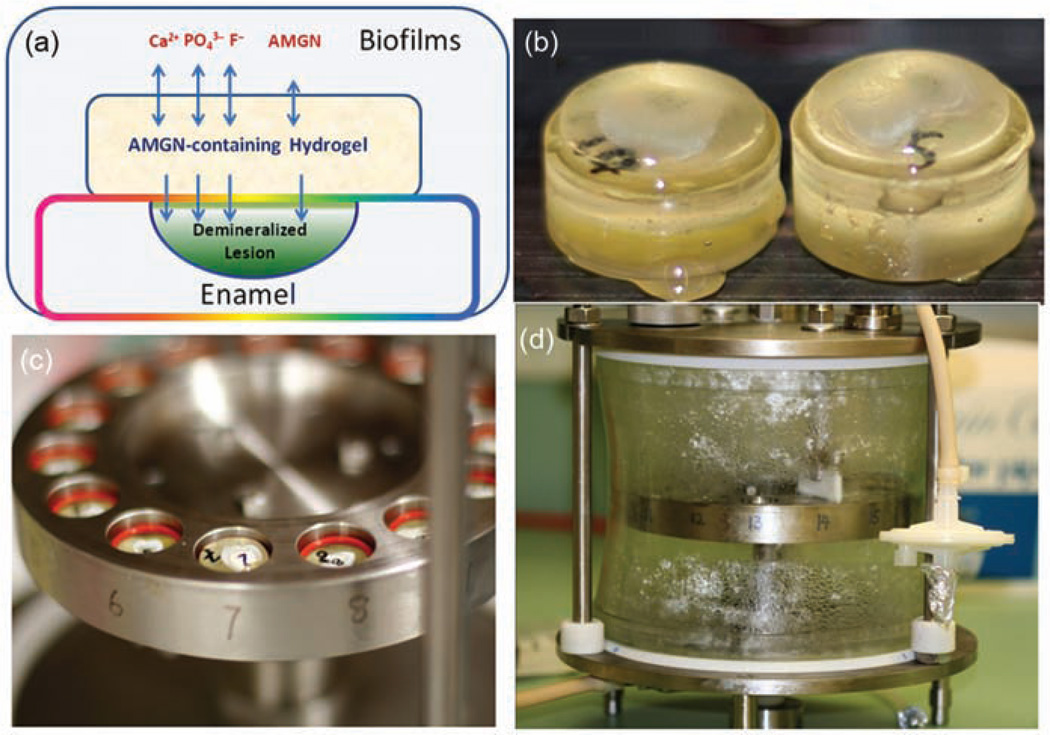
Figure 7.
The hydrogel-on-tooth specimens in a CDFF device for biofilm culture. (a) Illustration of a hydrogel-covered tooth lesion shows the ion and amelogenin transportation for remineralization, (b) the tooth specimens on plugs with remineralization hydrogel, (c) the flange with 15 holes where the plugs with O-ring can be inserted with a preset depth of 300 µm to the top, and (d) the sealed glass cylinder to ensure the desired atmosphere for biofilm formation. The Teflon scraper removes excess biofilm keeping a constant thickness of biofilm on the tooth/hydrogel surface.
CDFF: constant-depth film fermenter; AMGN: amelogenin.
Figure 8.
Enamel KHN changes after demineralization and remineralization (a) in hydrogel with a multispecies-simulated oral biofilm model in CDFF for 3 and 6 days and (b) after demineralization and repeated remineralization in hydrogel with a multispecies biofilm model in CDFF. Intact KHN is shown for the healthy tooth before demineralization. Etched KHN is shown for the tooth with artificial caries. The AM group is treated with remineralization hydrogel containing amelogenin and fluoride, F is treated with remineralization hydrogel containing fluoride but no amelogenin, and Ctrl is treated with blank hydrogel containing neither amelogenin nor fluoride. Error bars represent standard deviations, n = 10 and 6 for (a) and (b), respectively.
Demin: demineralization; CDFF: constant-depth film fermenter; KHN: Knoop hardness number.
The repetitive measurement of KHN after remineralization with amelogenin treatment (AM group) and the control remineralization group without amelogenin (F group) in the CDFF model is shown in Figure 8(b). For both treatments combined, the mean KHN increased with the number of cycles (F1,46 = 22.4, p < 0.001). Looking at each treatment separately, treated teeth in the AM group showed a significant increase in KHN after remineralization (F1,23 = 19.5, p < 0.001), but the increase in KHN for treated teeth in the F group did not achieve statistical significance (F1,23 = 3.3, p = 0.084). Across cycles, treated teeth in the AM group had a significantly greater increase in KHN compared to that of treated teeth in the F group (F1,46 = 14.5, p < 0.001). The comparison of the two treatments’ mean hardness for each number of cycles shows no significant difference after etching (29.2 ± 13.2 vs 33.2 ± 8.8, p = 0.867) and after the first treatment (75.5 ± 69.2 vs 40.9 ± 15.4, p = 0.147). However, after the second treatment cycle, the AM group had a significantly higher KHN compared to the F group (86.5 ± 35.0 vs 33.8 ± 9.4, p = 0.030). After the third and fourth cycles, the AM group had a significantly higher KHN compared to the F group (112.8 ± 68.4 vs 39.8 ± 13.4, p = 0.003 and 114.0 ± 69.7 vs 44.9 ± 8.7, p = 0.005 for cycles 3 and 4, respectively).
These results further indicate that the impact of oral biofilms on amelogenin degradation in the early stage of remineralization is not significant, and the amelogenin-modulated remineralization is still effective in the presence of multispecies biofilms. The continuous surface KHN increase in the AM group indicates the effectiveness of remineralization by repeated treatment.
After demineralization in a lactic acid buffer, enamel crystals become broken fragments, as observed in the FE-SEM image (Figure 4(c)). Nanocrystal bundles and smooth crystal surfaces were observed after 72 h of remineralization in hydrogel in the CDFF model (Figure 4(d)). ATR FT-IR spectrum on the hydrogel remineralized enamel (Figure 3(b) and (c)) demonstrates the HPO4 υ1 band (1020 cm−1) and υ4 band (600 and 554 cm−1), indicating the formation of HAP. Weak peaks for protein amide I, II, and III bands (1650, 1453, and 1256 cm−1) indicate the presence of amelogenin residue after being remineralized with amelogenin-containing hydrogel.
Cytotoxicity tests
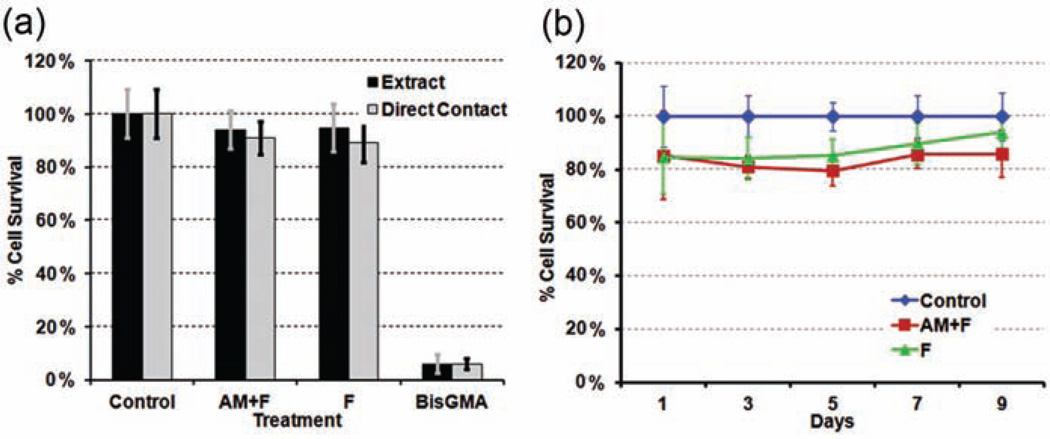
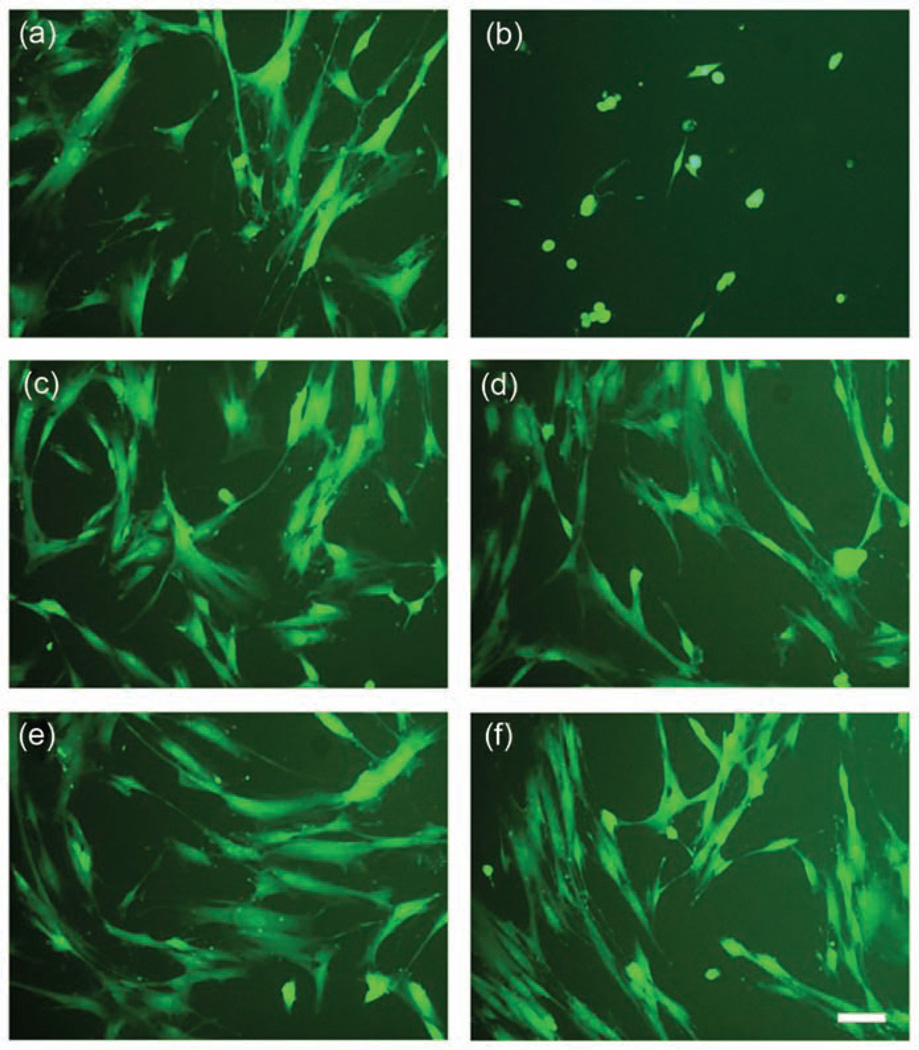
The relative cytotoxicity of amelogenin-containing hydrogels was examined using PDL fibroblasts. Neither hydrogel extracts nor growth of cells in direct contact with the hydrogels displayed any significant reduction in cell survival rate (p = 0.16 and 0.26, respectively) when compared to untreated cells grown in normal growth medium (Figure 9(a)). The addition of amelogenin to the hydrogels did not significantly reduce cell survival in extracts (p = 0.84) or direct contact experiments (p = 0.62). BisGMA at the concentration of 10−4 M (positive control) showed significant cytotoxicity (p < 0.001). An extended culture cell survival rate was observed up to 9 days showing no significant difference in PDL cell death between hydrogels with and without amelogenin. Although the survival rate was significantly lower than controls on a Petri dish from 1 to 7 days (n = 8, p < 0.05), there was no significant difference compared to controls after 9 days of culture (92% and 86% for F and AM hydrogels, respectively, n = 8, p > 0.1). The lower survival rate may be due to a reduced diffusion of medium nutrition through the hydrogel. In general, all hydrogels showed no cytotoxic potential to PDL cells within 9 days, since the survival rate in hydrogel contact coculture was above 80% at all times compared to control. A microscopic examination of fluorescently labeled PDL cells (Figure 10) revealed little change in cell shape or viability after 24 h of exposure to either hydrogel extracts or direct contact cultures to the hydrogels themselves. These results indicate that the novel remineralization hydrogels containing amelogenin display no cytotoxicity to human PDL fibroblasts within 9 days.
Figure 9.
Survival rate of gingival fibroblast (a) after culture with remineralization hydrogel extract and direct contact with remineralization hydrogels (n = 16) for 24 h and (b) after culture in direct contact with hydrogel for up to 9 days. BisGMA is the positive control 10−4 M.
Ctrl: cell culture in Petri dish; AM + F: hydrogel containing amelogenin and fluoride; F: hydrogel containing fluoride; BisGMA: bisphenol-A-glycidyldimethacrylate.
Figure 10.
Fluorescent microscopy images of live gingival fibroblast cells grown for 24 h in the presence of (a) control, (b) BisGMA, (c) AM + F hydrogel extract, (d) F hydrogel extract, (e) AM + F hydrogel direct contact, and (f) F hydrogel direct contact. The scale bar in (f) is 10 µm and applies to all images.
Control: cell culture in Petri dish; AM + F: hydrogel containing amelogenin and fluoride; F: hydrogel containing fluoride; BisGMA: bisphenol-A-glycidyldimethacrylate.
Discussion
To develop a hydrogel system for controlled release of amelogenin and other remineralization ions to promote enamel surface remineralization, several obstacles need to be overcome. First, the full-length amelogenin can be easily trapped in many traditional dental materials systems and lose its ability to modulate HAP crystal growth. The amelogenin molecule has an intrinsic bipolar characteristic, a proline-rich hydrophobic core, and a hydrophilic C-terminus tail, which behaves similar to amphiphilic molecules that easily bind to a charged matrix surface, such as chitosan and alginate. Second, the self-assembly of amelogenin appears to be strongly dependent on the pH of the environment.30 Considering that the pH in the oral cavity fluctuates dramatically, plus potential degradation by proteinases and peptidases of bacterial biofilms,31 both the release efficiency and functionality of amelogenin can be significantly altered.
Amelogenin self-assembly is also critical for its function in calcium phosphate mineralization. Recently, the self-assembled supermolecular particles have been determined by in situ atomic force microscopy (AFM),32 and those results suggest that amelogenin self-assembly is more complex and dynamic than previously expected. Although the chemical environment of in vivo enamel mineralization is not completely understood, amelogenin will inevitably interact with the charged biomacromolecular surface of proteins, lipids, and polysaccharides, as well as minerals. After investigating several candidates, we have found that agar, a nonionic polysaccharide, shows low retention of amelogenin, and can be used as a feasible loading–releasing system for both protein and ion release. Therefore, only the physical hydrogel was used in this study since the cross-linkers in chemically cross-linked hydrogels could cause damage of the protein and impede sufficient release.
To treat the early stages of caries in the clinic, topical fluoride varnishes (2.26% NaF), fluoride dentifrices (0.11% F), and a casein phosphopeptide–ACP (CPP-ACP) complex have been developed and used as tooth remineralizing agents.33,34 However, none of these products are capable of promoting organized calcium phosphate remineralization.25 In mineralization from a simulated body fluid, synthetic HAP in a coating is usually observed as plate-like crystals associated with an OCP precursor. The degree of supersaturation, IS, additive concentration, and temperature are important factors affecting crystal growth and crystal morphology.35 Unfortunately, in most cases, calcium, phosphate and/or fluoride-releasing dental materials only induce the formation of loosely structured HAP plates or granular calcium fluoride.36,37 Incorporation of 1–2 mg L−1 of fluoride in remineralization solutions may facilitate the formation of needle-like HAP.38 The formation of hyperbranched FAP crystals was observed using higher concentrations of F (>50 mg L−1).39 However, neither plate-like OCP nor hyperbranched FAP can restore the function (e.g. hardness) of the enamel or protect the underlying tooth structure from further demineralization. In this study, we have again demonstrated the possibility of improving surface hardness by growing organized enamel-like nanocrystals on demineralized enamel using amelogenin.
Simultaneous release of amelogenin, calcium, phosphate, and fluoride ions is feasible in an agar-based hydrogel system. However, the released ions may not be free ions but instead may be a complex of amorphous fluoridated calcium phosphate with amelogenin as suggested by previous researchers.9,40 The release profiles of Ca2+ and F− (Figure 1(c) and (d)) demonstrate that the addition of amelogenin can result in a higher release content, indicating that the protein may prevent crystallization inside hydrogel and stabilize the ACP complex. Since amelogenesis in vivo is a prolonged progress, where the dense layer of enamel crystal normally forms at a very low rate of a few micrometers per week,41 the remineralization of enamel may require repeated applications of our hydrogel in order to achieve sufficient enamel hardness.
Multispecies biofilms grown in CDFF are regarded as an artificial mouth model commonly used to simulate the oral environment.42 In this CDFF model, it is evident that the balance of enamel remineralization and demineralization is from bacteria generated acid. As shown, when samples were analyzed after 3 and 6 days of remineralization in the presence of hydrogels without replacing the hydrogel, the KHN after 3 days was significantly improved compared to demineralized enamel, but the KHN was reduced after additional 3 days without replacing the hydrogel (Figure 8(a)). This is presumably caused by amelogenin and F depletion from the hydrogel, which makes the enamel surface prone to acidic attack and demineralization. The repetitive application of remineralization hydrogel containing amelogenin can significantly increase enamel hardness (Figure 8(b)). This also has a major clinical implication as patients should apply the remineralization gel every night for several days. The thermoresponsive hydrogel with a sol/gel transition point of 32°C–45°C can prevent the amelogenin from being released into saliva, but it will be permeable to Ca2+, PO43−, and F− ions to facilitate ion exchange between saliva and the enamel surface, as illustrated in Figure 7(a). Such a gel-forming material will last up to 16 h on enamel surfaces and then could be removed by brushing and rinsing. In practice, repetitive use of such a gel would be required to attain adequate remineralization.
Although the final hardness of amelogenin hydrogel–induced remineralized enamel was lower than that of native enamel, the remineralized enamel treated with amelogenin-containing hydrogel showed significantly greater hardness than controls. The amelogenin residue in the remineralized crystals as detected by ATR FT-IR may limit its hardness. The application of enzymes such as enamelysin and KLK443 to remove excess intercrystalline amelogenin could improve the crystal packing density. Future studies will focus on increasing the hardness by optimizing the hydrogel composition and investigating mechanisms of the protein–ACP complex release. The controlled release of mineralization-promoting protein and ions from a polymer matrix can modulate biomineral formation and provide a new biomimetic approach for preparing artificial biomineralized structures and regenerating mineralized tissue.
Conclusion
In this exploratory study, biocompatible amelogenin–containing remineralization hydrogels were prepared to release Ca2+, F−, and amelogenin. This remineralization hydrogel applied to artificial enamel caries lesions, displayed remineralization activity on early artificial caries in a cyclic treatment model and multispecies oral biofilm model. Repetitive application of the hydrogel significantly improved enamel hardness continuously over time. These results demonstrate the feasibility of developing new amelogenin-containing remineralization dental materials for applications in caries management.
Acknowledgement
We would like to thank Dr Tung-Sung Tseng for helpful discussions of data analysis.\
Funding
This study was supported by NIH/NCRR CoBRE pilot grant (5P20RR020160-05) to Yuwei Fan and NIH/NIDCR grant to Zezhang T Wen (1R01DE19452) and Xiaoming Xu (1R01DE019203).
References
- 1.Moradian-Oldak J, Du C, Falini G. On the formation of amelogenin microribbons. Eur J Oral Sci. 2006;114(Suppl 1):289–296. doi: 10.1111/j.1600-0722.2006.00285.x. discussion 327–329, 382. [DOI] [PubMed] [Google Scholar]
- 2.Margolis HC, Beniash E, Fowler CE. Role of macromolecular assembly of enamel matrix proteins in enamel formation. J Dent Res. 2006;85:775–793. doi: 10.1177/154405910608500902. [DOI] [PubMed] [Google Scholar]
- 3.Iijima M, Moradian-Oldak J. Control of octacalcium phosphate and apatite crystal growth by amelogenin matrices. J Mater Chem. 2004;14:2189–2199. [Google Scholar]
- 4.Iijima M, Moriwaki Y, Wen HB, et al. Elongated growth of octacalcium phosphate crystals in recombinant amelogenin gels under controlled ionic flow. J Dent Res. 2002;81(1):69–73. doi: 10.1177/002203450208100115. [DOI] [PubMed] [Google Scholar]
- 5.Wang LJ, Guan XY, Yin HY, et al. Mimicking the self-organized microstructure of tooth enamel. J Phys Chem C. 2008;112(15):5892–5899. doi: 10.1021/jp077105+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uskokovic V, Li W, Habelitz S. Amelogenin as a promoter of nucleation and crystal growth of apatite. J Cryst Growth. 2011;316(1):106–117. doi: 10.1016/j.jcrysgro.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen HB, Moradian-Oldak J. Modification of calcium-phosphate coatings on titanium by recombinant amelogenin. J Biomed Mater Res A. 2003;64(3):483–490. doi: 10.1002/jbm.a.10401. [DOI] [PubMed] [Google Scholar]
- 8.Du C, Falini G, Fermani S, Abbott C, et al. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons. Science. 2005;307(5714):1450–1454. doi: 10.1126/science.1105675. [DOI] [PubMed] [Google Scholar]
- 9.Yang XD, Wang LJ, Qin YL, et al. How amelogenin orchestrates the organization of hierarchical elongated microstructures of apatite. J Phys Chem B. 2010;114:2293–2300. doi: 10.1021/jp910219s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Liu Y, Perez T, et al. Kinetics of nanochain formation in a simplified model of amelogenin biomacromolecules. Biophys J. 2011;101(10):2502–2506. doi: 10.1016/j.bpj.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng ZJ, Wang XM, Cui FZ, et al. The enamel softening and loss during early erosion studied by AFM, SEM and nanoindentation. Biomed Mater. 2009;4(1):015020. doi: 10.1088/1748-6041/4/1/015020. [DOI] [PubMed] [Google Scholar]
- 12.Wang LJ, Tang R, Bonstein T, et al. Enamel demineralization in primary and permanent teeth. J Dent Res. 2006;85:359–363. doi: 10.1177/154405910608500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki H, Margolis HC. Enhanced enamel remineralization under acidic conditions in vitro. J Dent Res. 2008;87(6):569–574. doi: 10.1177/154405910808700612. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane NJ, Cai F, Huq NL, et al. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89(11):1187–1197. doi: 10.1177/0022034510376046. [DOI] [PubMed] [Google Scholar]
- 16.Lussi A. Dental erosion–novel remineralizing agents in prevention or repair. Adv Dent Res. 2009;21(1):13–16. doi: 10.1177/0895937409335592. [DOI] [PubMed] [Google Scholar]
- 17.Fan Y, Sun Z, Moradian-Oldak J. Effect of fluoride on the morphology of calcium phosphate crystals grown on acid etched human enamel. Caries Res. 2009;43:132–136. doi: 10.1159/000209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y, Nelson JR, Alvarez JR, et al. Amelogenin-assisted ex vivo remineralization of human enamel: effects of supersaturation degree and fluoride concentration. Acta Biomater. 2011;7:2293–2302. doi: 10.1016/j.actbio.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamane S, Sugawara A, Watanabe A, et al. Hybrid nanoapatite by polysaccharide nanogel-templated mineralization. J Bioact Compat Polym. 2009;24:151–168. [Google Scholar]
- 20.Kolenbrander PE, Palmer RJ, Jr, Periasamy S, et al. Oral multispecies biofilm development and the key role of cell-cell distance. Nature Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 21.Chang SY, Chung RJ, Chou FC, et al. Effect of Streptococcus mutans on mechanical properties of human dental structures. J Mater Res. 2009;24:2301–2306. [Google Scholar]
- 22.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90(3):294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 23.Bitoun JP, Nguyen AH, Fan Y, et al. Transcriptional repressor Rex is involved in regulation of oxidative stress response and biofilm formation by Streptococcus mutans. FEMS Microbiol Lett. 2011;320:110–117. doi: 10.1111/j.1574-6968.2011.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu OH, Fincham AG, Hu CC, et al. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res. 1999;78:743–750. doi: 10.1177/00220345990780030601. [DOI] [PubMed] [Google Scholar]
- 25.Oshiro M, Yamaguchi K, Takamizawa T, et al. Effect of CPP-ACP paste on tooth mineralization: an FE-SEM study. J Oral Sci. 2007;49:115–120. doi: 10.2334/josnusd.49.115. [DOI] [PubMed] [Google Scholar]
- 26.Shu M, Browngardt CM, Chen YY, et al. Role of urease enzymes in stability of a 10-species oral biofilm consortium cultivated in a constant-depth film fermenter. Infect Immun. 2003;71:7188–7192. doi: 10.1128/IAI.71.12.7188-7192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu M, Wong L, Miller JH, et al. Development of multi-species consortia biofilms of oral bacteria as an enamel and root caries model system. Arch Oral Bio. 2000;45(1):27–40. doi: 10.1016/s0003-9969(99)00111-9. [DOI] [PubMed] [Google Scholar]
- 28.Winer BJ. Statistical principles in experimental design. New York: McGraw-Hill; 1971. [Google Scholar]
- 29.Sculean A, Auschill TM, Donos N, et al. Effect of an enamel matrix protein (Emdogain (R)) on ex vivo dental plaque vitality. J Clin Periodontol. 2001;28(11):1074–1078. doi: 10.1111/j.1600-051X.2001.281113.x. [DOI] [PubMed] [Google Scholar]
- 30.Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, et al. pH triggered self-assembly of native and recombinant amelogenins under physiological pH and temperature in vitro. J Struct Biol. 2007;160:57–69. doi: 10.1016/j.jsb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potempa J, Sroka A, Imamura T, et al. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- 32.Chen CL, Bromley KM, Moradian-Oldak J, et al. In situ AFM study of amelogenin assembly and disassembly dynamics on charged surfaces provides insights on matrix protein self-assembly. J Am Chem Soc. 2011;133(43):17406–17413. doi: 10.1021/ja206849c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi K, Miyazaki M, Takamizawa T, et al. Effect of CPP-ACP paste on mechanical properties of bovine enamel as determined by an ultrasonic device. J Dent. 2006;34:230–236. doi: 10.1016/j.jdent.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds EC. Re-mineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–1595. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 35.Wang LJ, Nancollas GH. Calcium orthophosphates: crystallization and dissolution. Chem Rev. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petzold M, Berthold L, Cismak A, et al. The influence of different fluoride compounds and treatment conditions on dental enamel: a descriptive in-vitro study of the CaF2 precipitation and microstructure. Caries Res. 2001;35(Suppl 1):45–51. doi: 10.1159/000049110. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto M, Nakamura K, Kaga M, et al. Crystal growth by fluoridated adhesive resins. Dent Mater. 2008;24:457–463. doi: 10.1016/j.dental.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Iijima M, Tohda H, Suzuki H, et al. Effects of F− on apatite-octacalcium phosphate intergrowth and crystal morphology in a model system of tooth enamel formation. Calcif Tissue Int. 1992;50:357–361. doi: 10.1007/BF00301634. [DOI] [PubMed] [Google Scholar]
- 39.Wang ZQ, Ma GB, Liu XY. Will fluoride toughen or weaken our teeth? Understandings based on nucleation, morphology, and structural assembly. J Phys Chem B. 2009;113(51):16393–16399. doi: 10.1021/jp905846p. [DOI] [PubMed] [Google Scholar]
- 40.Beniash E, Metzler RA, Lam RS, et al. Transient amorphous calcium phosphate in forming enamel. J Struct Biol. 2009;166(2):133–143. doi: 10.1016/j.jsb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmer JP, Papagerakis P, Smith CE, et al. Regulation of dental enamel shape and hardness. J Dent Res. 2010;89(10):1024–1038. doi: 10.1177/0022034510375829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung D, Spratt DA, Pratten J, et al. Chlorhexidinereleasing methacrylate dental composite materials. Biomaterials. 2005;26:7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Sun Z, Carpiaux W, Fan D, et al. Apatite reduces amelogenin proteolysis by MMP-20 and KLK4 in vitro. J Dent Res. 2010;89(4):344–348. doi: 10.1177/0022034509360660. [DOI] [PMC free article] [PubMed] [Google Scholar]