Abstract
Aim:
To evaluate the biocompatibility of a new root canal irrigant Q mix™ 2 in 1 in comparison to 0.9% sterile saline, 3% sodium hypochlorite (NaOCl), 2% chlorhexidine (CHX), and 17% ethylenediaminetetraacetic acid (EDTA).
Materials and Methods:
Six circles were drawn on the dorsal skin of 24 male Wistar Albino rats, leaving 2cm between each circle. Using a syringe, 0.1mL of each root canal irrigant was injected subcutaneously into five circles. In the sixth circle, the needle of an empty syringe was introduced into the skin, but no irrigant was injected (control group). Evaluations were done at 2 hours, 48 hours, 14 days, and 30 days postprocedure. Tissue samples were excised, embedded in paraffin blocks, and 3 μm thick sections were obtained and stained with hematoxylin and eosin. The areas of inflammatory reaction were evaluated. From each tissue sample, five sections presenting the greatest inflammatory reactions were examined under a light microscope, and analyzed statistically by analysis of variance (ANOVA) and Tukey's test.
Results:
At the two-hour examination period, all the irrigants showed a slight increase in the number of inflammatory cells, at 48 hours, the number of inflammatory cells were increased significantly, and after 14 and 30 days, they were decreased gradually. Qmix™ 2 in 1 showed a smaller number of inflammatory cells than other irrigants tested.
Conclusion:
QMix™ 2 in 1 was shown to be less toxic to the rat subcutaneous tissue than 3% NaOCl, 2% CHX, and 17% EDTA.
Keywords: Biocompatibility, root canal irrigant, subcutaneous tissue
INTRODUCTION
The success of endodontic treatment depends on the eradication of micro-organisms from the root canal system and prevention of reinfection.[1] Mechanical instrumentation alone does not result in a bacteria-free root canal system, when the complex anatomy is considered.[2] Thus, irrigants are essential to ensure bacterial minimization and elimination of organic tissue remnants.[3,4] An ideal root canal irrigant must have maximum tissue-dissolving and antibacterial effect, and must induce mild or no inflammatory response in the tissues.[5–7]
Sodium hypochlorite (NaOCl), with its antibacterial and dissolving effects on the necrotic tissues, is the most popular root canal irrigant.[8] Although it effectively kills bacteria, it is deleterious if accidentally expressed into the periapical tissues.[9,10]
Chlorhexidine gluconate (CHX) is used as an endodontic irrigating solution because of its antimicrobial efficiency and substantivity,[11] but it has no tissue-dissolving property.[2]
Ethylenediaminetetraaceticacid (EDTA) has been advocated as an adjunct for root canal preparation due to its chelating action;[12] however, its antimicrobial efficacy is low.[13]
QMix™ 2 in 1 solution contains a mixture of a bisbiguanide antimicrobial agent, a polyaminocarboxylic acid calcium-chelating agent, and a surfactant, and has been found to be effective against bacterial biofilms.[14]
During root canal procedures, the irrigating solution will be in contact with pulpal and periapical tissues. Debris as well as irrigating solutions may also be pushed beyond the apical foramen and possibly cause periapical complications.[11] In this regard, QMix™ 2 in 1 may impact on the physiological healing process anticipated during and following root canal treatment.
Therefore, the aim of this study was to compare the response of rat subcutaneous tissue to QMix™ 2 in 1, 0.9% sterile saline, 3% NaOCl, 2% CHX, and 17% EDTA.
MATERIALS AND METHODS
Twenty-four male Wistar Albino rats weighing 250-270g were used for the in vivo experiments. The animals were housed in a temperature-controlled environment with water and food (Kakatiya University of Pharmaceutical Sciences, Warangal, India). All experiments were conducted in accordance with the guidelines of the National Institute of Health (NIH) on the welfare of experimental animals and after approval by the Ethics in Research Committee of the Mamata Dental College and Hospital, Khammam, India.
Subcutaneous tissue reaction to the following irrigating solutions was evaluated: 0.9% sterile saline (Parenteral Drugs (India) Limited, Asrawad, Indore, India), QMix™ 2 in 1 (Dentsply, Tulsa Dental Specialties, Tulsa, OK), 3% NaOCl (Prime Dental, Mumbai, India), 2% CHX (Ammdent, Mohali, India), and 17% EDTA (Ammdent, Mohali, India).
Under general anesthesia with 5% ketamine hydrochloride (Neon Laboratories Limited, Phalghar, Thane, India), the dorsal skin of the animals was shaved and cleaned with 10% iodine solution. Using a glass template, six circles were demarcated on the dermis of each rat leaving 2cm between each circle. Using a tuberculin syringe, 0.1mL of each root canal irrigant was injected subcutaneously into five circles. For the control group, the needle of an empty syringe was introduced in the sixth circle, but no irrigant was injected.
Evaluations were made 2 hours, 48 hours, 14 days, and 30 days after injection. In each examination period, six animals from experimental groups were sacrificed by anesthetic overdose. The tissue specimens were excised with a scalpeland stored in 10% formalin solution for 48 hours. Collected samples were divided into six groups with six samples in each group, according to the irrigant injected. A total of 36 samples was collected at each examination period.
Group 1: Samples collected from the site where needle prick was given (control)
Group 2: Samples collected from the site where 0.9% saline was injected
Group 3: Samples collected from the site where QMix™ 2 in 1 was injected
Group 4: Samples collected from the site where 3% NaOCl was injected
Group 5: Samples collected from the site where 2% CHX was injected
Group 6: Samples collected from the site where 17% EDTA was injected
The tissue samples were embedded in paraffin blocks. Sections of 3 μm thickness were cut and stained with hematoxylin and eosin. From each tissue sample, five sections presenting the greatest inflammatory reactions were examined with a light microscope (Nikon Eclipse E800) and the characteristic areas were photographed at 20X magnification. Areas of inflammatory reaction were evaluated quantitatively by counting the number of inflammatory cells (neutrophils, lymphocytes, macrophages) using specific software (Image Pro Plus version 6.8, National Institute of Nutrition, Tarnaka, Hyderabad, India). These numbers were statistically evaluated and compared by ANOVA and Tukey's test (Graph Pad Prism 3.0; Graph Pad Software) at 5% significance level.
RESULTS
The following results were drawn by comparing all the groups and these are shown in Table 1.
Table 1.
Mean number and standard deviation of inflammatory cells at the different time periods after injection
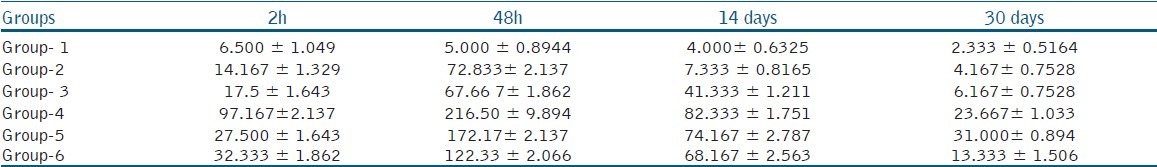
In the control group, there was no significant relation between the inflammatory reactions at different time periods.
In the sterile saline solution group, the number of inflammatory cells were increased in the 48-hour period and there was a significant difference between the two-hour and 48-hour results (P < 0.05) and there was a decrease in the mean number of inflammatory cells at 14 days and 30 days in comparison to the two earlier periods.
In the QMix™ 2 in 1 group, there was a significant increase in the number of inflammatory cells in the 48-hour interval and the number of inflammatory cells were gradually decreased after 14 and 30 days.
In the 3% NaOCl group, the average inflammatory reaction values were high and there was a significant difference between the two-hour and 48-hour time periods, and after 14 days, mild inflammatory reactions were seen.
In the 2% CHX gluconate group, inflammatory reaction reached the highest value at 48 hours with a significant difference between two hours and 48 hours periods (P < 0.05). At 14th and 30th day, there was a significant moderation in the values in comparison to the 48-hour results (P < 0.05).
In the 17% EDTA group, there was a significant increase in the number of inflammatory cells at 48 hours, and this gradually decreased at 14th and 30th day interval in comparison to the 48-hour results.
Comparison among the solutions at the evaluation periods
At two hours, only the 3% NaOCl group showed a higher inflammatory response (P < 0.05) compared to the control group.
At 48 hours, all experimental groups showed a statistically significant increase in inflammatory cells when compared to the control group (P < 0.05) [Figure 1].
Figure 1.
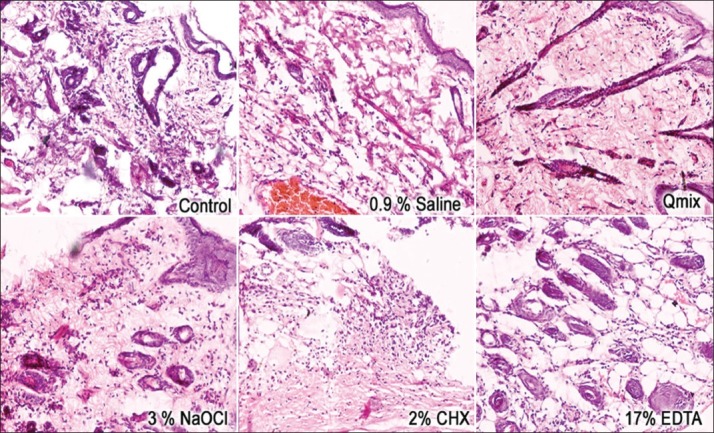
Reaction of rat subcutaneous connective tissue to different experimental groups after 48 hours (hematoxylin and eosin; ×20)
At 14 days, only the 0.9% sterile saline group showed a similar result to that of the control group (P > 0.05). At this time, the 3% NaOCl, 2.0% CHX, and 17% EDTA groups remained with a higher mean number of inflammatory cells compared to the control group (P < 0.05). The QMix™ 2 in 1 solution group presented a milder inflammatory response but still higher than that of the control group (P < 0.05).
At 30 days, the 2% CHX and3% NaOCl groups showed a significant mean number of inflammatory cells compared to the control group [Figure 2].
Figure 2.
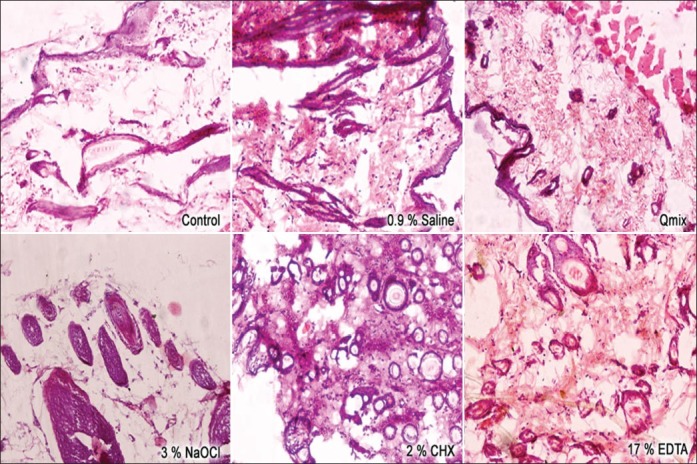
Reaction of rat subcutaneous connective tissue to different experimental groups after 30 days (hematoxylin and eosin; ×20)
DISCUSSION
The toxic effects of materials used in root canal treatment are of particular concern because damage or irritation to the periapical tissues could cause degeneration and delayed wound healing. Ideally a root canal irrigating solution should demonstrate low cytotoxicity.[11]
The growing technological evolution and continuous introduction of materials for different applications make the evaluation of the biological properties of these materials a mandatory condition. Materials must not have a deleterious effect when in contact with tissues, before they are marketed and used routinely in the clinic.[15] Following ISO/6876 and 10993-5 regulations,[16] in vitro cytotoxicity tests, such as tissue and cell culture assays, are important to provide initial evidence of cytotoxic effects. However, these tests lack the interaction of the material with the local cells and the surrounding tissues, including those cells which are attracted to the site of reaction.
Therefore, in vivo injection of materials in laboratory animals, which provides much more information about the inflammatory and immune responses developed by the test material, was used in the present study. It is clear that the data cannot be extrapolated to humans based on the results of the animal experiments. However, the introduction of different materials in the subcutaneous cellular tissue or bone from small laboratory animals is widely regarded as a valid screening test for their biological properties.[17]
The evaluation of inflammatory cell response in animal tissue is important because inflammation is the first part of the healing process, which justifies the study of the inflammatory progression after different stimuli, including irrigating solutions. The importance of these studies can be attributed to the fact that irrigant extrusion into surrounding periodontal tissues can occur in case of perforations and improper techniques or even in teeth with fully developed roots.[18] The toxicity of the root canal irrigants was tested by the same method used by Pashley and Yesilsoy et al.[19,20]
In the present study, group 1 (control group) induced mild or no reaction in the subcutaneous tissue and this process did not change at the different experimental periods. Likewise, other studies have reported only a very mild inflammatory reaction at the site of penetration with the needle of an empty syringe, suggesting that this reaction occurred due to a mechanical trauma caused by the puncture itself.[7,12]
Group 2 (0.9% sterile saline) showed a mild inflammatory response with a significant increase in the mean number of inflammatory cells only at 48hours. These findings were in agreement with Yesilsoy et al.,(1995)[20] suggesting that saline resulted in a favorable reaction in the connective tissues and can be considered as biocompatible as tissue repair occurred after 14 and 30 days.
Group 3 (QMix™ 2 in 1 solution) showed milder inflammatory reactions at two hours, and the number of inflammatory cells were increased significantly at 48 hours. After 14 and 30 days, the number of inflammatory cells decreased, which might be the symptom of repair following the injection of QMix™ 2 in 1.
Group 4 (3% NaOCl) and group 5 (2.0% CHX) presented a moderate inflammatory reaction of connective tissues. Although the mean number of inflammatory cells was considered significant at 48 hours and 14 days, a decrease in the number of inflammatory cells could be observed at 30 days, suggesting an ongoing repair process of connective tissues.[2] NaOCl has a pH of 11 to 12.5, which causes injury primarily by oxidation of proteins. In high concentrations, severe necrotic changes could be observed.[21]
Agarwal et al. (1997)[22] found that CHX disrupts the cell membrane of both crevicular and peripheral blood neutrophils at concentrations above 0.005% within five minutes, indicating that its inhibitory effect on neutrophil function is mostly due to its lytic properties. Group 6 (17% EDTA) showed the maximum number of inflammatory cells at 48 hours, and the number of inflammatory cells decreased after14 and 30 days. EDTA has been shown to inhibit the substrate adherence capacity of macrophages as well as the binding of vasoactive peptide to macrophage membranes in vitro.[23,24]
These results suggest that leakage or pushing of root canal irrigants into periapical tissues during cleaning and shaping of the root canal may alter the inflammatory response in the periapical lesions. Regeneration of tissue occurred at a slower rate in sites where 3% NaOCl, 2% CHX, and 17% EDTA was injected than those with QMix™ 2in 1.
CONCLUSION
The results of this study indicate that QMix™ 2 in 1 solution is less toxic to the rat subcutaneous tissue than 3% NaOCl, 2% CHX, and 17% EDTA. QMix™ 2 in 1 may therefore be considered safe for use as a final irrigant after NaOCl.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontic: Review. Int Endod J. 2009;42:288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 3.Hülsmann M, Hahn W. Complications during root canal irrigation-literature review and case reports. Int Endod J. 2000;33:186–93. doi: 10.1046/j.1365-2591.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams CE, Reid JS, Sharkey SW, Saunders WP. In vitro measurement of apically extruded irrigant in primary molars. Int Endod J. 1995;28:221–5. doi: 10.1111/j.1365-2591.1995.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined as endodontic irrigants. J Endod. 1998;24:472–6. doi: 10.1016/S0099-2399(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 6.Thomas AM, Chandra S, Pandey RK. Elimination of infection in pulpectomized deciduous teeth: Short term study using iodoform paste. J Endod. 1994;20:233–5. doi: 10.1016/S0099-2399(06)80284-0. [DOI] [PubMed] [Google Scholar]
- 7.Turkun M, Gokay N, Ozdemir N. Comparative investigation of the toxic and necrotic tissue dissolving effects of different endodontic irrigants. J Dent Faculty of Istanbul Uni. 1998;32:87–94. [Google Scholar]
- 8.Siqueira JF, Jr, Machado AG, Silveira RM, Lopes HP, de Uzeda M. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal in vitro. Int Endod J. 1997;30:279–32. doi: 10.1046/j.1365-2591.1997.00096.x. [DOI] [PubMed] [Google Scholar]
- 9.Haapasalo M, Udnaes T, Endal U. Persistent, recurrent, and acquiredinfection of the root canal systempost-treatment. Endod Topics. 2003;6:29–56. [Google Scholar]
- 10.Oncag O, Hosgor M, Hilmioglu S, Zekioglu O, Eronat C, Burhanoglu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:446–50. doi: 10.1067/moe.2001.116812. [DOI] [PubMed] [Google Scholar]
- 12.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Hulsmann M, Heckendorff M, Lenon A. Review on chelating agents in root canal treatment: Mode of action and indications for their use. Int Endod J. 2003;36:810–30. doi: 10.1111/j.1365-2591.2003.00754.x. [DOI] [PubMed] [Google Scholar]
- 14.Dai L, Khechen K, Khan S, Gillen B, Loushine BA, Wimmer CE, et al. The Effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod. 2011;37:80–4. doi: 10.1016/j.joen.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence WH, Malik M, Autian J. Development of a toxicity program for dental materials and products. J Biomed Mater Res. 1974;8:11–34. doi: 10.1002/jbm.820080104. [DOI] [PubMed] [Google Scholar]
- 16.Camps J, About I. Cytotoxicity testing of endodontic sealers: A new method. J Endod. 2003;29:583–6. doi: 10.1097/00004770-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Martínez Lalis R, Esaín ML, Kokubu GA, Willis J, Chaves C, Grana DR. Rat subcutaneous tissue response to modified portland cement, a new mineral trioxide aggregate. Braz Dent J. 2009;20:112–17. doi: 10.1590/s0103-64402009000200004. [DOI] [PubMed] [Google Scholar]
- 18.Gomes-Filho JE, Aurélio KG, Costa MM, Bernabe PF. Comparison of the biocompatibility of different root canal irrigants. J Appl Oral Sci. 2008;16:137–44. doi: 10.1590/S1678-77572008000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effect of NaOCl on vital tissue. J Endod. 1985;11:525–8. doi: 10.1016/S0099-2399(85)80197-7. [DOI] [PubMed] [Google Scholar]
- 20.Yesilsoy C, Whitaker E, Cleveland D, Philips E, Trope M. Antimicrobial and toxic effects of established and potential root canal irrigant. J Endod. 1995;21:513–5. doi: 10.1016/s0099-2399(06)80524-8. [DOI] [PubMed] [Google Scholar]
- 21.Gatot A, Arbelle J, Leiberman A, Yanai-Inbar I. Effects of sodium hypochlorite on soft tissues after its inadvertent injection beyond the root apex. J Endod. 1991;17:573–4. doi: 10.1016/S0099-2399(06)81725-5. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S, Piesco NP, Peterson DE, Charon J, Suzuki JB, Godowski K, et al. Effects of sanguinarium, chlorhexidine and tetracycline on neutrophil viability and functions in vitro. J Periodont Res. 1997;32:335–44. doi: 10.1111/j.1600-0765.1997.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 23.Segura JJ, Calvo JR, Guerrero JM, Jimenez A, Sampedro C, Llamas R. EDTA inhibits in vitro substrate adherence capacity of macrophages: Endodontic implications. J Endod. 1997;23:205–8. doi: 10.1016/S0099-2399(97)80046-5. [DOI] [PubMed] [Google Scholar]
- 24.Segura JJ, Calvo JR, Guerrero JM, Sampedro C, Jimenez A, Llamas R. The disodium salt of EDTA inhibits the binding of vasoactive intestinal peptide to macrophage membranes: Endodontic implications. J Endod. 1996;22:337–40. doi: 10.1016/S0099-2399(96)80213-5. [DOI] [PubMed] [Google Scholar]


