Abstract
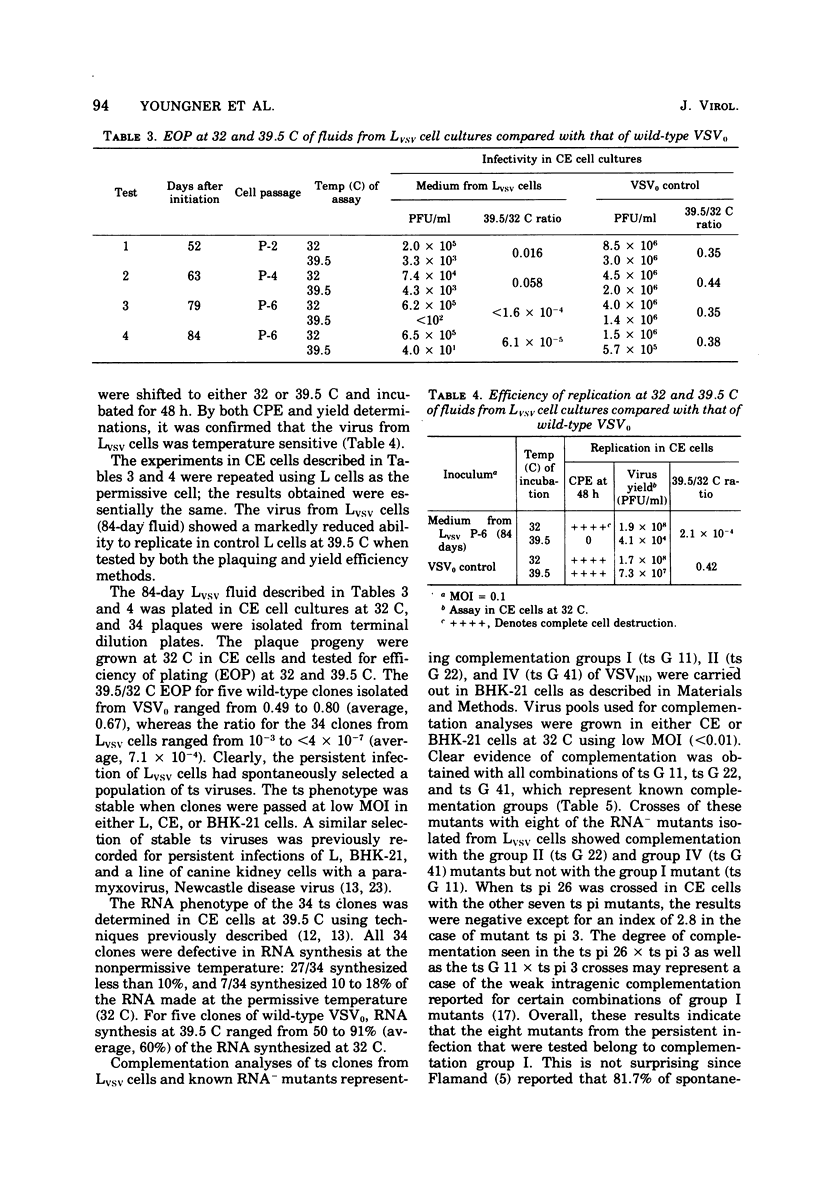
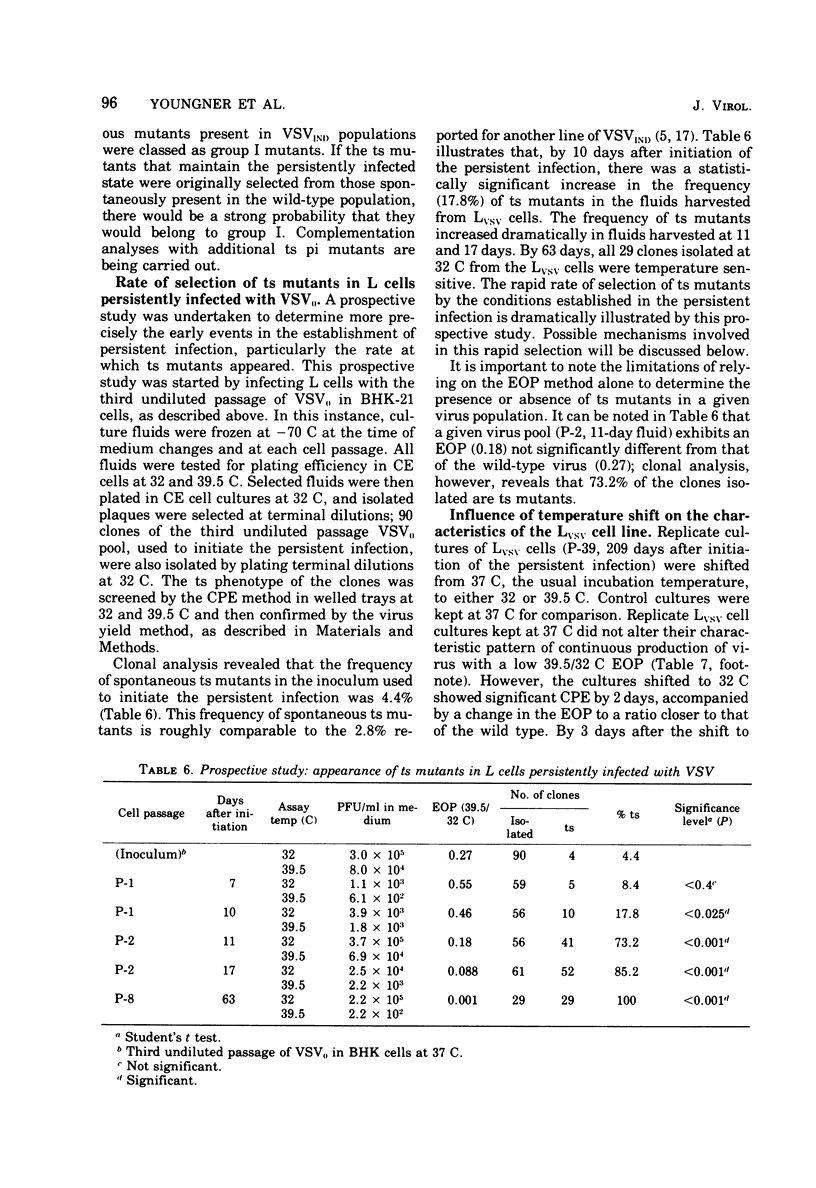
Noncytocidal persistent infections at 37 C of mouse L cells (Lvsv) with infective B particles of vesicular stomatitis virus (VSV) could be established only in the presence of large numbers of defective interfering (DI) particles. Under these conditions, there was a rapid spontaneous selection of temperature-sensitive (ts) virus. At 10 days there was an increase to 17.8% in the frequency of ts clones in the virus population; by 17 days this frequency had reached 85.2%, and by 63 days 100% of the clones isolated were ts at 39.5 C, the nonpermissive temperature used. All 34 of the clones isolated from the 84-day fluid had an RNA-phenotype, and 8 clones that were tested all belonged to VSV complementation group I. When tested by an interference assay, Lvsv fluids did not contain significant numbers of DI particles (less than 1 DI/PFU). Furthermore, persistent infection of L cells at 37 C could be initiated under conditions in which few, if any, DI particles were present by using low input multiplicities (10(-4) and 10(-5) of a clonal isolate of an RNA-group I mutant obtained from Lvsv cells. On the basis of these and other results, a mechanism is proposed to explain the role of ts mutants in both the establishment and maintenance of the persistently infected state.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Youngner J. S. Inhibition of pseudorabies virus replication by vesicular stomatitis virus. II Activity of defective interfering particles. J Virol. 1976 May;18(2):534–541. doi: 10.1128/jvi.18.2.534-541.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Youngner J. S. Inhibition of pseudorabies virus replication by vesicular stomicles virus I. Activity of infectious and inactivated B particles. J Virol. 1976 May;18(2):526–533. doi: 10.1128/jvi.18.2.526-533.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmilo A. J., Stanners C. P. Mutant of vesicular stomatitis virus which allows deoxyribonucleic acid synthesis and division in cells synthesizing viral ribonucleic acid. J Virol. 1972 Oct;10(4):605–613. doi: 10.1128/jvi.10.4.605-613.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Selection of temperature-sensitive mutants during persistent infection: role in maintenance of persistent Newcastle disease virus infections of L cells. J Virol. 1973 Sep;12(3):481–491. doi: 10.1128/jvi.12.3.481-491.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive defect of mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1973 Sep;12(3):472–480. doi: 10.1128/jvi.12.3.472-480.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1972 Feb;9(2):200–206. doi: 10.1128/jvi.9.2.200-206.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Conditional lethal mutants of vesicular stomatitis virus. Curr Top Microbiol Immunol. 1975;69:85–116. doi: 10.1007/978-3-642-50112-8_2. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. W., Iinuma M. Recovery of infectious proviral DNA from mammalian cells infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3230–3234. doi: 10.1073/pnas.72.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants isolated from hamster and canine cell lines persistently infected with Newcastle disease virus. J Virol. 1975 Nov;16(5):1332–1336. doi: 10.1128/jvi.16.5.1332-1336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Thacore H. R., Kelly M. E. Sensitivity of ribonucleic acid and deoxyribonucleic acid viruses to different species of interferon in cell cultures. J Virol. 1972 Aug;10(2):171–178. doi: 10.1128/jvi.10.2.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanov V. M., Bogomolova N. N., Gavrilov V. I., Andzhaparidze O. G., Deryabin P. G., Astakhova A. N. Infectious DNA of tick-borne encephalitis virus. Arch Gesamte Virusforsch. 1974;45(3):215–224. doi: 10.1007/BF01249684. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Parfanovich M. I. Integration of measles virus nucleic acid into the cell genome. Arch Gesamte Virusforsch. 1974;45(3):225–234. doi: 10.1007/BF01249685. [DOI] [PubMed] [Google Scholar]



