Abstract
Background:
Two of the most critical factors affecting the prognosis of an avulsed tooth after replantation are extra oral dry time and the storage medium in which the tooth is placed before treatment is rendered. However, the ability of a storage/transport medium to support cell viability can be more important than the extra oral time to prevent ankylosis and replacement resorption.
Aim:
Purpose of this study was evaluation and comparison of efficacy of a new storage medium, oral rehydration solution (ORS) with coconut water, and propolis in maintaining the viability of periodontal ligament (PDL) cells by using a collagenase-dispase assay.
Materials and Methods:
40 teeth were selected with intact crown which were advised for Orthodontic extraction having healthy PDL. Teeth were then randomly divided into three experimental storage solution groups. Other 10 were divided into positive and negative control groups (5 each).
Statistical Analysis and Result:
The results were statistically analyzed with analysis of variance and multiple range by using post hoc tests. The results of the prevailing study indicated that coconut water group demonstrated a significantly higher number of viable PDL cells than propolis 50%, and ORS. There was no significant difference between coconut water and propolis 50% groups.
Keywords: Coconut water, oral rehydration solution, propolis
INTRODUCTION
Dento-alveolar trauma is a very common occurrence out of all injuries and frequently associated with avulsion injury. Avulsion injury is associated with compromised esthetic.[1] The reported incidence of tooth avulsions ranges from 1% to 16% of all traumatic injuries occurring in permanent dentition.[2]
Avulsion injury, one of the most severe forms of dental trauma, is characterized by complete displacement of the tooth from its alveolar socket. Because of the complexity of avulsion, neurovascular supply is severely compromised and usually results in loss of pulp vitality. A vital periodontal membrane (PDM) has been found to be of ultimate importance for the successful healing of replanted teeth.
Two of the most critical factors affecting the prognosis of an avulsed tooth after replantation are extra oral dry time and the storage medium in which the tooth is placed. However, the ability of a storage/transport medium to support cell viability is more important than the extra oral time to prevent ankylosis and replacement resorption.[3]
Different storage mediums recommended are saliva, saline, milk, viaspan, HBSS, gatorade, propolis, coconut water.[4,5]
Propolis, a substance obtained from the honeybee extract, is a potent antimicrobial, antioxidant, anti-inflammatory, antibacterial, antifungal, antiviral, and tissue regenerative agent.[6] In general, propolis is composed of 50% resin and vegetable balsam, 30% wax, 10% essential and aromatic oils, 5% pollen, and 5% various other substances including organic debris. Martin, Pileggi and Φzan et al.[7] found propolis to be a superior transport medium to HBSS or milk in terms of maintaining periodontal ligament (PDL) cell viability after avulsion.
The biologically pure, tender coconut water is readily accepted by the body because it is sterile and thus, used as a blood plasma substitute. Gopikrishna et al.[8] found that coconut water was superior to HBSS or milk in terms of maintaining PDL cell viability after avulsion and storage.
Another storage media oral rehydration solution (ORS) which contains sodium 75 mmol [1725 mg], glucose 75 mmol [13.5 gm, 1.35%], potassium 20 mmol [782 mg], chloride 65 mmol [2801 mg], and base (citrate) 10 mmol has also been tested as its content having similarity with HBSS and Gatorade. It is a hypotonic solution with osmolarity between 270 and 300 mOsm/L.[9]
Hence, the purpose of this study was evaluation and comparison of efficacy of three different storage medium—ORS, coconut water and propolis in maintaining the viability of PDL cells by using a collagenase-dispase assay.
MATERIALS AND METHODS
40 teeth were selected with intact crown and close apices which were advised for orthodontic extraction having healthy PDL.
After a traumatic extractions, the teeth were held with forceps from the coronal region, and the coronal 3 mm of PDL was scraped with a curette to remove cells that might have been damaged.
The teeth were then randomly divided into three experimental storage solution groups,
Positive and negative control groups consisted of five samples each.
Tender coconut water (Group 1):
Freshly open tender coconut water was used for each sample.
Preparation of propolis 50% (Group 2):
Propolis was made into 50% concentration using 0.4% ethanol solution.
Propolis 50% was prepared by adding 50 mg ground propolis per 250 ml of the 0.4% ethanol solution. Before submersion of teeth in propolis, solutions were shaken for 15 minutes.
Preparation of ORS (Group 3):
1 teaspoon of ORS powder was taken in 200 ml (one glass) of distal water and stirred.
New experimental solution was made every time.
Teeth in experimental groups were dried for 30 minutes (including time taken for curetting coronal PDL cells), followed by a 45-minute immersion in one of the three storage solution groups.
The teeth in positive control group after extraction was immediately treated with dispase and collagenase.
The teeth in negative control group were bench-dried for 8 hours, with no follow- up storage solution time, and then placed in the dispase and collagenase.
After drying and soaking of each experimental teeth, 2.5 ml of stock solution containing grade II dispase and collegenase were added to teeth and incubated for 30 min at 37°C. After incubation, 50 μL of fetal bovine serum was added to each tube with help of micropipette.
All tubes were then centrifuged for 4 minutes at 1000 rpm and supernatant was removed with sterile micropipettes.
The cells were labeled with 0.4% trypan blue for determination of viability. The number of viable PDL cells was counted under light microscope with hemocytometer at 40× magnification.
Statistical analysis
Results were statistically analyzed using analysis of variance and post hoc tests [Table 1].
The level of significance was 5% (P < 0.05).
Statistical analysis showed a significant difference among the groups. Tukey test showed significantly higher number of viable PDL cells in coconut water group than propolis 50% and ORS.
There was no significant difference between coconut water and propolis 50% groups. However, both coconut water and propolis 50% groups demonstrated a significantly higher number of viable PDL cells than ORS [Table 2].
All experimental solution groups were significantly lower than positive control and higher than negative control group.
Table 1.
Standard deviation of test groups
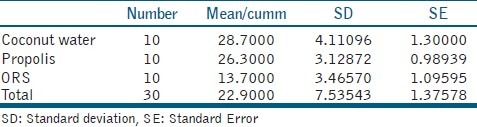
Table 2.
Post hoc test

DISCUSSION
Avulsion injuries are the worst of dentoalveolar injuries. Usually, avulsion involves single tooth, and the most frequently avulsed tooth is maxillary central incisor.
The healing pattern of an avulsed tooth after replantation will depend upon the healing potential of each cellular component of the tissues involved. Prognosis of a replanted tooth will depend on minimal damage to the PDL, which is critical for regeneration of the attachment apparatus and also to protect root from resorption.[10,11]
Extra oral time and storage conditions are the most crucial factors in determining the viability of the remaining PDL cells, and thus the survivability of the avulsed tooth. Studies have shown that an avulsed tooth can be replanted without complications after 1-3 hours of being placed in suitable storage conditions.[5]
An ideal storage medium would be one that is capable of preserving the viability, mitogenicity, and clonogenic capacity of the damaged PDL in order to facilitate repopulation of the denuded root surface thereby preventing further root resorption.[12] The storage medium should have a physiological osmolarity and pH and should be maintained at an appropriate temperature to allow optimal cell growth or survival. Finally, the ideal storage media should be readily available for use in emergency situations.[5,13]
This study focused on evaluation and comparison of efficacy of three different storage media-ORS, coconut water and propolis in maintaining the viability of PDL cells by using a collagenase-dispase assay.
The biologically pure, tender coconut water is readily accepted by the body because it is sterile and thus used as a blood plasma substitute. It also helps to replace fluids, electrolytes (potassium, calcium, and magnesium), and sugar lost from the body during heavy physical exercise. Gopikrishna et al.[8] found that coconut water was superior to HBSS or milk in terms of maintaining PDL cell viability after avulsion and storage. It has high potassium content and contains antioxidants linked to a variety of health benefits. Cytokinins in coconut water may be among its most beneficial components.[8,14]
Propolis is a multifunctional material used by bees in the construction and maintenance of their hives. Recently, it has attracted much attention as a useful substance applied in medicine and cosmetics because of its antibacterial and antifungal activities. In general, propolis is composed of 50% resin and vegetable balsam, 30% wax, 10% essential and aromatic oils, 5% pollen, and 5% various other substances.[7]
Also Ozan et al.[5] evaluated the effect of propolis on survival of PDL and found propolis to be a superior transport medium to HBSS or milk in terms of maintaining PDL cell viability after avulsion and storage.
ORS is an easily available rehydration solution. It contains sodium 75 mmol [1725 mg], glucose 75 mmol [13.5 gm, 1.35%], potassium 20 mmol [782 mg], chloride 65 mmol [2801 mg], and base (citrate) 10 mmol. It is a hypotonic solution with osmolarity between 270 and 300 mOsm/L.[9]
Various techniques have been used to quantitate the number of viable PDL cells like a stepwise by Reinholdt et al., Chromogenic stain by Soder et al.[14]
In the current study to minimize the exposure of cells to active trypsin and to preserve maximum cell viability, the root surface was treated with collagenase and dispase grade II as was performed in the work by Pileggi et al.[15]
This procedure allowed rapid cell retrieval and maintained maximum cellular integrity, as was demonstrated by the positive control samples. This method is more representative of the actual clinical situation because the cells are not subjected to long processing times to determine their viability status. Collagenase and dispase assay provides a combination of collagenolytic and proteolytic enzymes required for tissue disaggregation.[16,17]
Also, we used fetal bovine serum as growth supplement for cell culture media because of its high content of embryonic growth promoting factors. When used at appropriate concentrations, it supplies many defined and undefined components that have been shown to satisfy specific metabolic requirements for the culture of cells in vitro.[18]
The trypan blue exclusion staining technique was used because it is quick, easily performed, and distinctively differentiates nonviable cells from viable cells. It is based on the principle that live cells possess intact cell membranes that exclude certain dyes.[19]
In this test, a cell suspension is simply mixed with dye and then visually examined to determine whether cells take up or exclude dye. In the protocol presented here, a viable cell will have a clear cytoplasm whereas a nonviable cell will have a blue cytoplasm.[20,21]
The results of the prevailing study indicated that coconut water group demonstrated a significantly higher number of viable PDL cells than propolis 50%, and ORS. There was no significant difference between coconut water and propolis 50% groups.
Ideal requirements for a storage media as suggested by Blomof[5] are 290-330 mOsm/L and a pH of 6.6 to 7.8. Even though the osmolarity of ORS is comparable the pH is significantly less than the ideal which may be the reason for the comparatively lesser number of viable PDL cells in this study.
It remains to be explored, whether the modification of ORS by addition of common day to day product could increase its pH to the required level to optimize its efficacy as storage media.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lee JY, Vann WF, Jr, Sigurdsson A. Management of avulsed permanent incisors: A decision analysis based on on changing concepts. Pediatr Dent. 2001;23:357–60. [PubMed] [Google Scholar]
- 2.Blomlöf L, Lindskog S, Andersson L, Hedström KG, Hammarström L. Storage of experimentally avulsed teeth in milk prior to replantation. J Dent Res. 1983;62:912–6. doi: 10.1177/00220345830620081301. [DOI] [PubMed] [Google Scholar]
- 3.Gopikrishna V, Baweja PS, Venkateshbabu N, Thomas T, Kandaswamy D. Comparision of coconut water, propolis, HBSS, and milk on PDL cell survival. J Endod. 2008;34:587–9. doi: 10.1016/j.joen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 4.James F, Wesley MS. Evaluation of infant rehydration solutions; product evaluation. 2004. [Last accessed on 2011 Jul 08]. pp. 1–4. Available from: http://www.aicompanies.com/documents/file/InfantRehydrate.pdf .
- 5.Ozan F, Polat ZA, Er K, Ozan U, Değer O. Effect of propolis on survival of periodontal ligament cells: New storage media for avulsed tooth. J Endod. 2007;33:570–3. doi: 10.1016/j.joen.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shaher A, Wallace J, Agarwal S, Bretz W, Baugh D. Effect of propolis on human fibroblasts from the pulp and periodontal ligament. J Endod. 2004;30:359–61. doi: 10.1097/00004770-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Campbell-Falck D, Thomas T, Falck TM, Tutuo N, Clem K. The intravenous use of coconut water. Am J Emerg Med. 2000;18:108–11. doi: 10.1016/s0735-6757(00)90062-7. [DOI] [PubMed] [Google Scholar]
- 8.Gopikrishna V, Thomas T, Kandaswamy D. A quantitative analysis of coconut water: A new storage media for avulsed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e61–5. doi: 10.1016/j.tripleo.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Blomlöf L. Storage of human periodontal ligament cells in a combination of different media. J Dent Res. 1981;60:1904–6. doi: 10.1177/00220345810600111301. [DOI] [PubMed] [Google Scholar]
- 10.Hiremath G, Kidiyoor K. Avulsion and storage media. Inves and Clinical Dent. 2011:1–6. doi: 10.1111/j.2041-1626.2010.00043.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes M, Westphalen V. Study of storagenmedia for avulsed teeth. Brazil Dent Traumatol. 2009;1:69–76. [Google Scholar]
- 12.Al-Nazhan S, Al-Nasser A. Viability of human periodontal ligament fibroblasts in tissue culture after exposure to different contact lens solutions. J Contemp Dent Pract. 2006;7:37–44. [PubMed] [Google Scholar]
- 13.Chamorro MM, Regan JD, Opperman LA, Kramer PR. Effect of storage media on human periodontal ligament cell apoptosis. Dent Traumatol. 2008;24:11–6. doi: 10.1111/j.1600-9657.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 14.Almas K. Propolis as a natural remedy: An update. Saudi Dental Journal. 2001;13:1. [Google Scholar]
- 15.Highmedia-Collegenase and dispase enzyme product description. [Last accessed on 2011 Jul 08]. Available from: http://www.e-labdoc.roche.com/LFR_PublicDocs/ras/10269638001_en_13.pdf .
- 16.Sigma FETAL BOVINE SERUM (FBS) Product Numbers F2442, product description. [Last accessed on 2011 Jul 21]. Available from: http://www.sigmaaldrich.com/etc/medialib/docs/Sigma/Product_Information_Sheet/f2442pis.Par.0001.File.tmp/f2442pis.pdf .
- 17.Technical Reference Guide Protocol for Performing a Trypan Blue Viability Test. [Last accessed on 2011 Aug 23]. Available from: http://www.bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_BenchGuides_Protocol_for_Performing_a_Trypan_Blue_Viability_Test.pdf .
- 18.Longo-Sorbello Giuseppe S. A., Say&m Guray, Banerjee Debabrata, Bertino Joseph R. Cytotoxicity and Cell Growth Assays, cell and tissue culture: assorted techniques. Elsevier Science (USA) 2006:315–324. [Google Scholar]
- 19.Immunocytometry solution-Hemocytometer Counting and Cell Viability. [Last accessed on 2011 Jul 23]. Available from: http://www.groups.molbiosci.northwestern.edu/morimoto/research/Protocols/II.%20Eukaryotes/A.%20Cell%20Culture/3b.%20Hemacytometer.pdf .
- 20.Kenny DJ, Barrett EJ, Casas MJ. Avulsions and intrusions: The controversial displacement injuries. J Can Dent Assoc. 2003;69:308–13. [PubMed] [Google Scholar]
- 21.Singla A, Garg S. Reimplantation: Clinical implications and outcome of dry storage of avulsed teeth. J Clin Exp Dent. 2010;2:38–42. [Google Scholar]


