Abstract
Complete debridement and disinfection of the root canal system are fundamental requirements for successful endodontic treatment. Despite the morphological challenges of the internal root anatomy, root canal irrigants play an important role in the optimization of the root canal preparation, which is essentially a chemo-mechanical procedure. Enterococcus faecalis is one of the most resistant microorganisms that dominants the microbial ecosystem of persistent periradicular lesions in retreatment cases. For that reason, many in vitro and in vivo studies evaluated and compared the antibacterial activity of sodium hypochlorite and chlorhexidine at varying concentrations using different experimental models against this microorganism. However, many controversies with regard to the ideal irrigant and concentration do in fact exist. Hence, this review aims to discuss the antibacterial activity of these two main root canal irrigants against Enterococcus faecalis using the agar diffusion and direct contact methods and the possible modulating factors responsible for inconsistent findings among different studies. In addition, the disinfection potential of both chemical agents on gutta percha and Resilon cones are also discussed. The source of this review was conducted through an electronic literature search using PubMed database from December 1997 until December 2011, which analyze the related laboratory investigations of both irrigants, published in major endodontic journals.
Keywords: Agar diffusion, chlorhexidine, direct contact, enterococcus faecalis, irrigants, sodium hypochlorite
INTRODUCTION
Complete debridement and adequate elimination of microbial irritants, including microorganisms and their toxins, is a fundamental prerequisite for successful endodontic therapy.[1] The ability to reach this goal by mechanical instrumentation is unlikely to be achieved due to the complex root canal anatomy which provides an ideal environment for microorganisms to survive and continue their pathological process.[2] Accordingly, many studies emphasized the crucial role of chemical irrigants in the optimization of root canal disinfection, and it was concluded that a chemo-mechanical preparation would pave the way for proper debridement and most favorable disinfection of the root canals.[3–6]
Potent antimicrobial activity, dissolving of remaining pulp tissues with no systemic hazards, reducing instrument friction during mechanical preparation and availability are among the main requirements for an ideal root canal irrigant.[1,7] Sodium hypochlorite (NaOCl) has widely been accepted as a root canal irrigant since its first reported use by Walker in 1936.[8] It mainly acts as a potent antimicrobial agent[9,10] and an effective organic solvent for vital, necrotic and fixed tissue.[1,11] Despite its fulfillment of other desirable properties including availability and low cost, it has a few drawbacks. Apart from its unpleasant taste, tendency to bleach clothes and it is potentially corrosive,[1,12] there is a concern regarding its noxious effects if concentrated solutions were inadvertently forced into the periapical tissues during irrigation or leaked through the rubber dam.[12] Besides, some controversies do exist with regard to its antimicrobial activity at lower concentrations, which are advocated as an attempt to reduce its toxic reactions.[9,10,13–15]
Chlorhexidine gluconate (CHX) is routinely used in dentistry as a mouth rinse in the prevention and treatment of periodontal disease and caries.[16] It has potent and substantive antimicrobial activity against some resistant bacteria such as Enterococcus faecalis.[9,10,17–19] and lower cytotoxicity than NaOCl.[17,20] CHX also shows a promising potential use as a root canal irrigant and medicament.[9,18] However, there is a general agreement that CHX can serve only as an adjunct irrigant or as a final rinse rather than a reasonable substitute for NaOCl.[1,2,7] This is due to its inability to dissolve organic/necrotic tissue remnants and its lesser antibacterial activity against Gram-negative rather than Gram-positive bacteria, by which the former microorganisms are the one that predominantly found in primary endodontic infections.[1,2,7] Although CHX has been commonly held as less caustic than NaOCl, it has been reported to cause irritation to the skin.[21] In addition, its cytotoxic effects on human osteoblasts might indicate its ability to impair the regenerative potential of the periapical tissues.[22]
Enterococcus faecalis (E. faecalis) is a Gram positive facultative anaerobic bacterium found in the human normal flora. In endodontics, E. faecalis is rarely present in primary apical periodontitis, and is dominant in the microbial ecosystem of persistent periradicular lesions after root canal treatment.[23,24] It is ecologically tolerant and has the ability to survive harsh conditions as it exhibits considerable genetic polymorphisms and can bind to dentin and resist the action of calcium hydroxide, especially when a high pH is not maintained.[24] Apart from the virulence of E. faecalis, the complex anatomical variations of the root canal system, including accessory canals, inter-canal communications and apical ramifications, which favor bacterial growth and the inherent limitations of endodontic materials would add more challenges for obtaining a disinfected state of the root canal system [Figure 1]. However, high concentrations, different formulations, applications and combinations of some root canal irrigants have been shown to reveal some promise.[1,23]
Figure 1.
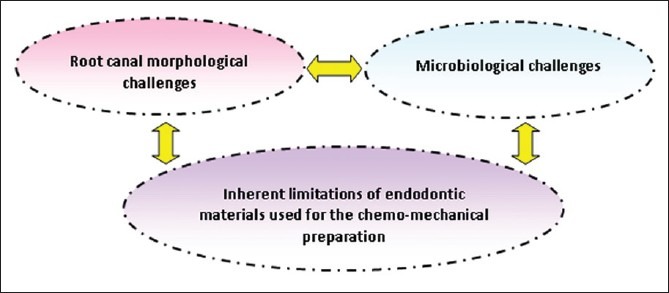
Challenges for the optimization of chemo-mechanical preparation during root canal treatment
Many laboratory investigations have been carried out to evaluate and compare the antimicrobial effectiveness of root canal irrigants including NaOCl and CHX. Despite the methodological differences between those studies, many of them followed the agar diffusion and direct contact methods as the most common and simple but relevant protocols for evaluating the antibacterial activity of a given chemical agent [Tables 1 and 2]. Nevertheless, some controversies and conflicting results do exist when those protocols were applied to compare the antibacterial activity of the same chemical agents, but in different applications and/or concentrations against the same microorganism. Therefore, the purpose of this article is to present an overview of antibacterial activity of sodium hypochlorite and chlorhexidine gluconate against E. faecalis using the agar diffusion and direct contact methods and discuss the possible contributing factors responsible for the heterogenic findings. Studies evaluating the antibacterial action of CHX and NaOCl on gutta percha and Resilon cones using both methods are also discussed.
Table 1.
Summary of in vitro studies that performed agar diffusion test to compare between the antibacterial activity of NaOCl and CHX against E. faecalis (BSA: Bovine Serum Albumin)

Table 2.
Summary of in vitro studies that performed direct contact test to compare between the antibacterial activity of NaOCl and CHX against E. faecalis. (MBEC: Minimal Biofilm Eradication Concentration, BSA: Bovine Serum Albumin)
Search methodology
An electronic literature search from December 1997 until December 2011 was conducted in the PubMed search engine to identify laboratory investigations published on the antibacterial activity of NaOCl and CHX at varying concentrations against E. Faecalis using the following keywords: “sodium hypochlorite”, “chlorhexidine” and “Enterococcus faecalis”. Articles from major endodontic journals (Journal of Endodontics, International Endodontic Journal, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology (now named as Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology) and Australian Endodontic Journal) were identified. The pooled data were then analyzed and the studies that followed the agar diffusion and direct contact methods were finally selected.
Agar diffusion test
Definition
Agar diffusion test is one of the most commonly used tests to study the antimicrobial activity of endodontic irrigants.[8] It involves the placement of paper disks, previously saturated with the chemical agent onto the agar surface sub-cultured with a selected microorganism. After a certain period of time, zones of inhibition of variable diameters form around the paper disks. These zones of inhibition signify the presence of antimicrobial activity of the given chemical agent against the selected microorganism/s.[36]
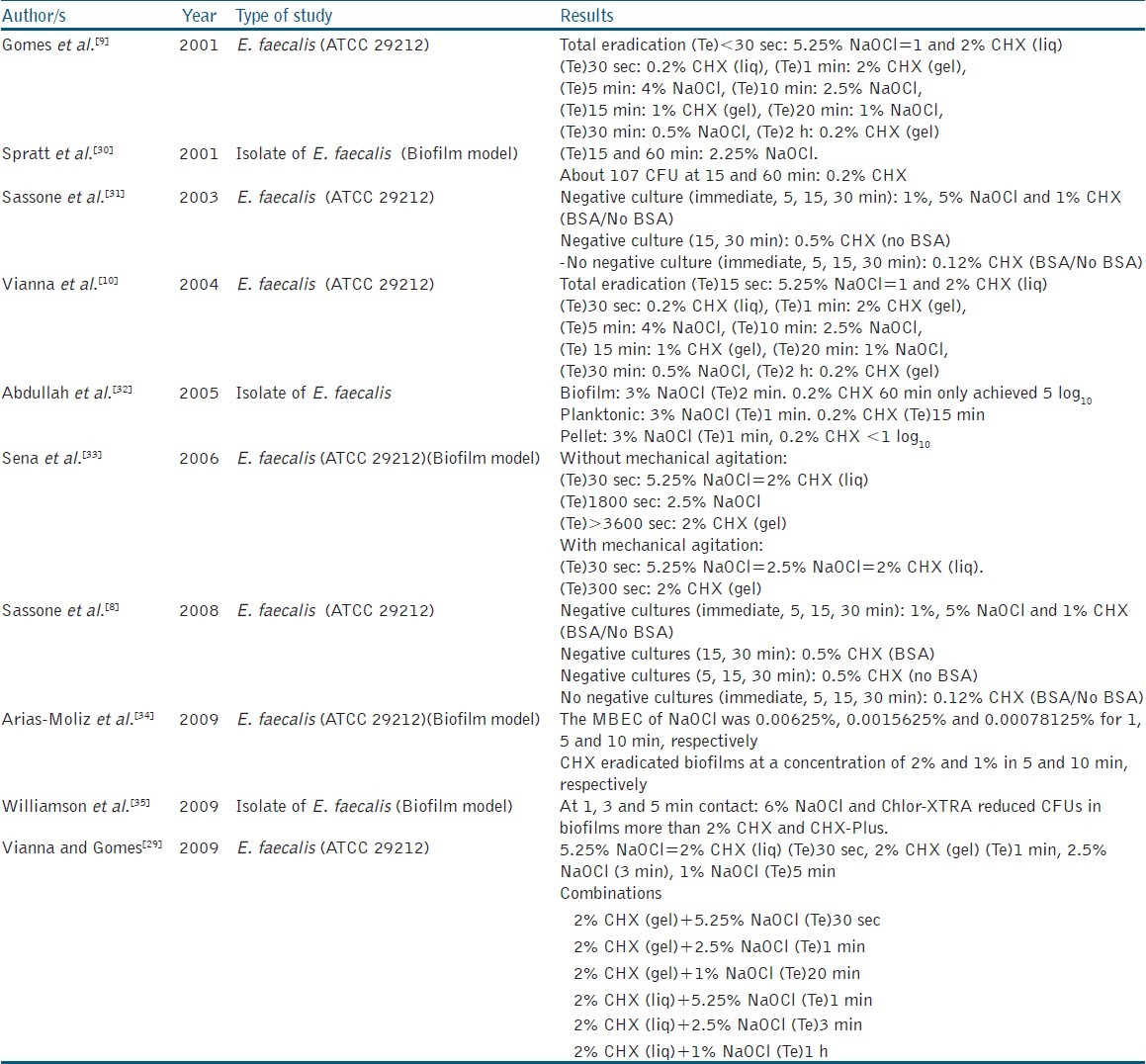
The agar diffusion method has been followed in some in vitro studies to evaluate the antibacterial activity of NaOCl and CHX at varying concentrations. Table 1 summarizes these reported studies which measured the inhibition zones of NaOCl and CHX against isolated or commercial strains of E. faecalis.
Methods used for measuring the inhibition zones
Based on the studies summarized in Table 1, the zones of microbial growth inhibition have been measured using the following methods:
-
(a)
The diameter is measured using a transparent ruler with or without 6 mm (the diameter of the paper disc) as the cut off value.[8,25,26]
-
(b)
The shortest distance between the outer margin of the material and the initial point of bacterial growth.[29,37]
-
(c)
The largest diameter.[28]
Leonardo et al.[27] used 0.05% triphenyl tetrazolium chlorate in 1.0% agar gel to provide an accurate identification of the inhibition halos whereby only the viable microorganisms will be stained by this tetrazolium-based compound.
Comparison between NaOCl and CHX at various concentrations
Siqueira et al.[25] and Ayhan et al.[26] found that higher concentrations of NaOCl (4-5.25%) were more effective than 1-2% CHX in eradicating E. faecalis. On the contrary, Vianna and Gomes[29] observed the larger inhibition zones of 2% CHX either in the liquid or the gel form compared to that of 5.25% NaOCl. While Siqueira et al.[25] and Ayhan et al.[26] followed the traditional methodological procedure of the agar diffusion test by applying 6 mm paper discs soaked of the test solutions onto the agar surface, Vianna and Gomes[29] introduced hollow stainless steel tubes containing 40 μL of the test irrigant rather than the paper discs, onto the agar surface.[25,26,29] This modified application might be the cause for the inconsistent finding demonstrated in that study. Davis et al.[28] formulated a well-controlled study by determining the amount of each chemical agent needed to saturate 6 mm paper prior running the experiment (15 and 20 μL for NaOCl and CHX, respectively). In contrast to the results demonstrated by previous studies, they found that the difference between the antibacterial activity of 5.25% NaOCl and 2% CHX against E. faecalis (ATCC 4082), cultured in both aerobic and anaerobic conditions was insignificant. This quantification was only mentioned in one study by Ayhan et al.[26] who soaked the paper discs in 15 μL of both test irrigants.
Interestingly, Sassone et al.,[8] compared the effectiveness of agar diffusion method by adding bovine serum albumin (BSA) as an organic load. This is to simulate the organic content within the root canal that could influence the antimicrobial capability of such substances. This study revealed that all solutions (NaOCl 1%, 5%; CHX 0.12%, 0.5%, 1%) demonstrated a potent antimicrobial activity against E. faecalis when BSA was absent. When BSA was present, neither concentration of NaOCl created an inhibition zone while all CHX solutions exhibited some zone of growth inhibition. The authors reported the likely explanation for this finding was the possible formation of a high molecular weight substance, as a result of a reaction between NaOCl and BSA, which prevent the diffusion of the irrigating solution through the media. In contrast, this was not observed with CHX as it has a good diffusing ability through the agar gel.[8] Based on these results, Sassone et al.[8] concluded that the agar diffusion method has many critical aspects that might impair its reliability and simulation to the clinical situation. However, it is worth noting that Sassone et al.[8] used 7 mm paper discs and soaked them with only 10 μL of the test irrigants for 1 minute, which is different from the previous studies by Ayhan et al.[26] and Davis et al.[28]
Using the same method, some studies demonstrated that NaOCl at the concentrations of 2.5% and below has lower antibacterial activity than 2% CHX against E. faecalis.[25,26,27,29] However, Siqueira et al.[25] reported that the efficacy of 2.5% NaOCl was equivalent to that of 0.2% CHX.
Combination of NaOCl and CHX
The combination of NaOCl and CHX has been advocated during the chemo-mechanical procedure to provide a synergistic antimicrobial activity.[20] During this combined application, CHX is frequently recommended as the final rinse owing to the importance of its antimicrobial substantivity.[7,38] Kuruvilla and Kamath[20] reported that the antimicrobial effect of 2.5% NaOCl and 0.2% CHX used in combination was better than that of using either component alone. In contrast, Vianna and Gomes[29] found that the use of 2% CHX alone showed the strongest antimicrobial action but the combination of NaOCl with CHX dramatically reduced the antibacterial activity of CHX. On the other hand, the combination of all concentrations of NaOCl to both CHX gel and liquid demonstrated a greater zone of inhibition on almost all the agar plates as opposed to the acting of NaOCl of equivalent concentration alone. A decrease in the antimicrobial activity also was observed when 2% CHX liquid were combined with higher concentration of NaOCl (2.5 and 5.25%). This observation could be explained by the large quantity of brown precipitate formed when higher concentrations of NaOCl were used presenting some difficulty for the irrigants to diffuse through the agar plates thus creating smaller zone of inhibition. In addition to the discoloration potential of this precipitate to tooth structure[39,40] and its ability to act as a barrier that obscure the contact of the chemical agent to microorganisms during root canal treatment, there are some concerns raised regarding the presence of carcinogenic parachloroaniline (PCA) in its chemical composition.[40–43]
Modulating factors that might affect the results of the agar diffusion method among different studies
Despite the common application of the agar diffusion method, many factors, other than the actual antibacterial activity of the tested material, might affect the reliability and reproducibility of the agar diffusion method. These factors include the chemical agent's formulation (liquid or gel), its molecular size, solubility and diffusion ability of the material through the agar medium, contact between the test material and the gel, inoculum density, agar viscosity, storage conditions of the agar plates and incubation time. Furthermore, the soaking and application procedures might also affect the diffusion of the material into the agar[29,36] [Figure 2a].
Figure 2.
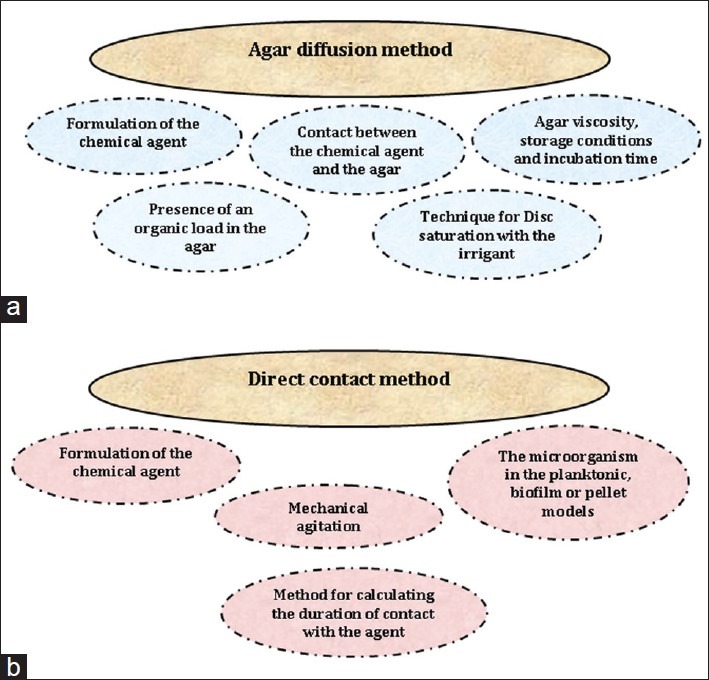
Possible modulating factors that may cause inconsistencies results of the agar diffusion and direct contact methods when NaOCl and CHX are examined at a given concentration
Direct contact method
Definition
Direct contact method is a different experimental approach for evaluating the antibacterial activity of a given chemical agent, in which a fixed volume of this agent is mixed with a bacterial suspension for a certain time. The antibacterial activity is verified through culture of the resulting mixture in a nutrient medium and thus, the presence or absence of bacterial growth is evaluated for each bacterial species, chemical agent and time.[8,31] Apart from that, a few studies presented their findings by means of time taken (contact time) to produce negative cultures, which signified the 100% growth inhibition of certain microorganisms in the presence of certain irrigants.[9,10,29]
It is worth noting that the direct contact method can be further characterized in the literature based on the use of planktonically grown microorganisms or a biofilm model. The use of simple biofilm models have been investigated to evaluate the antimicrobial efficacy since the conditions used in laboratory tests do not reflect the in vivo condition where bacteria grow as biofilms on tooth surface.[44] Most of the in vitro studies use planktonic cultures for testing the antimicrobial efficacy of endodontic irrigants. Depending on the concentration of the substance tested and the susceptibility of the microorganism, the latter can be eliminated in seconds using the planktonic cells and the direct contact methods. Unfortunately, such killing effect may not happen clinically. Therefore, it is believed that the use of biofilm model could reproduce a better picture of bacteria organization to simulate an in vivo condition as closely as possible compared to the use of its planktonic counterparts [Table 2].[30,33]
Comparison between NaOCl and CHX at various concentrations
Planktonic model
Some studies compared the antibacterial activity between NaOCl and CHX in the liquid and gel forms against E. faecalis, and the results revealed that 5.25% NaOCl and 2% CHX liquid took about 15 to 30 seconds to eliminate the microorganisms whereas 2% CHX gel took about 1 minute to produce 100% inhibition of E. faecalis growth.[9,10,29] Meanwhile, slightly lower NaOCl concentration at 2.5-4% took about 3 to 10 minutes to produce negative cultures.[9,10,29] and further dilution of NaOCl (0.5-1%) showed a delayed activity that can reach up to 30 minutes to achieve negative culture upon direct contact, suggesting that the antibacterial activity of NaOCl is proportional to its concentration being more potent at higher concentrations.[9,10,29]
It was also observed that CHX gel at lower concentrations (1% and 0.2%) were significantly less effective, and produced complete inhibition after 15 minutes and 2 hours, respectively.[9,10] In contrast, CHX liquid at lower concentrations (1% and 0.2%) exerted their antibacterial activity against E. faecalis within similar time range taken as for 2% CHX liquid (15 to <30 seconds).[9,10] The possible explanation for this finding could be related to the nature of CHX liquid that mixes well with the bacterial suspension, thus immediately exerting its bacterial action. On the other hand, the gel formulation is more difficult to mix, preventing the direct contact between the bacterial cells and CHX, thus requiring a longer time to exert its antibacterial action, though its action is more substantive.[9] It is also worth noting the use of CHX gel during mechanical instrumentation may have some advantages over the liquid form as it acts as a lubricant and has the ability to remove dentin debris and remaining tissues.[18]
A study by Sassone et al.[8] found that the presence of bovine serum albumin (BSA) in the direct contact test did not affect the antibacterial activity of 1% and 5% NaOCl solutions against E. faecalis. Meanwhile, 1% CHX was effective throughout all time intervals indicated (immediate, 5, 15 and 30 minutes) whereas 0.5% CHX showed some delay in the antibacterial activity, especially in the presence of BSA. Conversely, 0.12%, CHX was not effective against E. faecalis independent of time or addition of BSA.
Biofilm model
NaOCl and CHX have been demonstrated as an effective antimicrobial agents against biofilm populations.[30,32,34,35] Nevertheless, different concentrations of NaOCl, ranging from 0.00625% to 6% have shown to be the most significantly effective antibacterial agent which killed E. faecalis within 1 minute[34,35] and 2-3 minutes.[32,35] In contrast, 2% and 0.2% CHX took 5 minutes and only reducing the colony forming units (CFU) to 107 after 1 hour, respectively.[30,32,34,35]
The related literature shows that 5.25% NaOCl exerts its antibacterial action within 30 seconds, which shows no difference in terms of time taken when E. faecalis is grown in either a biofilm model[33] or a planktonic suspension.[9] Nevertheless, 2% CHX gel shows a significant delay in its antibacterial action from 1 minute[9] to >1 hour[33] when the results are compared on planktonic suspension and biofilm model, respectively. These findings are supported by the fact that the organization of bacteria within biofilms confers a range of phenotypic properties that are different or not evident in their planktonic counterparts, and thus are far less susceptible to antimicrobial killing.[32,44] The rationale behind this relies on the nature of biofilm itself, which is defined as the communities of microorganisms attached to a surface, embedded in extra-cellular matrix of polysaccharides.[44] The development of an organized community with complex differentiation and functional heterogeneity would constitute a protected mode of growth that allows the microorganisms to survive in a variety of harsh environments.[45] In an attempt to overcome this challenge, Sena et al.[33] proved that mechanical agitation of CHX gel can be able to reduce the time taken to inhibit the growth of E. faecalis from 1 hour to 15 minutes. This mechanical agitation probably has disrupted the normal organization of the biofilm thus providing a more effective exposure of the well-protected microorganism to the chemical agent.
Pellet model
Interestingly, Abdullah et al.[32] investigated the efficacy of 3% NaOCl and 0.2% CHX on E. faecalis using planktonic suspension, biofilm and pellet. The results showed that complete eradication has occurred after 1 minute of exposure to NaOCl in both planktonic suspension and pellet, but the time was doubled for the biofilm. Meanwhile, CHX took 15 minutes to produce 100% bacterial killing in planktonic suspension, which was not demonstrated in the biofilm and pellet throughout all time intervals. Abdullah et al.[33] commented that the resistance of E. faecalis pellet to CHX is attributed to the inability of CHX to penetrate into the center of the pellet while NaOCl possessed a potent antibacterial action against E. faecalis regardless of growth condition or presentation of the bacterial cells because of its tissue dissolving properties. As such, its antibacterial action may be less inhibited by the exo-polysaccharides matrix in the biofilm or the multiple layers of bacterial cells in the pellet form. Indeed, the difference in susceptibility towards CHX between E. faecalis biofilm and planktonic suspension is also attributed to the complex structural and dynamic resistance mechanisms organized in the biofilm.[44,46]
Combination of NaOCl and CHX
Vianna and Gomes[29] reported that both 2% CHX gel and liquid forms constitute the best performance when combined with 5.25% NaOCl, which revealed 100% growth inhibition of E. faecalis at 30 seconds. The significantly lowest antibacterial activity was demonstrated when 2% CHX at both the liquid and gel formulations were combined with only 1% NaOCl. In this instance, these combinations took as long as 20 minutes and 1 hour to exert their maximum antibacterial activity, respectively. The possible explanation for the shortened killing time of E. faecalis when higher concentrations of NaOCl are combined with CHX would be that larger amounts of (PCA) precipitate are formed that would cause considerable chemical changes in the liquid media thus leading to a more rapid microbial death.[29]
Modulating factors that might affect the results of the direct contact method among different studies
Due to the presence of different methodological procedures amongst these laboratory investigations, it is difficult to come out with an accurate comparison of the inherent antibacterial activity between NaOCl and CHX. Different physical forms of the antimicrobial agents, method for calculating the duration of contact with the agent, the use of mechanical agitation and the different presentation of microorganisms (planktonic, biofilm or pellet) are all modulating factors that may affect the results [Figure 2b]. Despite the prime importance of proposing a standardized protocol, it seems that the debate between the most effective antibacterial activity and least cytotoxic concentration of a given chemical agent would remain.
Disinfection of gutta percha and Resilon cones
Although gutta percha cones usually are introduced into the market in sterile and firmly sealed packages, their subjection to the dental operating environment shows an increased likelihood for contamination by a variety of microorganisms such as cocci, rods and yeasts.[47,48] Gomes et al.[47] found that gutta percha cones can be easily contaminated during handling by gloves, without using sterile gauze. Indeed, this contamination may cause re-infection to the prepared root canals and periapical tissues, thus affecting the rate of clinical success.[48] Gutta percha cones cannot be sterilized by the usual moist and dry heat sterilization due to their thermoplastic nature.[47] Owing to this inherent property, some studies evaluated the ability of chemical disinfectants such as NaOCl and CHX to provide a rapid and potent disinfection to gutta percha and Resilon cones against E. faecalis [Table 3].
Table 3.
Summary of in vitro studies examined the antibacterial activity of gutta percha and/or Resilon cones against E. faecalis after the soaking with NaOCl and CHX
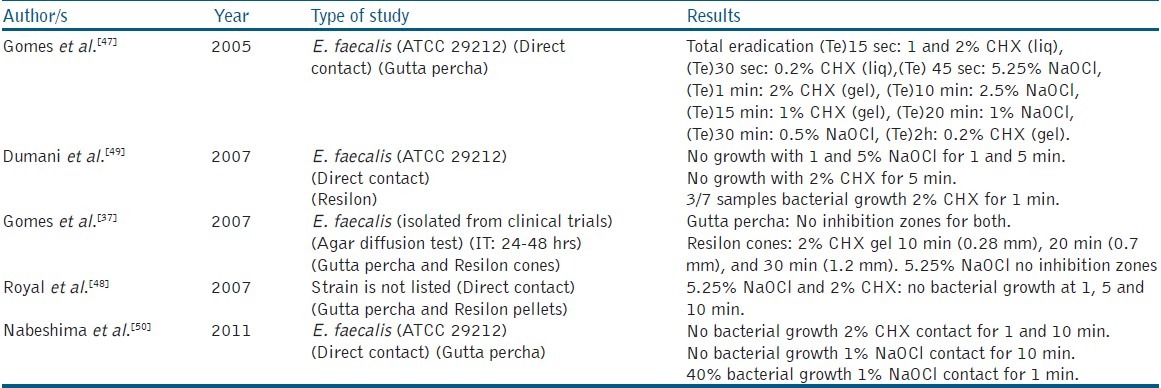
Using the agar diffusion method, Gomes et al.[37] examined the residual antibacterial effect of NaOCl and CHX gel against E. faecalis, and found that neither of them was able to present any residual antibacterial effects, due to their inability to bind on gutta percha cones after rinsing by sterile saline solution and vortexing for 1 minute before adding onto the agar surface. Nevertheless, the antibacterial property was found effective when Resilon cones were treated with CHX gel.
By applying the direct contact test, Gomes et al.[47] Royal et al.[48] and Nabeshima et al.[50] demonstrated the rapid (1 minute or less) and entire disinfection of gutta percha cones from E. faecalis using high concentrations of NaOCl (5.25%) and CHX gel (2%). On the contrary, Dumani et al.[49] found that 2% CHX was only effective (100% eradication) with Resilon cones after 5 minutes of contact. Lower concentrations of NaOCl (<2.5%) showed contradictory results with gutta percha and Resilon cones.[47,49,50] It is worth noting that Resilon has been reported to undergo color changes when combined with NaOCl or CHX, and forms a pink precipitate with the latter.[48]
Concluding remarks
Sodium hypochlorite and chlorhexidine possess an effective antibacterial action against E. faecalis and their level of effectiveness depends mainly upon a) their concentration and form, b) different models of the microorganism and c) experimental procedures including manipulation and duration of contact with the microorganism. Besides the possible toxic interaction, the synergistic antibacterial activity of NaOCl/CHX combination remains controversial.
Although the agar diffusion and direct contact methods are considered as valuable tools for evaluating and comparing the antibacterial activity of chemotherapeutic agents, a comprehensive and detailed standard protocol is required to ensure high levels of reliability and reproducibility among different researchers.
Disinfection of gutta percha and Resilon cones with high concentrations of 5.25% NaOCl or 2% CHX would maintain the sterility of the prepared root canal system. However, the chemical interaction between Resilon cones and both irrigants requires further investigations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am. 2010;54:291–312. doi: 10.1016/j.cden.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadi Z, Abbott PV. Antimicrobial substantivity of root canal irrigants and medicaments: A review. Aust Endod J. 2009;35:131–9. doi: 10.1111/j.1747-4477.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- 3.Siqueira JF, Jr, Rocas IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26:331–4. doi: 10.1097/00004770-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Siqueira JF, Jr, Guimaraes-Pinto T, Rocas IN. Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intra-canal medication with calcium hydroxide on cultivable bacteria in infected root canals. J Endod. 2007;33:800–5. doi: 10.1016/j.joen.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Martinho FC, Chiesa WM, Marinho AC, Zaia AA, Ferraz CC, Almeida JF, et al. Clinical investigation of the efficacy of chemomechanical preparation with rotary nickel-titanium files for removal of endotoxin from primarily infected root canals. J Endod. 2010;36:1766–9. doi: 10.1016/j.joen.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Siqueira JF, Jr, Alves FR, Almeida BM, de Oliveira JC, Rocas IN. Ability of chemomechanical preparation with either rotary instruments or self-adjusting file to disinfect oval-shaped root canals. J Endod. 2010;36:1860–5. doi: 10.1016/j.joen.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Sassone LM, Fidel RA, Murad CF, Fidel SR, Hirata R., Jr Antimicrobial activity of sodium hypochlorite and chlorhexidine by two different tests. Aust Endod J. 2008;34:19–24. doi: 10.1111/j.1747-4477.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- 9.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 10.Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 11.Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30:785–7. doi: 10.1097/00004770-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Hülsmann M, Rödig T, Nordmeyer S. Complications during root canal irrigation. Endod Top. 2009;16:27–63. [Google Scholar]
- 13.Carson KR, Goodell GG, McClanahan SB. Comparison of the antimicrobial activity of six irrigants on primary endodontic pathogens. J Endod, 2005;31:471–3. doi: 10.1097/01.don.0000148868.72833.62. [DOI] [PubMed] [Google Scholar]
- 14.Berber VB, Gomes BP, Sena NT, Vianna ME, Ferraz CC, Zaia AA, et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int Endod J. 2006;39:10–7. doi: 10.1111/j.1365-2591.2005.01038.x. [DOI] [PubMed] [Google Scholar]
- 15.Cāmara AC, de Albuquerque MM, Aguiar CM, de Barros Correia AC. In vitro antimicrobial activity of 0.5%, 1%, and 2.5% sodium hypochlorite in root canals instrumented with the ProTaper Universal system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e55–61. doi: 10.1016/j.tripleo.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 16.Cervone F, Tronstad L, Hammond B. Antimicrobial effect of chlorhexidine in a controlled release delivery system. Endod Dent Traumatol. 1990;1:33–6. doi: 10.1111/j.1600-9657.1990.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 17.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, et al. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 18.Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27:452–5. doi: 10.1097/00004770-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Wang CS, Arnold RR, Trope M, Teixeira FB. Clinical efficiency of 2% chlorhexidine gel in reducing intracanal bacteria. J Endod. 2007;33:1283–9. doi: 10.1016/j.joen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24:472–6. doi: 10.1016/S0099-2399(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 21.Foulkes DM. Some toxicological observations on chlorhexidine. J Periodontal Res Suppl. 1973;12:55–60. doi: 10.1111/j.1600-0765.1973.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee TH, Hu CC, Lee SS, Chou MY, Chang YC. Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int Endod J. 2010;43:430–5. doi: 10.1111/j.1365-2591.2010.01700.x. [DOI] [PubMed] [Google Scholar]
- 23.Portenier I, Waltimo TMT, Haapasalo M. Enterococcus faecalis - the root canal survivor and ‘star’ in post treatment disease. Endod Top. 2003;6:135–59. [Google Scholar]
- 24.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 25.Siqueira JF, Jr, Batista MM, Fraga RC, de Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998;24:414–6. doi: 10.1016/S0099-2399(98)80023-X. [DOI] [PubMed] [Google Scholar]
- 26.Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endod J. 1999;32:99–102. doi: 10.1046/j.1365-2591.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 27.Leonardo MR, da Silva LA, Filho MT, Bonifacio KC, Ito IY. In vitro evaluation of the antimicrobial activity of a castor oil-based irrigant. J Endod. 2001;27:717–9. doi: 10.1097/00004770-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Davis JM, Maki J, Bahcall JK. An in vitro comparison of the antimicrobial effects of various endodontic medicaments on Enterococcus faecalis. J Endod. 2007;33:567–9. doi: 10.1016/j.joen.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Vianna ME, Gomes BP. Efficacy of sodium hypochlorite combined with chlorhexidine against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:585–9. doi: 10.1016/j.tripleo.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J. 2001;34:300–7. doi: 10.1046/j.1365-2591.2001.00392.x. [DOI] [PubMed] [Google Scholar]
- 31.Sassone LM, Fidel R, Fidel S, Vieira M, Hirata R., Jr The influence of organic load on the antimicrobial activity of different concentrations of NaOCl and chlorhexidine in vitro. Int Endod J. 2003;36:848–52. doi: 10.1111/j.1365-2591.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 32.Abdullah M, Ng YL, Gulabivala K, Moles DR, Spratt DA. Susceptibilties of two Enterococcus faecalis phenotypes to root canal medications. J Endod. 2005;31:30–6. doi: 10.1097/01.don.0000136205.80807.5a. [DOI] [PubMed] [Google Scholar]
- 33.Sena NT, Gomes BP, Vianna ME, Berber VB, Zaia AA, Ferraz CC, et al. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J. 2006;39:878–85. doi: 10.1111/j.1365-2591.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 34.Arias-Moliz MT, Ferrer-Luque CM, Espigares-Garcia M, Baca P. Enterococcus faecalis biofilms eradication by root canal irrigants. J Endod. 2009;35:711–4. doi: 10.1016/j.joen.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Williamson AE, Cardon JW, Drake DR. Antimicrobial susceptibility of monoculture biofilms of a clinical isolate of Enterococcus faecalis. J Endod. 2009;35:95–7. doi: 10.1016/j.joen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Tobias RS. Antibacterial properties of dental restorative materials: A review. Int Endod J. 1988;21:155–60. doi: 10.1111/j.1365-2591.1988.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 37.Gomes BP, Berber VB, Montagner F, Sena NT, Zaia AA, Ferraz CC, et al. Residual effects and surface alterations in disinfected gutta-percha and Resilon cones. J Endod. 2007;33:948–51. doi: 10.1016/j.joen.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Rosenthal S, Spångberg L, Safavi K. Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:488–92. doi: 10.1016/j.tripleo.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Krishnamurthy S, Sudhakaran S. Evaluation and prevention of the precipitate formed on interaction between sodium hypochlorite and chlorhexidine. J Endod. 2010;36:1154–7. doi: 10.1016/j.joen.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed HMA, Abbott PV. Discolouration potential of endodontic procedures and materials: a review. Int Endod J. 2012;45:883–97. doi: 10.1111/j.1365-2591.2012.02071.x. [DOI] [PubMed] [Google Scholar]
- 41.Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33:966–9. doi: 10.1016/j.joen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Thomas JE, Sem DS. An in vitro spectroscopic analysis to determine whether para-chloroaniline is produced from mixing sodium hypochlorite and chlorhexidine. J Endod. 2010;36:315–7. doi: 10.1016/j.joen.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowicki JB, Sem DS. An in vitro spectroscopic analysis to determine the chemical composition of the precipitate formed by mixing sodium hypochlorite and chlorhexidine. J Endod. 2011;37:983–8. doi: 10.1016/j.joen.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. J Med Micrbiol. 1996;44:79–87. doi: 10.1099/00222615-44-2-79. [DOI] [PubMed] [Google Scholar]
- 45.O’Tootle GA, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 46.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 47.Gomes BP, Vianna ME, Matsumoto CU, Rossi Vde P, Zaia AA, Ferraz CC, et al. Disinfection of gutta-percha cones with chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:512–7. doi: 10.1016/j.tripleo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Royal MJ, Williamson AE, Drake DR. Comparison of 5.25% sodium hypochlorite, MTAD, and 2% chlorhexidine in the rapid disinfection of polycaprolactone-based root canal filling material. J Endod. 2007;33:42–4. doi: 10.1016/j.joen.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 49.Dumani A, Yoldas O, Isci AS, Koksal F, Kayar B, Polat E. Disinfection of artificially contaminated Resilon cones with chlorhexidine and sodium hypochlorite at different time exposures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e82–5. doi: 10.1016/j.tripleo.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Nabeshima CK, Machado ME, Britto ML, Pallotta RC. Effectiveness of different chemical agents for disinfection of gutta-percha cones. Aust Endod J. 2011;37:118–21. doi: 10.1111/j.1747-4477.2010.00256.x. [DOI] [PubMed] [Google Scholar]


