Abstract
Patients with restless leg syndrome present with sensory symptoms similar to peripheral neuropathy. While there is evidence of abnormalities of dopaminergic pathways, the peripheral nervous system has been studied infrequently. We studied conventional nerve conduction studies, quantitative thermal sensory testing and sympathetic skin response in 57 patients with primary restless leg syndrome. Almost two third patients demonstrated abnormalities in the detailed testing of the peripheral nervous system. Sbtle abnormalities of the peripheral nervous system may be more common than previously believed.
Keywords: Quantitative sensory testing, restless legs syndrome, sympathetic skin response
Introduction
Patients with restless legs syndrome (RLS) present with predominantly sensory manifestations, which often resemble and sometimes are mistaken as those of peripheral neuropathy. Although there is ample evidence for involvement of dopaminergic pathways as well as iron metabolism in the etiopathogenesis of RLS, there are several unanswered questions in this area, and there is not yet much literature on involvement of the peripheral nervous system in patients with RLS. Moreover, a large proportion of patients with peripheral neuropathy of varied etiology may have a combination of neuropathic symptoms as well as RLS. We conducted this study to assess the prevalence of electrophysiological and psychophysiological evidence of involvement of small peripheral nerve fibers in consecutive patients of primary RLS, using quantitative thermal sensory testing, sympathetic skin response, and nerve conduction studies.
Materials and Methods
Over a period of 3 years from October 2003 to September 2006, all consecutive patients who presented to the sleep disorders clinic, neurology services at the All India Institute of Medical Sciences, fulfilling the IRLSSG criteria of RLS,[1] were evaluated in detail. Patients were interrogated for details of clinical phenomena involving limbs, their duration, intensity, aggravating and relieving factors, diurnal pattern of occurrence for association with other medical or neurological illnesses, drug history, and response to dopamine agonists through a pre-structured performa. All patients underwent investigation for detailed hematological and biochemical profile, mainly for evidence of anemia, low ferritin levels for hepatic or renal impairment. All patients with RLS, thus diagnosed, without any clinical evidence of or family history of peripheral neuropathy formed the study population. Patients with pregnancy or any medical, hematological, or neurological disorders, known as secondary causes of RLS, were excluded. After detailed clinical evaluation and assessment of severity by the RLS rating scale,[2] patients were subjected to nerve conduction studies on upper limb and lower limb on one side, followed by examination with quantitative thermal sensory testing (QST) by method of limits.[3] Routine nerve conduction studies, carried out on a Medelec Synergy Ò EMG/EP system, included motor conduction and F-wave studies for Median, Ulnar, common peroneal and posterior tibial nerves and sensory conduction studies for Median, Ulnar, and Sural nerves on one side in patients with symmetrical distribution of symptoms and evaluation of a third limb in patients with asymmetrical symptoms. The sympathetic skin response (SSR) was tested for in all patients, with assessment using electrode placement over an upper limb and the ipsilateral lower limb.
QST was carried out using a Medoc TSA-II Neurosensory analyzer at one or more sites, with method of Limits. Subjects were seated comfortably, on a chair in a quiet room, with ambient temperature of 24°-25° Celsius. The site used for testing the lower limb was dorsolateral border of foot, and the site used in the upper limb was the hypothenar eminence. The modalities tested were cold sensation and warm sensation. Patients were instructed in detail, the nature of the test and the need to react attentively and promptly to change in temperatures. The test was performed by placing a 30 mm × 30 mm thermode over the skin of the site to be tested, and averages of 4 readings of threshold temperature for each sensory modality were noted. Note was also made if the modality was identified incorrectly. Based on values obtained from the control population, previously reported, the mean threshold temperature ± 2 SD for each modality was considered as the upper (or lower) limit of normal.
Results
A total of 105 patients (52 males, 53 females) with RLS were seen from September 2003 to August 2006. Among these, 57 patients (38 females, 19 males) with an average age of 43 ± 14.5 years could be classified as primary RLS. The average duration of symptoms in lower limbs was 5.4 years. All patients revealed no abnormality on detailed medical and neurological examination. Current drug treatment included dopamine agonists (57 patients), Clonazepam (2 patients), Topiramate for migraine (5 patients), SSRIs (5 patients), and oral iron (3 patients) [Table 1].
Table 1.
Clinical details of patients with primary RLS (n = 57)
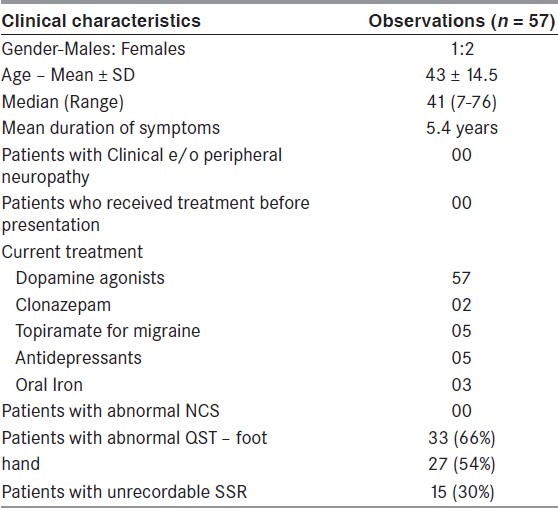
Nerve conduction studies were found normal in all patients. QST could be performed in 50 patients and revealed abnormal thresholds for both sensations in lower limbs and for at least one modality of sensation (cold or warm) in upper limbs in 33 patients (66%). Fifteen patients (30%) among these (all of whom had abnormalities on QST) also showed a non-recordable SSR, both on upper limb and lower limb recordings.
Thermal thresholds
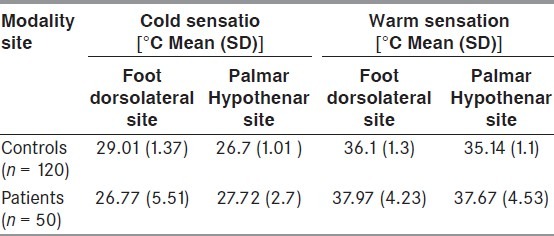
The average cold sensation thresholds among patients over the foot (dorsolateral) site were 26.77 ± 5.51 while over the palmar (hypothenar) site were 27.72 ± 2.7. The average warm sensation threshold over the foot site was 37.97 ± 4.23 and 37.67 ± 4.53. [Table 2].
Table 2.
Thermal thresholds in patients with RLS as compared with those in normal controls
Discussion
This study is among the few studies, in which thermal threshold abnormalities have been studied for and identified. This is the largest study till now with 57 patients suffering from primary RLS, studied with quantitative assessment of thermal sensory abnormalities, and nearly 66% patients were observed to manifest with significant abnormalities.
We included 37 female and 19 male patients in the age group of 43 ± 14 years. Our patients had fairly long standing symptoms with an average duration of about 5 years. We had meticulously excluded conditions, which could be associated with secondary RLS. Nerve conduction studies conducted on all patients revealed no abnormalities. In one of the earliest of the few studies, addressing the subject of peripheral nerve involvement in patients with primary RLS, Iannaconne S et al. examined 8 consecutive patients. All known causes for peripheral neuropathy and other neurological abnormalities were ruled out. The age and sex distribution was similar to that in our study. In this study also, nerve conduction studies were normal in all patients.[4] In another study by Polydefkis M et al. on 22 consecutive patients presenting with RLS, one among whom already had symptoms of large fiber peripheral neuropathy, 5 patients were detected to have abnormal Sural sensory nerve action potential amplitudes. Also, 3 patients, who clearly had unilateral radicular symptoms and backache, were found to have reduced deep peroneal compound muscle action potential amplitudes.[5] The patients included in this study were older (average age 62.2 ± 5.9). They did not exclude patients with known peripheral neuropathy or clinical features of neuropathy although patients with any comorbid illness like diabetes, uremia, and Vit B12 deficiency were excluded. The less stringent inclusion criteria possibly account for the observation of abnormal lower limb sensory conduction abnormalities, which may also not necessarily be suggestive of presence of large fiber neuropathy considering the older age group of the patients.
In their review on secondary RLS, Ondo W reported electrophysiologic evidence of neuropathy using standard EMG/NCV techniques in 37/98 (36.6%) of RLS patients; most having mild-to-moderate sensory axonal neuropathies of diverse etiology. The presence of neuropathy was much higher in patients who did not have a family history, compared with those who did have a family history: 22/31 (71%) vs. 15/67 (24%), P < 0.001.[6] These observations are valuable; however, since we carefully included patients with primary RLS, excluding all patients with any identifiable secondary causes, comparison would be improbable.
We observed abnormalities in at least one modality on quantitative thermal sensory testing among 66% of patients examined in this study. Schattschneider S et al. found abnormalities in thermal thresholds in 72% patients with secondary RLS (n = 22) and 55% patients with idiopathic RLS (n = 20). In addition to the QST, they also examined peripheral C fiber function with quantitative nerve axon reflex testing (QNART) and found no abnormalities in this among patients with primary RLS as compared to significant abnormalities among patients with secondary RLS. The authors attribute these differences to abnormal central somatosensory processing among patients with primary RLS while these are attributed to small fiber neuropathy in patients with secondary RLS.[7] In a recent study, 21 patients with primary RLS were compared with 13 patients with secondary RLS (with co-existent small fiber neuropathy confirmed by skin biopsy) and 20 normal controls, the patients with primary RLS showed hyperalgesia to blunt pressure, pinprick, and vibratory hyperesthesia while patients with secondary RLS, associated with small fiber neuropathy, showed thermal hypoesthesia to cold (A delta-fiber mediated) and warm (C-fiber mediated) and hyperalgesia to pinprick.[8] These authors conclude that static mechanical hyperalgesia in primary and secondary restless legs syndrome is consistent with the concept of central disinhibition of nociceptive pathways, which might be induced by conditioning afferent input from damaged small fiber neurons in secondary restless legs syndrome. None of our patients had symptoms of small fiber neuropathy, yet abnormalities on thermal thresholds were observed in a sizeable proportion of patients similar to that observed in these 2 studies discussed. Our limitation was inability to perform any further investigations to localize the level of involvement within the sensory pathways. This work, however, should form a basis for further investigation into the prevalence of small fiber neuropathy among patients diagnosed with primary RLS and also confirm findings observed by these investigators in small number of patients. It is possible that even among patients with primary RLS, who do not progress into overt small or large fiber neuropathy, there are abnormalities in the peripheral nervous system as shown by Iannaconne et al.[4]
Lastly, we also observed the sympathetic skin response, a non-specific tool to assess integrity of the sympathetic nervous system, to be abnormal among 30% of patients studied. This has not been reported yet in any previous studies. This observation in conjunction with thermal threshold abnormalities might also strengthen the possibility of involvement of the small unmyelinated fibers within the peripheral nervous system, especially since previous investigators have found almost similar findings as ours among patients with primary RLS.
Conclusion
This study reveals that a large proportion, almost two-thirds, of patients with primary RLS demonstrates abnormal thermal thresholds and SSR with a possibility of significantly prevalent abnormalities of small peripheral nerve fibers.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Allen RP, Hening WA, Montplaisir J, Picchietti D, Trenkwalder C, Walters AS Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology: A report from the RLS diagnosis and epidemiology workshop at the national institute of health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. International Restless Legs Syndrome Study Group.Validation of the International restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 3.Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20:198–204. doi: 10.1002/(sici)1097-4598(199702)20:2<198::aid-mus10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Iannaccone S, Zucconi M, Marchettini P, Ferini-Strambi L, Nemni R, Quattrini A, et al. Evidence of peripheral axonal neuropathy in primary restless legs syndrome. Mov Disord. 1995;10:2–9. doi: 10.1002/mds.870100103. [DOI] [PubMed] [Google Scholar]
- 5.Polydefkis M, Allen RP, Hauer P, Earley CJ, Griffin JW, McArthur JC. Subclinical sensory neuropathy in late-onset restless legs syndrome. Neurology. 2000;55:1115–21. doi: 10.1212/wnl.55.8.1115. [DOI] [PubMed] [Google Scholar]
- 6.Ondo W. Secondary Restless legs syndrome. In: Chaudhari KR, Odin R, Olanow CW, editors. Restless legs syndrome. London and New York: Taylor& Francis; 2009. pp. 57–84. [Google Scholar]
- 7.Schattschneider J, Bode A, Wasner G, Binder A, Deuschl G, Baron R. Idiopathic restless legs syndrome: Abnormalities in central somatosensory processing. J Neurol. 2004;251:977–82. doi: 10.1007/s00415-004-0475-3. [DOI] [PubMed] [Google Scholar]
- 8.Bachmann CG, Rolke R, Scheidt U, Stadelmann C, Sommer M, Pavlakovic G, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. 2010;133(Pt 3):762–70. doi: 10.1093/brain/awq026. [DOI] [PubMed] [Google Scholar]


