Abstract
Context:
Impairment of initiating sequential movements and processing of proprioception contribute to characteristic Parkinson's disease (PD) gait abnormalities. Many studies have used a single external cue or 2 different cues to correct PD gait.
Aim:
An aim of this study was to determine the influence of paired proprioceptive cues on gait parameters of individuals with PD.
Setting and Design:
Double-blind randomized controlled trial.
Materials and Methods:
Subjects were 30 PD patients who had mild to moderate impairment according to the United Parkinson's Disease Rating Scale (UPDRS). They were randomly assigned to either a routine physiotherapy program or treadmill training with vibratory stimuli applied to the feet plantar surfaces and proprioceptive neuromuscular facilitation (PNF) as well as the same physiotherapy program. All Participants received a 45-minutes session of low intensity physiotherapy program, 3 times a week, for 8 weeks. The duration of treadmill training was 5 minutes at baseline and 25 minutes at the end of treatment. Walking speed and distance were recorded from the treadmill control panel for both groups before and immediately after the end of treatment. The Qualysis ProReflex motion analysis system was used to measure cadence, stride length, hip, knee, and ankle joints’ angular excursion.
Results:
The cadence, stride length, and lower limb joints’ angular excursion showed a significant improvement in both groups (P ≤ 0.05). These improvements in spatio-temporal parameters and angular excursion were higher in the study group than in the control group (P ≤ 0.05).
Conclusion:
Potentiated proprioceptive feedback improves parkinsonian gait kinematics, the hip, knee, and ankle joints’ angular excursion.
Keywords: Gait, Parkinson's disease, proprioceptive cues
Introduction
Parkinson's disease (PD) is characterized by deficits in motor control, such as difficulties in movement initiation, scaling movement amplitudes, or modulating muscle activity.[1] Bradykinesia is especially pronounced when individuals with PD attempt to perform internally initiated walking.[2] This affects PD patients’ ability to initiate or alter the gait pattern and to adapt to disturbances during walking.[3,4] The severity of gait disorders in PD patients varies according to disease duration, different phases of the anti-parkinsonian medication cycle, the environmental circumstances in which walking occurs, the presence of external cues, and the extent to which a PD patient uses attentional strategies to bypass the defective basal ganglia (BG) in order to regulate the walking pattern.[5]
The most characteristic gait disorder affects speed and cadence,[6,7] causing reduced stride length with increased step-to-step variability, with insufficient hip, knee, and ankle flexion.[8] These gait problems increase the incidence of falls[9] and risk of bone fracture among PD patients.[10] Beyond the acute trauma, falls may result in fear of falling, nursing home admission, and social problems such as self-imposed restrictions on activities of daily living.[9,10]
Laboratory-based studies show that applying auditory (rhythmic beeps or metronome) or visual cues (rhythmically flashing lights or floor marks) are effective at normalizing gait in PD.[7,11–13] In addition, it has been found that while double tasks during walking interfere with gait performance,[14] applying external rhythms during a dual task may actually facilitate parkinsonian gait.[13,15,16]
Both auditory and visual cue modalities, although highly effective in controlled situations, have the potential disadvantage that the user is not able to use them during daily activities without bystanders noticing. In addition, noisy or brightly-lit environments may restrict the practical utility of both modalities. Therefore, it is important to study the effect of proprioceptive cue modalities consisting of miniature hidden vibrating devices that apply rhythmic vibrations to the skin synchronized with the step. These stimuli are synchronously applied with the steps while walking on a treadmill, which imitates the real life situations and provide an additional proprioceptive cueing.[6] This study examined the influence of paired proprioceptive cues on gait parameters of individuals with PD. The hypothesis was that enhanced proprioceptive stimuli (vibratory stimuli and treadmill) may improve spatio-temporal parameters of gait and lower limb angular excursion and consequently increase the quality of life of patients with PD.
Subjects and Methods
Thirty levodopa-dependent PD patients (9 females, 21 males), with ages ranging from 49 to 70 years (62.5 ± 6.1 years), represented the sample of the study. The participants were recruited from the Department of Neurology at the Faculty of Medicine, Cairo University, Egypt. The patients were randomly assigned into 2 equal groups (G1 and G2). With their eyes closed, subjects were randomized by self-selecting a card corresponding to one of the 2 groups. Subjects and examiner were blinded during randomization, except for a nurse who prepared the groups cards. All the patients were subjected to clinical neurological examination, which included a review of medical history, mentation (using Mini Mental State Examination), a motor examination, and assessment of activities of daily living using the United Parkinson's Disease Rating Scale (UPDRS), by a well-trained neurologist [Figure 1]. The individuals who were able to walk independently for 6 minutes on the treadmill (Enraf-Nonius B.V. Germany) without interruption, suffered from mild to moderate disability according to UPDRS ADL/motor scores with duration of illness ranging from 3 to 5 years with interest in participating, and ability to give their informed consent participated in this study. Patients with any of the following criteria were excluded from the study: Any other neuro-musculoskeletal disorder, which could potentially cause gait disorders;[17] epilepsy; mental and cognitive impairment; marked rigidity (more than 3 according to the rigidity UPDRS subscale; poor visuo-spatial abilities; dyskinesias; anorexia; uncontrolled blood pressure, or cardiovascular disorders.[18,19] For all participating subjects, all PD medications were kept stable during the course of the study and assessment, and treatment were done in “on” stage of medications. This study was approved by the research review boards of the Faculty of Medicine, Cairo University, Egypt.
Figure 1.
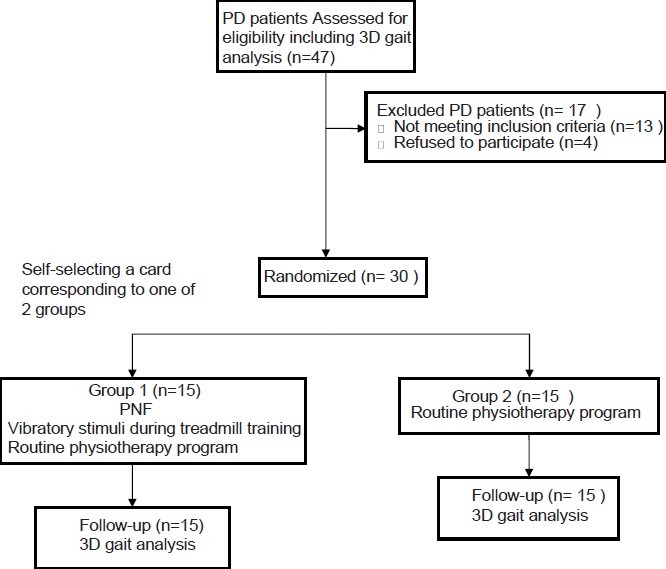
Reporting trials diagram
Qualysis ProReflex motion capture (Qualysis Medical AB, Sweden) system in the motion analysis laboratory of the Faculty of Physical therapy Cairo University, Egypt was used for gait analysis of all participants after completion of the physiotherapeutic programs. The patients in the control group (G2) were treated with an individually designed physiotherapy program of mild intensity exercises conducted by a neuro-physiotherapist. This low-intensity exercise program consisted of: Passive prolonged stretch; balance training and weight shifting exercises; and graduated active exercises for the axial muscles to maintain/increase the muscle strength. The functional training included: Standing up and sitting down; turning around using a large arc of movement, or using full body movements, the “clock turn strategy;” traditional gait training including instructions to walk with long steps, even stride length and swinging the arms. The patients were instructed to walk at least 2 times a week for 30 min; focus on maintaining long strides and adequate ground clearance, maintain upright posture by consciously attending to standing upright, checking posture in a mirror and reinforce physiotherapy strategies in the home and community. Participants attended 45minutes sessions 3 times per week for 8 weeks.
The participants in the study group (G1) received proprioceptive neuromuscular facilitation techniques and vibratory stimuli during walking on the treadmill in addition to the same designed physiotherapy program as G2. The vibratory devices (VDs) (OPTEC Co. Ltd., Japan) were inserted into the patients’ shoes and were activated in the push off-phase of the gait. The VDs deliver supra-threshold stimulation through 1 little toe sensor, which stimulates 3 VDs below the heel and forefoot without delay. The patients were asked to pay attention to the vibratory stimuli which are activate when the foot is in the swing phase. The time of gait training on the treadmill started at 6 minutes followed by 5 minutes rest. The walking speed was selected according to the ability of each patient, and then its progression is self-selected by each subject. The treadmill walking time was increased gradually from 6 to 25 minutes at the end of the program. Participants in G1 attended 51-70 minutes sessions, 3 times per week for 8 weeks, including the low and moderate intensity exercise programs.
An International Business Machines (IBM) compatible PC was used to store and analyze the 3D data. The data were imported to Q-gait software for analysis. The kinematic parameters of gait, including cadence and stride length, were calculated. Hip/ knee flexion and ankle dorsiflexion angles during different gait sub-phases were also measured. The walking distance and speed were recorded by the treadmill before treatment for both groups and at the end of the treatment programs.
Statistical analysis
The Social Package for Social Sciences (SPSS) version 16.0 (SPSS Inc, Chicago, IL, USA) was used to analyze the data. Descriptive statistics such as means, standard deviations as well as percentages were used to describe the participants. Statistical measures of the mean scores and standard deviation were calculated for the baseline and post-intervention for each participant. The simple t test was used to compare the means of the cadence, stride length, walking distance, and speed measured in G1 and G2. It was also used to compare the pre/post measures of the hip, knee, and ankle angular excursion. The between-group comparison was also performed using the same test. The results were accepted as significant at values of P ≤ 0.05.
Results
Patients’ demographics are summarized in Table 1. All 30 participants were PD patients who had suffered 4.0 ± 0.9 years and 3.8 ± 0.9 years in G1 and G2, respectively, prior to enrollment. The mean age in G1 was 61.4 ± 7.28 years, and it was 63.2 ± 5.6 years in G2. The stage of PD was 2-3 according to Modified Hoehn and Yahr staging. The mean values of UPDRS motor and ADL scores in G1 during the “on” state were 31.2 ± 4.1 and 24.8 ± 3.09, respectively, and 30.2 ± 3.9 and 25.4 ± 2.9, respectively, in G2. The assessment at baseline showed no significant differences between the participants in the 2 groups with respect to their demographic characteristics (P > 0.05). The cardinal features of PD as presented in both groups were as follows: Bradykinesia 100% in both groups, tremor 46.6% in G1 and 33.4% in G2, rigidity 66.6% in G1 and 60.0% in G2, postural instability 73.3% in G1 and 80.0% in G2.
Table 1.
Demographic and clinical data of the participants
The statistical analysis of the study group (G1) showed a significant increase in the cadence after 6 weeks of treatment (P = 0.001) [Table 2]. A significant difference was also observed in the control group (G2) after the intervention (P = 0.001). The between-group differences were also significant with the increase in cadence noted in G1 being more than that of G2 (P = 0.01). The mean values of the stride length in G1 and G2 before/after applying physical therapy programs are shown in Table 2. Post-treatment measures of the stride length revealed a significant increase within groups. The between-groups difference was also significant with the increase in stride length observed in G1, being more than that of G2 (P = 0.01). The interventions effects on walking distance and speed are shown in Table 2. These parameters showed significant improvements in both groups with more improvement shown by the patients who received the paired proprioceptive feedback (G1).
Table 2.
Spatiotemporal parameters of gait measured before/after treatments (mean ± standard deviation)
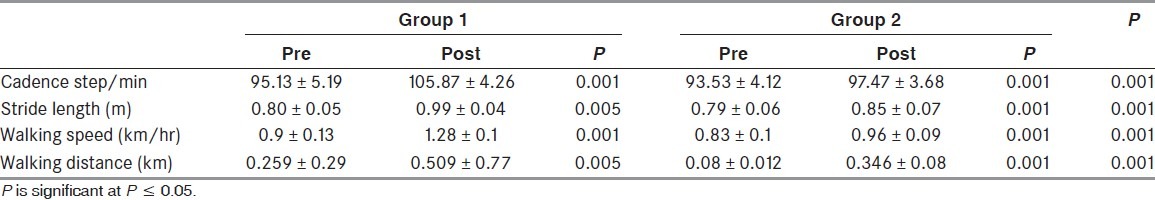
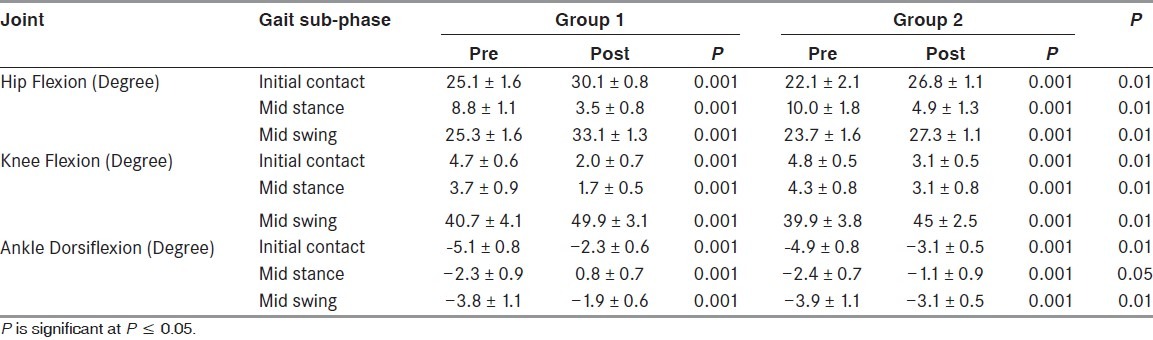
Table 3 details the hip flexion, knee flexion, and ankle dorsiflexion angles in the 3 different sub-phases of gait in both groups. The most notable differences are the significant increases in the hip and knee flexion during the mid-swing sub-phase after treatment (P = 0.001) compared to before treatment. Also, ankle dorsiflexion was significantly improved in the mid-swing sub-phase (P = 0.001) compared to before treatment. In the control group, hip and knee flexion during the mid-swing sub-phase were higher than before treatment (P = 0.001). Also, the ankle dorsiflexion during the mid swing was higher than before treatment (P = 0.01). It is obvious that the improvement shown by patients in G1 was better than that of patients in G2 throughout the measured angular excursion (P = 0.01).
Table 3.
Lower limb angular excursion of hip, knee, and ankle (in degrees) measured before/after treatments (mean ± standard deviation)
Discussion
The results of this study prove the positive effect of the different physical therapy programs (those performed by in both groups) on various aspects of PD patients’ pattern of gait. This agrees with the research outcomes[20] that attribute this to the efficacy of exercises in increasing the availability of dopamine in the striatum and protecting neurons from further neuronal degeneration by activating endogenous antioxidant systems in the brain. Exercise also reduces the behavioral impairments elicited by the dopaminergic neurotoxins and the loss of dopamine neurons. This is also in a close agreement with Tajiri et al.,[21] and Al-Jarrah et al.[22] who showed in histological studies that potentiated proprioceptive feedback-induced alterations in both dopaminergic and glutamatergic neurotransmission.
The results of the current study confirm our hypothesis that application of vibratory stimuli during treadmill training has a positive effect on the angular excursion and spatiotemporal parameters of gait. The improvements in G1 were better than those in G2.This can be attributed to the effect of using external somatosensory cues. These cues trigger conscious control mechanisms and attention strategies and reduce automatic control during walking. The automatic control of movement involves the supplementary motor area (SMA), which receives its major input from the BG. In PD patients, the conscious control of walking bypasses the inefficient SMA and activates the dorsolateral premotor control system. This system receives its major input from the sensory cortical area. This agrees with previous studies.[23–25] We attribute the potent effect of external stimuli on the gait pattern of PD patients to the efficacy of attention in generating cortical plasticity in the primary somatosensory and motor cortex, and improvement in the motor memory. Holschneider et al.[26] stated that long-term exercise training elicits plastic changes in the brain. Such changes would result in a combination of increases in the efficiency of neural processing (sensorimotor cortex, striatum, vermis) and enforcement of the cerebellar-cortical circuit.
The improvement in the PD gait parameters in the present study can also be ascribed to the potent effect of external cues on activating an alternate pathway involving the cerebellum, sensorimotor cortex, and lateral premotor cortex.[27,28] In this pathway, the cerebellum is responsible for movement timing, and the premotor cortex may be responsible for scaling the motor activity when facilitated by somatosensory cues related to the task (walking). This means that the recruitment of these structures can compensate for an inefficient BG in PD.
Lewis et al.[29] ascribed the improvement in PD patients’ pattern of gait in response to somatosensory cues to the dominancy of the cerebellar-cortical pathways. In the normal condition, externally generated tasks are mainly processed through cerebellar-cortical circuitry, with minimal recruitment of the BG-cortical circuitry. Internally generated tasks are mainly encoded in the BG-cortical pathway, with minimal recruitment of cerebellar-cortical circuitry. In PD patients, externally generated tasks are also processed primarily via the cerebellar-cortical pathway with an enhanced activity in the cerebellum and lateral premotor cortex areas. Internally generated tasks in PD patients neither adequately activate the BG-cortical pathway nor recruit the cerebellar-cortical pathway. These results contradict Yogev et al.[30] who reported that external cues support a more automatic form of motor control. This inconsistency may result from the use of dual tasks in the study of Yogev et al.[30] These dual tasks may disturb the patient's attention to and focus on walking, which elicit the automatic control of walking.
The results of the present study can be ascribed to the facilitation of the frontal-lobe cognitive strategies (working memory) by externally cueing the sequential movement (gait). This agrees with Campos-Sousa et al.[31] and Wegen et al.[32] The frontal lobe areas can select a motor program in response to an external stimulus, and send it to the primary motor cortex, which is responsible for the execution of sequential movements. External cues also contribute to sensorimotor integration that requires organizing and processing of proprioceptive inputs in working memory.[33] This explanation contradicts Yogev et al.[30] who stated that intrinsic regulation of sequential movements by the BG cannot be adequately compensated by the parkinsonian nervous system. Holschneider et al.,[26] using brain imaging, showed that there are increases in perfusion in the prefrontal cortex, deep cerebellar nuclei, thalamus, and hippocampus, which compensate for a functionally disrupted BG, which support our findings.
The improvement in the gait pattern of PD patients can be attributed to stretch of the hip flexors and ankle plantar flexor muscles by the moving treadmill running-belt.[34] This stretch increases the gait-related unconscious proprioceptive impulses from these muscles. Augmentation of the sensory inputs stimulates the gait central pattern generator. The hip joint flexion and ankle joint plantar flexion at the end of the stance phase are facilitated by increased efferent impulses. It has also been reported that treadmill walking facilitates many forms of proprioceptive information.[35] It rhythmically stimulates pressure load receptors of the feet, muscle spindles, and Golgi tendon organs. Vestibular input from the vestibular system and input from neck muscle proprioceptors are activated during treadmill training. These rhythmic inputs are transferred to neuronal circuits and increase the strength of inputs converging on pyramidal tract neurons.
The results of the current study show that vibration has a positive effect on the angular displacement of the lower limb joints during walking. This can be attributed to enhancement of cortico-spinal excitability and increased tonic activities in the foot muscles in response to vibration applied to PD patients. This result agrees with Ivanenko et al.[35] and Thompson et al.[36] who reported that increased tonic activities in the muscles can induce a tonic vibration reflex within the triceps surae muscle group. It is also in agreement with Bogaerts et al.[37] who proved that vibration is associated with an extensive sensory stimulation and that efficient use of the proprioceptive feedback loop could modify the cortico-spinal output and muscles activities.
Limitations
An important limitation of this study was the small sample size. Nevertheless, we were able to the show benefits of using paired proprioceptive cues on the gait pattern of the participants of PD. This trend of changes we observed warrants further study with a larger sample size to enable a more discerning statistical analysis. Studying sensorimotor area excitability after the application of external cues is needed to reveal the central effect of such stimuli and the role of central sensorimotor integration on the gait impairment in individuals with PD.
Conclusions
Potentiated proprioceptive feedback improves parkinsonian gait kinematics and the angular excursion of lower limb joints. The vibrating devises can be easily implanted within the patient's shoes to be used all over the day as a reminder for proper gait pattern. Additionally, a well-designed physiotherapeutic programs, conducted under the supervision of a well-trained physiotherapist, are desirable for parkinsonian rehabilitation.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 2.Johnson TR, Griffin JW, McArthur JW. Philadelphia: Mosby-Elsevier; 2006. Current therapy in neurologic disease; pp. 245–64. [Google Scholar]
- 3.Roiz R, Cacho EW, Pazinatto MM, Reis JG, Cliquet A, Jr, Barasnevicius-Quagliato EM. Gait analysis comparing Parkinson's disease with healthy elderly subjects. Arq Neuropsiquiatr. 2010;68:81–6. doi: 10.1590/s0004-282x2010000100018. [DOI] [PubMed] [Google Scholar]
- 4.Mirek E, Rudziñska M, Szczudlik A. The assessment of gait disorders in patients with Parkinson's disease using the three-dimensional motion analysis system Vicon. Neurol Neurochir Pol. 2007;41:128–33. [PubMed] [Google Scholar]
- 5.Michel J, Benninger D, Dietz V, van Hedel HJ. Obstacle stepping in patients with Parkinson's disease: Complexity does influence performance. J Neurol. 2009;256:457–63. doi: 10.1007/s00415-009-0114-0. [DOI] [PubMed] [Google Scholar]
- 6.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease. Arch Phys Med Rehabil. 2007;88:1154–58. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Baker K, Rochester L, Nieuwboer A. The effect of cues on gait variability-reducing the attentional cost of walking in people with Parkinson's disease. Parkinsonism Relat Disord. 2008;4:314–20. doi: 10.1016/j.parkreldis.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Harris MH, Holden MK, Cahalin LP, Fitzpatrick D, Lowe S, Canavan PK. Gait in older adults: A review of the literature with an emphasis toward achieving favorable clinical outcomes, part ii. Gait Posture. 2008;8:37–45. [Google Scholar]
- 9.Williams DR, Watt HC, Lees AJ. Predictors of falls and fractures in bradykinetic rigid syndromes: A retrospective study. J Neurol Neurosurg Psychiatry. 2006;77:468–73. doi: 10.1136/jnnp.2005.074070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloem BR, Hausdorff MJ, Visser EJ, Giladi N. Falls and freezing of gait in Parkinson's disease: A review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–84. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 11.van Wegen E, Lim L, Goede de C, Nieuwboer A, Willems A, Jones D, et al. The effects of visual rhythms and optic flow on stride patterns of patients with Parkinson's disease. Parkinsonism Relat Disord. 2006;12:21–7. doi: 10.1016/j.parkreldis.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Suteerawattananon M, Morris GS, Etnyre BR, Jankovic J, Protas EJ. Effects of visual and auditory cues on gait in individuals with Parkinson's disease. J Neurol Sci. 2004;219:63–9. doi: 10.1016/j.jns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.del Olmo MF, Cudeiro J. Temporal variability of gait in Parkinson disease: Effects of a rehabilitation programme based on rhythmic sound cues. Parkinsonism Relat Disord. 2005;11:25–3. doi: 10.1016/j.parkreldis.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, et al. Attending to the task: Interference effects of functional tasks on walking in Parkinson's disease and the roles of cognition, depression, fatigue, and balance. Arch Phys Med Rehabil. 2004;85:1578–85. doi: 10.1016/j.apmr.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, et al. The effect of external rhythmical cues (auditory and visual) on walking during a functional task in homes of people with Parkinson's disease. Arch Phys Med Rehabil. 2005;86:999–1006. doi: 10.1016/j.apmr.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: The RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–40. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MD, Shaikh S, Chau T. Effect of treadmill walking on the stride interval dynamics of human gait. Gait Posture. 2009;30:431–35. doi: 10.1016/j.gaitpost.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Pelosin E, Faelli E, Lofrano F, Avanzino L, Marinelli L, Bove M, et al. Effects of treadmill training on walking economy in Parkinson's disease: A pilot study. Neurol Sci. 2009;30:499–504. doi: 10.1007/s10072-009-0141-8. [DOI] [PubMed] [Google Scholar]
- 19.Stolze H, Klebe S, Baecker C, Zechlin C, Friege L, Pohle S, et al. Prevalence of gait disorders in hospitalized neurological patients. Mov Disord. 2005;20:89–94. doi: 10.1002/mds.20266. [DOI] [PubMed] [Google Scholar]
- 20.Reisi P, Babri S, Alaei H, Sharifi MR, Mohaddes G, Noorbakhsh SM, et al. Treadmill running improves long-term potentiation (LTP) defects in streptozotocin-induced diabetes at dentate gyrus in rats. Pathophysiology. 2010;17:33–38. doi: 10.1016/j.pathophys.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, et al. Exercise exerts neuro- protective effects on Parkinson's disease model of rats. Brain Res. 2010;1310:200–7. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 22.Al-Jarrah M, Jamous M, Al Zailaey K, Bweir SO. Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson's disease. NeuroRehabilitation. 2010;26:369–73. doi: 10.3233/NRE-2010-0574. [DOI] [PubMed] [Google Scholar]
- 23.Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–8. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond MJ, Cameron JL, Leaka RK, Mirnics K, Russell VA, Smeyne RJ, et al. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord. 2009;15(Suppl 3):S42–5. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
- 25.Wulf G, Landers M, Lewthwaite R, Töllner T. External focus instructions reduce postural instability in individuals with Parkinson disease. Phys Ther. 2009;89:162–8. doi: 10.2522/ptj.20080045. [DOI] [PubMed] [Google Scholar]
- 26.Holschneider DP, Yang J, Guo Y, Maarek JM. Reorganization of functional brain maps after exercise training: Importance of cerebellar-thalamic-cortical pathway. Brain Res. 2007;118:496–507. doi: 10.1016/j.brainres.2007.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 28.Elsinger CL, Harrington DL, Rao SM. From preparation to online control: Reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31:1177–87. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, et al. Task specific influences of Parkinson's disease on the striatothalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience. 2007;147:224–35. doi: 10.1016/j.neuroscience.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson's disease: Which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–56. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 31.Campos-Sousa IS, Campos-Sousa RN, Ataíde L, Jr, Soares MM, Almeida KJ. Executive dysfunction and motor symptoms in Parkinson's disease. Arq Neuropsiquiatr. 2010;68:246–51. doi: 10.1590/s0004-282x2010000200018. [DOI] [PubMed] [Google Scholar]
- 32.van Wegen E, de Goede C, Lim I, Rietberg M, Nieuwboer A, Willems A, et al. The effect of rhythmic somatosensory cueing on gait in patients with Parkinson's disease. J Neurol Sci. 2006;248:210–4. doi: 10.1016/j.jns.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd R, Carr J. Treadmill walking in neurorehabilitation. Neurorehabil Neural Repair. 1999;13:171–3. [Google Scholar]
- 34.Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. 2008;89:1221–9. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha motoneuron activation in humans walking at different speeds. J Neurophysiol. 2006;95:602–18. doi: 10.1152/jn.00767.2005. [DOI] [PubMed] [Google Scholar]
- 36.Thompson C, Bel’anger M, Fung J. Effects of bilateral Achilles tendon vibration on postural orientation and balance during standing. Clin Neurophysiol. 2007;118:2456–67. doi: 10.1016/j.clinph.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Bogaerts A, Verschueren S, Delecluse C, Claessens AL, Boonen S. Effects of whole body vibration training on postural control in older individuals: A 1 year randomized controlled trial. Gait Posture. 2007;26:309–16. doi: 10.1016/j.gaitpost.2006.09.078. [DOI] [PubMed] [Google Scholar]


