Abstract
Thromboembolism is a rare complication in patients with myotonic dystrophy. While immobilization of patients with advanced disease predisposes to high risk for venous thromboembolism, hypercoagulability could account for venous thromboembolism in patients without impaired mobilization. We report a patient with myotonic dystrophy type 1 who developed pulmonary thromboembolism unrelated to immobilization.
Keywords: Hypercoagulability, myotonic dystrophy, pulmonary thromboembolism
Introduction
Myotonic dystrophy type 1 is an autosomal-dominant disorder characterized by progressive skeletal muscle weakness, wasting, myotonia and non-muscular manifestations. Respiratory failure is one of the life-threatening complications, which is usually caused by pulmonary infection, heart failure or diaphragmatic weakness.
Pulmonary thromboembolism (PTE) is a rare cause of respiratory failure, and immobility in advanced cases might lead to venous thromboembolism (VTE). However, VTE could also be associated with hypercoagulability of which causes are not well defined.
We describe a patient with myotonic dystrophy type 1 who developed PTE without immobility, with a brief review of the literature.
Case Report
A 38-year-old man was transferred to our emergency room for shortness of breath. Two hours before admission, he began to have sudden onset of dyspnea, and was intubated in another hospital. He reported to be healthy before this episode, and systemic review was negative. He did not have a remarkable past medical history, including surgery, allergy or specific medications. However, he had a family history of ischemic stroke; his father died of complication of ischemic stroke at the age of 65 years and his brother had ischemic stroke at the age of 39 years.
In the emergency department, he had respiratory distress and tachycardia, with an oxygen saturation of 81% even under the intubated state. The blood pressure fell to an unmeasurable level, and the heart rate was 102 beats/min. There were crackles in both lungs on auscultation. Chest X-ray revealed mediastinal widening and cardiomegaly with bilateral infiltrations. Computed tomography (CT) of the chest showed dilated pulmonary trunk, but there was no evidence of pulmonary thrombus, aortic dissection or lymphadenopathy. The patient was admitted to the intensive care unit with a tentative diagnosis of acute respiratory failure due to severe pneumonia.
On hospital day 3, chest X-ray showed decreased infiltration and vital signs were stabilized. However, he developed sudden onset of tachypnea and hypotension on the next day. Arterial blood gas analysis results while breathing ambient air were pH 7.21, PaO2 50.0 mmHg, PaCO2 71.4 mmHg and oxygen saturation 76%. A follow-up chest CT revealed a large thrombus in the right main pulmonary artery and marked dilatation of the pulmonary arteries [Figure 1]. In addition, atrophy of pectoralis and paravertebral muscles was detected [Figure 2]. Transthoracic echocardiogram (TTE) showed pulmonary arterial hypertension (right ventricular systolic pressure 60 mmHg) with D-shaped left ventricle. However, echocardiographic evidence of a thrombus was not identified.
Figure 1.
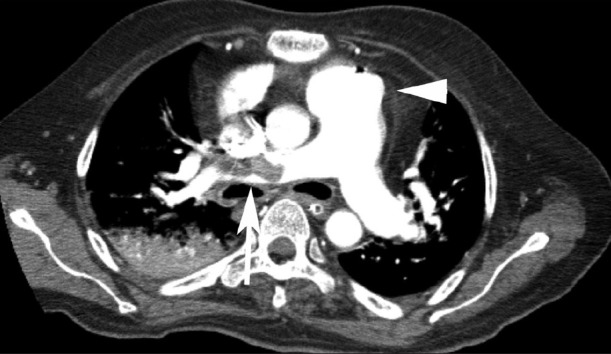
Chest computed tomography showed a large thrombus in the right main pulmonary artery (arrow) and dilatation of pulmonary arterial trunk (arrow head)
Figure 2.
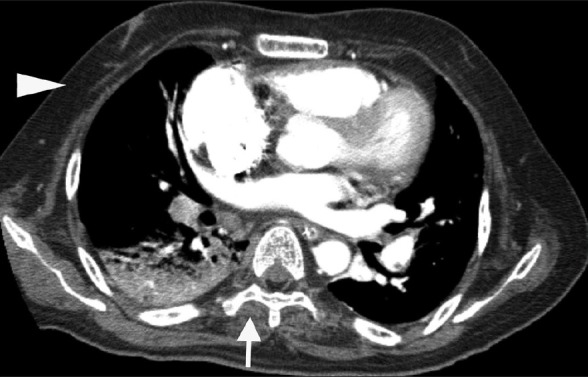
Chest computed tomography revealed atrophy of pectoralis (arrow head) and paravertebral (arrow) muscles, which were replaced by fat tissue
The patient received thrombolytic therapy followed by subcutaneous low-molecular weight heparin, and his symptoms improved with the treatment. Screening tests for hypercoagulable state including protein C, protein S and anti-thrombin III were normal. Lupus anticoagulant, anti-cardiolipin antibodies and factor V Leiden mutation tests were also negative.
During the admission, neurologic examinations performed by a consultant neurologist revealed mild ptosis in both eyes, frontal baldness, temporal wasting, elongated face, weakness of distal upper extremities and percussion myotonia. Electromyography showed typical myotonic discharges in proximal and distal extremities, consistent with myotonic dystrophy. The diagnosis of myotonic dystrophy type 1 was subsequently confirmed by an expansion of CTG repeat on chromosome 19. He was discharged to a rehabilitation facility, at which time he was functioning independently with nocturnal non-invasive ventilation.
Discussion
Acute respiratory failure may occur in patients with myotonic dystrophy undergoing anesthesia, and it may rarely be the revealing symptom undergoing surgery.[1] Recently, a case study of three patients found that ventilatory failure was the initial presenting feature of myotonic dystrophy without the provocative challenge of anesthesia or surgery.[2] Our patient is unusual in that he presented with acute respiratory distress associated with PTE unrelated to immobilization.
Some forms of muscular dystrophies are associated with a thromboembolism and risk of PTE.[3–5] The cause of thromboembolism in muscular dystrophies is not clear, but several factors may explain this predisposition to thrombosis. Patients with dystrophies are more sedentary, and sedentary lifestyle may lead to thromboembolism. Also, an elevation of serum fibrinogen degradation products (FDP) and plasma D-dimer was observed in Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD), and FDP levels significantly correlated with creatine kinase in these forms of dystrophies.[5] However, myotonic dystrophy with mild muscle degeneration did not show elevation of these parameters.[5] These findings suggest that enhanced coagulation and fibrinolysis may be secondary to muscle degeneration and related to severity of muscle fiber necrosis.
In a BMD patient with recurrent thrombosis, an upregulation of utrophin (dystrophin-related protein) was found on muscle cell membrane and in the intramuscular small vessels. Utrophin is present in various tissues, including endothelial cells, vascular smooth muscle and platelet membrane. The utrophin upregulation may have an unexpected influence on coagulation factors such as thrombomodulin, because the most characteristic finding in the skeletal muscle of this BMD patient was a lower expression of thrombomodulin on the muscle cell membrane compared with other forms of muscular dystrophies with less-frequent thrombotic attacks.[6,7] However, a direct cause-and-effect relationship has not been proven, and there is limited information about thromboembolic risk factors in myotonic dystrophy.
The initial chest CT of our patient showed dilated pulmonary artery, which might suggest chronic pulmonary hypertension, associated with recurrent PTE. Patients with chronic insidious myopathies are more sedentary, and lack of physical activity can contribute to the development of thrombosis. However, immobilization is not sufficient to explain the predisposition to thromboembolism because our patient did not have difficulty doing basic activities of daily living. Another hypothesis to explain the cause of thromboembolism is hypercoagulable state. Two episodes of deep vein thrombosis (DVT) of the lower extremities were reported in a patient with myotonic dystrophy. By that time, this patient was able to perform the normal activities of daily living, but work-up for DVT revealed double heterozygous type I protein C and factor V Leiden.[8] Additional study was presented of a patient with myotonic dystrophy who had respiratory difficulty due to heart failure and multiple PTE. The patient was healthy before this event, denied family history of thromboembolism and had a negative hypercoagulable work-up.[9] In our case, the facts that the patient had asymptomatic recurrent thromboembolic events and family history of cerebrovascular attacks support the possibility of predisposition to hypercoagulable state despite a negative standard hypercoagulable work-up. We believe that unknown factors play an important role in the development of hypercoagulability in myotonic dystrophy type 1 patients without impaired mobilization, and further investigation is required to evaluate thrombotic risk factors in these patients.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Mathieu J, Allard P, Gobeil G, Girard M, De Braekeleer M, Begin P. Anesthetic and surgical complications in 219 cases of myotonic dystrophy. Neurology. 1997;49:1646–50. doi: 10.1212/wnl.49.6.1646. [DOI] [PubMed] [Google Scholar]
- 2.Souayah N, Tick Chong PS, Dreyer M, Cros D, Schmahmann JD. Myotonic dystrophy type 1 presenting with ventilatory failure. J Clin Neuromuscul Dis. 2007;9:252–5. doi: 10.1097/CND.0b013e3181520095. [DOI] [PubMed] [Google Scholar]
- 3.Hanajima R, Kawai M. Incidence of cerebral infarction in Duchenne muscular dystrophy. Muscle Nerve. 1996;19:928. [PubMed] [Google Scholar]
- 4.Riggs T. Cardiomyopathy and pulmonary emboli in terminal Duchennne's muscular dystrophy. Am Heart J. 1990;119:690–3. doi: 10.1016/s0002-8703(05)80302-3. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Takenaka M, Miyai I, Yamamoto Y, Matsumura T, Nozaki S, et al. Coagulation and fibrinolysis disorder in muscular dystrophy. Muscle Nerve. 2001;24:399–402. doi: 10.1002/1097-4598(200103)24:3<399::aid-mus1012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi I, Niiyama T, Uchida Y, Inose M, Nakagawa M, Arimura K, et al. Multiple episodes of thrombosis in a patient with Becker muscular dystrophy with marked expression of utrophin on the muscle cell membrane. Acta Neuropathol. 1999;98:313–6. doi: 10.1007/s004010051086. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Komiya T, Kawai M. [Hypercoagulable state in Duchenne muscular dystrophy] Rinsho Shinkeigaku. 1997;37:374–8. [PubMed] [Google Scholar]
- 8.Cobos E, Phy M, Keung YK. Thrombotic risk of muscular dystrophy: Protein C deficiency, factor V Leiden, and myotonic dystrophy. Clin Appl Thromb Hemost. 1999;5:185–6. doi: 10.1177/107602969900500308. [DOI] [PubMed] [Google Scholar]
- 9.McDonnell M, Alcantar J, Wachsner RY, Meymandi SK. Cardiomyopathy and multiple pulmonary emboli in a patient with myotonic dystrophy. Congest Heart Fail. 2008;14:106–10. doi: 10.1111/j.1751-7133.2008.07562.x. [DOI] [PubMed] [Google Scholar]


