Abstract
Background
Bacterial vaginosis is uncommon in women who are virgins. We estimated effects of sexual debut on vaginal bacterial colonization.
Methods
Women who were virgins and aged 18–22 enrolled in a study of human papillomavirus acquisition were followed every 4 months for up to 2 years. Vaginal swabs from before and after sexual debut, or two independent visits for those remaining virginal were tested by quantitative polymerase chain reaction for Lactobacillus crispatus, L. jensenii, L. iners, Gardnerella vaginalis, and the bacterial vaginosis-associated species Atopobium vaginae, Megasphaera spp., Leptotrichia spp, Sneathia, spp BVAB1, BVAB2, and BVAB3.
Results
We evaluated 97 women: 71 who became sexually active and 26 who remained virginal. At first sampling, 22/26 (85%) of women who remained virginal were colonized with Lactobacillus species compared to 22/26 (85%) at follow-up (p > 0.99). G. vaginalis was present in 12/26 (46%) initially, and 11/26 (42%) at follow-up (p > 0.99). Among women who became sexually active, colonization with Lactobacillus species remained stable: 65/71 (92%) vs. 66/71 (93%) (p > 0.99), while colonization with G. vaginalis increased [28/71 (39%) vs 40/71 (56%); p = 0.02]. Among women who did not initiate sexual activity during the study, 2/26 (8%) had any bacterial vaginosis-associated species detected at both the first and second visits(p > 0.99). Among women who became sexually active during the study 15/71 (21%) were colonized with bacterial vaginosis-associated species initially, compared to 13/71 (18%) after sexual debut (p = 0.77).
Conclusions
Among women who were virgins, vaginal colonization with bacterial vaginosis-associated bacterial species is uncommon and does not change after sexual debut.
Background
Bacterial vaginosis (BV) is a syndrome characterized by a shift in the bacterial composition of the vaginal microbiota from a Lactobacillus predominant ecosystem to a mixed, diverse, primarily anaerobic community.(1) BV is most prevalent in women of reproductive age(2) and has clearly been linked to sexual activity,(3) though the mechanism for this link is unclear. Several possibilities have been proposed including transmission of a specific bacterial pathogen(4), transmission of a phage that attacks the protective lactobacilli(5) or use of vaginal lubricants that disrupt the normal ecosystem.(6) Initial studies suggested that women who had not had penile-vaginal intercourse were unlikely to have BV(7, 8); however more recent studies have shown that non-penetrative sexual contact – such as oral sex, use of sex toys, or mutual masturbation (9) – can increase the risk of altered vaginal microbiota.
BV has a high rate of recurrence after treatment (10) and is linked to adverse reproductive health outcomes, such as preterm delivery, pelvic inflammatory disease and acquisition of sexually transmitted infections(11); thus prevention is important. Understanding how sexual behavior may transmit or facilitate development of BV may be an important part of developing a prevention strategy. A cross-sectional study of young Australian women showed that many of the BV-associated bacterial species, such as Megasphaera species, Leptotrichia and the Clostridia-like Bacterial Vaginosis Associated Bacterium (BVAB) 1, 2 and 3, are less prevalent in sexually naïve women.(12) Little is known about the normal vaginal microbiota in adolescents and sexually inexperienced women, and whether initiation of sexual activity induces a change from a healthy Lactobacillus-dominant microbiota toward one that might increase the risk of developing BV. We undertook this study to examine whether initiation of sexual intercourse in a virginal population was associated with shifts in the vaginal microbiota that might indicate how sex predisposes women to develop BV.
Methods
Study cohort and design
This analysis used samples and data collected from 18–22 year-old women who were virgins enrolled in a longitudinal study of HPV acquisition in Seattle, Washington, between December 2000 and March 2007.(13) Both the parent study and this analysis were approved by the University of Washington Institutional Review Board, and all participants provided written informed consent. Participants were eligible for the parent study if they reported never having penile-vaginal intercourse, or a first episode of penile-vaginal intercourse in the last 3 months. All participants were college students at the University of Washington in Seattle. Only participants who had never had penile-vaginal intercourse at enrollment (167 of 250 enrolled participants) were eligible for inclusion in this analysis. Two visits were selected: one visit before and one after sexual debut (if the latter occurred during study follow-up), or two visits within two years of each other if the participant remained a virgin.
Study participants were seen every 4 months and completed a questionnaire about general health, contraception, vaginal medication use in the 7 days prior to the visit, sexual activity and genital symptoms. Participants completed on-line diaries about sexual activity and condom use in the month prior to each study visit. A pelvic examination was performed by a clinician at each visit, where the appearance of the external genitalia, vagina and cervix was examined. Testing for human papillomavirus (HPV) was performed at all visits as previously described.(13) BV was evaluated using Amsel’s clinical criteria at all visits. (14) Any ulcerative lesions were cultured for herpes virus, and gonorrhea and Chlamydia were tested for at each visit by culture of cervical swabs.
Laboratory assays
Self-collected vaginal swabs were placed in 1 mL of specimen transport medium (STM) and stored at −80 degrees Celsius until DNA extraction was performed, using the Qiagen DNA Blood mini kit (Qiagen Inc., Valencia CA). Previous studies have established that self-collected swabs provide similar results to provider-collected swabs, both in detection of bacterial presence and bacterial quantity.(15) Extracted DNA was tested in a quantitative PCR assay using primers targeting the human 18S rRNA gene to validate that successful DNA extraction occurred. An internal amplification control PCR using exogenous DNA from a jellyfish gene was used to test for presence of PCR inhibitors (16). Samples were then subjected to taxon-directed 16S rRNA gene quantitative PCR (qPCR) assays for the detection and quantification of Lactobacillus crispatus, L. jensenii, L. iners, Gardnerella vaginalis, Atopobium vaginae, Leptotrichia/Sneathia spp combined assay), Megasphaera spp., BVAB1, BVAB2 and BVAB3, which have been described elsewhere (17, 18). The assays use a TaqMan format, and are run on an ABI 7500 Thermocycler (Applied Biosystems, Foster City, CA) or Eppendorf Mastercycler ep Realplex thermal cycler (Eppendorf, Westbury, NY). Negative reactions were assigned a value at the lower limit of detection for the assay.
Statistical analysis
Demographic characteristics were compared between women who remained virginal and those who became sexually active using Student’s t-test, chi-square test or Mann-Whitney. Differences in prevalence of individual species at the first and second visits were assessed using McNemar’s test for paired data. Log-transformed quantities of bacteria were compared using t-test or paired t-test. For analyses of acquisition of BV-associated bacterial colonization at the follow-up visit, women colonized with any of these species at baseline were excluded. BV-associated species were defined as Atopobium vaginae, Leptotrichia/Sneathia spp, Megasphaera spp., BVAB1, BVAB2 and BVAB3. Gardnerella vaginalis was considered a commensal organism, as it can be found in a significant proportion of women both with and without BV. (19) Fisher’s exact test was used to assess the association between initiation of penile-vaginal sexual intercourse and the presence of individual bacterial species at the follow-up visit. Other potential confounding factors were also evaluated for association with colonization, including race, use of hormonal contraception and menstrual phase using Fisher’s exact test. Menstrual phase was defined as proliferative if a visit was within 14 days of the last menstrual period, luteal if the visit was more than 14 days but less than 35 days since the last menstrual period, and oligomenorrheic if the last menstrual period was more than 35 days prior to the visit. Based on our available sample size for this analysis of 73 (after exclusion of 17 women with baseline colonization by BV-associated species), we would have 80% power to detect a two-fold difference in the proportion of women colonized by BV-associated species, with an alpha of 0.05.
Results
The parent study enrolled 167 virginal participants, of whom 108 (65%) became sexually active with male partners and 59 (35%) did not. Of these enrolled virgins, 97 had available samples from at least two time points, including 71 women who became sexually active during the study and 26 who remained virgins. There were no significant differences in demographic characteristics at enrollment between these two groups of women (Tables 1 and 2): the majority were white and were not using any hormonal birth control methods at enrollment. Two women reported vaginal medication use in the 7 days prior to their study visit, both used vaginal anti-fungal creams for treatment of yeast. Between the initial and subsequent visit chosen for this sub-analysis, women who initiated sexual intercourse became more likely to use birth control (primarily condoms and hormonal methods) (p < 0.01) and 88% reported only one new sexual partner (Table 2). Two women who reported remaining virginal also reported condom use, but did not report any penile-vaginal sexual contact suggesting that condoms were used during non-penile vaginal sexual activity. Among women who became sexually active, the mean number of penile-vaginal sex acts in the 4 weeks prior to the follow-up visit was 5.5 ± 7.1 (range 0–31). Only 46 women reported using condoms, at a mean of 3.4 ± 4.2 sex acts during that time, or a mean of 54% of sex acts. No participants were diagnosed with a sexually transmitted infection other than HPV, and no participants developed BV. There was a significant increase in the number of women testing positive for HPV in the group that became sexually active. (p = 0.03)
Table 1.
Comparison of Demographic and Clinical Characteristics at Initial Visit Between Women Who Remained Virginal Throughout the Study and Those Who Became Sexually Active
| Participants Who Remained Virginal (n = 26) |
Participants Who Became Sexually Active (n = 71) |
P | |
|---|---|---|---|
| Age (mean ± SD) | 20 ± 1.2 (range 18–23) |
19.5 ± 1.1 (range 18–22) |
.27 |
| Ethnicity | .37 | ||
| White | 16 (62%) | 46 (64%) | |
| Black | 1 (4%) | 0 (0%) | |
| Asian | 6 (23%) | 14 (20%) | |
| Hispanic | 0 (0%) | 3 (4%) | |
| Other | 2 (8%) | 8 (11%) | |
| Age at menarche | 12.5 ± 1.4 (range 10–16) |
12.5 ± 1.2 (range 10–17) |
.83 |
| Smoker | 3 (12%) | 11 (15%) | .69 |
| Ever used tampons | 21 (81%) | 66 (92%) | .28 |
| Ever douched | 0 | 0 | n/a |
Table 2.
Comparison of Demographic and Clinical Characteristics at the Initial and Follow-up Visits Between Women Who Remained Virginal Throughout the Study and Those Who Became Sexually Active
| Initial Visit | Follow-up Visit |
Initial Visit |
Follow-up Visit |
P | |
|---|---|---|---|---|---|
| Months between visits (median, IQR) | 12 (8, 12) (Range 4–20) |
8 (4, 12) (Range 3–36) |
0.39* | ||
| Vaginal medication use in last week | 0 | 0 | 2 (3%) | 0 | - |
| STD diagnosis† | 0 | 0 | 0 | 0 | - |
| Birth control method | < 0.01‡ | ||||
| None | 25 (96%) | 22 (85%) | 70 (97%) | 6 (8%) | |
| Condoms | 0 | 2 (8%) | 0 | 25 (35%) | |
| Withdrawal | 0 | 0 | 0 | 2 (3%) | |
| Hormonal | 0 | 0 | 2 (3%) | 38 (53%) | |
| Other | 1(4%) | 1(4%) | 0 | 1(1%) | |
| Number of new partners | < 0.01‡ | ||||
| 0 | n/a | 26 | n/a | 0 | |
| 1 | 0 | 63 (88%) | |||
| 2 | 0 | 6 (8%) | |||
| 3 | 0 | 3 (4%) | |||
| Days since last vaginal intercourse (median, IQR) | n/a | n/a | n/a | 5 (2, 16) (range 1–43) |
- |
| Vaginal | 0.08‡ | ||||
| Less | 18 (69%) | 13 (50%) | 36 (50%) | 35 (49%) | |
| Normal | 5 (19%) | 8 (31%) | 32 (44%) | 30 (41%) | |
| More | 1 (4%) | 2 (8%) | 3 (4%) | 6 (8%) | |
| pH (mean + SD) | 4.2 ± 0.3 | 4.1 ± 0.3 | 4.1 ± 0.2 | 4.3 ± 1 | |
| Any HPV | 1 (4%) | 0 | 3 (4%) | 11 (15%) | 0.03‡ |
Data are n(%) unless otherwise specified.
IQR, interquartile range, STD, sexually transmitted disease; SD, standard deviation; HPV, human papillomavirus.
P value for Mann-Whitney test comparing medians between women who remained virginal and those who became sexually active.
This included testing for gonorrhea, Chlamydia, and herpes simplex virus.
P value for chi-square test comparing values at follow-up between women who remained virginal and those who became sexually active.
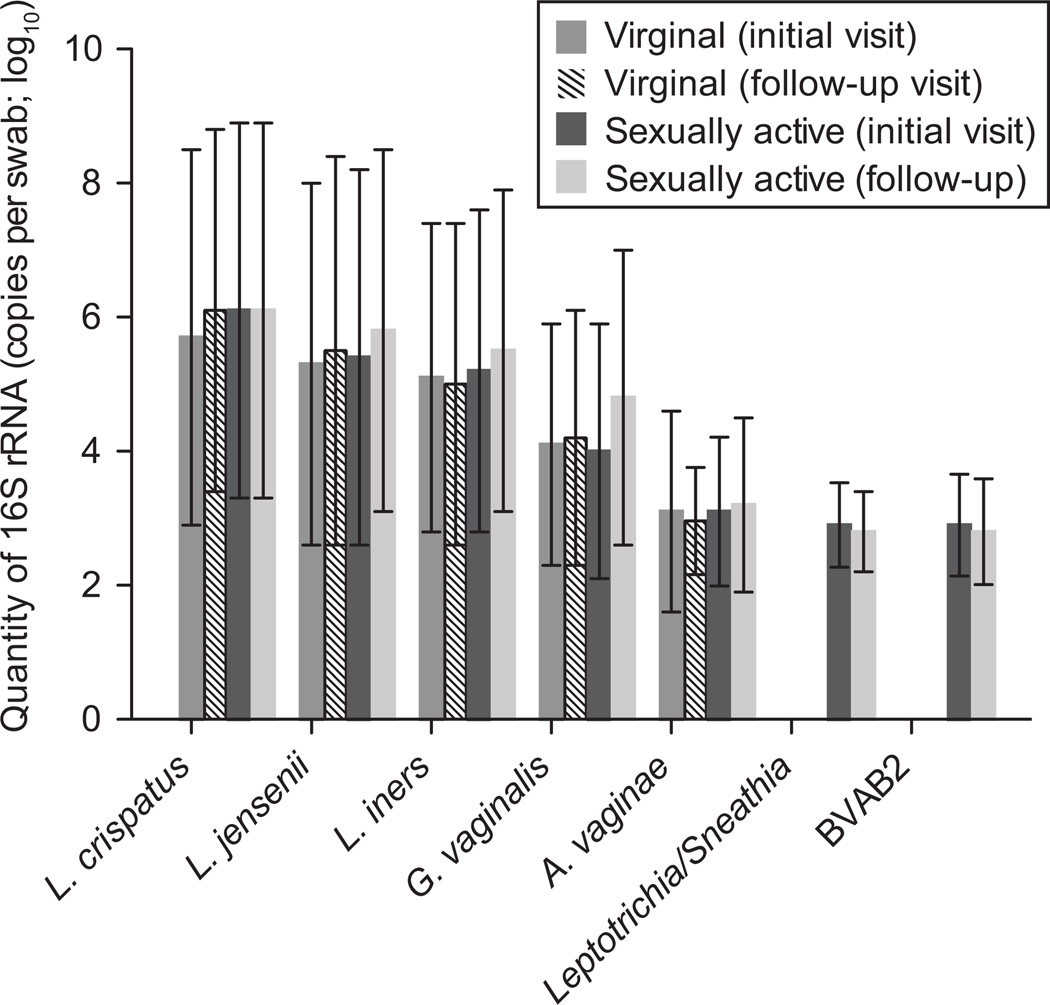
Very few participants had any of the BV-associated species (Atopobium, Leptotrichia, Sneathia, Megasphaera, BVAB1, BVAB2 or BVAB3) detected, either at the initial or follow-up visit (Table 3). Over half had at least one Lactobacillus species detected at both visits, and approximately 40% in each group had G. vaginalis at the initial visit. While prevalence of these species did not change in the women who remained virginal, there was a significant increase in prevalence of G. vaginalis in the group that became sexually active, from 28/71 (39%) at the initial visit to 40/71 (56%) at follow up (p = 0.02).(Table 3) There were no significant differences in quantity of bacteria between women who remained virginal and those who became sexually active, or between the initial visit and follow-up visit in either group (Figure 1).
Table 3.
Presence of Commensal Lactobacillus species, G. vaginalis, and Bacterial Vaginosis-Associated Species at Initial and Follow-up Visits in Women Who Remained Virginal Throughout the Study and Those Who Became Sexually Active
| Participants Who Remained Virginal (n = 26) |
Participants Who Became Sexually Active (n = 71) |
|||||
|---|---|---|---|---|---|---|
| Detection | Initial Visit | Follow-up Visit |
P* | Initial Visit | Follow-up Visit |
P* |
| Lactobacillus crispatus | 14 (54%)† | 15 (58%)† | > 0.99 | 44 (62%)† | 45 (63%)† | 1.00 |
| Lactobacillus jensenii | 13 (50%) | 14 (54%) | > 0.99 | 37 (52%) | 44 (62%) | 0.12 |
| Lactobacillus iners | 15 (63%) | 14 (58%) | > 0.99 | 39 (55%) | 43 (61%) | 0.45 |
| Gardnerella vaginalis | 12 (46%) | 11 (42%) | > 0.99 | 28 (39%) | 40 (56%) | 0.02 |
| Atopobium vaginae | 2 (8%) | 2 (8%) | > 0.99 | 8 (11%) | 11 (15%) | 0.45 |
| Leptotrichia/Sneathia | 0† | 0† | - | 6 (8%)‡ | 2 (3%)‡ | 0.22 |
| Megasphaera spp. | 0 | 0 | - | 0 | 1 (1) | - |
| BVAB1 | 0 | 0 | - | 0 | 1 (1) | - |
| BVAB2 | 0 | 0 | - | 4 (6%)‡ | 2 (3%)‡ | 0.5 |
| BVAB3 | 0† | 0† | - | 0† | 0§ | - |
| Any Lactobacillus spp present | 22 (85%) | 22 (85%) | > 0.99 | 65 (92%) | 66 (93%) | > 0.99 |
| Any bacterial vaginosis-associated spp. present | 2 (8%) | 2 (8%) | > 0.99 | 15 (21%) | 13 (18%) | 0.77 |
Data are n(%) unless otherwise specified.
P for McNemar test comparing prevalence of bacterium at initial and follow-up visits.
Missing data for one participant.
Missing data for three participants.
Missing data for two participants.
Figure 1.
Quantity of bacteria measured by quantitative polymerase chain reaction (PCR) at initial and follow-up visits in women who remained virginal throughout the study and those who became sexually active. No statistically significant differences were found.
The majority of participants had similar patterns of colonization at both visits, no matter whether they remained virginal or became sexually active. Of participants who remained virginal throughout the study, 22/26 (85%) had at least one Lactobacillus species detected at both visits, and both of the 2 women colonized with Atopobium vaginae at enrollment were also colonized at follow-up. Of the 12 women colonized with G. vaginalis at enrollment, 10 (83%) remained colonized at follow-up. Among women who became sexually active, 62 (87%) were colonized with at least one Lactobacillus species at both visits. If analysis is restricted to just L. crispatus and L. jensenii (both common H2O2-producers) 53 (75%) had persistent colonization. Colonization with BV-associated species was more dynamic in this group: of the 6 women colonized with either Leptotrichia or Sneathia at enrollment, only 1 maintained that colonization at follow-up, of the 4 colonized with BVAB2 only 2 were positive at follow-up, and while 2/8 women colonized with Atopobium vaginae lost colonization at follow-up, 5 additional women gained colonization. Presence of BV-associated species at the initial or follow-up visits was not associated with presence of HPV at follow-up (p = 0.18 and p = 0.84, respectively).
After excluding women colonized with BV-associated bacteria at baseline, only 5 women gained colonization with these species at follow-up. The likelihood of colonization by any BV-associated species at the follow-up visit was not significantly associated with race, use of hormonal contraception, phase of the menstrual cycle, or initiation of sexual intercourse. (Table 4) However, of the 80 women with no BV-associated bacteria detected at baseline, the 5 who were colonized at follow-up were all women who had become sexually active.(Table 5) Among the 53 women without baseline colonization who completed online diary information, the 5 who gained colonization with BV-associated bacteria had a mean of 2 ± 3.5 penile-vaginal sex acts in the 4 weeks prior to their clinic visit, compared to 5.3 + 7 in 48 women who did not gain colonization with any of these species. Only 34 women reported condom use, 2 who gained colonization with BV-associated species reported using condoms in a mean of 63% of acts, while the remaining 32 reported use in 55% of acts.
Table 4.
Associations With Colonization by Bacterial Vaginosis-Associated Bacterial Species at Follow-up, Among Women Without Colonization at Baseline
| n | Bacterial Vaginosis - Associated Bacteria Absent at Follow-up (n = 67) |
Bacterial Vaginosis - Associated Bacteria Present at Follow-up (n = 5) |
P* | |
|---|---|---|---|---|
| Ethnicity | > 0.99 | |||
| White | 47 | 44 (66%) | 3 (60%) | |
| Nonwhite | 26 | 23 (34%) | 2 (40%) | |
| Hormonal contraception | 0.66 | |||
| Yes | 34 | 31 (46%) | 3 (60%) | |
| No | 39 | 36 (54%) | 2 (40%) | |
| Menstrual phase | 0.86 | |||
| Proliferative | 23 | 20 (30%) | 2 (40%) | |
| Luteal | 25 | 23 (34%) | 2 (40%) | |
| Oligomenorrheic | 24 | 23 (34%) | 1 (20%) | |
| Initiated sexual intercourse | 0.31 | |||
| Yes | 52 | 47 (70%) | 5 (100%) | |
| No | 21 | 20 (30%) | 0 |
Data are n(%) unless otherwise specified.
Fisher exact test
Table 5.
Association Between Detection of Bacterial Vaginosis-Associated Bacteria at Follow-up, Stratified by Presence of Bacterial Vaginosis-Associated Bacteria at Baseline
| No Bacterial Vaginosis - Associated Bacteria Present at Baseline (n = 78) |
Bacterial Vaginosis -Associated Bacteria Present at Baseline (n = 17) |
|||||
|---|---|---|---|---|---|---|
| Remained Virginal |
Initiated Sex |
P* | Remained Virginal |
Initiated Sex |
P* | |
| Bacterial vaginosis-associated bacteria present at follow-up | 0 | 5 | 0.31 | 2 | 8 | 0.49 |
| No bacterial vaginosis-associated bacteria at follow-up | 22 | 51 | 0 | 7 | ||
Data are n unless otherwise specified.
Fisher exact test
Discussion
Our study offers a unique perspective on the effects of sexual intercourse on the vaginal microbiota by comparing samples from before and after initiation of sexual intercourse. In this cohort of sexually inexperienced women, we found that both BV and vaginal colonization with BV-associated bacterial species was rare, and that the vaginal microbiota of women who were virgins appeared to be stable over the follow-up interval. Women who became sexually active during the study were more likely to gain colonization with G. vaginalisbut there was no loss of colonization with Lactobacillus species after initiation of sexual intercourse, nor increase in colonization with the fastidious BV-associated species.
While BV is associated with sexual activity,(2) the mechanism by which women lose vaginal Lactobacillus colonization and gain the polymicrobial community associated with BV is unclear. Eschenbach et al studied the vaginal microbiota in sexually experienced women within 8–12 hours after one episode of intercourse with or without a condom, and saw an increase in culture-based detection of E. coli(20) but did not evaluate the fastidious BV-associated organisms described here. Fethers et al used similar qPCR techniques to evaluate colonization in a cross-sectional study that combined sexually naïve and sexually experienced women.(12) They found that G. vaginalis, Sneathia and BVAB3 were associated with more frequent penile-vaginal sex, as well as with unprotected sex. Women with no sexual experience, or who always used condoms were much less likely to be colonized with the BV-associated species that were tested for in their study. Of note, their analysis did not assess changes in the microbiota over time. Our study confirms that vaginal colonization with BV-associated bacterial species is rare in sexually inexperienced women, and does not significantly increase with initiation of penile-vaginal sexual activity. Although we did see an increase in the proportion of women colonized with G. vaginalis among those who became sexually active, this microbe is widely prevalent in women with and without BV(21), and for the purposes of this analysis is considered a commensal organism.
Our group has previously shown that the vaginal microbiota in sexually experienced women can be quite dynamic when sampled daily, due in part to the influence of menses.(18) Other groups have shown significant variation in vaginal microbiota over time as well.(22, 23) Interestingly, in our data, women who remained virginal had very stable microbiota, while those who became sexually active began to show some variability in bacterial composition – though not to the extent reported in older women. This suggests that sexual activity, as well as menses, may play a role in disrupting or changing the vaginal microbial environment, though via what mechanism is not clear.
In a previous analysis from a similar cohort that excluded girls who remained virginal, presence of HPV was associated with diagnosis of BV, and more often preceded diagnosis of BV than the opposite (BV preceding HPV).(24) In that analysis the median time to first detection of HPV was 4 months, while median time to first BV diagnosis was 12 months. Some have hypothesized that HPV infection, or the immune response, may predispose women to develop BV. In this analysis we had too few women with HPV at baseline to draw any firm conclusions, but did not find any association between HPV and detection of BV-associated bacteria.
Our study is limited in that we did not collect information on non-penetrative sexual contact; therefore, we were unable to evaluate any associations with these species and non-penetrative types of sexual activity, which have clearly been associated with an increased risk of BV. As our participants who became sexually active during the study had higher prevalence of BV-associated bacteria at the first visit, we suspect that these women were already participating in non-penetrative behaviors. This is consistent with a previous analysis of this cohort showing that women who initiated vaginal intercourse during the study had higher incidence of HPV infection prior to sexual debut, suggesting a non-penile exposure.(25) Self-report of sexual activity could be incorrect, as some women may be uncomfortable disclosing sexual information to researchers. In addition, our sample size is small, due to the unique nature of the cohort, and thus affords limited power to detect any differences. Our participants are also a low-risk population for development of BV: primarily white, well-educated, non-smokers with few sexual partners. However, this allowed us to come closer to isolating the effect of sex alone on the vaginal microbiota. We tested for 11 individual bacterial species, both healthy commensals and well-described BV-associated species, but this is not a comprehensive assessment of all potential species that may colonize the vagina.
Sexual activity is clearly linked to the development of BV, but likely via a more complex mechanism than some other sexually transmitted infections. Some have hypothesized that the change in vaginal pH due to semen is what drives the shift in microbiota that results in BV. We did not have adequate numbers of women with reported information on condom use to draw any conclusions about the effect of semen, independent of sexual activity. Interestingly, the women who did gain colonization with BV-associated bacteria reported fewer penile-vaginal sex acts, and higher percentage of condom use with those acts.
Young, sexually inexperienced women are unlikely to have vaginal colonization with BV-associated bacterial species, and the composition of the vaginal microbiota does not change rapidly after initiation of sexual activity. This suggests that the association between sex and the development of BV is complex.
Acknowledgments
Supported in part by pilot funding from the University of Washington Institute for Translational Health Sciences, and by R01AI038383. Dr. Mitchell is supported by a K08 award (NIAID, 1K08AI087969 – 01)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. The New England journal of medicine. 2005 Nov 3;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 2.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004 associations with symptoms, sexual behaviors, and reproductive health. Sexually transmitted diseases. 2007 Nov;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 3.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008 Dec 1;47(11):1426–1435. doi: 10.1086/592974. [DOI] [PubMed] [Google Scholar]
- 4.Schwebke JR. Bacterial vaginosis: are we coming full circle? The Journal of infectious diseases. 2009 Dec 1;200(11):1633–1635. doi: 10.1086/648093. [DOI] [PubMed] [Google Scholar]
- 5.Pavlova SI, Kilic AO, Mou SM, Tao L. Phage infection in vaginal lactobacilli: an in vitro study. Infectious diseases in obstetrics and gynecology. 1997;5(1):36–44. doi: 10.1155/S1064744997000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Thomas KK, Agnew K, Ringwood K. Prevalence and risks for bacterial vaginosis in women who have sex with women. Sexually transmitted diseases. 2010 May;37(5):335–339. [PMC free article] [PubMed] [Google Scholar]
- 7.Bump RC, Buesching WJ., 3rd Bacterial vaginosis in virginal and sexually active adolescent females: evidence against exclusive sexual transmission. American journal of obstetrics and gynecology. 1988 Apr;158(4):935–939. doi: 10.1016/0002-9378(88)90097-x. [DOI] [PubMed] [Google Scholar]
- 8.Yen S, Shafer MA, Moncada J, Campbell CJ, Flinn SD, Boyer CB. Bacterial vaginosis in sexually experienced and non-sexually experienced young women entering the military. Obstetrics and gynecology. 2003 Nov;102(5 Pt 1):927–933. doi: 10.1016/s0029-7844(03)00858-5. [DOI] [PubMed] [Google Scholar]
- 9.Fethers KA, Fairley CK, Morton A, Hocking JS, Hopkins C, Kennedy LJ, et al. Early sexual experiences and risk factors for bacterial vaginosis. The Journal of infectious diseases. 2009 Dec 1;200(11):1662–1670. doi: 10.1086/648092. [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. The Journal of infectious diseases. 2006 Jun 1;193(11):1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 11.Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. The Journal of infectious diseases. 2010 Dec 15;202(12):1907–1915. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fethers K, Twin J, Fairley CK, Fowkes FJ, Garland SM, Fehler G, et al. Bacterial Vaginosis (BV) Candidate Bacteria: Associations with BV and Behavioural Practices in Sexually-Experienced and Inexperienced Women. PLoS One. 2012;7(2):e30633. doi: 10.1371/journal.pone.0030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winer RL, Hughes JP, Feng Q, O'Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. The New England journal of medicine. 2006 Jun 22;354(25):2645–2654. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 14.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. The American journal of medicine. 1983 Jan;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 15.Menard JP, Fenollar F, Raoult D, Boubli L, Bretelle F. Self-collected vaginal swabs for the quantitative real-time polymerase chain reaction assay of Atopobium vaginae and Gardnerella vaginalis and the diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis. 2012 Apr;31(4):513–518. doi: 10.1007/s10096-011-1341-8. [DOI] [PubMed] [Google Scholar]
- 16.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC infectious diseases. 2008;8:73. doi: 10.1186/1471-2334-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in Vaginal Bacterial Concentrations with Intravaginal Metronidazole Therapy for Bacterial Vaginosis as Assessed by Quantitative PCR. Journal of clinical microbiology. 2009 Jan 14; doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. Journal of clinical microbiology. 2007 Oct;45(10):3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eschenbach DA, Patton DL, Hooton TM, Meier AS, Stapleton A, Aura J, et al. Effects of vaginal intercourse with and without a condom on vaginal flora and vaginal epithelium. The Journal of infectious diseases. 2001 Mar 15;183(6):913–918. doi: 10.1086/319251. [DOI] [PubMed] [Google Scholar]
- 21.Marrazzo JM, Thomas KK, Fiedler TL, Ringwood K, Fredricks DN. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Annals of internal medicine. 2008 Jul 1;149(1):20–28. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwebke JR, Richey CM, Weiss HL. Correlation of behaviors with microbiological changes in vaginal flora. The Journal of infectious diseases. 1999 Nov;180(5):1632–1636. doi: 10.1086/315065. [DOI] [PubMed] [Google Scholar]
- 23.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012 May 2;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao C, Hughes JP, Kiviat N, Kuypers J, Lee SK, Adam DE, et al. Clinical findings among young women with genital human papillomavirus infection. American journal of obstetrics and gynecology. 2003 Mar;188(3):677–684. doi: 10.1067/mob.2003.164. [DOI] [PubMed] [Google Scholar]
- 25.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. American journal of epidemiology. 2003 Feb 1;157(3):218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]