Abstract
Background
The impact of the addition of gemcitabine (G) to 5-FU chemoradiation (CRT) on 5-year overall survival (OS) in resected pancreatic adenocarcinoma are presented with updated results of a phase III trial.
Methods
Following resection of pancreatic adenocarcinoma patients were randomized to pre and post CRT 5-FU vs. pre and post CRT G. 5-FU = continuous (CI) at 250 mg/m2/day. G = 1000 mg/m2 weekly; both given over 3 weeks pre and 12 weeks post - CRT. CRT = 50.4 Gy with CI 5-FU. Primary endpoint was survival for all patients and for pancreatic head tumor patients.
Results
Four hundred and fifty-one patients were eligible. Univariate analysis showed no difference in OS. Pancreatic head tumor patients (n=388) had a median survival and 5-year OS of 20.5 months and 22% with G vs. 17.1 months and 18% with 5-FU. On multivariate analysis, patients on the G arm with pancreatic head tumors experienced a trend towards improved OS (p=0.08). First site of relapse local recurrence in 28% of patients vs. distant relapse in 73%.
Conclusion(s)
The sequencing of 5-FU CRT with G as done in this trial is not associated with a statistically significant improvement in OS. Despite local recurrence being approximately half of that reported in previous adjuvant trials, distant disease relapse still occurs in ≥ 70% of patients. These findings serve as the basis for the recently activated EORTC/US Intergroup RTOG 0848 phase III adjuvant trial evaluating the impact of CRT after completion of a full course of G.
Keywords: Pancreas, Adenocarcinoma, Adjuvant Chemotherapy and Adjuvant Radiation Therapy
Background
The potential impact of gemcitabine (G) in the adjuvant setting following resection of pancreatic adenocarcinoma has only recently been tested and reported in phase III trials. (1-4) Oettle et al had reported initially an increase in 3-year progression-free survival (PFS) with the use of adjuvant G without radiation therapy (RT)(1). This subsequently translated into an increase in overall survival (OS) at 5 years with further follow-up (2).
Reported patterns of failure analysis have demonstrated a 50 – 85 % component of local tumor relapse associated with liver and/or systemic tumor relapse following resection of pancreatic adenocarcinoma (5-9). This rationale for inclusion of use of RT in the postoperative adjuvant setting has been substantiated to a limited extent by phase III trials and by large institutional results (10-13). In 2008, we reported the initial results of the US Intergroup phase III adjuvant trial evaluating the impact of the addition of G to fluorouracil-based chemoradiation (CRT) following the resection of pancreatic adenocarcinoma (4).
At 3 years, the addition of G to fluorouracil-based CRT improved the median and 3-year OS in patients with resected pancreatic head adenocaracimona at p=0.05 on multivariate analysis. At the time of initial analysis, there was uncertainty whether this survival advantage would last over time. Therefore we report the long term results of this trial.
METHODS
Eligibility
This Intergroup trial was conducted by the following U.S. National Cancer Institute (NCI) sponsored cooperative groups: the Radiation Therapy Oncology Group (RTOG), the Eastern Corporative Oncology Group, and the Southwest Oncology Group, inclusive of Canadian affiliates. RTOG served as the lead group with the trial designation RTOG 97-04. The eligibility criteria included histologically confirmed adenocarcinoma of the pancreas and gross total tumor resection, confirmed by central review of patients operative and pathology reports. In addition, a postoperative computed tomographic (CT) imaging was required within 3 weeks of randomization to exclude patients who had evidence of persistent/recurrent local disease or metastatic disease prior to therapy. Surgical resection margins were categorized as negative, microscopically positive, or unknown (defined as those having no comment regarding margins in the pathology report). Eligibility requirements also included stages T 1-4, N 0-1, and M 0 according to the 1997 staging criteria of the American Joint Commission on Cancer (14). If there were no identifiable lymph nodes within the resection specimen, the patient was ineligible.
Patients were required to have a Karnofsky performance status of ≥ 60 and adequate hematologic, renal, and hepatic function as defined by the following: a white blood cell count of ≥ 3,000/mm3, a platelet count ≥ 100,000/mm3, serum bilirubin and creatinine ≤ 1.5 X the upper limit of normal, a serum aspartate aminotransferase concentration ≤ 5 X the upper limit of normal, and a documented caloric intake of > 1500 kcal per day. Submission of a CA 19-9 serum tumor marker was required for central testing and review. Protocol therapy was required to begin 3-8 weeks after resection and within 5 days of randomization.
All patients required written and informed consent according to institutional and federal guidelines.
Treatment Plan
After undergoing tumor resection, patients were randomly assigned to either 5-FU (arm 1) or G (arm 2) pre and post CRT. Randomization was performed 3-8 weeks after surgery by a dynamic balancing procedure that included stratification according to tumor diameter (< 3 cm vs. ≥ 3 cm), nodal status (negative vs. positive) and surgical margins (negative vs. positive vs. unknown). Pre-CRT chemotherapy in arm 1 consisted of 5-FU, 250 mg/m2/day, continuous infusion for 3 weeks; in arm 2, G 1000 mg/m2 as a 30 minute infusion once weekly for 3 weeks. Between 1-2 weeks after completion of pre-CRT chemotherapy, CRT was initiated and was the same for both arms. CRT consisted of 50.4Gy with continuous infusion 5-FU, 250 mg/m2/day throughout RT. Post-CRT chemotherapy was initiated 3-5 weeks after completion of CRT; arm 1 consisted of 3 months of continuous infusion 5-FU [(4 weeks on + 2 weeks off) X 2] and in arm 2, 3 months of G [(3 weeks on + 1 week off) X 3]. Continuous infusion 5-FU was given 7 days each week. RT was delivered in 28 fractions, 5 days per week, to the tumor bed, and regional nodes. The tumor bed was defined by preoperative CT imaging.
Local pancreatic, celiac, mesenteric, paraortic, pancreatic, duodenal and hepatoportal lymph nodes were included in the RT fields (15). After an initial dose of 45Gy, the final 5.4Gy dose was limited to the tumor bed as defined by the preoperative tumor volume. At least 4-MV photons and a minimum 3-4 field approach were used. Doses were limited to < 60% of hepatic volume receiving > 30Gy.
At least two thirds of one functioning kidney was spared from RT fields and the spinal cord was limited to < 45Gy. Prospective QA of RT was required for all. This was inclusive of submission of preoperative abdominal CT scan and RT fields to be used for central review and approval prior to the start of CRT.
Follow-up of patients
First follow-up was required at 4 weeks after completion of CRT and prior to start of post-CRT chemotherapy. Follow-up continued to occur at 3-month intervals for 1 year, then at 6 month intervals for 2 years and then annually. Follow-up consisted of physical examination, complete blood count, liver function testing, chest x-ray, and CT scanning as clinically indicated. Elevation in CA-19-9 level in and of it itself was not to be considered a criterion for disease recurrence.
Statistical Considerations
Overall survival (OS) was the primary endpoint for this trial. Secondary endpoints were disease-free survival (DFS) and toxicity, which was scored with the U.S. NCI Common Toxicity Criteria version 2.0 for chemotherapy and the RTOG morbidity scoring scheme for RT. All endpoints were pre-specified in the original design of the trial and all analyses were conducted on an intent-to-treat basis.
Failure for OS was defined as death due to any cause and was measured from date of randomization to date of death or last follow-up for censored patients. Failure for DFS was defined as local, regional or distant relapse, appearance of a second primary or death due to any cause and measured from date of randomization to date of first failure or last follow-up for censored patients.
Local relapse was defined as recurrence at the primary resection site; regional relapse was defined as recurrence in regional lymph nodes associated with the primary resection site, and all other relapses were defined as distant. Patients who did not fail an endpoint were censored as of their last follow-up.
Patients were stratified by nodal status (uninvolved vs. involved), tumor diameter (< 3cm vs. ≥ 3cm) and surgical margin status (negative vs. positive vs. unknown). The permuted block randomization method was used with patient factors balanced according to the permuted block randomization method (16). At an original expected accrual of 5 patients/month, 330 patients were targeted to detect a 33% reduction in the hazard rate of overall survival for the CRT+ G arm as compared to the CRT+5-FU arm (increase in median survival from 18 to 27 months; hazard ratio (HR), 0.67) with 80% power and a 2-sided alpha of 0.05, assuming an exponential distribution. In early 2001, based on unexpectedly rapid accrual (13 patients/month), and after approval by the U.S. Intergroup, the RTOG data monitoring committee, and the NCI, the sample size was increased to find a smaller treatment effect with more power. Four-hundred and seventy analyzable patients would provide 85% power to detect a 28% decrease in the hazard rate of overall survival (increase in median survival from 18 to 25 months; HR, 0.71) for all patients and 80% power in patients with pancreatic head lesions.
All analyses were performed using SAS/STAT® software (17). Chi-squared tests were used to compare differences among pretreatment characteristics between treatment arms.
For CA19-9, the variable was categorized as < 180 vs. ≥ 180 to be consistent with a protocol specified CA19-9 analysis to be done subsequently and based on published literature (18). Z-tests were used to test for differences in binomial proportions of grade ≥ 2 late (defined as >90 days from the end of treatment) toxicities (worst overall, worst non-hematologic and worst hematologic).
OS and DFS were estimated univariately with the Kaplan-Meier method (19) and treatment arms were compared using the log-rank test (20). Multivariate analyses were performed with Cox proportional hazard models (21) to test for treatment differences (between arms) while adjusting for the stratification variables of nodal involvement (no vs. yes), tumor diameter (< 3 vs. ≥ 3 cm) and margin status (negative vs. positive and negative vs. unknown), as well as any other variables that were imbalanced between the treatment arms. A tumor diameter of 3 cm was used for stratification based on published large institutional experience (22). All tests were done at a significance level of 0.05. All variables are coded such that a HR > 1 indicates an increased risk for the second level of the variable and a HR < 1 a benefit for the second level of the variable.
FINDINGS
Demographic Characteristics
Between July 1998 and July 2002, 538 patients were registered from 164 RTOG/ECOG/SWOG institutions. Eighty-six patients (16%) were ineligible for reasons previously reported (4) and 1 patient withdrew consent. Of the remaining 451 patients, 230 were randomly assigned to CRT + 5-FU and 221 to CRT + G. Pretreatment factors (Table 1) were similar between groups with the exception of T stage.
Table 1.
Pretreatment Patient Characteristics
| CRT + 5-FU (n=230) | CRT + Gemcitabine (n=221) | ||||
|---|---|---|---|---|---|
| Age | |||||
| Median | 62 | 61 | |||
| Min-Max | 36-81 | 33-84 | |||
| n | % | n | % | Chi-square p-value | |
| Gender | |||||
| Male | 139 | 60 | 117 | 53 | 0.11 |
| Female | 91 | 40 | 104 | 47 | |
| Primary Tumor Location | 0.40 | ||||
| Head | 201 | 87 | 187 | 85 | |
| Non-Head | 29 | 13 | 34 | 15 | |
| Tumor Grade | 0.26 | ||||
| Well differentiated | 40 | 17 | 28 | 13 | |
| Moderately differentiated | 124 | 54 | 117 | 53 | |
| Poorly/Undifferentiated | 54 | 23 | 67 | 30 | |
| Unknown | 12 | 5 | 9 | 4 | |
| Largest tumor dimension of primary | 0.33 | ||||
| <3 cm | 100 | 43 | 86 | 39 | |
| ≥ 3cm | 130 | 57 | 135 | 61 | |
| N-stage (surgical) | 0.37 | ||||
| N0 | 82 | 36 | 70 | 32 | |
| N1 | 148 | 64 | 151 | 68 | |
| Surgical Margins | 0.49 | ||||
| Negative | 102 | 44 | 86 | 39 | |
| Positive | 75 | 33 | 77 | 35 | |
| Unknown* | 53 | 23 | 58 | 26 | |
| T-stage (surgical) | 0.013 | ||||
| T1/T2 | 68 | 30 | 43 | 19 | |
| T3/T4 | 162 | 70 | 178 | 81 | |
| CA19-9 Category† | 0.76 | ||||
| < 180 | 113 | 88 | 107 | 86 | |
| ≥ 180 | 16 | 12 | 17 | 14 | |
Not mentioned/commented on pathology report
Pretreatment CA19-9 values for Lewis Antigen positive patients (negative patients do not express CA19-9)
The CRT + G arm had a higher percentage of patients with T3/T4 disease (81%) as compared to those randomized to the CRT + 5-FU arm (70%, p=0.013). The majority (86%) of tumors were of the pancreatic head. Sixty-six percent of patients had nodal metastases. Thirty-four percent of patients had microscopically positive margins and 25% had pathology reports with no comment regarding the status of surgical margins (unknown).
Treatment Tolerance/Toxicity
Treatment delivery/tolerance and acute toxicity was acceptable and has been previously reported (4). Grade 2 or higher late toxicities are summarized in (Table 2). There was no statistically significant difference in worst late non-hematologic, hematologic, or overall toxicities between the two arms.
Table 2.
Late Grade 2+ Toxicities
| Toxicity | CRT + 5FU | CRT + Gemcitabine | z-score | Two-sided p-value |
|---|---|---|---|---|
| Worst hematologic | 4.5% | 3.3% | 0.6466 | 0.5179 |
| Worst non-hematologic | 16.8% | 19.2% | -0.6507 | 0.5152 |
| Worst overall | 18.6% | 21.0% | -0.6274 | 0.5304 |
Outcomes
The protocol specified that the initial treatment results be reported after either there had been 355 total deaths in both arms or there had been 190 deaths on the 5-FU containing arm. This analysis has been performed with 368 deaths, with 188 occurring on the 5-FU arm. All patients have been potentially followed for a minimum of 6.6 years.
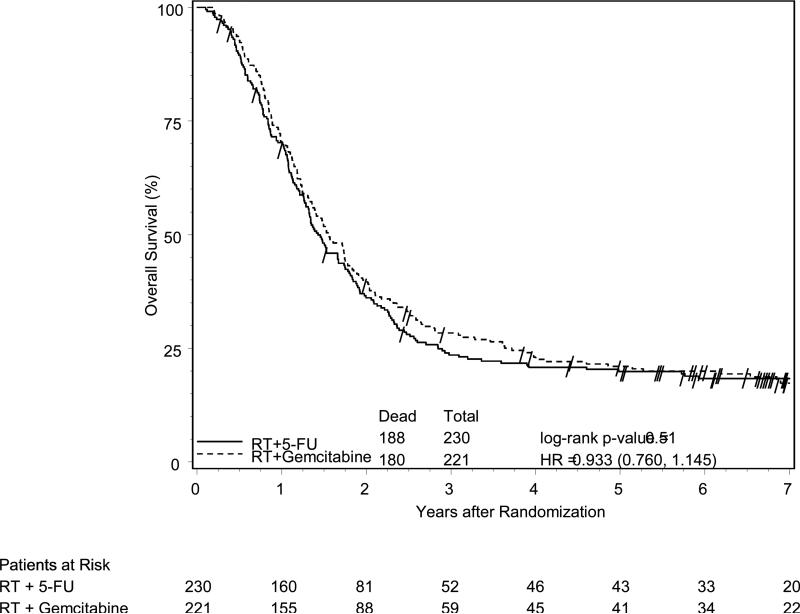
With a median follow-up time of 1.48 years (min-max, 0.1-9.1) for all patients and 6.98 years (min-max, 0.3-9.1) for alive patients, there was no statistically significant difference in OS (Figure 1A) or DFS between treatment arms. While treatment crossover was not allowed, among the 230 patients in the 5-FU arm, 95 (41%) received additional ‘salvage’ chemotherapy after any failure; with 81% (77/95) receiving G as salvage therapy.
Figure 1A.
Overall survival among all eligible patients, according to treatment group assignment. No significant difference between treatment arms was observed.
Among the 221 patients in the G arm, 77 (35%) received additional ‘salvage’ chemotherapy after any failure; with 60% (46/77) receiving additional G as salvage therapy.
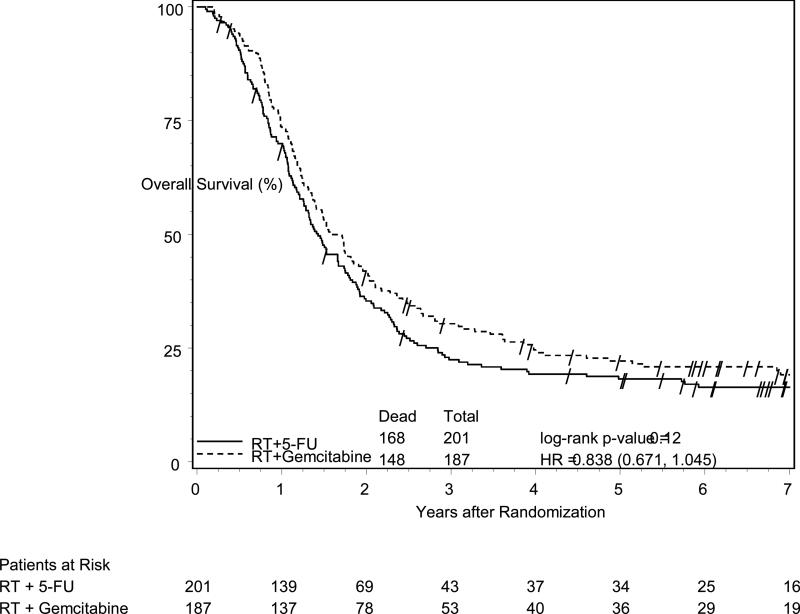
Patients with pancreatic head tumors had a median survival time (MST) of 20.5 months and a 5-year survival of 22% (95% CI: 16%-29%) on the G arm vs. 17.1 months and 18% (95% CI: 13%-24%) on the 5-FU arm (log-rank p=0.12, HR=0.84, 95% CI (0.67 -1.05), (Figure 1B). On multivariate analysis for pancreatic head patients, after adjusting for the protocol specified stratification variables of nodal status, which strongly effected survival (p=0.003), tumor diameter, and surgical margin status, the treatment effect shows a trend (HR=0.82, 95% CI (0.65 – 1.02), p=0.08) towards improved OS for the G arm (Table 3). Although T stage was imbalanced between the arms, it was not a significant factor on multivariate analysis.
Figure 1B.
Overall survival among all eligible patients with pancreatic head tumors, according to treatment group assignment. Patients had a median and 5-year survival of 20.5 months and 22% in the gemcitabine arm vs. 17.1 months and 18% in the 5-FU arm (p=0.12, HR=0.84, 95%CI (0.67 -1.05).
Table 3.
Multivariate Analysis for Overall Survival: All Eligible Patients with Tumor Location of Head
| Adjustment Variables | Comparison | Adjusted HR | p-value† |
|---|---|---|---|
| Treatment | Gemcitabine vs. 5-FU | 0.82 (0.65, 1.02) | 0.08 |
| Nodal Involvement | No vs. Yes | 1.45 (1.13, 1.85) | 0.003 |
| Tumor Diameter | <3 vs. ≥ 3 cm | 1.20 (0.95, 1.51) | 0.12 |
| Surgical Margin Status | Negative | 1.0 (Ref level) | -- |
| Positive | 1.05 (0.81, 1.37) | 0.69 | |
| Unknown | 0.96 (0.72, 1.29) | 0.80 |
Abbreviations: 5-FU, fluorouracil; HR, hazard ratio; C.I., confidence interval
p-value from Chi-square test using the Cox proportional hazards model.
The pattern of tumor relapse was recorded as the site of the first relapse only, and these sites were categorized as local, regional, or distant (Table 4). Eighty-nine percent (n=205/230) failed on the 5-FU arm and 88% (n=195/221) failed on the G arm. Distribution of relapse was similar among all patients and among patients with pancreatic head tumors. Local relapse occurred in 30% of patients on the 5-FU arm and 25% on the G arm. Regional relapse was similar on both arms at 8% vs. 7%. Distant relapse was ≥ 70% in both arms.
Table 4.
Site of First Relapse*
| CRT + 5-FU | CRT + Gemcitabine | |||||||
|---|---|---|---|---|---|---|---|---|
| All Patients (n=205) | Pancreatic Head (n=181) | All Patients (n=195) | Pancreatic Head (n=163) | |||||
| Site | n | % | n | % | n | % | n | % |
| Local | 61 | 30 | 55 | 30 | 49 | 25 | 40 | 25 |
| Regional | 16 | 8 | 15 | 8 | 14 | 7 | 13 | 8 |
| Distant | 143 | 70 | 125 | 69 | 149 | 76 | 127 | 78 |
Because patients could have simultaneous sites of first relapse, the total number of relapses are greater then the number of patients in each group and the percents will add to >100%.
INTERPRETATION
The 5-year results of this study demonstrate that the previously observed improvement in 3-year OS with the addition of G to adjuvant fluorouracil based CRT for patients with pancreatic head adenocarcinoma is no longer seen at 5 years.
While the OS curves remain separated throughout 5 years (Figure 1B) only a trend towards statistical significance is now seen. Thus, in the context of this study design, any improvement in survival associated with the use of G appears to be temporary and marginal at best.
Although the 5-year results in this study did not reach statistical significance, the observed marginal difference in survival between study arms favoring the G arm deserves further comments in the context of the conduct and design of this trial. First, 41% of the patients on the 5-FU arm received salvage chemotherapy after any recurrence, of which 81% of patients received G. This ‘cross-over’ like occurrence may have diluted differences in survival between the two study arms. Secondly, a greater proportion of patients on the G arm had T3/T4 disease (81% vs. 70%, p=0.013).
In addition and perhaps most importantly, is the associated potential negative impact on the survival benefit which could be seen with G due to the prolonged interruption of G related to the fundamental design of this study. In the G arm, patients were given a single cycle of G, followed by a required 1 – 2 weeks break before initiation of 5.5 weeks of 5-FU based CRT. This was then followed by a required 3 – 5 week break to allow for recovery from acute toxicity and restaging before resumption of G given in three additional cycles. Thus, between the first cycle of G and resumption of the final three cycles of G, patients were without G therapy for at least 9.5 weeks and for as long as 12.5 weeks. Given the known exponential rate of tumor recurrence associated with this disease, this prolonged interruption of G therapy may well have been a significant factor contributing to the temporary and eventually marginal survival benefit seen with use of G in this study.
The 5-year results from this study must also be reviewed in the context of the recently updated Phase III CONKO-001 trial and the recently reported Phase III ESPAC-3 trial (2, 3). The CONKO trial randomized patients with completely resected pancreatic cancer to six total cycles of G versus surgery alone. Patient selection and eligibility requirements clearly differed between RTOG 9704 and the CONKO trial (23), including frequency of R0 resections (42% - RTOG vs. 83% - CONKO) and the CONKO requirement for CA19-9 to be < 2.5 x upper limit of normal (~ 90). RTOG 97-04 did not exclude patients by CA19-9, with 21% having values > 90. CONKO-001 initially demonstrated only a statistically significant improvement in disease-free survival without an improvement in overall survival (1). However, the recently reported 5-year results in now demonstrate a statistically significant improvement in overall survival with a median and 5-year survival rate of 22.8 months and 21% in the G arm and 20.2 months and 9% in the surgery alone arm (p=0.005) (2). The use of 6 total cycles of G in the CONKO trial should be viewed in contrast to our study, which in addition to the prolonged interruption of G therapy, made use of only four total cycles of G.
While the CONKO trial evaluated the impact of adjuvant G compared to surgery alone, the recently reported ESPAC-3 trial was a direct comparison of G to 5-FU in the adjuvant setting. This trial randomized patients with completely resected pancreatic carcinoma to six cycles of G versus six cycles of 5-FU. In this adjuvant trial where patients did not receive RT by design, direct comparison of G versus 5-FU demonstrated no significant difference in survival. (3) While RTOG 9704 represents the only other phase III trial making a direct comparison of G to 5-FU in the adjuvant setting, all patients received CRT by design.
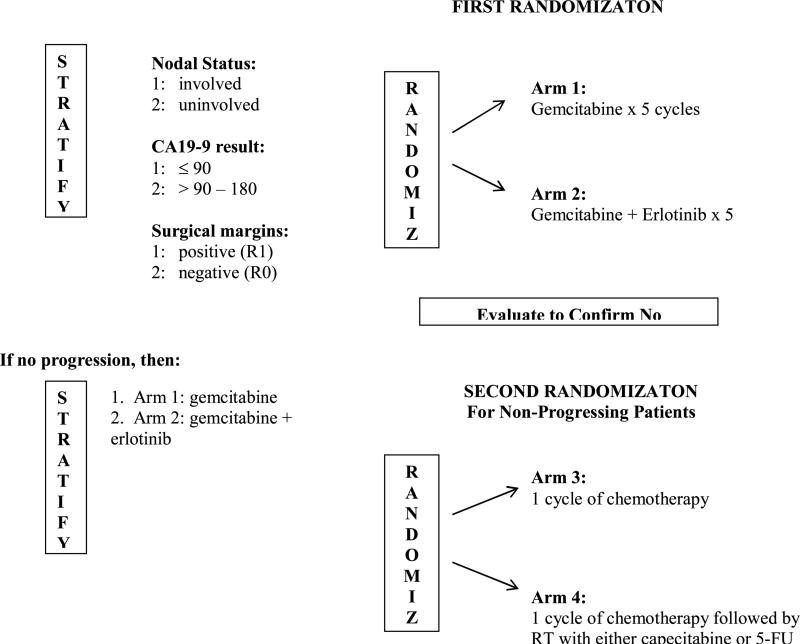
When viewed in total, the findings from RTOG 9704, CONKO-001, and ESPAC-3, as well as previously reported phase III postoperative adjuvant therapy trials for pancreatic adenocarcinoma, would suggest the following: 1) the survival benefit associated with G is observed with use of 6 cycles; 2) the survival benefit seen with G in comparison to 5-FU appears to be temporary/marginal at best and seen only with the inclusion of CRT; 3) while the local recurrence rate of 28% seen in RTOG 9704, which made use of central RT QA (22), is the lowest reported to date and is approximately half of that reported in previous phase III adjuvant CRT trials (10,11, 24), distant disease relapse remains the primary mode of failure occurring in greater than 70% of patients. These fundamental observations based on level one evidence serve as the basis of the international EORTC/US Intergroup/RTOG 0848 which is a phase III trial evaluating adjuvant therapy in patients with resected pancreatic adenocarcinoma, the schema of which is shown in Figure 2. Patient stratification will include CA19-9 level ( < 90 vs. > 90 – 180). By design this trial will have two randomizations. The first randomization will evaluate the impact of the addition of erlotinib to G. The testing of erlotinib is based on results in the locally advanced and metastatic setting demonstrating a survival benefit with use of erlotinib in combination with G (25).
Figure 2.
Schema for RTOG 0848: A Phase III Trial Evaluating Both Erlotinib and Chemoradiation as Adjuvant Treatment for Patients with Resected Head of Pancreas Adenocarcinoma
After five cycles of G based therapy patients will undergo re-imaging and those who have no evidence of disease progression will undergo a second randomization evaluating the impact of CRT. CRT will be given after completion of the sixth and final cycle of G. During this sixth and final cycle central RT QA will be performed. This trial will also allow the use of intensity modulated radiation therapy (IMRT) in addition to Trans-Atlantic central RT QA. Quality of life, patient reported outcomes, and correlative molecular science analyses are also planned.
Acknowledgements
Supported by RTOG U10 CA21661 and CCOP U10 CA37422 grants from the NCI.
Footnotes
Presented at the 51st Annual Scientific Meeting of the American Society of Therapeutic Radiology and Oncology, Chicago, IL, November, 2009.
The authors have no conflict of interest
References
- 1.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant Chemotherapy with Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Trial. JAMA. 2007;297(3):267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 2.Neuhaus P, Riess H, Post S, et al. CONKO-001: Final Results of the Randomized, Prospective, Multicenter Phase III Trial of Adjuvant Chemotherapy with Gemcitabine versus Observation in Patients with Resected Pancreatic Cancer (PC). J Clin Oncol. 2008;26(suppl LBA4504) [Google Scholar]
- 3.Neoptolemos J, Buchler M, Stocken DD, et al. ESPAC-3(v2): A multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folonic acid (5-FU/FA) versus gemcitabine (GEM) in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(suppl18) [Google Scholar]
- 4.Regine WF, Winter K, Abrams RA, et al. Fluorouracil vs. Gemcitabine Chemotherapy Before and After Fluorouracil- based Chemoradiation Following resection of Pancreatic Adenocarcinoma: A Randomized Controlled Trial. JAMA. 2008;299(9):1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 5.Nicecki SS, Sarr MG, Colby TV, et al. Long-term Survival after Resection for Ductal Adenocarcinoma of the Pancreas. Is it really Improving? Ann Surg. 1995;221(1):59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirokowski RJ, Believernicht SW, Lawrence W, Jr., et al. Pancreatic and Periampullary Carcinoma: Experience with 200 Patients over a 12-year Period. Am J Surg. 1982;143(2):189–193. doi: 10.1016/0002-9610(82)90064-2. [DOI] [PubMed] [Google Scholar]
- 7.Gudjonsson B. Cancer of the Pancreas: 50 Years of Surgery. Cancer. 1987;60(9):2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Tepper J, Nardi G, Suit H. Carcinoma of the Pancreas. Review of MGH Experience from 1963-1973. Analysis of Surgical Failure and its Implications for Radiation Therapy. Cancer. 1976;37(3):1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::aid-cncr2820370340>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Griffin JF, Smalley SR, Jewell W, et al. Patterns of Failure after Curative Resection of Pancreatic Carcinoma. Cancer. 1990;66(1):56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Kalser MH, Ellenberg SS. Pancreatic Cancer. Adjuvant Combined Radiation and Chemotherapy Following Curative Resection. Arch Surg. 1985;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 11.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant Radiotherapy and 5-fluorouracil after Curative Resection of Cancer of the Pancreas and Periampullary Region: Phase III Trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230(6):776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant Radiotherapy and Chemotherapy for Pancreatic Carcinoma: The Mayo Clinic Experience (1975-2005). J Clin Oncol. 2008;26(21):3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 13.Herman JM, Schwartz MJ, Hsu CC, et al. Analysis of Fluorouracil Based Adjuvant Chemotherapy and Radiation after Pancreaticoduodenectomy for Ductal Adenocarcinoma of the Pancreas: Results of a Large Prospectively Collected Database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26(21):3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming ID, Cooper JS, Henson DE, et al. American Joint Committee on Cancer Manual for Staging of Cancer. 5th ed. Lippincott-Raven; Philadelphia, PA: 1997. Exocrine pancreas. pp. 122–123. [Google Scholar]
- 15.Regine WF. Postoperative Adjuvant Therapy: Past, Present, and Future Trial Development. In: Evans DB, Pisters PWT, Abbruzzese JL, editors. Pancreatic Cancer. Springer; New York, USA: 2006. pp. 235–242. [Google Scholar]
- 16.Zelen M. The Randomization and Stratification of Patients to Clinical Trials. J Chron Dis. 1974;27(7-8):365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 17.SAS/STAT® . Version 9.2. SAS Institute Inc.; Cary, NC: 2009. [Google Scholar]
- 18.Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of Recurrence and Survival by Post-Resection CA 19-9 Values in Patients with Adenocarcinoma of the Pancreas. Ann Surg Oncol. 1958;4(7):551–556. doi: 10.1007/BF02305535. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparameteric estimation from incomplete observations. J Amer Statist Assoc. 1958;53(282):457–481. [Google Scholar]
- 20.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. 1966;50(3):163–170. Rep. [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables. J Royal Stat Soc Series B. 1972;34(2):187–229. [Google Scholar]
- 22.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticduodenectomy for Pancreatic Adenocarcinoma: Postoperative Adjuvant Chemoradiation Improves Survival. A Prospective, Single-Institution Experience. Ann Surg. 1997;225(5):621–636. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson A. Adjuvant Therapy for Pancreatic Cancer. JAMA. 2007;297(3):311–313. doi: 10.1001/jama.297.3.311. [DOI] [PubMed] [Google Scholar]
- 24.Neoptolemos JP, Stocken DD, Friess H, et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy After Resection of Pancreatic Cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 25.Moore MJ, Goldstin D, Hamm, et al. Erlotinib Plus Gemcitabine compared with Gemcitabine Alone in Patients with Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]