Abstract
A novel polyelectrolyte nanocapsule system composed of biopolymers, chitosan and heparin has been fabricated by the layer-by-layer technique on silica nanoparticles followed by dissolution of the silica core. The nanocapsules were of the size range 200 ± 20 nm and loaded with the positively charged anticancer drug doxorubicin with an efficiency of 89%. The loading of the drug into the capsule happens by virtue of the pH-responsive property of the capsule wall, which is determined by the pKa of the polyelectrolytes. As the pH is varied, about 64% of the drug is released in acidic pH while 77% is released in neutral pH. The biocompatibility, efficiency of drug loading, and enhanced bioavailability of the capsule system was confirmed by MTT assay and in vivo biodistribution studies.
Keywords: drug delivery, layer-by-layer, electrostatic interaction, biocompatible
Introduction
Controlled drug release1 is one of the most sought-out properties the scientific community has been striving for since the latter half of the 20th century. The incentive for such a drive is mainly owing to the vast advantages that it provides such as improved efficacy, reduced side effects and enhanced patient well-being. One of the major component for the creation of a potent controlled drug-delivery system are polymers such as polysodium 4-styrene sulfonate, polyallylamine hydrochloride,2 chitosan, dextran sulfate,3 etc. With the inception of various synthesis techniques, polymers with unique properties have been produced, which have opened the frontiers for designing drug-delivery systems with different release mechanisms and applications.
Numerous techniques have been devised for the fabrication of nanocapsules over the years. Suspension polymerization involves a water-insoluble monomer dispersed as droplets by steric stabilizer to produce polymer particles as a dispersed solid phase. However, their drawback is that nanosized particles cannot be fabricated.4 An improvement on this was emulsion polymerization in which discrete monomer-polymer particles are dispersed in a continuous aqueous phase, though there were issues due to the influences of numerous factors such as temperature, stirring, emulsifier and solvents.5 Using dendrimers proved to be a promising method considering its ability to produce well-defined nanosized structures, but it is both tedious and expensive.6 Of the various techniques utilized for fabrication of controlled drug-delivery devices, layer-by-layer (LbL) assembly is one of the easiest and facilitates creation of functional surfaces. It involves sequential adsorption of oppositely charged PE onto the surface of a sacrificial template, which is subsequently dissolved.7–10 The driving force for LbL is the electrostatic interaction between the oppositely charged polyelectrolytes (PE). The resulting capsules are found to have well-orchestrated size, shape, and wall thickness. Though capsules of various size ranges have been formulated, nanocapsules were deemed the most suitable. The advantage of nanocapsule is its ability to evade the first-pass metabolism, thereby effectively reducing the dosage required for therapy.11 It is observed that cancerous tissues have leaky vasculature which allows the nanocapsules to reach the site without much hindrance (enhanced permeability and retention effect) unlike microcapsules.12
Chitosan (CS) is a hydrophilic, positively charged polysaccharide13 made up of a high ratio of D-glucosamine to N-acetyl glucosamine units obtained by the alkaline N-deacetylation of chitin and reacts with negatively charged PE by electrostatic forces.14 Owing to its attractive properties such as biodegradability, biocompatibility, antimicrobial activity, etc, it is used in fabrication of microspheres and microcapsules for controlled release of drugs,15,16 as scaffolds for tissue-engineering applications,17,18 and as hydrogels for membrane preparation.19 Heparin (HP) is a polyanionic mucopolysaccharide composed of repeating disaccharide units of glucosamine and uronic acid linked by 1–4 interglycosidic bonds having a mean molecular weight of 15 kDa.20 Heparin has always been found to be an effective anticoagulant and plays a significant role in gastric ulcer healing by virtue of its ability to increase nitric oxide synthesis and facilitate mucosal cell proliferation by stimulating growth factors.21
Several research teams have been working on drug-delivery systems with reasonable success, but the development of nanocapsules have been beset with problems owing to difficulties in optimizing the properties. It is generally observed that CS having a pKa of 6.5 is found susceptible to acidic conditions, thereby reducing its applicability.22 In order to resolve this issue, we decided to electrostatically link CS to HP by the LbL technique. Herein we report the preparation and stimuli responsive behavior of a novel sustained drug-delivery system made up of the said PEs with silica as the sacrificial template.
Experimental section
Materials
Chitosan (CS; Mw = 6500 kDa), HP, doxorubicin hydrochloride (C27H29NO11HCl, FW = 580), Dulbecco’s modified Eagle’s medium (DMEM) and fetal calf serum were purchased from Sigma Aldrich (Bangalore, India). Hydrofluoric acid (HF) was purchased from Thomas Baker Ltd (Bangalore, India). Acetic acid (CH3COOH), ammonium fluoride (NH4F), sodium chloride (NaCl), sodium hydroxide (NaOH), and hydrochloric acid (HCl) were obtained from Rankem, RFLC Ltd (Bangalore, India). Mouse melanoma cell line (B16-F10) and MCF-7 were obtained as a kind gift from Prof Annapurni Rangarajan, Molecular Reproduction and Developmental Genetics Department (MRDG), Indian Institute of Science, Bangalore, India, and MTT was purchased from HiMedia (Bangalore, India). Double autoclaved MilliQ water (Millipore, Billerica, MA, USA) was used for all the experiments.
Results
Capsule fabrication
Initially, stock solutions of CS and HP were prepared at concentrations of 1 mg/mL in 1 M sodium chloride. The process was carried out at pH 5.6, taking into account the pKa values of CS and HP. A silica template (220 ± 20 nm) was chosen as the sacrificial core. Since it is negatively charged, positively charged CS electrostatically binds to it, forming the first layer. The template was incubated for 15 minutes followed by centrifugation at 4000 rpm for 5 minutes (MIKRO 200R; Hettich Zentrifugen, Tuttlingen, Germany) and washed thrice with pH 5.6 water to remove the unadsorbed PE. Subsequently, the second layer (HP) was deposited followed by centrifugation and rinsing as described above. The process was continued alternatively with CS/HP until six layers were formed. Finally, the silica template was removed using a buffer (0.2 M HF + 0.8 M NH4F) over a period of 1.5 hours followed by five washings. The hollow capsules were stored in water at −4°C for further study.
Drug-loading studies
Doxorubicin (1 mg/mL) was chosen as the model drug and 400 μL was incubated in 200 μL of capsules overnight in pH 8 water at room temperature. The nanocapsules were then immersed in pH 5 for 60 minutes at room temperature for effective locking of the capsule layers to retain the loaded doxorubicin. Following this, the sample was washed twice with distilled water and subjected to centrifugation at 2000 rpm for 5 minutes to remove the unencapsulated drug. The amount of doxorubicin loaded within the capsules was calculated by using a spectrophotometer (NanoDrop ND1000; Thermo Scientific, Wilmington, DE, USA) by measuring the absorbance of the supernatant at 496 nm.
Drug release studies
Doxorubicin release studies were carried out in acidic pH over a period of 48 hours. The supernatant was taken out at stipulated time periods (0.5, 1, 2, 4, 8, 16, 24, and 48 hours) and release rate was quantified by measuring the absorbance at 496 nm using the NanoDrop spectrophotometer.
Characterization of nanocapsules
About 5 μL of capsules were dried overnight on a clean silicon wafer and subjected to gold sputtering to ensure electrical conductivity (JFC 1100E ion-sputtering device; JEOL, Tokyo, Japan) and analyzed by field emission-scanning electron microscopy (FE-SEM; FEI-SIRION, Eindhoven, The Netherlands). Similarly, the sample was placed on a carbon-coated 300 mesh copper grid (Toshniwal Bros SR Pvt Ltd, Bangalore, India) for field emission-transmission electron microscopy (FE-TEM; Tecnai F30; FEI, Eindhoven, The Netherlands).
Zeta potential measurement
Zeta potential of the outer surface of each layer was measured using Zetasizer Nano ZS (Malvern, Southborough, MA, USA) in order to ensure the alternate deposition of CS and HP. Each value so obtained was in effect the average of three parallel measurements. The concentrations of both PEs are 1 mg/mL in 1 M sodium chloride prior to combining and the pH is 5.6 taking into account the pKa of the PE, ie, CS (6.5) and HP (4). We maintain this pH throughout the whole assembly process.
Confocal microscopy
Confocal images were taken using a LSM confocal scanning system (Carl Zeiss, Jena, Germany) equipped with 100× oil immersion objective and numerical aperture of 1.4. For visualization, doxorubicin was used because of its fluorescent property, which was electrostatically adsorbed into the capsule and has an excitation wavelength of 496 nm. This gave an indication regarding the degree of encapsulation in the capsule at various pH. In case of cell-line studies, B16-F10 and MCF-7 were treated with doxorubicin-loaded CS–HP nanocapsules for 30 minutes, washed repeatedly, fixed with 4% paraformaldehyde, and visualized under a confocal microscope.
MTT assay
The ability of viable cells to reduce a soluble yellow tetrazolium salt, MTT to blue formazan crystals is the principle behind MTT assay. The CS–HP bare nanocapsules and doxorubicin-loaded nanocapsules were assessed for in vitro toxicity by MTT assay in MCF-7 cell line. The cell lines were maintained in DMEM supplemented with 10% fetal calf serum at 37°C and 5% CO2 and seeded in a 96-well plate at a cell density of 5 × 104 cells/mL. After 14 hours, various concentrations of empty nanocapsules, doxorubicin-loaded nanocapsules, and free doxorubicin were added to the cells. After 48 hours of incubation, 20 μL of MTT dye (5 mg/mL) was added to each well and kept for 4 hours at 37°C. The percentage of cell viability was determined at 570 nm relative to nontreated cells by measuring the absorbance of the colored solution obtained by solubilization of the insoluble formazan.
In vivo distribution of doxorubicin-encapsulated nanocapsules
BALB/c mice (6–8 weeks) were bred and housed at the Central Animal Facility, Indian Institute of Science, Bangalore, India. All procedures were carried out as per the rules laid down by the Institute. Mice were assigned into two groups (n = 21) and injected with 10 mg/kg of free doxorubicin or doxorubicin-encapsulated nanocapsules by intravenous injection in the tail vein. Blood was collected by retro-orbital puncture at 1, 2, 4, 8, 12, 24, and 48 hours after the injection (n = 3 at each time point) and plasma was collected by centrifugation at 2500 rpm for 20 minutes and frozen at −20°C until assayed. Doxorubicin was extracted with acidic alcohol (0.3 M HCl: ethanol, 3:7, V/V) and detected with a spectrofluorometer (Optizen 3220UV; Mecasys Co. Ltd, Daejeon, Korea) 470 nm excitation and 590 nm emission wavelengths. Bioavailability from 0 to 48 hours was calculated from the area under curve in the blood concentration versus time curve (AUC0–48) using the linear trapezoidal rule in GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA).
Discussion
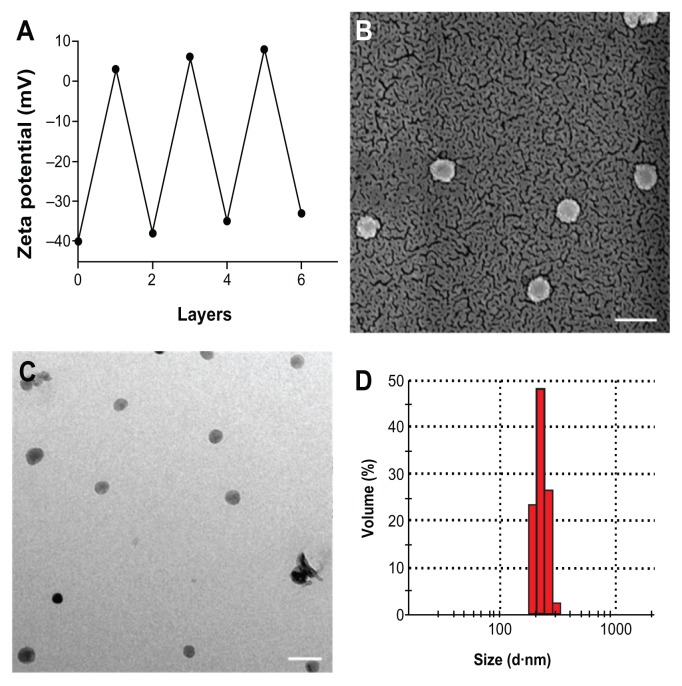
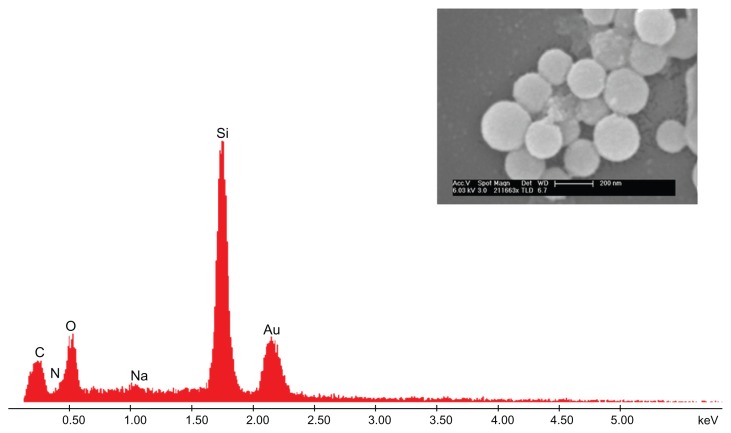
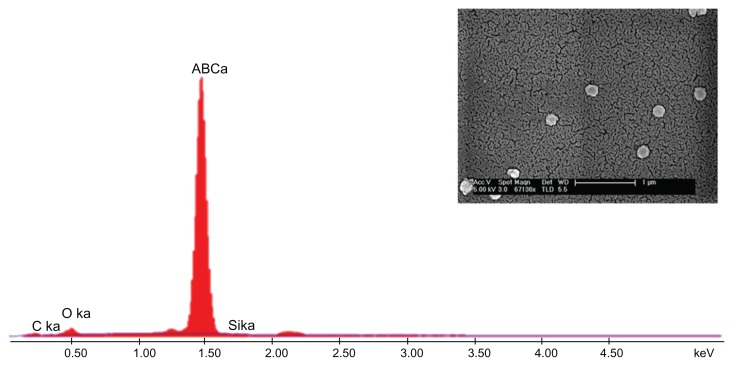
As mentioned earlier, the first step involved fabrication of CS–HP nanocapsules by the LbL technique and the whole process was carried out at pH 5.6 (pKa values: CS, 6.5; HP, 4), in order to ensure that majority of the functional groups are in the charged state, NH3+ and SO42−, respectively. Since the sacrificial template SiO2 is negatively charged as confirmed by zeta potential (−40 mV), CS was chosen as the first layer which adsorbs onto it by electrostatic interaction. This was followed by deposition of HP and the process was continued until six layers were deposited. Zeta potential measurements after each layer suggested a charge reversal, which confirmed adsorption of PEs (Figure 1A). After the deposition of six layers, the template was dissolved by buffer (NH4F + HF) which complexes the Si2+ ions leading to the formation of hollow nanocapsules. The capsules so formed were found to be in the range of 200 ± 20 nm ascertained by SEM, TEM, and dynamic light scattering (DLS) (Figure 1B–D). Energy dispersive X-ray spectrometry (EDS) and SEM were also done for both the core intact CS-HP nanocapsules and hollow nanocapsules (Figures 2 and 3).
Figure 1.
(A) Zeta potential variation as a function of layer number during the LbL process. The measurements were carried out at room temperature by suspending the particles in deionized water of pH 5.6. (B) SEM and (C) TEM images of CS-HP nanocapsules after core dissolution. (D) Size determined by dynamic light scattering.
Note: Scale bar is 1 μm.
Abbreviations: CS, chitosan; HP, heparin; LbL, layer-by-layer; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Figure 2.
CS-HP nanocapsule.
Notes: EDS indicates the presence of silica, and inset SEM image shows the nanocapsule to have a rough surface indicative of deposition.
Abbreviations: CS, chitosan; EDS, energy-dispersive X-ray spectrometry; HP, heparin; SEM, scanning electron microscopy.
Figure 3.
Hollow CS-HP nanocapsule.
Notes: EDS of empty capsules (see inset) shows no silica peak indicating complete removal of silica core.
Abbreviations: CS, chitosan; EDS, energy-dispersive X-ray spectrometry; HP, heparin; SEM, scanning electron microscopy.
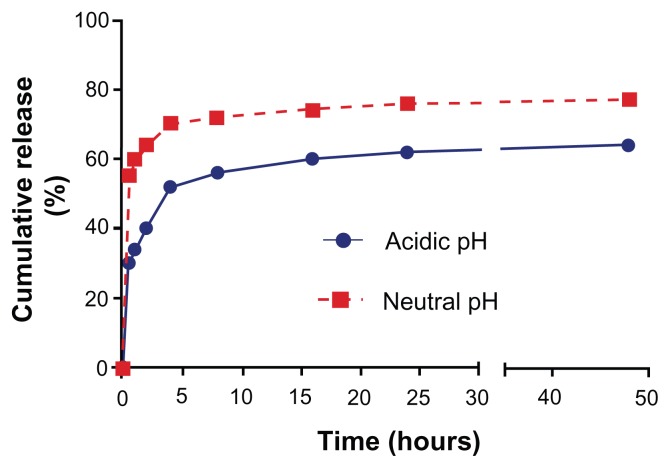
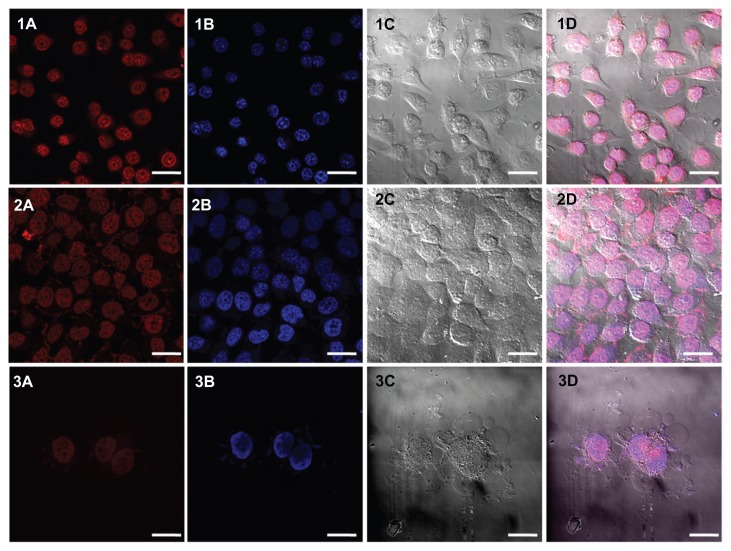
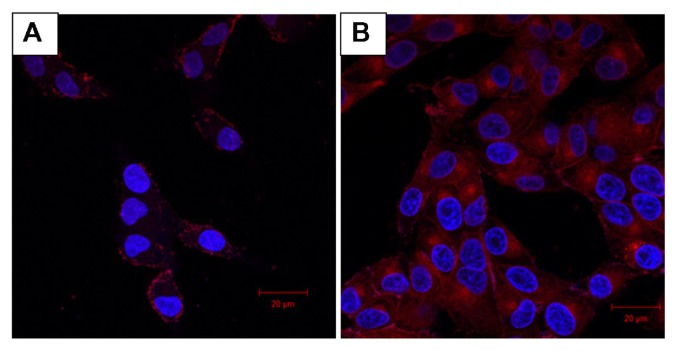
The empty capsules were incubated with doxorubicin 1 mg/mL, which enters the capsule by virtue of the pores formed on the capsules. Loading was done at a pH higher than the pKa of CS so that the electrostatic interaction between the PE layers diminishes due to deprotonation of amino groups. The loading studies carried out using ultraviolet-spectroscopy indicated that 89% of the drug was loaded into the hollow nanocapsules (358.8 μg out of 400 μg). Drug release studies were carried out in acidic and neutral pH over a period of 48 hours and it was observed that 77% release was obtained in acidic pH as opposed to 64% in neutral pH (Figure 4). This increased release percent in acidic pH makes it a better choice for use in cancerous cells owing to its more acidic nature. Subsequently, confocal laser scanning microscopy was used as the cell nucleus was stained with DAPI, which has an emission maximum at 461 nm (blue) (Figure 5 [1A–3A]). On release of doxorubicin from the capsules after incubation with the cells for more than 30 minutes, the nucleus is found to be stained red with an emission maximum of 496 nm (Figure 5 [1B–3B]). Doxorubicin forms complexes with DNA by intercalation between base pairs, and inhibits topoisomerase II activity by stabilizing the DNA-topoisomerase II activity.23 After 5 hours of incubation, the cells lines show blebs which are indicative of apoptosis suggesting the cytotoxic activity of doxorubicin24 (Figure 5 [3C]). For the purpose of comparison with doxorubicin-loaded nanocapsules, confocal images of free doxorubicin loaded into the cells are also provided (Figure 6).
Figure 4.
Drug release studies in acidic pH (4.8) and neutral pH (7.4).
Figure 5.
CLSM images of (1) B16-F10 and (2 and 3) MCF-7 cells incubated with CS-HP nanocapsules. While (1) and (2) show images of cells incubated with nanocapsules for 1 hour, the cells in (3) were incubated for 5 hours.
Notes: (A) Capsules loaded with doxorubicin appear red in color. (B) Nucleus stained blue using DAPI. (C) Bright field image ([3] MCF-7 cells show blebs, which is characteristic of apopotosis) and (D) combined field image. Scale bar is 5 μm.
Abbreviations: CLSM, confocal laser scanning microscopy; CS, chitosan; HP, heparin.
Figure 6.
(A) Confocal image of CS-HP nanocapsule loaded with doxorubicin after 1 hour shows the nanocapsules located on the cell membrane as dots. (B) Confocal image of free doxorubicin after 1 hour shows it to be evenly distributed.
Abbreviations: CH, chitosan; HP, heparin.
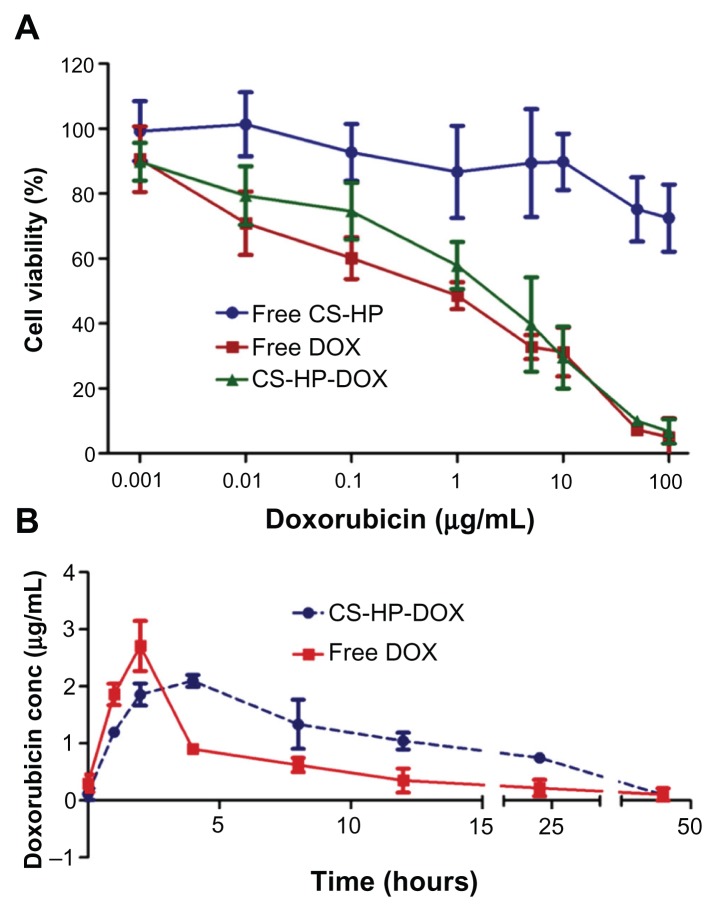
Being a novel system, the capsules are assessed for in vitro toxicity by MTT assay using MCF-7 cell line. These cells were exposed to a series of equivalent concentrations of free doxorubicin and doxorubicin-encapsulated nanocapsules for 48 hours to compare the cytotoxic activity of encapsulated and free drug. The percentage of viable cells was quantified using MTT assay. Empty nanocapsules showed no toxicity even at higher concentrations (Figure 7A), which proved the biocompatible nature of the nanocapsules. There was no significant difference in the cell viability between free doxorubicin and doxorubicin-encapsulated nanocapsules. These results indicate that the encapsulation of doxorubicin can be used for in vivo studies to better understand the physiological effect of the loaded nanocapsules.
Figure 7.
(A) MTT assay for cytotoxic assessment in MCF-7 cell line. Cytotoxicity effect of different concentration of empty capsules (free CS-HP), doxorubicin-loaded nanocapsules (CS-HP-DOX), and free-doxorubicin were checked using MTT assay. Data represents mean ± standard deviation. (B) Biodistribution studies done by injecting a single dose of 10 mg/kg doxorubicin in free form and encapsulated in nanocapsule. Serum was collected at different time periods and doxorubicin concentration was measured.
Abbreviations: CS, chitosan; DOX, doxorubicin; HP, heparin.
Biodistribution studies were carried out to understand the pharmacokinetics of the nanocapsule-loaded doxorubicin and free doxorubicin. BALB/c mice were injected intravenously with free doxorubicin or nanocapsule-loaded doxorubicin. At different time intervals, serum was collected and doxorubicin concentration was determined after extraction. It is observed that over a period of 24 hours, the concentration of free doxorubicin reduces to 0.25 μg mL−1, while that of nanocapsule-loaded doxorubicin is 0.75 μg mL−1 in serum. This clearly suggests an increase in the circulation time of doxorubicin when it was loaded in nanocapsules (Figure 7B). This can be due to the slow and complete release of doxorubicin from the capsules before being eliminated, and also due to the fact that the nanoparticles gets accumulated in the tumor tissues due to their enhanced permeability and retention effects. This increased circulation time can provide better efficiency of the drug in vivo.
From AUC0–48, bioavailability was calculated and compared for free and nanocapsule-loaded doxorubicin. There is a twofold increase (100% increase, ie, 38.66 μg hour mL−1: 19.32 μg hour mL−1) in bioavailability for nanocapsule-loaded doxorubicin compared with free doxorubicin. This substantial increase in bioavailability for drug-loaded nanocapsules ensures that this system can reduce the frequency and dosage of drug required for treating any pathological condition. In short, this system negates the toxicity and adverse side effects prevalent with free drug.
Conclusion
Our results clearly prove that we have successfully fabricated novel CS–HP nanocapsules of the size range 200 ± 20 nm. By removal of the sacrificial template, we were able to obtain hollow nanocapsules of good integrity and dispersity in water. The capsules were characterized by several techniques along with MTT assay, which conclusively proved the biocompatibility of the system. As discussed earlier, the loading of the hollow capsules depends primarily on the pKa of CS and HP and therefore, by varying the choice of PE, we can alter the application modality. It was observed that the doxorubicin-loaded capsules had much enhanced biodistribution as opposed to free doxorubicin. This property will play a significant role in drastically reducing the adverse effects presently plaguing the free drugs.25,26
Acknowledgments
We would like to extend our gratitude to Nanoscience Initiative, IISc for providing a microscopy facility, the Department of Microbiology, IISc for their confocal facility and Central Animal facilities for providing us with animals for in vivo studies. This study is financially supported by the Department of Biotechnology, Government of India.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Han X, Zhu Y. (PAH/PSS)5 microcapsules templated on silica core: Encapsulation of anticancer drug DOX and controlled release study. Colloids Surf A Physiochem Eng Asp. 2005;264(2):49–54. [Google Scholar]
- 3.Itoh Y, Matsusaki M, Kida T, Akashi M. Enzyme-responsive release of encapsulated proteins from biodegradable hollow capsules. Biomacromolecules. 2006;7(10):2715–2718. doi: 10.1021/bm060289y. [DOI] [PubMed] [Google Scholar]
- 4.Kwak NS, Baek Y, Hwang TS. The synthesis of poly(vinylphosphonic acid-co-methacrylic acid) microbeads by suspension polymerization and the characterization of their indium adsorption properties. J Hazard Mater. 2012;(0):203–204. 213–220. doi: 10.1016/j.jhazmat.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Oh HG, Shin H, Jung H, Lee BH, Choe S. Control of molecular weight of polystyrene using the reverse iodine transfer polymerization (RITP) – Emulsion technique. J Colloid Interf Sci. 2011;353(2):459–466. doi: 10.1016/j.jcis.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Navarro M, Rojo J. Synthetic strategies to create dendrimers: Advantages and drawbacks. In: Jesus MdlF, Grazu V., editors. Frontiers of Nanoscience. Amsterdam, The Netherlands: Elsevier; 2012. pp. 143–156. [Google Scholar]
- 7.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 8.Donath E, Sukhorukov GB, Caruso F, Davis SA, Möhwald H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew Chemi Int Ed Engl. 1998;37(16):2201–2205. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2201::AID-ANIE2201>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Sukhorukov GB, Donath E, Lichtenfeld H, et al. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf A Physicochem Eng Asp. 1998;137(1–3):253–266. [Google Scholar]
- 10.Caruso F, Caruso RA, Möhwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282(5391):1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 11.Kwan KC. Oral bioavailability and first-pass effects. Drug Metab Dispos. 1997;25(12):1329–1336. [PubMed] [Google Scholar]
- 12.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev. 2001;47(1):83–97. doi: 10.1016/s0169-409x(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 14.Kas HS. Chitosan: Properties, preparations and application to microparticulate systems. J Microencapsul. 1997;14(6):689–711. doi: 10.3109/02652049709006820. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima Y, Handa T, Kasai A, Takenaka H, Lin SY. The effects of thickness and hardness of the coating film on the drug release rate of theophylline granules coated with chitosan-sodium tripolyphosphate complex. Chem Pharm Bull (Tokyo) 1985;33(6):2469–2474. doi: 10.1248/cpb.33.2469. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki T, Komuro T, Yomota C, Okada S. Usage of chitosan as a pharmaceutical material effectiveness as an additional additives of sodium alginate. J Pharmacol Exp Ther. 1999;290:789–796. [PubMed] [Google Scholar]
- 17.Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–3928. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Gao C, Mao Z, et al. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24(26):4833–4841. doi: 10.1016/s0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 19.Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):35–52. doi: 10.1016/s0939-6411(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 20.Andersson LO, Barrowcliffe TW, Holmer E, Johnson EA, Söderström G. Molecular weight dependency of the heparin potentiated inhibition of thrombin and activated factor X. Effect of heparin neutralization in plasma. Thromb Res. 1979;15(3–4):531–541. doi: 10.1016/0049-3848(79)90159-2. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Wang HY, Cho CH. Association of heparin with basic fibroblast growth factor, epidermal growth factor, and constitutive nitric oxide synthase on healing of gastric ulcer in rats. J Pharmacol Exp Ther. 1999;290(2):789–796. [PubMed] [Google Scholar]
- 22.Liu W, Sun S, Cao Z, et al. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials. 2005;26(15):2705–2711. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Fornari FA, Randolph JK, Yalowich JC, et al. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45(4):649–656. [PubMed] [Google Scholar]
- 24.Massart C, Barbet R, Genetet N, Gibassier J. Doxorubicin induces Fas-mediated apoptosis in human thyroid carcinoma cells. Thyroid. 2004;14(4):263–270. doi: 10.1089/105072504323030915. [DOI] [PubMed] [Google Scholar]
- 25.Bisht S, Maitra A. Dextran–doxorubicin/chitosan nanoparticles for solid tumor therapy Wiley. Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(4):415–425. doi: 10.1002/wnan.43. [DOI] [PubMed] [Google Scholar]
- 26.Levi-Schaffer F, Bernstein A, et al. Reduced toxicity of daunorubicin by conjugation to dextran. Cancer Treat Rep. 1982;66(1):107–114. [PubMed] [Google Scholar]