Abstract
There has been mounting evidence of a causal role for telomere dysfunction in a number of degenerative disorders. Their manifestations encompass common disease states such as idiopathic pulmonary fibrosis and bone marrow failure. Although these disorders seem to be clinically diverse, collectively they comprise a single syndrome spectrum defined by the short telomere defect. Here we review the manifestations and unique genetics of telomere syndromes. We also discuss their underlying molecular mechanisms and significance for understanding common age-related disease processes.
Telomeres are DNA–protein structures that protect chromosome ends from degradation and fusion1. They are therefore essential for the maintenance of genomic integrity. Several observations have established the role of telomeres in cellular ageing. Because the replication machinery cannot fully copy to DNA ends, telomeres shorten progressively with cell division, eventually triggering senescence2. This observation has raised the idea that the length of telomeres may serve as a ‘molecular clock’ and that the accumulation of short telomeres with age in humans may contribute to age-dependent processes3. A compensatory mechanism, primarily the enzyme telomerase, in some settings adds back additional telomeric DNA4,5. This Review highlights how recent discoveries in human genetics have synergized with the field of telomere biology to unequivocally establish a causal role for telomere dysfunction in several specific, common and recognizable disease contexts.
The field of telomere research started with a focus on fundamental cellular mechanisms and in simple model organisms (reviewed in Ref. 6). Observations in the 1990s linked telomere biology to cancer, which was the first human disease in which telomeres were thought to have a role. Telomerase activity was found to be upregulated in most cancers7, and since then telomerase has been pursued as a target in cancer treatment8. In the past decade, discoveries in the area of human genetics have defined a new and emerging field; its findings establish a clear role for telomere dysfunction in diverse degenerative disease states. This field was sparked around the turn of the twenty-first century when the genetic basis of a rare disorder known as dyskeratosis congenita was elucidated. Unbiased positional cloning techniques identified mutations in the dyskeratosis congenita 1 (DKC1) gene, which encodes a conserved protein called dyskerin, but initially the function of dyskerin was not known9. Insights into telomerase biochemistry then revealed that it was as an essential component of the telomerase enzyme10,11. This early work has since led the path to the identification of a number of mutated telomerase and telomere genes using both unbiased and candidate gene approaches. During the past 5 years, the range of diseases affected by telomere length disequilibrium has been greatly extended and now encompasses prevalent disorders that have previously been poorly understood. Among these, the lung disease idiopathic pulmonary fibrosis (IPF) — best known until recently by its ‘idiopathic’ adjective — is the most common manifestation of telomere-mediated disease12. The cumulative evidence that we review here highlights a group of diverse disorders that share the molecular defect of short telomere length. Because of their overlapping clinical features, it has been proposed to consider them as a single syndrome spectrum13, and here we refer to them as ‘the telomere syndromes.’ The telomere syndromes are highly relevant for understanding age-related disease because the short telomere defect that they share is acquired with age. Moreover, as we will discuss, they probably represent the most prevalent monogenic premature ageing disorders.
In this Review, we aim to highlight the clinical spectrum of the telomere syndromes and present a model within which to understand their diverse clinical manifestations. Because, as we discuss, telomere length is heritable, the unique inheritance patterns that distinguish these syndromes from other Mendelian disorders are also discussed. Finally, we examine how telomere biology provides insights into disease mechanisms in several important contexts in which novel therapeutic paradigms are needed.
Telomeres and telomerase
Telomere structure
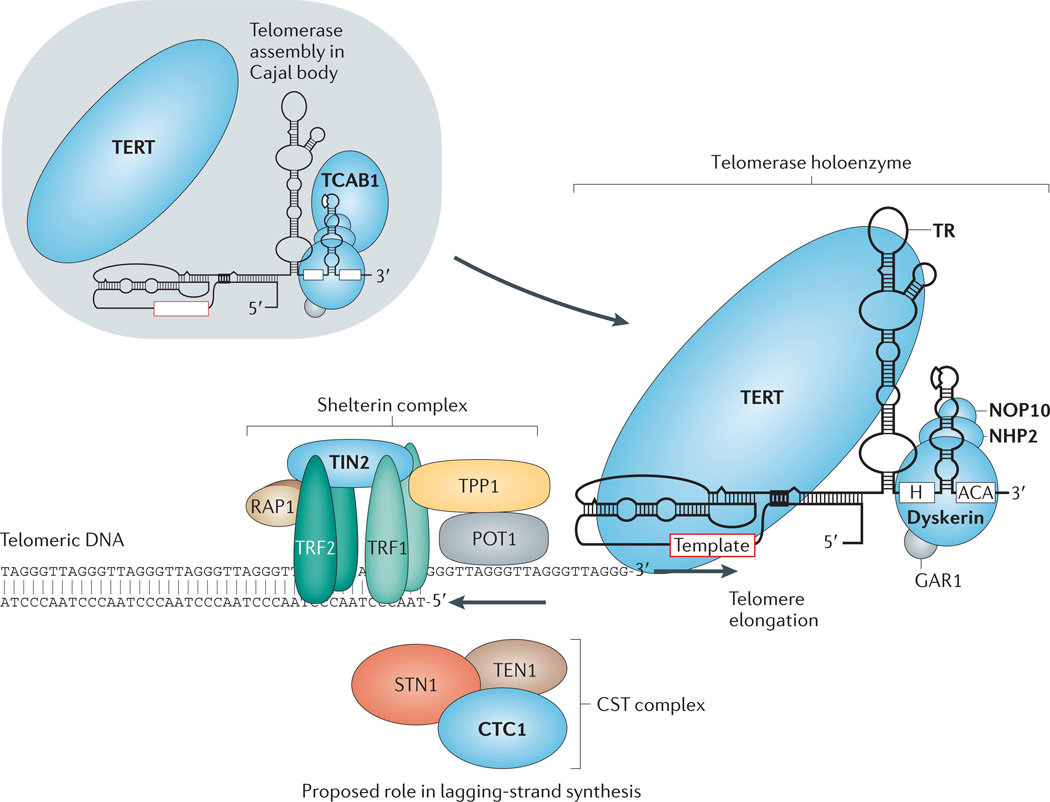
In humans and other vertebrates, the telomeric DNA sequence is a tract of tandem repeats of the six-nucleotide unit sequence TTAGGG14,15. These sequences extend for thousands of bases at chromosome ends, averaging 10 kb in a newborn human’s cord blood16. Telomere DNA is bound by a specialized group of protective proteins collectively called shelterin17. The human shelterin complex includes six proteins: telomere repeat binding factor 1 (TRF1), TRF2, repressor/activator protein 1 (RAP1), TRF1-interacting nuclear protein 2 (TIN2), TIN2-interacting protein 1 (TPP1) and protection of telomeres 1 (POT1) (Fig. 1). The combination of intact shelterin components and a telomere of sufficiently long tract length is essential to protect a chromosome end from eliciting DNA damage responses, deleterious degradation or participation in genomedestabilizing recombination or fusion events (reviewed in Ref. 18). Another complex that is relevant to the disease processes discussed below, known as CST, comprises three proteins — conserved telomere protection component 1 (CTC1), suppressor of cdc thirteen 1 (STN1) and telomeric pathway with STN1 (TEN1) — and is also found at human telomeres19,20 (Fig. 1). CST binds single-stranded DNA, and CTC1 is thought to function in telomere and non-telomere lagging-strand synthesis21,22. Although the telomere-protective roles of budding yeast CST are extensively characterized, the functional specificity of CST at mammalian telomeres remains unclear, and CST may have more general roles in DNA replication21,22. As described below, mutations in genes that code for some of these telomere proteins have recently been shown to cause a subset of telomere syndromes.
Figure 1. Telomerase and telomere components involved in human monogenic telomere syndromes.
Components for which mutations have been identified in telomere syndromes are indicated in bold type and shaded in blue. Shelterin complex components are made up of six component proteins — telomere repeat-binding factor 1 (TRF1), TRF2, repressor/activator protein 1 (RAP1), TRF1-interacting nuclear protein 2 (TIN2), TIN2-interacting protein 1 (TPP1) and protection of telomeres 1 (POT1) — which are essential for telomere protection and for regulating telomere elongation. The telomerase enzyme complex is comprised of TERT (the reverse transcriptase) and TR (the essential RNA component that contains a template for telomere repeat addition). TR contains a 3′H/ACA box motif that binds the dyskerin protein, which is part of a larger dyskerin complex that also consists of NHP2, NOP10 and GAR1. Note that for simplicity, one dyskerin complex is shown per TR molecule, although two copies are now thought to bind each TR. Telomerase Cajal body protein 1 (TCAB1) binds a Cajal body localization motif in TR and has a role in TR trafficking and biogenesis. In the Cajal body, TR and TERT assemble into a functional holoenzyme complex. The CST complex has three components — conserved telomere protection component 1 (CTC1), suppressor of cdc thirteen 1 (STN1) and telomeric pathway with STN1 (TEN1) — which are thought to function in part in telomere lagging-strand synthesis. Figure adapted, with permission, from Ref. 13 © (2009) Annual Reviews.
Telomerase structure
Telomerase is the specialized DNA polymerase that synthesizes new telomere sequences onto chromosome ends4,5,23. Telomerase has two conserved components that carry out the function of telomere repeat addition: the core telomerase protein, TERT, which contains the telomerase reverse transcriptase domain, and an essential RNA component, TR (also known as TERC), which complexes with TERT and provides the template for telomeric sequence synthesis23–25. The human telomerase holoenzyme is thought to assemble in the Cajal body, where TERT and TR form a ribonucleoprotein enzyme complex26. Although TERT and TR are sufficient to generate enzyme activity in vitro, telomerase relies on other proteins for its assembly, trafficking and regulation26,27. The best-characterized mammalian telomerase accessory component is the dyskerin protein11. Dyskerin forms a core complex with three smaller proteins NHP2, NOP10 and GAR1 (Ref. 28). Dyskerin binds to an H/ACA box RNA structural motif within TR (as well as small nucleolar RNAs) and is essential for TR stability and telomerase function in vivo10,11,29 (Fig. 1). Another protein, the telomerase Cajal body protein 1 (TCAB1), binds TR and regulates its trafficking30,31 (Fig. 1). As described below, mutations in the genes that encode the essential telomerase components — namely, TERT, TR and DKC1 — are a common cause of human telomere syndromes.
Elongation of telomeres by telomerase is tightly regulated
Telomere elongation is cell-cycle-regulated; telomerase elongates telomeric DNA by adding telomere repeats during S phase and into M phase32,33. Responding to cis-regulatory mechanisms mediated through the telomere DNA–protein complex, telomerase preferentially elongates the shortest telomeres, so only a subset of telomeres may be elongated in any cell cycle34. The extent of telomere elongation is highly sensitive to telomerase levels, as is evident by the haploinsufficiency for genes encoding telomerase components that is seen in yeast, mouse and human cells35–41. Even at wild-type levels, telomerase generally elongates only a few telomeres in any given cell cycle or generation34,36. This may be due to unbalanced stoichiometry between telomerase and its substrates, as well as to other telomerase-independent processes. Even in somatic human cells that are naturally enriched for telomerase activity, such as haematopoietic stem cells (HSCs), telomeres typically become shorter with each cell division and with age42,43. In human male germ cells, telomere lengths remain stable or even elongate with age3. The mechanisms by which telomeres are maintained in germ cell lineages, which are enriched for telomerase7, are not fully understood.
The limitation on the telomerase synthesis reaction on human telomeres favours telomere shortening as a general default state and means that telomere shortening generally accompanies cell proliferation. The constitutional telomere length in humans and mice is likely to be set early in embryonic development, when telomerase activity is transiently upregulated44.
Some biological rationales that underlie the tight regulation of telomerase levels include the possibility that short telomeres have a protective role as an innate tumour suppressive mechanism in long-lived, multicellular organisms by limiting the replicative potential of cells45. This idea is consistent with the fact that telomerase activity is upregulated in most human cancers, which probably contributes to sustaining their immortality7. Limiting telomerase levels may also prevent unwanted telomere addition at DNA double-strand-break sites, which could happen if telomerase competes with appropriate DNA repair mechanisms46,47. Another compatible explanation may be that the telomere ‘clock mechanism’ is under evolutionary selection because by possibly limiting organismal lifespan, it ensures a constant infusion of a diverse genetic pool into the population of some species.
Telomere length measurement
To understand the links between genetic defects in telomere maintenance, telomere length and disease pathogenesis, it is important to be able to quantify telomere length. Appreciation for a unified genetic mechanism for a number of syndromes discussed below has been in part due to advances in telomere length measurement and in assembling the normal ranges. Measuring telomere length has unique considerations because in a given cell there are many telomeres, and each telomere has a unique length. A complete telomere length profile therefore reflects the mean telomere length as well as the length distribution within a given cell type (for example, of the 92 telomeres in a human interphase cell). Studies in mice and yeast have shown that it is not the average but the shortest telomeres that determine cell responses, suggesting that only one, or a few, overtly short telomeres trigger the DNA damage response and the consequent checkpoint cascade34,48,49. For human fibroblasts, a threshold of five dysfunctional short telomeres seems to be needed to trigger a senescence response in vitro50. The shortest telomeres hence have a genetically dominant role in determining cellular phenotypes. In human studies, measuring the shortest telomeres in a population of cells is not readily feasible for practical reasons, and the mean telomere length in leukocytes or leukocyte subsets is typically measured instead. When examining telomere measurements within a mixed cell population, it is important to note that this is a surrogate marker of the mean in a heterogeneous cell population and that telomere length is mosaic, reflecting the telomere length regulation and replicative history of each cell type51,52. Methods that are commonly used to measure telomere length include restriction fragment analysis, fluorescence in situ hybridization and PCR-based techniques. These methods are reviewed in detail elsewhere53. In the monogenic syndromes discussed below, telomere length measurement on peripheral blood lymphocytes using fluorescence in situ hybridization and flow cytometry is a useful diagnostic tool in certain clinical settings54–57.
The monogenic telomere disorders
Several discoveries made during the past decade have underscored the importance of telomere length maintenance in human disease. The initial discoveries primarily came from studies of rare monogenic disorders of childhood but have more recently come to encompass common disorders that manifest well into adulthood. Together they can be appreciated as a single spectrum caused by defects in telomere maintenance. Synergistic with these genetic discoveries has been a deepening understanding of telomerase and telomere biology that has helped to define their underlying pathophysiology. In this section, we highlight the clinical manifestations as well as the unique genetics of the monogenic telomere syndromes.
Although the Mendelian telomere disorders have diverse clinical presentations, they share an underlying defect of short telomere length. Their grouping together as a syndrome spectrum is crucial for clinical decisions because the short telomere defect in affected individuals is present in germ cells and thus simultaneously affects multiple organs even when one disease presentation predominates. The mutant telomere genes, their prevalence in disease contexts and the genetic mechanisms by which mutant genes cause telomere shortening are summarized in Table 1.
Table 1.
Disease spectrum, frequency of gene mutations and mechanism of telomere shortening in telomere syndromes
| Gene | First diagnosis | Mutation frequency (%) |
Mechanism of telomere shortening | Refs |
|---|---|---|---|---|
|
TERT; TR |
Familial IPF | 8–15 |
|
36, 38, 39, 41, 54, 56, 68, 73, 74, 77, 111, 116 |
| Sporadic IPF | 1–3 | |||
| Aplastic anaemia | 3–5 | |||
| Autosomal dominant dyskeratosis congenita | 10* | |||
| Familial MDS–AML | 20 | |||
| DKC1 | De novo dyskeratosis congenita | ? |
|
9, 11, 124, 125 |
| X-linked recessive dyskeratosis congenita | 15–25* | |||
| Hoyeraal–Hreiderasson syndrome | ? | |||
| TINF2 | De novo dyskeratosis congenita | 15–25* |
|
62, 126, 127 |
| Autosomal-dominant dyskeratosis congenita | Rare | |||
| Hoyeraal–Hreiderasson syndrome | Rare | |||
| Revesz syndrome | Rare | |||
| NOP10 | Autosomal-recessive dyskeratosis congenita | ‡ | Presumed loss of telomerase function | 61 |
| NHP2 | Autosomal-recessive dyskeratosis congenita | ‡ | Presumed loss of telomerase function | 60 |
| TCAB1 | Autosomal-recessive dyskeratosis congenita | ‡ | Impaired TR trafficking; loss-of-function | 63 |
| CTC1 | Coats plus syndrome | 90 | Loss-of-function | 22, 64, 66, 67 |
| Autosomal-recessive dyskeratosis congenita | ? |
Refers to frequency of total dyskeratosis congenita patients.
Only two cases have been reported for each of these genes in the literature to date. AML, acute myeloid leukaemia; CTC1, conserved telomere protection component 1; DKC1, dyskeratosis congenita 1; IPF, idiopathic pulmonary fibrosis; MDS, myelodysplastic syndrome; TCAB1, telomerase Cajal body protein 1; TINF2, TRF1-interacting nuclear factor 2.
Childhood-onset telomere disorders
Dyskeratosis congenita was the first disorder to be linked to a mutant telomere gene9,11,38. Its incidence is thought to be rare, estimated at 1 in 1 million individuals. Dyskeratosis congenita is classically defined by mucocutaneous features: skin hyperpigmentation, oral leukoplakia and nail dystrophy58. The primary causes of mortality are bone marrow failure, pulmonary fibrosis and cancer58. The underlying biology of the cancer-prone state in dyskeratosis congenita is discussed later in this Review. Although affected individuals do not generally have overt progeroid features, they develop progressive and apparently irreversible organ failure. The organ failure patterns resemble many features of age-related disease13. In most cases (80%) of dyskeratosis congenita, the organ failure occurs first in the bone marrow in aplastic anaemia58,59.
Dyskeratosis congenita is characterized by the short telomere defect11,38,55. However, its genetic basis and mode of inheritance vary, and X-chromosome-linked-recessive, autosomal-dominant, autosomal-recessive and de novo cases have all been described58,59. Eight mutant genes have been identified in dyskeratosis congenita9,38,39,60–64, and all of them encode telomerase or telomere protein components (summarized in Table 1). Mutations in the X chromosome DKC1 gene, which encodes dyskerin, and TRF1-interacting nuclear factor 2 (TINF2), which encodes the shelterin component TIN2, are the most commonly identifiable in classic dyskeratosis congenita59. Heterozygous TINF2 mutations usually manifest in the first decade of life as severe de novo dyskeratosis congenita cases and are thus only rarely transmitted in an autosomal-dominant fashion62. Mutations in TERT and TR account for less than 10% of autosomal-dominant dyskeratosis congenita38,39. Altogether, mutations in four genes — DKC1, TINF2, TERT and TR — account for most dyskeratosis congenita cases with an identifiable genetic defect. Mutations in the telomerase accessory proteins NHP2 (Ref. 60), NOP10 (Ref. 61) and TCAB1 (Ref. 63) have been reported in rare autosomal-recessive families. A recent case report identified biallelic mutations in CTC1 in a patient with dyskeratosis congenita64. In ~40% of cases, dyskeratosis congenita remains genetically uncharacterized.
Two other rare disorders with onset in infancy have been linked to severe telomere dysfunction and mutant telomerase and telomere genes (Table 1). Hoyeraal–Hreidarsson syndrome is characterized by developmental delay, immunodeficiency and cerebellar hypoplasia58. Revesz syndrome is characterized by bilateral exudative retinopathy62. These more severe telomere syndromes are associated with extensive telomere shortening and often first present with complications related to immunodeficiency, and bone marrow failure develops later in life, similarly to dyskeratosis congenita65 (Fig. 2). Recently, biallelic mutations in CTC1 were identified in patients with Coats plus syndrome, which is characterized in part by exudative retinopathy and intracranial calcifications66,67. Both of these features can be seen in Hoyeraal–Hreidarsson and Revesz syndromes. CTC1 mutation carriers have a short telomere length relative to age-matched controls, but whether this is a pure telomere disorder or a compound defect that is also related to non-telomere functions of CTC1 remains to be fully determined22,66. On the basis of their overlapping clinical findings, Hoyeraal–Hreidarsson and Revesz syndromes (and probably Coats plus syndrome) represent the same spectrum of disease because retinopathy, intracranial calcifications and bone marrow abnormalities can be seen in all three disorders and occur in the setting of very short telomere length. The differing nomenclature of these disorders probably reflects in part the clinical complications that were first documented before elucidation of the shared underlying telomere defect.
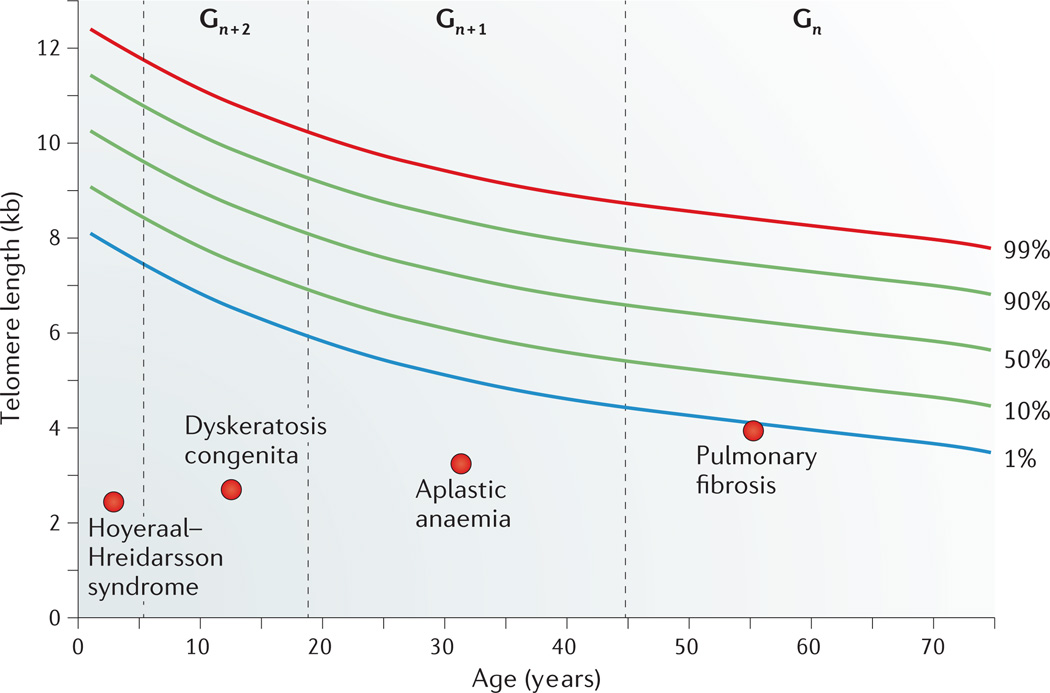
Figure 2. Age-dependent manifestations of telomere syndromes.
A schematic drawing that illustrates the typical range of telomere lengths by age in, for example, peripheral blood lymphocytes. At every age, telomere length displays a normal distribution that is defined by the percentile lines labelled on the right. Telomere length in individuals with four different clinical presentations across the age range is indicated. The dashed lines represent a typical age range in which these disorders may first manifest, and ‘Gn’, ‘Gn + 1’ and ‘Gn + 2’ designate three successive generations manifesting with earlier-onset and evolving disease type owing to progressive telomere shortening.
Adult-onset manifestations of telomere-mediated disease
Telomere disorders most commonly manifest as adult-onset disease and as a consequence of germline TERT or TR loss-of-function mutations13. The frequency of these mutations is highest in individuals with the progressive disorder IPF, which accounts for 8–15% of familial and 1–3% of sporadic cases54,56,68,69. In families, pulmonary fibrosis displays autosomal-dominant inheritance with age-dependent penetrance. In IPF patients with telomerase mutations, the mean age of onset is 51 years, although IPF can manifest as late as the ninth decade of life70,71. IPF is therefore one of the latest adult-onset presentations of a Mendelian disorder. Because IPF affects an estimated 100,000 individuals in the United States alone, lung disease is the most prevalent manifestation of mutant telomere genes12. In addition, 3–5% of aplastic anaemia patients carry mutant TERT and TR genes41,72,73. Liver cirrhosis, which is a known complication of dyskeratosis congenita, can also be a first adult-onset presentation of mutant telomerase genes39,74. The spectrum of monogenic telomere-mediated disorders is therefore broad, and the historically coined classic dyskeratosis congenita criteria identify only a small subset (perhaps less than 5%) of affected individuals12.
The telomere syndrome concept
The phenotypic heterogeneity caused by mutant telomere genes (summarized in Table 1) may initially give the impression that telomere shortening causes isolated cases of IPF, aplastic anaemia or dyskeratosis congenita. However, affected individuals often have subclinical disease concurrently in other organs, even when symptoms related to a single disorder predominate13,39. For example, patients with IPF who have mutant telomerase genes are at an increased risk of developing bone marrow failure and liver disease54,71. Conversely, individuals with telomere-related aplastic anaemia have an increased incidence of fatal pulmonary fibrosis when they are exposed to pulmonary toxic drugs in the bone marrow transplant setting, even though they may have previously had no symptoms58,71. Indeed, the co-occurrence of IPF and bone marrow failure, along with liver cirrhosis, is specific to and highly predictive of a germline telomere maintenance defect13,39,71. The shared underlying telomere defect in aplastic anaemia and IPF brings together clinical entities that were previously considered to be disparate and defines a recognizable syndrome complex that predominantly manifests in adults, in contrast to dyskeratosis congenita. Recognizing the adult manifestations of the telomere syndrome spectrum is important for diagnostic and treatment decisions in several common, important clinical settings12,56.
Telomere syndromes predispose to cancer
Patients with telomere syndromes are cancer-prone. In classic dyskeratosis congenita, where it has been studied, cancer diagnoses have been estimated to occur at an 11-fold increased incidence relative to the population in one series75. However, the cancer-related mortality is limited to approximately 10% of dyskeratosis congenita cases, with the remaining mortality attributable to the degenerative disease phenotypes described above. The cancer spectrum in dyskeratosis congenita has a predilection for high-turnover tissues with an increased incidence of squamous cell carcinomas of the skin, upper aerodigestive and anogenital tracts75. In addition, both dyskeratosis congenita and IPF patients are prone to developing haematological malignancies, commonly manifesting as myelodysplasia or acute myeloid leukaemia70,75,76. Acute myeloid leukaemia is a known complication of aplastic anaemia, but it may possibly be a first presentation of a germline mutation in TERT or TR in a subset of familial leukaemia cases (20%)77 and, at a much lower frequency, sporadic cases78.
The biological basis underlying the increased cancer incidence in dyskeratosis congenita may involve more than one mechanism. Defects in telomere function that lead to the genomic instability that is seen in some animal models have been implicated as one possible pathway79, although in most mouse studies, short telomeres have been found to be cancer-protective rather than cancer-promoting80. Telomere length limits the long-term proliferative capacity of the adaptive immune system in patients with telomere syndromes81, and this immunodeficiency in turn may lead to a failure of cancer surveillance. It is also possible that the increased cancer incidence may be intrinsic to the organ failure state itself, rather than to the telomere defect per se82. This latter model could explain the inherent increased risk of malignant transformation in both acquired and inherited forms of aplastic anaemia.
Genotype–phenotype correlations
Telomere length is a modifier of disease severity
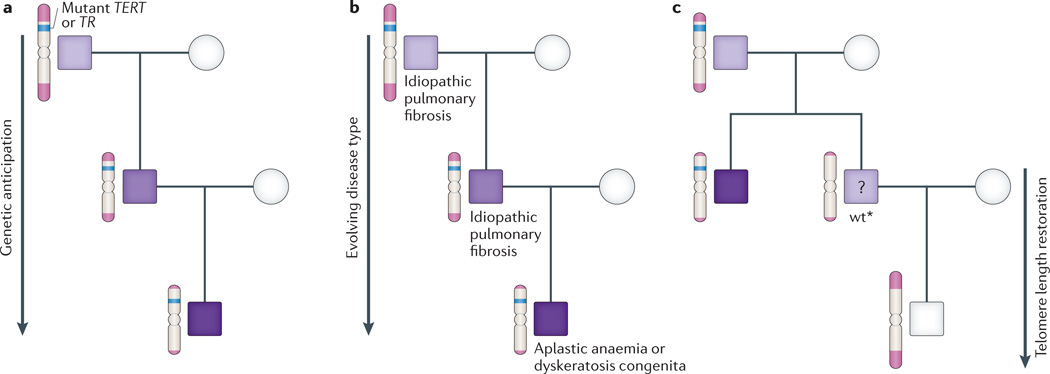
Mutations in TERT and TR are the most commonly identifiable defects in monogenic telomere syndromes. They manifest in an autosomal-dominant inheritance pattern because complete or partial loss-of-function of one allele is sufficient to perturb telomere maintenance38,39,41. The dominant pattern of inheritance in affected families distinguishes these essential telomerase genes, with their strict gene dosage requirement, as a rare exception among the usually recessive DNA repair disorders. They are therefore also substantially more prevalent. The most compelling evidence for the causal role of telomere shortening as a primary modifier of disease is that families with mutant telomerase genes display genetic anticipation39,83. In an autosomal-dominant family with a single mutant telomerase gene, adults with long telomeres may not develop disease until the seventh decade, their immediate offspring may show disease in mid-life, and in turn their progeny may be severely affected in childhood39. This anticipation closely parallels the progressive telomere shortening with each successive generation and occurs because telomeres shorten in germ cells, and the shortened telomeres are transmitted to progeny along with the mutant telomerase gene (Fig. 3a). Genetic anticipation due to telomere shortening was initially described in telomerase-null mice that similarly display progressively worsening phenotypes in late generations84,85. Aside from trinucleotide repeat expansion, telomere shortening is the only other characterized molecular mechanism of genetic anticipation in autosomal-dominant disorders.
Figure 3. Unique genetics of autosomal-dominant telomere syndromes.
a | Schema of a typical autosomal-dominant family with an inherited mutation in TERT (the reverse transcriptase) or TR (the telomerase RNA) showing earlier-onset disease with each generation, as illustrated by the darkening shades of purple. b | Disease type evolves in autosomal-dominant families from lung-predominant, which commonly manifests as idiopathic pulmonary fibrosis (IPF), to bone-marrow-failure-predominant, which presents as aplastic anaemia or dyskeratosis congenita. c | In mouse and human families, progeny of telomerase mutation carriers inherit the short telomeres even when they do not carry the mutant telomerase gene and are designated wt*. Mice with the wt* genotype have mild telomere-mediated phenotypes, but it remains unclear whether this is the situation for human cases (as represented by the question mark). The telomere length in human families is restored in progeny of these individuals. Figure adapted, with permission, from Ref. 12 © (2012) Elsevier.
Telomere length is a modifier of disease type
In autosomal-dominant families, the phenotype also predictably evolves with progressive telomere shortening. In earlier generations, pulmonary fibrosis is usually the primary manifestation, whereas in later generations, bone marrow failure in younger individuals is seen as a first complication71. On the basis of this evolving pattern, dyskeratosis congenita — with its classic mucocutaneous features — is predicted to manifest eventually in subsequent generations71 (Fig. 3b). Thus the entire spectrum of telomere-mediated disease can be seen within a single family, although the number of generations across which the disease evolves from a lung-predominant to a bone-marrow-predominant phenotype depends in part on the degree of telomerase loss-of-function and probably on the initial telomere length in the founder39,76. This unfolding pattern of disease in autosomal-dominant telomere syndromes is unique among Mendelian disorders. It can pose clinical challenges towards recognizing the genetic basis because in families, older generations may not develop disease until long after a young family member is diagnosed, and the disease type may be different. This heterogeneity also makes for complex clinical genetic counselling discussions. The extent of telomere shortening also correlates with the disease type and severity beyond autosomal-dominant families13,56,62,86 (Fig. 2). Hence, the degree of telomere shortness is a determinant of both disease severity and type.
Telomere length is a heritable trait
Evidence from mouse studies
Even when the telomere maintenance genes are intact, there is compelling evidence from model systems that telomere length — ‘the telotype’ — is a uniquely inherited genotype36,82,87. In most Mus musculus laboratory strains, the mean telomere length is ~50 kb. This contrasts with Mus castaneus mice, a wild-derived strain with a shorter mean telomere length (~15 kb) and a homogeneous telomere length distribution comparable to humans. M. castaneus therefore offers an opportunity to model telomere dynamics relevant to humans in a defined genetic background36,40,88. When quantified, telomere length is short in wild-type offspring of parent M. castaneus mice that have short telomeres, indicating that telomere length is inherited36,82 (Fig. 3c). Because these offspring mice are wild-type at every gene locus but have short telomeres, they are referred to as ‘wt*’ in the literature36,82. wt* mice provide a model for testing whether short telomeres on their own are sufficient to cause disease. Indeed, wt* mice show the same degenerative phenotypes as telomerase-null mice, albeit in a milder form36,82. Importantly, the telomere-related phenotypes can persist in wt* mice for several generations, until the telomere length set point is restored82 (Fig. 3c). The influence of parental telomere length in progeny occurs because telomere elongation, even at wild-type levels of telomerase, is only incremental during development, and telomerase restores the length of some, but not all, short telomeres82. Therefore, telomere length is inherited as a unique genotype, and its inheritance can be uncoupled from the telomerase and telomere gene loci.
Heritability of telomere length in humans
The heritability of telomere length is also supported by human studies. Progeny of individuals with TERT or TR mutations are known to have short telomeres relative to age-matched controls even when they do not inherit a mutant gene70,76,89 (Fig. 3c). It still remains unclear, however, whether the telomere shortening in these individuals is a risk factor for telomere-mediated phenotypes. In addition to studies in families, a number of population-based twin and non-twin studies have shown that parental telomere length (especially the paternal) influences offspring telomere length with heritability estimates ranging from 0.36–0.84 (reviewed in Ref. 90). These observations collectively support that telomere length is a genetically determined trait and that short telomeres are probably sufficient to cause disease phenotypes within similar thresholds to those seen in monogenic disorders. Because telomere length is polymorphic in populations and a shorter length accumulates with age, it is intriguing to consider that the short telotype may contribute to the missing heritability for disorders that are considered to have complex inheritance, especially those that have an age-dependent penetrance.
Mechanisms of telomere-mediated disease
Dissecting the underlying molecular mechanisms of telomere-mediated disease has important clinical implications because currently available treatments are limited and do not reverse the underlying pathology except with organ transplantation13. On a molecular level, telomere dysfunction is thought to be a consequence of shortening the telomeric DNA repeat tracts to the extent that they no longer support a functional shelterin complex18. The short, dysfunctional telomeres trigger a DNA damage response that resembles the response elicited by DNA double-strand breaks91–94. The consequent signalling cascades activate checkpoints that induce either cellular senescence or apoptosis, and in at least some contexts both processes occur simultaneously82,85,91,95. The downstream effector pathways of telomere-induced senescence are largely p53-dependent96, but there may be involvement of the retinoblastoma protein (RB) pathway in some settings97. The effector pathway involved may be cell- or tissue-type-specific, similarly to the responses to non-telomeric DNA damage98.
Although short telomeres cause similar cellular responses (namely, apoptosis and senescence) in different tissues, telomere-mediated disease manifests as apparently diverse disease processes. This is evident in the contrast between the bone marrow failure state of aplastic anaemia and the scar-forming phenotype of IPF. The telomerase-knockout mouse has provided an important model for understanding telomere-mediated disease mechanisms because there is a remarkable convergence with human disease phenotypes (an overview is shown in Table 2)36,82,84,85,97,99–111. Although it was initially clear that high-turnover tissues are particularly sensitive to telomere length85,101,112, more recently it has been recognized that telomere dysfunction causes prominent phenotypes in slow-turnover tissues97,99,100,103 (Table 2). Clinically, this is most apparent in the high penetrance of pulmonary disease in telomerase mutation carriers, even though the lung has a slow mitotic rate56,99. We propose that at least three primary mechanisms explain the diverse telomere-mediated disease phenotypes. These are discussed separately below within the organ context in which they have been considered, although almost certainly overlapping mechanisms have a role.
Table 2.
Organ-specific disease phenotypes in humans and mice with short telomeres
| Tissue type | Disease manifestations in humans with telomere syndromes |
Telomere phenotypes in mice with short telomeres |
|---|---|---|
| High-turnover tissues | ||
| Skin | Premature hair greying58,59 | Premature hair greying101 |
| Bone marrow | Aplastic anaemia111,116 | |
| Immune | ||
| Intestinal epithelium | Enterocolitis65,110 | |
| Slow-turnover tissues | ||
| Lung | Emphysema with cigarette smoke99 | |
| Liver | Cryptogenic liver fibrosis–cirrhosis13,74 | Fibrotic fatty liver changes after injury102 |
| Bone | Decreased threshold for osteoporosis in Wrn-deficient mice107 | |
| Endocrine | Uncertain | |
| Cardiac muscle | Dilated cardiomyopathy100 | |
| Skeletal muscle | Decreased threshold for Duchenne myopathy105 | |
| Cancer | ||
| Multiple tissue types | Gastrointestinal microadenoma36 | |
AML, acute myeloid leukaemia; ER, endoplasmic reticulum; HSC, haematopoietic stem cell; MDS, myelodysplastic syndrome; NK, natural killer; Wrn, Werner syndrome ATP-dependent helicase.
Stem cell failure in highly proliferative tissues
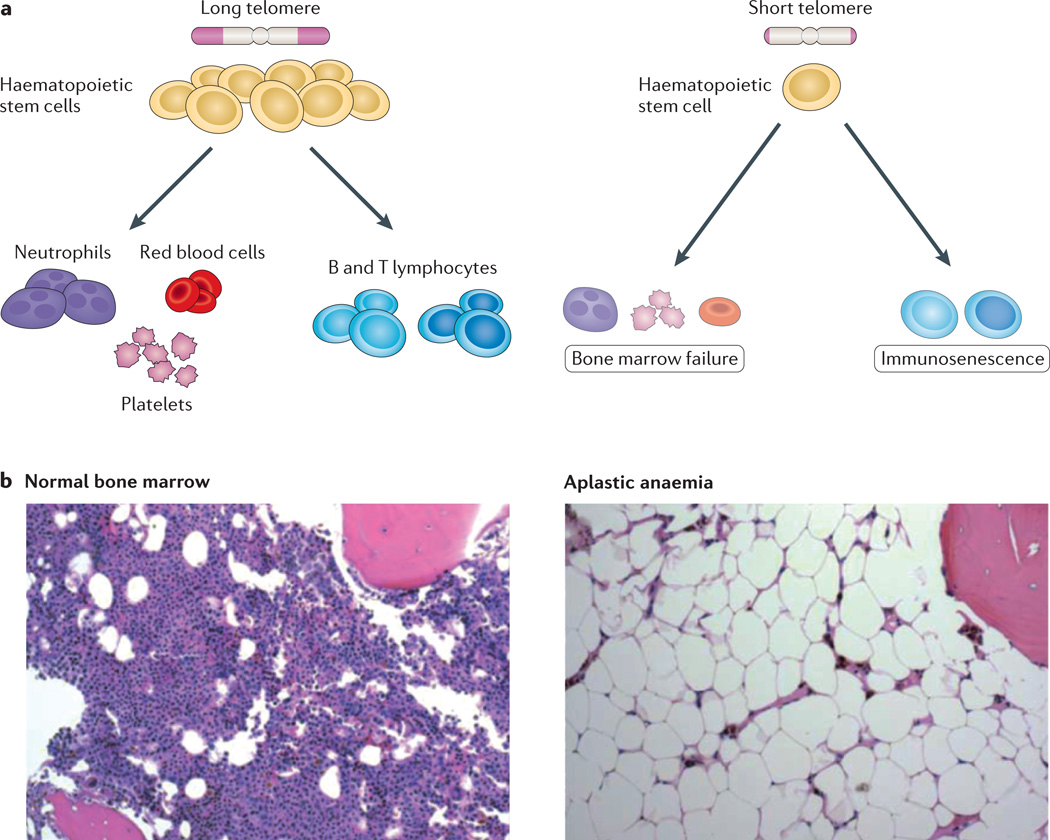
Cell turnover is remarkably high in the haematopoietic compartment, where an estimated 109 cells are produced every hour113. It is therefore not surprising that telomere-associated disease manifests so prominently in the bone marrow. Haematopoiesis relies on the self-renewal and differentiation capacity of a well-characterized oligoclonal stem cell compartment. In both humans and mice, there is clear evidence that short telomeres cause quantitative and qualitative defects in HSCs, which manifest as stem cell exhaustion36,41,71,114–117 (Fig. 4). The cellular mechanisms and the downstream effectors of telomere-mediated stem cell failure are not completely known, but deletion of cyclin-dependent kinase inhibitor 1a (Cdkn1a), which encodes p21, a transcriptional target of p53, partially rescues the HSC self-renewal defect in mice118. In humans, the aplastic anaemia phenotype can be reversed with allogeneic stem cell transplant, indicating that this telomere-related stem cell failure state is primarily cell-autonomous. Telomere dysfunction also causes stem cell failure phenotypes in other tissues. For example, in the intestinal epithelium, it manifests as villous atrophy due to a loss of crypt stem cells36,101,118.
Figure 4. Short telomeres cause haematopoietic stem cell failure.
a | Simplified schema of the haematopoiesis hierarchy with intact telomere length (left panel) and in the presence of telomere dysfunction (right panel). Telomere dysfunction causes both quantitative and qualitative defects in haematopoietic stem cells, which cause a decrease in mature blood forms. Defects in lymphopoiesis also cause immune defects. b | Histopathology of normal bone marrow biopsy shows intact marrow cellularity and haematopoietic cellular elements (left panel). In the right panel, a photomicrograph of a bone marrow biopsy from an individual with aplastic anaemia shows acellular marrow and replacement of the marrow parenchyma by fat. Panel b reproduced, with permission, from Ref. 13 © (2009) Annual Reviews.
Multiple hits are additive to telomere dysfunction in slow-turnover tissues
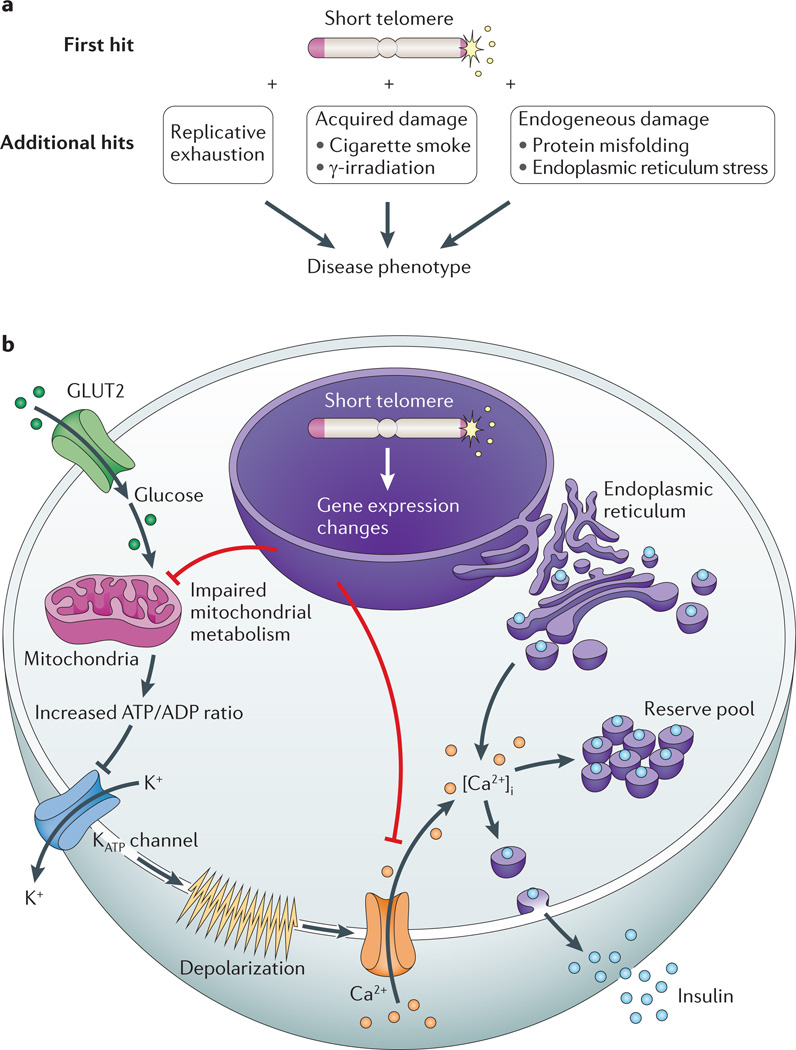
In contrast to the bone marrow, in the lung, cell turnover is very slow (2% per week in murine alveolar epithelial cells)99. Moreover, the telomere defect alone is not sufficient to induce spontaneous lung disease in mice99. Instead, a second insult is required, and when mice with short telomeres are experimentally exposed to cigarette smoke, which is known to accelerate disease onset in human telomerase mutation carriers, they develop lung disease99. In this setting, the cigarette smoke causes additive damage to dysfunctional telomeres in lung epithelial cells. The cumulative effect is likely to reach a threshold of cellular damage that develops into an airspace destruction phenotype recognized as emphysema. The requirement for a ‘second hit’ in the lung may be related to the fact that its basal proliferation rate is very slow, and therefore replicative exhaustion is delayed. Indeed, in mouse models, short telomeres have been shown to lower the disease threshold to other exogenous and endogeneous damage, such as γ-irradiation119 and endoplasmic reticulum stress due to protein misfolding97 (Fig. 5a). A model that requires multiple additional hits for phenotypes to manifest in slow-turnover tissues could explain why telomere-mediated lung disease represents an attenuated, adult-onset phenotype that is commonly seen after middle age12,99.
Figure 5. Mechanisms of telomere-mediated disease in slow-turnover tissues.
a | Telomere length lowers the threshold to endogenous and exogenous damage, which is hypothesized to precipitate disease in slow-turnover tissues. b | A schematic of an insulin-producing β-cell showing the mechanism of a telomere-mediated insulin exocytosis defect. Glucose passively enters β-cells through the glucose transporter type 2 (GLUT2; also known as SLC2A2). Following glycolysis, ATP is generated by oxidative phosphorylation in the mitochondria. The net cytosolic change of the ATP/ADP ratio leads to closure of ATP-dependent K+ channels and opening of Ca2+ channels. The influx of extracellular Ca2+ results in an increase in the concentration of free, intracellular Ca2+ ([Ca2+]i), which triggers the release of insulin from both reserve and back-up pools. Short telomeres cause gene expression changes in pancreatic islets, which are associated with global metabolic dysregulation, mitochondrial dysfunction and concurrent defects in glucose-dependent and glucose-independent Ca2+ handling.
How telomere dysfunction causes tissue remodelling, as seen in fibrosis (in the lung and liver), as well as air space destruction in emphysema is not fully understood. Because telomere dysfunction causes epithelial defects in other tissues, it has been hypothesized that the fibrotic phenotypes may be consequences of epithelial stem cell exhaustion56. One model to explain the progressive fibrosis that characterizes these disorders is that epithelial senescence or apoptosis stimulates a lung-remodelling response56,99. Indeed, some types of cellular senescence, including the telomere-induced replicative type, are associated with an in vitro senescence-associated secretory phenotype (SASP), where cytokines and proteases are detected in culture media120. Differential secretory profiles have also been detected in sera from mice with short telomeres121. Therefore, telomere dysfunction in epithelial cells, or perhaps another cell type, might cause parenchymal organ remodelling, which manifests as fibrosis and/or emphysema46,54,99.
Exocytosis defects in pancreatic β-cells
Although telomere dysfunction causes obvious histopathology in the bone marrow and parenchymal organs, there is evidence that in some settings, short dysfunctional telomeres compromise organ homeostasis even when cell mass is preserved. This mechanism has been elucidated in adult mouse β-cells of the pancreatic islets, which have a fairly slow turnover (4% per week). Short telomeres cause spontaneous insulin secretion defects in vivo and in vitro, even when the β-cell mass is intact97 (Fig. 5b). Although they appear morphologically normal, mouse β-cells with dysfunctional telomeres show the hallmarks of senescence. They have an activated DNA damage response, impaired proliferation, p16INK4A upregulation and altered gene expression97. The dysregulation of gene expression affects pathways that are essential for insulin secretion signalling and exocytosis, including mitochondrial function and Ca2+ handling97. Therefore, it seems that telomere-induced senescence represents more than a loss of replicative potential and can limit cellular function even when cells appear morphologically intact97. Gene expression changes due to telomere shortening also occur in vitro in fibroblasts undergoing replicative senescence122. Notably, impairment of insulin secretion in the presence of an intact β-cell mass also occurs in the early stages of human age-related diabetes123. The fact that the gene expression changes that occur during telomere-induced senescence cause functional changes in the absence of structural tissue disruption highlights a novel mechanism by which senescence and its associated gene expression changes cause disease. Shortened dysfunctional telomeres in other mouse cell types, such as cardiac myocytes and hepatocytes, have also been associated with decreased mitochondrial copy number and defective oxidative metabolism103. In these tissues, the metabolic dysfunction appears to be due to the effect of downregulation of PGC1α, which is a transcriptional co-activator that is important for mitochondrial biogenesis103. Future studies may unravel a role of telomere dysfunction in other secretory tissues, such as the nervous system, which like the endocrine pancreas manifests an age-dependent functional decline.
Summary and implications
Telomere syndromes represent an archetype of premature-ageing syndromes because the short telomere defect they share is progressively acquired with age in humans. Their recognition as a syndrome spectrum has important clinical implications because it brings together seemingly unrelated disease states that share the shortened telomere pathology, as well as overlapping phenotypes. There is compelling evidence that telomere length is heritable, and because it is a measurable genotype it may eventually be shown to account for previously missing heritability of a subset of age-dependent complex disorders. Beyond its canonical phenotypes in high-turnover tissues, telomere dysfunction is sufficient to cause — and indeed commonly causes — degenerative phenotypes in slow-turnover tissues. These additional phenotypes now extend the scope of telomere-mediated disease into clinical contexts of prevalent conditions, such as IPF and diabetes.
The now-evident connections between telomere biology and disease are expected to evolve in the coming years. As the tools for understanding the basis of Mendelian disorders continue to abound, novel genes in uncharacterized telomere syndrome cases almost certainly will emerge. Such discoveries would enrich the understanding of how telomeres are maintained. With the increasing clinical appreciation of new disease patterns and the availability of genetic testing, defining how telomere biology can inform individualized medicine decisions will be important. Another important challenge that emerges from the clinical connections we have discussed here will be how to integrate the science of telomeres into advancing the understanding of a number of untreatable conditions.
Relevantly to the topic of this Review, we emphasize that telomere biology research started with a curiosity-driven focus on the molecular mechanisms of chromosome biology. Connections to disease followed decades after the fundamental foundations were laid, in contrast to much human genetics-initiated investigation. Many of the early discoveries came from studying simple organisms, such as the protozoan Tetrahymena thermophila and yeasts, in which clinical implications could not have been prospectively envisioned6. The emerging appreciation for the scope of telomere-mediated disease now in turn poses new possibilities to unravel still-puzzling fundamental aspects of telomeres that can inform clinical paradigms.
Acknowledgements
Much of the basic biology that is relevant to disease and that is discussed in this Review has been studied in a number of model organisms, and we acknowledge that owing to space limitations we could not reference that important work comprehensively. We are grateful to several colleagues and laboratory members for helpful discussions and comments on the manuscript. M.A. acknowledges research support from the US National Institutes of Health Heart, Lung and Blood Institute (NHLBI), the US National Cancer Institute (NCI) and the Maryland Stem Cell and Flight Attendants Medical Research Foundations. E.H.B. acknowledges support from the US National Institute of General Medical Sciences (NIGMS) and the NCI.
Glossary
- Senescence
Classically defined as a permanent arrest in the cell cycle in G0.
- Pulmonary fibrosis
A scarring disorder of the lung in which alveolar structures are replaced with extracellular matrix components such as collagen.
- Cajal body
A small subnuclear organelle that contains the telomerase ribonucleoprotein complex, as well as other newly assembled ribonucleoproteins.
- Haploinsufficiency
A state in a diploid organism whereby one normal gene copy is insufficient for normal function.
- Oral leukoplakia
White patches in the mucosa of the mouth; this is often considered to be a precancerous state.
- Nail dystrophy
Abnormal or absent finger nails.
- Exudative retinopathy
A condition in which white–yellow spots are seen in the retina, indicating damage to retina blood vessels. When it is an isolated finding, it is often referred to as Coats disease.
- Coats plus syndrome
A syndrome defined by multiple congenital anomalies that are beyond the retinal abnormalities of Coats disease patients.
- Aplastic anaemia
A bone marrow failure state characterized by low blood counts and a paucity of haematopoietic cells in the bone marrow.
- Genetic anticipation
A pattern by which a certain phenotype manifests at an earlier age and with increasing severity with successive generations in autosomal-dominant disorders.
- Heritability
A quantification of the genetic component contributing to a specific trait.
- Missing heritability
The state in which the specific genotypes underlying the inheritance of a certain trait are not known.
- Allogeneic stem cell transplant
Transplant of stem cells, most frequently bone-marrow-derived, from an alternative donor to replace a failed organ.
- Cell-autonomous
An effect that is intrinsic to a specific cell type and not to an independent factor beyond that cell type.
- Crypt stem cells
Cells in the intestinal crypt that are responsible for the regenerative capacity of the epithelial protective barrier in the intestine.
- Tissue remodeling
The process by which tissue structures change, often in the setting of recovery from injury or healing.
- Senescence-associated secretory phenotype (SASP)
The phenomenon by which cultured senescent cells secrete growth factors, cytokines and proteases.
Footnotes
Competing interests statement
The authors declare competing financial interests: see Web version for details.
References
- 1.Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture) Angew. Chem. Int. Edn Engl. 2010;49:7405–7421. doi: 10.1002/anie.201002387. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 3. Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114.. References 2 and 3 showed that telomere length shortens in human cells with age and limits their in vitro replicative potential.
- 4.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 5.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 7. Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428.. In this study, telomerase activity was found to be enriched in cancer and germ cells, suggesting that telomerase has a role in extending replicative potential.
- 8.Buseman CM, Wright WE, Shay JW. Is telomerase a viable target in cancer? Mutat. Res. 2012;730:90–97. doi: 10.1016/j.mrfmmm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heiss NS, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature Genet. 1998;19:32–38. doi: 10.1038/ng0598-32.. This study identified mutations in the X-linked DKC1 gene as a first cause of dyskeratosis congenita.
- 10.Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell. Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141.. This study showed that dyskerin is essential for telomerase RNA stability and that dyskeratosis congenita cell lines have a short telomere length, linking this disease with defects in telomere maintenance.
- 12.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat. Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armanios M. Syndromes of telomere shortening. Annu. Rev. Genom. Hum. Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyzis RK, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl Acad. Sci. USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allshire RC, et al. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988;332:656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri H, et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- 17.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 18.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 19.Surovtseva YV, et al. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol. Cell. 2009;36:207–218. doi: 10.1016/j.molcel.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake Y, et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol. Cell. 2009;36:193–206. doi: 10.1016/j.molcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Price CM, et al. Evolution of CST function in telomere maintenance. Cell Cycle. 2010;9:3157–3165. doi: 10.4161/cc.9.16.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu P, et al. CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J. 2012;31:2309–2321. doi: 10.1038/emboj.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 24.Lingner J, et al. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 25.Feng J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 26.Podlevsky JD, Chen JJ. It all comes together at the ends: telomerase structure, function, and biogenesis. Mutat. Res. 2012;730:3–11. doi: 10.1016/j.mrfmmm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb. Perspect. Biol. 2011;3:a003558. doi: 10.1101/cshperspect.a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 30.Venteicher AS, et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tycowski KT, Shu MD, Kukoyi A, Steitz JA. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol. Cell. 2009;34:47–57. doi: 10.1016/j.molcel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diede SJ, Gottschling DE. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases α and δ. Cell. 1999;99:723–733. doi: 10.1016/s0092-8674(00)81670-0. [DOI] [PubMed] [Google Scholar]
- 33.Marcand S, Brevet V, Mann C, Gilson E. Cell cycle restriction of telomere elongation. Curr. Biol. 2000;10:487–490. doi: 10.1016/s0960-9822(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira MT, Arneric M, Sperisen P, Lingner J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell. 2004;117:323–335. doi: 10.1016/s0092-8674(04)00334-4. [DOI] [PubMed] [Google Scholar]
- 35.Erdmann N, Liu Y, Harrington L. Distinct dosage requirements for the maintenance of long and short telomeres in mTert heterozygous mice. Proc. Natl Acad. Sci. USA. 2004;101:6080–6085. doi: 10.1073/pnas.0401580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hao LY, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020.. In reference 36, using CAST/EiJ mice that have telomere length similar to humans, this paper established that telomerase haploinsufficiency is sufficient to cause dyskeratosis-congenita-like phenotypes. It also showed that telomere length is inherited and can cause degenerative phenotypes even when telomerase is wild-type.
- 37.Mozdy AD, Cech TR. Low abundance of telomerase in yeast: implications for telomerase haploinsufficiency. RNA. 2006;12:1721–1737. doi: 10.1261/rna.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vulliamy T, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 39. Armanios M, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl Acad. Sci. USA. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102.. References 38 and 39 identified mutations in the essential telomerase genes, TERT and TR, in dyskeratosis congenita.
- 40.Strong MA, et al. Phenotypes in mTERT+/− and mTERT+/− mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol. Cell. Biol. 2011;31:2369–2379. doi: 10.1128/MCB.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamaguchi H, et al. Mutations in TERT , the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980.. This study identified TERT mutations in patients with aplastic anaemia.
- 42.Chiu CP, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 43.Vaziri H, et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc. Natl Acad. Sci. USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Greider CW. Telomerase RNA levels limit the telomere length equilibrium. Cold Spring Harb. Symp. Quant. Biol. 2006;71:225–229. doi: 10.1101/sqb.2006.71.063. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Monson EK, Teng SC, Schulz VP, Zakian VA. Pif1p helicase, a catalytic inhibitor of telomerase in yeast. Science. 2000;289:771–774. doi: 10.1126/science.289.5480.771. [DOI] [PubMed] [Google Scholar]
- 47.Makovets S, Blackburn EH. DNA damage signalling prevents deleterious telomere addition at DNA breaks. Nature Cell Biol. 2009;11:1383–1386. doi: 10.1038/ncb1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9.. This study established that the shortest telomeres have a genetically dominant influence on cellular phenotypes.
- 49.McEachern MJ, Blackburn EH. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 1996;10:1822–1834. doi: 10.1101/gad.10.14.1822. [DOI] [PubMed] [Google Scholar]
- 50.Kaul Z, Cesare AJ, Huschtscha LI, Neumann AA, Reddel RR. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 2011;13:52–59. doi: 10.1038/embor.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J. Immunol. Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rufer N, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-Caveats and a critical assessment of the available technologies and tools. Mutat. Res. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alder JK, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alter BP, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157.. References 54–56 showed that lymphocyte telomere length using flow cytometry and fluorescence in situ hybridization could be a useful diagnostic marker in identifying individuals with telomere-related disease.
- 57.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nature Protoc. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 58.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatr. Transplant. 2007;11:584–594. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 59.Savage SA, Alter BP. Dyskeratosis congenita. Hematol. Oncol. Clin. North Am. 2009;23:215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vulliamy T, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc. Natl Acad. Sci. USA. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walne AJ, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Savage SA, et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004.. This paper was the first to show that mutations in a shelterin component are a cause of dyskeratosis congenita.
- 63.Zhong F, et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keller RB, et al. CTC1 mutations in a patient with dyskeratosis congenita. Pediatr. Blood Cancer. 2012;59:311–314. doi: 10.1002/pbc.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jyonouchi S, Forbes L, Ruchelli E, Sullivan KE. Dyskeratosis congenita: a combined immunodeficiency with broad clinical spectrum—a single-center pediatric experience. Pediatr. Allergy Immunol. 2011;22:313–319. doi: 10.1111/j.1399-3038.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- 66.Anderson BH, et al. Mutations in CTC1 , encoding conserved telomere maintenance component 1, cause Coats plus. Nature Genet. 2012;44:338–342. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]
- 67. Polvi A, et al. Mutations in CTC1 , encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am. J. Hum. Genet. 2012;90:540–549. doi: 10.1016/j.ajhg.2012.02.002.. References 66 and 67 identified CTC1 mutations in Coats plus syndrome. Reference 66 identified short telomere length in these patients, suggesting a role for telomere dysfunction in the pathophysiology of this syndrome.
- 68. Tsakiri KD, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl Acad. Sci. USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104.. References 56 and 68 identified mutations in the essential telomerase genes in familial pulmonary fibrosis.
- 69.Cronkhite JT, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diaz de Leon A, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT ) mutations. PLoS ONE. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du HY, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2008;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yamaguchi H, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335.. This study identified TR mutations in patients with bone marrow failure.
- 74.Calado RT, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS ONE. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alder JK, et al. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS Genet. 2011;7:e1001352. doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirwan M, et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum. Mutat. 2009;30:1567–1573. doi: 10.1002/humu.21115. [DOI] [PubMed] [Google Scholar]
- 78.Calado RT, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc. Natl Acad. Sci. USA. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nature Rev. Cancer. 2008;8:450–458. doi: 10.1038/nrc2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knudson M, Kulkarni S, Ballas ZK, Bessler M, Goldman F. Association of immune abnormalities with telomere shortening in autosomal-dominant dyskeratosis congenita. Blood. 2005;105:682–688. doi: 10.1182/blood-2004-04-1673. [DOI] [PubMed] [Google Scholar]
- 82.Armanios M, et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vulliamy T, et al. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nature Genet. 2004;36:447–449. doi: 10.1038/ng1346.. References 83 and 39 (above) showed that mutations in the essential telomerase genes cause genetic anticipation in autosomal-dominant dyskeratosis congenita.
- 84.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA . Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 85. Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345.. References 84 and 85 are two landmark papers that established that telomerase itself is not essential and that short telomeres cause apoptosis and cell loss in high-turnover tissues.
- 86.Alter BP, et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97:353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Makovets S, Williams TL, Blackburn EH. The telotype defines the telomere state in Saccharomyces cerevisiae and is inherited as a dominant non- Mendelian characteristic in cells lacking telomerase. Genetics. 2008;178:245–257. doi: 10.1534/genetics.107.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldman F, et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc. Natl Acad. Sci. USA. 2005;102:17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat. Res. 2012;730:68–74. doi: 10.1016/j.mrfmmm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 92.Enomoto S, Glowczewski L, Berman J. MEC3, MEC1, and DDC2 are essential components of a telomere checkpoint pathway required for cell cycle arrest during senescence in Saccharomyces cerevisiae . Mol. Biol. Cell. 2002;13:2626–2638. doi: 10.1091/mbc.02-02-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.IJpma A, Greider CW. Short telomeres induce a DNA damage response in Saccharomyces cerevisiae . Mol. Biol. Cell. 2003;14:987–1001. doi: 10.1091/mbc.02-04-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nautiyal S, DeRisi JL, Blackburn EH. The genome-wide expression response to telomerase deletion in Saccharomyces cerevisiae . Proc. Natl Acad. Sci. USA. 2002;99:9316–9321. doi: 10.1073/pnas.142162499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hemann MT, et al. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol. Biol. Cell. 2001;12:2023–2030. doi: 10.1091/mbc.12.7.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chin L, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 97.Guo N, et al. Short telomeres compromise β-cell signaling and survival. PLoS ONE. 2011;6:e17858. doi: 10.1371/journal.pone.0017858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Alder JK, et al. Telomere length is a determinant of emphysema susceptibility. Am. J. Respir. Crit. Care Med. 2011;183:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leri A, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO. J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rudolph KL, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 102.Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253–1258. doi: 10.1126/science.287.5456.1253. [DOI] [PubMed] [Google Scholar]
- 103. Sahin E, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787.. References 97 and 103 implicate short telomeres as a cause of mitochondrial dysfunction, a pathogenic mechanism that highlights a role for telomere dysfunction in slow-turnover tissues.
- 104.Herrera E, Martinez AC, Blasco MA. Impaired germinal center reaction in mice with short telomeres. EMBO. J. 2000;19:472–481. doi: 10.1093/emboj/19.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sacco A, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pignolo RJ, et al. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell. 2008;7:23–31. doi: 10.1111/j.1474-9726.2007.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang S, et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 108.Minamino T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 109.Lee BW, Yap HK, Quah TC, Chong A, Seah CC. T cell immunodeficiency in dyskeratosis congenita. Arch. Dis. Child. 1992;67:524–526. doi: 10.1136/adc.67.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sznajer Y, et al. Further delineation of the congenital form of X-linked dyskeratosis congenita (Hoyeraal- Hreidarsson syndrome) Eur. J. Pediatr. 2003;162:863–867. doi: 10.1007/s00431-003-1317-5. [DOI] [PubMed] [Google Scholar]
- 111.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 112.Herrera E, et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO. J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Metcalf D. The Molecular Control of Blood Cells. Harvard Univ. Press; 1988. pp. 1–22. [Google Scholar]
- 114.Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- 115.Goldman FD, et al. Characterization of primitive hematopoietic cells from patients with dyskeratosis congenita. Blood. 2008;111:4523–4531. doi: 10.1182/blood-2007-10-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fogarty PF, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA . Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 117.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 118.Choudhury AR, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 119.Wong KK, et al. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nature Genet. 2000;26:85–88. doi: 10.1038/79232. [DOI] [PubMed] [Google Scholar]
- 120.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang H, et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc. Natl Acad. Sci. USA. 2008;105:11299–11304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang H, Pan KH, Cohen SN. Senescencespecific gene expression fingerprints reveal cell-typedependent physical clustering of up-regulated chromosomal loci. Proc. Natl Acad. Sci. USA. 2003;100:3251–3256. doi: 10.1073/pnas.2627983100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J. Clin. Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Knight SW, et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br. J. Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- 125.Yaghmai R, et al. Overlap of dyskeratosis congenita with the Hoyeraal-Hreidarsson syndrome. J. Pediatr. 2000;136:390–393. doi: 10.1067/mpd.2000.104295. [DOI] [PubMed] [Google Scholar]
- 126.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiang YJ, Kim SH, Tessarollo L, Campisi J, Hodes RJ. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Mol. Cell. Biol. 2004;24:6631–6634. doi: 10.1128/MCB.24.15.6631-6634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]