Abstract
Mutations generate sequence diversity and provide a substrate for selection. The rate of de novo mutations is therefore of major importance to evolution. We conducted a study of genomewide mutation rate by sequencing the entire genomes of 78 Icelandic parent-offspring trios at high coverage. Here we show that in our samples, with an average father’s age of 29.7, the average de novo mutation rate is 1.20×10−8 per nucleotide per generation. Most strikingly, the diversity in mutation rate of single-nucleotide polymorphism (SNP) is dominated by the age of the father at conception of the child. The effect is an increase of about 2 mutations per year. After accounting for random Poisson variation, father’s age is estimated to explain nearly all of the remaining variation in the de novo mutation counts. These observations shed light on the importance of the father’s age on the risk of diseases such as schizophrenia and autism.
The rate of de novo mutations and factors that influence it have always been a focus of genetics research1. However, investigations of de novo mutations through direct examinations of parent-offspring transmissions were previously mostly limited to studying specific genes2,3 or regions4–7. Recent studies that employed whole genome sequencing8,9 are important but too small to adequately address the question of diversity in mutation rate. To better understand the nature of de novo mutations we designed and conducted a study as follows.
Samples and mutation calls
As a part of a large sequencing project in Iceland10–12 (Methods Summary), we sequenced 78 trios, a total of 219 distinct individuals, to over 30X average coverage (Fig. 1). Forty-four of the probands (offspring) have autism spectrum disorder (ASD), and 21 are schizophrenic (SZ). The other thirteen probands were included for various reasons, including the construction of multi-generation families. The probands include five cases where at least one grandchild was also sequenced. In addition, 1859 other Icelanders, treated as population samples, were whole genome sequenced (all > 10X, 469 > 30X). These were used as population samples to help filtering out artifacts. Sequence calling was performed for each individual using GATK (Methods Summary). The focus here is on SNP mutations. The investigation was restricted to autosomal chromosomes.
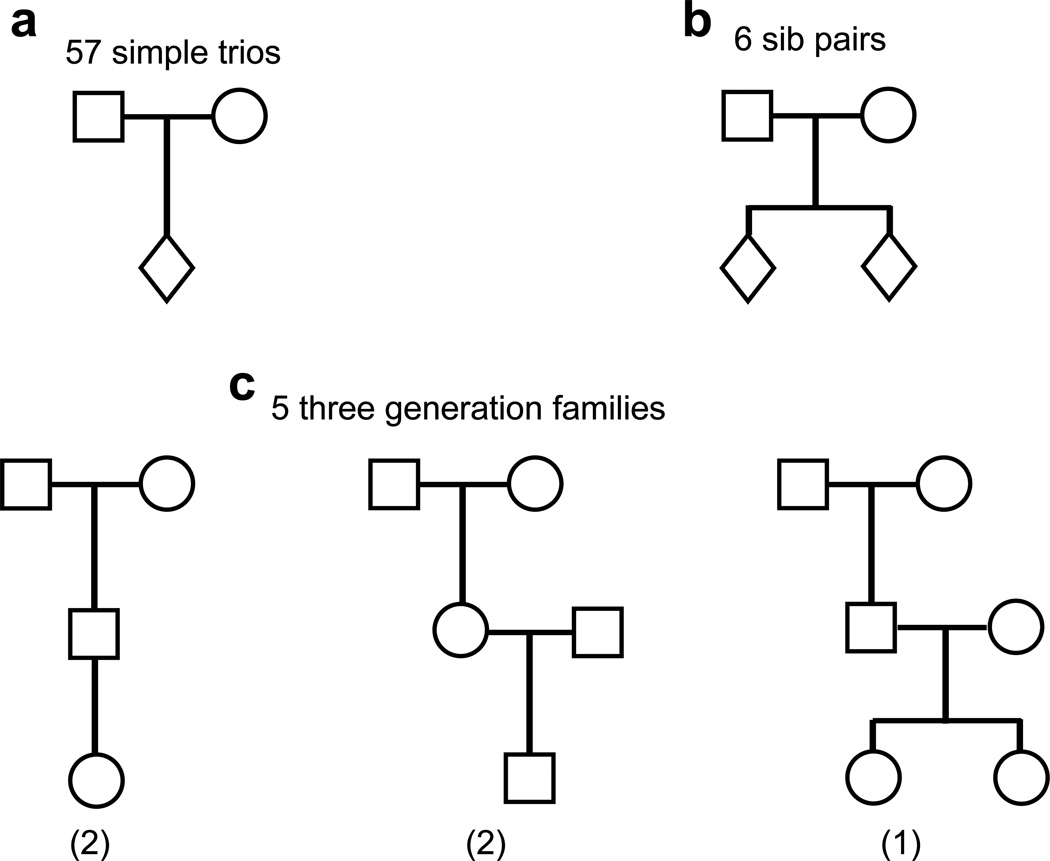
Figure 1. A summary of the family types.
a Fifty-seven simple trios. b Six sib pairs accounting for 12 trios. c Five three generation families accounting for 9 trios.
Criteria for calling a de novo SNP mutation were as follows. (i) All variants that have likelihood ratio lik(AR)/lik(RR) or lik(AA)/lik(RR) > 104, where R denotes the reference allele and A the alternative allele, in any of the 1859 population samples, were excluded. Some recurrent mutations could have been filtered out, but the number should be small. The de novo mutation calls further satisfy the conditions that (ii) there are at least 16 quality reads for the proband at the mutated site, (iii) the likelihood ratio lik(AR)/lik(RR) is above 1010, and (iv) for both parents the ratio lik(RR)/lik(AR) is above 100. Applying criterion (i) through (iv) gave 6221 candidate mutations. Further examination led us to apply additional filtering (v) by including only SNPs where the number of A allele calls is above 30% among the proband’s quality sequence reads. This was considered necessary because there was an abnormally high number of putative mutation calls where, despite extremely high lik(AR)/lik(RR) ratios for the proband, the fraction of A calls were low (Supplementary Fig. 1). Applying (v) eliminated 1,285 candidate mutations (Supplementary Information). With high coverage, the false negatives resulting from (v) is estimated to be a modest 2% (Supplementary Information). After 3 more candidates were identified as false positives by Sanger sequencing (see section on validation below), a total of 4,933 de novo mutations, or an average of 63.2 per trio, were called. (The de novo mutations are listed individually is in Supplementary Table 1.)
Parent of origin and father’s age
For the five trios where a child of the proband was also sequenced, parent of origin of each de novo mutation called was determined as follows. If the paternal haplotype of the proband was transmitted to his/her child, and the child also carries the mutation, then the mutation was considered to be paternal in origin. If the child carrying the paternal haplotype of the parent does not have the mutation, then it is inferred that the mutation is on the maternal chromosome of the proband. Similar logic was applied when the child inherited the maternal haplotype of the proband. In the five trios, the average number of paternal and maternal mutations is 55.4 and 14.2 respectively (Table 1). If mutations were purely random with no systematic difference between trios, their number should be Poisson distributed with the variance equal to the mean. The data, however, exhibit over dispersion (Table 1). This is much more striking for the paternal mutations (variance = 428.8, P = 1.2 ×10−5) than the maternal mutations (variance = 48.7, P = 0.016). Moreover, the number of paternal mutations has a monotonic relationship with the father’s age at conception of the child. Here, the mean number of paternal mutations is substantially higher than the mean number of maternal mutations (ratio = 3.9), but the difference is even greater for the variance (ratio = 8.8). Hence, variation of de novo mutation counts in these individuals is mostly driven by the paternal mutations.
Table 1.
De novo mutations observed with parental origin assigned
| Number of de novo mutations in proband | |||||
|---|---|---|---|---|---|
| Father's Age (yrs) |
Mother's Age (yrs) |
Paternal Chromosome |
Maternal Chromosome |
Combined | |
| Trio 1 | 21,8 | 19.3 | 39 | 9 | 48 |
| Trio 2 | 22.7 | 19.8 | 43 | 10 | 53 |
| Trio 3 | 25.0 | 22.1 | 51 | 11 | 62 |
| Trio 4 | 36.2 | 32.2 | 53 | 26 | 79 |
| Trio 5 | 40.0 | 39.1 | 91 | 15 | 106 |
| Mean | 29.1 | 26.5 | 55.4 | 14.2 | 69.6 |
| SD | 8.4 | 8.8 | 20.7 | 7.0 | 23.5 |
| Variance | 70.2 | 77.0 | 428.8 | 48.7 | 555.3 |
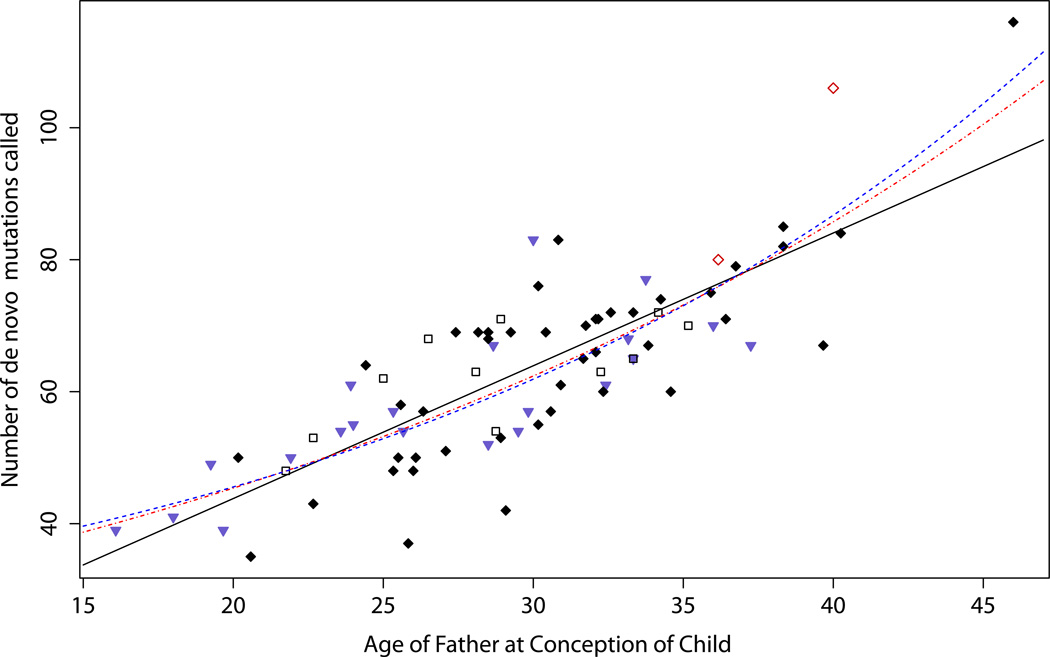
Relationships between parents’ age and the number of mutations (paternal and maternal combined as they could not be reliably separated without data from a grandchild) were examined using all 78 trios (Fig. 2). The number of mutations increases with father’s age (P = 3.6×10−19) with an estimated effect of 2.01 mutations per year (standard error (SE) = 0.17). Mother’s age is substantially correlated with father’s age (r = 0.83) and, not surprisingly, is also associated with the number of de novo mutations (P = 1.9×10−12). However, when father’s age and mother’s age were entered jointly in a multiple regression, father’s age remained highly significant (P = 3.3×10−8), but not mother’s age (P = 0.49). Based on existing knowledge about the mutational mechanisms in sperm and eggs2, the results support the notion that the increase in mutations with parental age manifests itself mostly, maybe entirely, on the paternally inherited chromosome.
Figure 2. Father‘s age and number of de novo mutations.
Number of de novo mutations called is plotted against father's age at conception of child for the 78 trios. The solid black line denotes the linear fit. The broken red curve is based on an exponential model fitted to the combined mutation counts. The broken blue curve corresponds to a model where maternal mutations are assumed to have a constant rate of 14.2 and paternal mutations assumed to increase expoenentially with father‘s age. ♦ proband autistic;  proband schizophrenic;
proband schizophrenic;  proband a parent of an autistic case; □ others.
proband a parent of an autistic case; □ others.
Given a particular mutation rate, due to random variation, the number of actual mutations is expected to have a Poisson distribution. After taking Poisson variation into account, with a linear fit (effect = 2.01 mutations per year), father’s age explains 94.0% (90% confidence interval (CI): [80.1%, 100%]) (Supplementary Information) of the remaining variation in the observed mutation counts. When an exponential model is fitted (red curve in Fig. 2), the number of paternal and maternal mutations combined is estimated to increase by 3.23% per year. This model explains 96.6% (90% CI: [83.2%, 100%]) of the remaining variation. A third model fitted (blue curve in Fig. 2) assumes that the maternal mutation rate is a constant at 14.2 and paternal mutations increase exponentially. This explains 97.1% (90% CI: [84.3%, 100%]) of the remaining variation and the rate of paternal mutations is estimated to increase by 4.28% per year, which corresponds to doubling every 16.5 years and increasing by 8 fold in 50 years. Seventy six of the 78 trios have father’s age between 18 and 40.5, a range where the differences between the 3 models are modest. Hence, while it appears that the number of paternal de novo mutations increases at a rate that accelerates with father’s age, more data at the upper age range are needed to better evaluate the nature of the acceleration.
Validation and the nature of errors
Among the de novo mutations originally called, two were observed twice, both in siblings, one on chromosome 6 and one on chromosome 10. These cases were examined by Sanger sequencing. The one on chromosome 6 is not actually de novo as it was seen in the mother also. The one on chromosome 10 was confirmed, i.e. it was observed in both siblings, who share the paternal haplotype in this region, but not the parents. This supports the theory that de novo mutations in different sperms of a man are not entirely independent2. Our trios include seven sibpairs with 921 de novo mutations called. A false de novo mutation call for one sib resulting from a missed call in the parent would show up in the other sib also about 50% of the time. Only one such false positive was detected indicates that this type of error accounts for a small percentage (2/(920/2) = 0.43%) of the called mutations. To evaluate the overall number of false positives, 111 called de novo mutations were randomly selected for Sanger sequencing. Eleven failed primer design. Six did not produce results of good quality in at least one member of the corresponding trio (Supplementary Information). For the remaining 94 cases, 93 were confirmed as de novo mutations, i.e. the mutated allele was observed in the proband but not in the parents. One false positive, where the putative mutation was not observed in the proband, was identified. The 17 cases that could not be verified are more likely to be located in genomic regions that are more difficult to analyse and hence probably have higher false-positive rates than average. Even so, the overall false-positive rate for the de novo mutation calls cannot be high.
The variance of the number of false positives is as important as the mean. False positives that are Poisson distributed, while adding noise, would not create bias for the effect estimates in either the linear or exponential models for father’s age, nor would they bias the estimate of the fraction of variance explained after accounting for Poisson variation. In general, they do not create substantial bias for analyses of differences and ratios. However, if the variance of the false positives is higher than the mean, resulting from systematic effects that affect trios differently, such as DNA quality and library construction, it would increase the unexplained variance and reduce the fraction of variance explained by father’s age. The candidates filtered out by criterion (v), if kept, would have introduced false positives of this kind (Supplementary Information). Since father’s age explains such a high fraction of the systematic variance of the currently called de novo mutations, false positives with this property cannot be common. A similar discussion about false negatives13 is in Supplementary Information.
Father’s age and diseases
Consistent with other epidemiological studies14,15, in Iceland, the risk of schizophrenia increases significantly with father’s age at conception (n = 569, P = 2×10−5). Father’s age is also associated with the risk of ASD. The observed effect is limited to non-familial cases (n = 631, P = 5.4×10−4), defined as those whose closest ASD relative is farther than cousins. The epidemiological results, the effect of father’s age on de novo mutation rate shown here, together with other studies that have linked de novo mutations to autism and schizophrenia, including three recent studies of autism through exome sequencing4–6, all point to the possibility that, as a man ages, the number of de novo mutations in his sperm increases, and the chance that a child would carry a deleterious mutation (not necessarily limited to SNP mutations) that could lead to autism or schizophrenia increases proportionally. However, this scenario does not imply that the relationship observed here between mutation rate and father’s age would have been much different if the probands studied were chosen to be all non-ASD/SZ cases instead. For example, assume that autism/schizophrenia is in each case caused by only one de novo mutation. Then autism/schizophrenia cases would on average have more de novo mutations than population samples. The magnitude could be substantial if the distribution of father’s age has a large spread in the population, but then most of the difference would be due to the cases having older fathers. If we control for the age of the father at the conception of the individual, this difference in the average number of de novo mutations between autistics/schizophrenics and controls would be reduced to approximately one (Supplementary Information).
Mutations by type and by chromosome
Examination of the 4933 de novo mutations revealed that 73 are exonic, including two stopgain SNP and 60 nonsynonymous SNP (Supplementary Table 2). One non-familial schizophrenic proband carries a de novo stopgain mutation (p.Arg113X) in the gene NRXN1, previously associated with schizophrenia16–20. One non-familial autistic proband has a stopgain de novo mutation (p.R546X) in the cullin 3 gene (CUL3). De novo loss of function mutations in CUL3 have been reported to cause hypertension and electrolyte abnormalities21. Recently, a separate stopgain de novo mutation (p.E246X) in CUL3 was reported in an autistic case5. Another one of our mutations is a nonsynonymous variant (p.G900S) two bases from a splice site in the EPH receptor B2 (EPHB2), a gene implicated in the development of the nervous system. A de novo stopgain mutation (p.Q858X) in this gene has recently been described in another autistic case6. Given the small number of loss of function de novo mutations we and others have reported (approximately 70 genes in the three autism exome scans4–6), the overlap is unlikely to be a coincidence. Hence, CUL3 and EPHB2 can be added to the list of genes that are relevant for ASD. Effective genome coverage, computed by discounting regions that have either very low (< ½ genome average) or very high (> 3 times genome average) local coverage, the latter often a symptom of misaligning reads, was estimated to be 2.63 billion basepairs (Supplementary Information). From that, 4933 mutations correspond to a germline mutation rate of 1.20×10−8 per nucleotide per generation, falling within the range between 1.1×10−8 and 3.8×10−8 previously reported3,7,8,22,23. Tables 2 and 3 summarize the nature of the de novo mutations with respect to sequence context. Approximately two-thirds (3344/4933 = 67.8%) are transitions. Moreover, there is clear difference between mutation rates at CpG and non-CpG sites. CpG dinucleotides are known to be mutational hotspots in mammals, ostensibly because spontaneous oxidative deamination of methylated cytosines leads to an increase in transition mutations24. The observed rate of transitions here is 18.2 times that at non-CpG sites, somewhat higher but not inconsistent with previous estimates of 13.3 (Ref. 23) and 15.4 (Ref. 3). Transversion rate is also higher at CpG sites, 2.55 fold that at non-CpG sites. Most of this increased transversion rate at CpG sites is presumably due to general mutation bias favouring mutations that decrease G+C content. The rate of mutations that change a strong (G:C) basepair to a weak one (A:T) is 2.15 times higher than mutations in the opposite direction. This mutational pressure in the direction of A+T is observed for both transitions (ratio = 2.24) and transversions (ratio = 1.82) and cannot be solely explained by CpG mutations. Father’s age does not appear to affect the ratios between the rates of these different classes of mutations, i.e. as a man ages rates of all mutation types increase by a similar factor.
Table 2.
Germline mutation rates at CpG and non-CpG sites
| Type of mutation | N | Rate per base per generation |
|---|---|---|
| Transition at non-CpG | 2489 | 6.18×10−9 |
| Transition at CpG | 855 | 1.12×10−7 |
| Transversion at non-CpG | 1516 | 3.76×10−9 |
| Transversion at CpG | 73 | 9.59×10−9 |
| All | 4933 | 1.20×10−8 |
Mutation rates are per generation per base. For non-CpG sites, the effective number of bases examined is taken as 2.583 billion, whereas for CpG sites the number is 48.80 million. These numbers take into account the variation of local coverage in sequencing (Supplementary Information).
Table 3.
Strong to Weak and Weak to Strong Mutation rates
| Mutation Type | S->W (N) rate | W->S (N) rate | S->W rate / W->S rate |
|---|---|---|---|
| Transition | (2025) 1.21×10−8 | (1319) 5.42×10−9 | 2.24 |
| Transversion | (446) 2.67×10−9 | (358) 1.47×10−9 | 1.82 |
| All | (2471) 1.48×10−8 | (1677) 6.89×10−9 | 2.15 |
N denotes observed mutation counts and mutation rates are calculated per generation per base. For Strong (G:C) to Weak (A:T), the effective number of sites examined is taken as 1.071 billion, and for Weak to Strong the number is 1.560 billion.
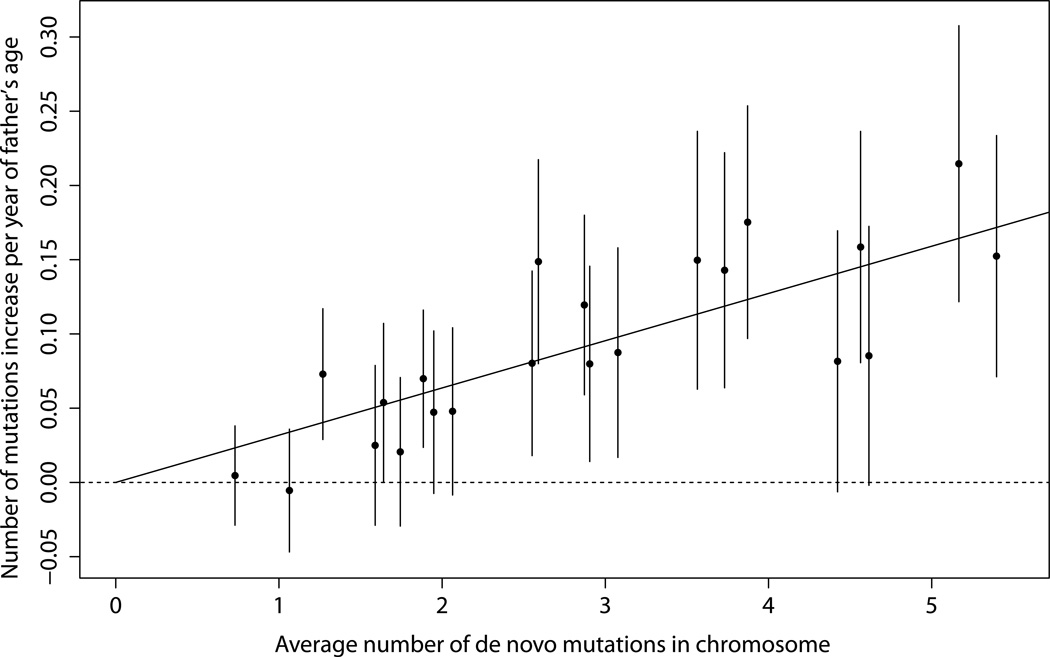
The average number of mutations for each chromosome separately and the effect of father’s age are displayed in Fig. 3. The effect of father’s age is significant (P < 0.05) for 14 of the 22 chromosomes when evaluated individually. The solid line in the figure corresponds to a model where the linear effect of father’s age is proportional to the mean number of mutations on the chromosome, or that father’s age has a uniform multiplicative effect across the chromosomes. All 22 95% CIs overlap the line, indicating that the results are consistent with the model.
Figure 3. Effect of father‘s age by chromosome.
By chromosome, the estimated increase in the number of de novo mutations per year of father‘s age is plotted against the average number of mutations observed. 95% confidence intervals are given. The solid straight line corresponds to the model where the additive effect of father‘s age on the number of de novo mutations is assumed to be proportional to the mean number of mutations on the chromosome. From left to right, the points correspond to chromsome 21, 22, 19, 20, 15, 17, 18, 14, 16, 13, 12, 9, 10, 11, 8, 7, 6, 3, 5, 4, 2, and 1.
Discussion
Recombination rate is higher for women than men, and children of older mothers have more maternal recombinations that those of young mothers25. However, men transmit a much higher number of mutations to their children than women. Furthermore, even though our data also show some over dispersion in the number of maternal de novo mutations, it is the age of the father that is the dominant factor in determining the number of de novo mutations in the child. Seeing an association between father’s age and mutation rate is not surprising2, but the large linear effect of over two additional mutations per year, or the estimated exponential effect of paternal mutations doubling every 16.5 years, is striking. Even more so is the fraction of the variation it explains, which limits the possible contribution by other factors, such as the environment and the genetic and non-genetic differences between individuals, to mutation rate on a population level. Given the results, it may no longer be meaningful to discuss average mutation rate in a population without consideration of father’s age. Also, even though factors other than father’s age do not appear to contribute substantially to the mutation rate diversity in our data, it does not mean that hazardous environmental conditions could not cause a meaningful increase in mutation rate. Rather, the results indicate that, to estimate such an effect for a specific incident, it is crucial to take the father’s age into account.
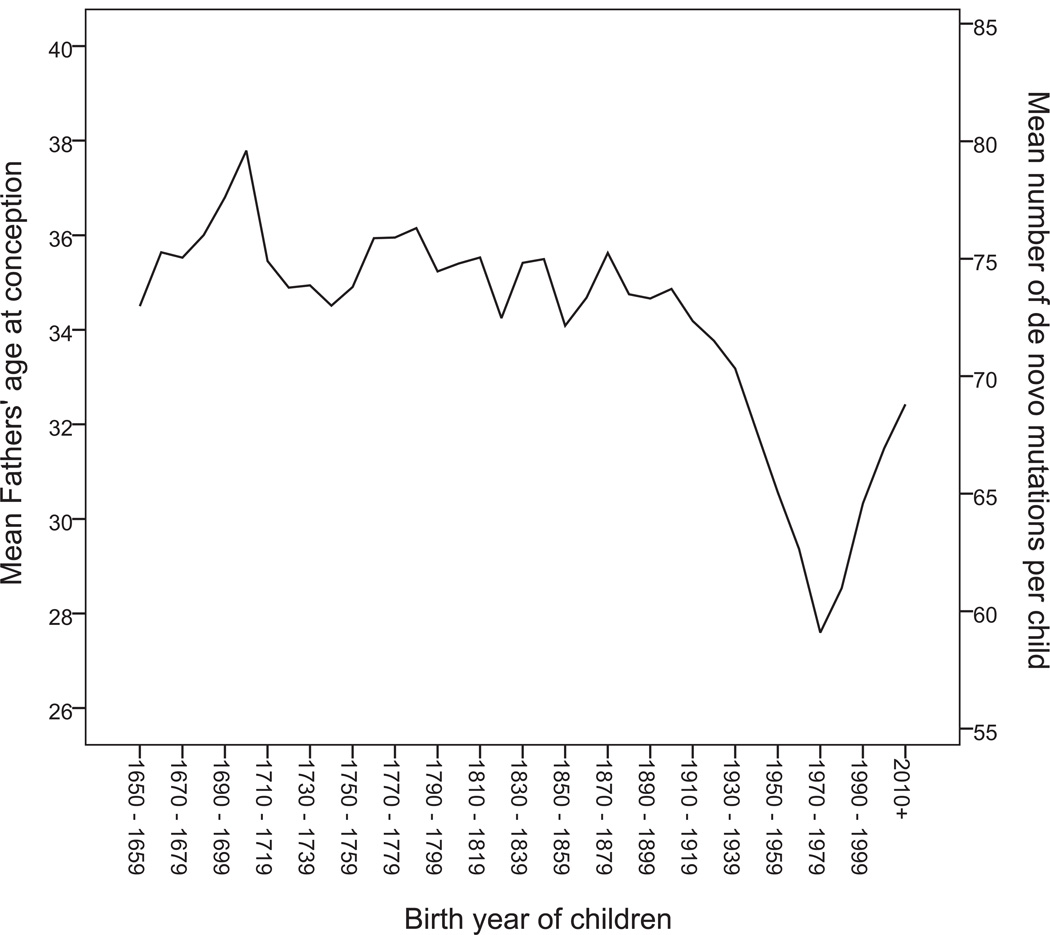
It is well known that demographic characteristics shape the evolution of the gene pool through the forces of genetic drift, gene flow and natural selection. With the results here, it is now clear that demographic transitions that affect the age at which males reproduce can also have a considerable impact on the rate of genomic change through mutation. Recent transition of Icelanders from a rural agricultural to an urban industrial way of life, which engendered a rapid and sequential drop in the average age of fathers at conception from 34.9 years in 1900 to 27.9 years in 1980, followed by an equally swift climb back to 33.0 years in 2011, primarily due to the impact of higher education and the increased use of contraception (Fig 4). Based on the fitted linear model, while individuals born in 1900 carried on average 73.7 de novo mutations, those born in 1980 carried on average only 59.7 such mutations (a decrease of 19.1%), while the mutational load of individuals born in 2011 has increased by 17.2% to 69.9. Demographic change of this kind and magnitude is not unique to Iceland, and it raises the question of whether the reported increase in ASD diagnosis lately is at least partially due to an increase in the average age of fathers at conception. Also, the observations here are likely to have important implications for the use of genetic variation to estimate divergence times between species or populations, since the mutation rate cannot be treated as a constant scaling factor, but rather must be considered along with the paternal generation interval as a time-dependent variable.
Figure 4. Demographics of Iceland and de novo mutations.
The deCODE Genetics genealogy database was used to assess fathers' age at conception for all available 752,343 father-child pairs, where the child's birthyear was ≥1650. The mean age of fathers at conception (left vertical axis) is plotted by birthyear of child, grouped into 10 year intervals. Based on the linear model fitted for the relationship between father's age and the number of de novo mutations, the same plot, using the right vertical axis, shows the mean number of expected mutations for each 10 year interval.
Methods Summary
Whole genome sequence data for this study were generated using the Illumina GAllx and HiSeq2000 instruments. The sequencing reads were aligned to the hg18 reference genome with BWA26 and duplicates were marked with Picard [http://picard.sourceforge.net/]. Quality score recalibration, indel realignment and SNP/Indel discovery was then performed on each sample separately, using GATK 1.2 (Ref. 27). Likelihoods presented are based on the normalized Phred-scaled likelihoods that are calculated by the GATK variant calling. Statistical analysis was performed in part using the R statistical package. Estimates and confidence intervals for the fraction of variance explained after accounting for Poisson variation were calculated using Monte Carlo simulations (Supplementary Information). Variants were annotated using SNP effect predictor (snpEff2.0.5, database hg36.5) and Genome Analysis Toolkit 1.4-9-g1f1233b with only the highest-impact effect (Cingolani, P. “snpEff:Variant effect prediction”, http://snpeff.sourceforge.net, 2012). More details in Supplementary Information.
Supplementary Material
Acknowledgements
This research was partly funded by The National Institute of Health grant MH071425 (K. Stefansson); The European Community's Seventh Framework Programme, PsychCNVs project, grant agreement HEALTH-F2-2009-223423, and NextGene project, grant agreement IAPP-MC-251592; The European Community IMI grant EU-AIMS, grant agreement 115300.
Footnotes
Author Contributions A.K. and K.S. planned and directed the research. A.K. wrote the first draft and together with K.S., S.B., P.S., A.H. and U.T. wrote the final version. O.T.M. and U.T. oversaw the sequencing and lab work. G. Masson, G. Magnusson and G.S. processed the raw sequencing data. A.K. and M.L.F. analyzed the data with W.W., H.H., G.B.W., S.S., G.T. and D.F.G. providing assistance. P.S. and S.A.G. performed functional annotations. S.B. analyzed the mutations with respect to sequence content. A.S., Aslaug J. and Adalbjorg J. did the Sanger sequencing. A.H. investigated the contribution of demographics.
Author Information The authors from deCode genetics are employees of or own stock options in deCODE Genetics. Wendy S.W. Wong is an employee of Illumina Inc., a public company that develops and markets systems for genetic analysis; she receives stocks as part of her compensation.
References
- 1.Keightley PD. Rates and fitness consequences of new mutations in humans. Genetics. 2012;190:295–304. doi: 10.1534/genetics.111.134668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crow JF. The origins, patterns and implications of human spontaneous mutation. Nature reviews. Genetics. 2000;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 3.Kondrashov AS. Direct estimates of human per nucleotide mutation rates at 20 loci causing Mendelian diseases. Human mutation. 2003;21:12–27. doi: 10.1002/humu.10147. [DOI] [PubMed] [Google Scholar]
- 4.Neale BM, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012 doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012 doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012 doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue Y, et al. Human Y chromosome base-substitution mutation rate measured by direct sequencing in a deep-rooting pedigree. Current biology : CB. 2009;19:1453–1457. doi: 10.1016/j.cub.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad DF, et al. Variation in genome-wide mutation rates within and between human families. Nature genetics. 2011;43:712–714. doi: 10.1038/ng.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roach JC, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science. 2010;328:636–639. doi: 10.1126/science.1186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holm H, et al. A rare variant in MYH6 is associated with high risk of sick sinus syndrome. Nature genetics. 2011;43:316–320. doi: 10.1038/ng.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafnar T, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nature genetics. 2011;43:1104–1107. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 12.Sulem P, et al. Identification of low-frequency variants associated with gout and serum uric acid levels. Nature genetics. 2011;43:1127–1130. doi: 10.1038/ng.972. [DOI] [PubMed] [Google Scholar]
- 13.Keightley PD, et al. Analysis of the genome sequences of three Drosophila melanogaster spontaneous mutation accumulation lines. Genome research. 2009;19:1195–1201. doi: 10.1101/gr.091231.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophrenia bulletin. 2001;27:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161:334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- 16.Duong L, et al. Mutations in NRXN1 in a family multiply affected with brain disorders: NRXN1 mutations and brain disorders. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2012 doi: 10.1002/ajmg.b.32036. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier J, et al. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Human genetics. 2011;130:563–573. doi: 10.1007/s00439-011-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirov G, et al. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Human molecular genetics. 2008;17:458–465. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- 19.Levinson DF, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. The American journal of psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rujescu D, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Human molecular genetics. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyden LM, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch M. Rate, molecular spectrum, and consequences of human mutation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:961–968. doi: 10.1073/pnas.0912629107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genetics. 2000;156:297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulondre C, Miller JH, Farabaugh PJ, Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978;274:775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- 25.Kong A, et al. Recombination rate and reproductive success in humans. Nature genetics. 2004;36:1203–1206. doi: 10.1038/ng1445. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. Fast and accurate short read alignment with Burrows- Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.