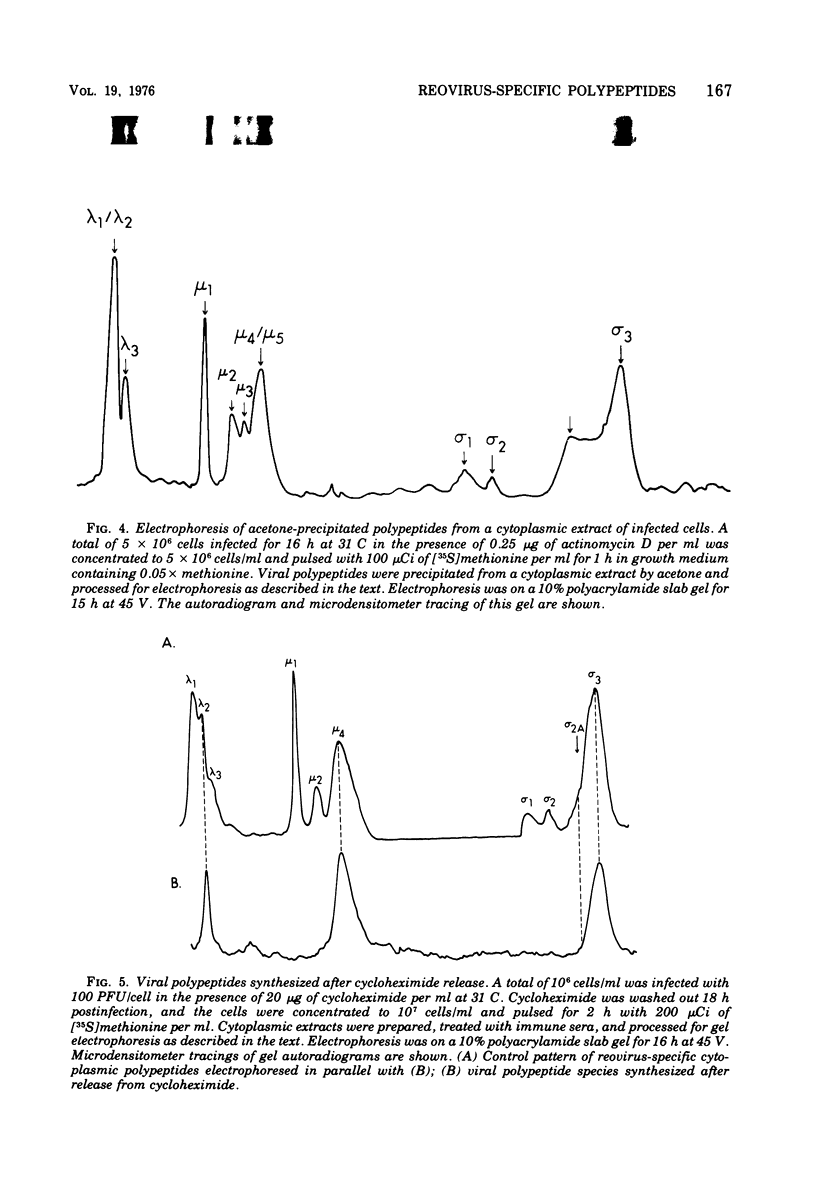
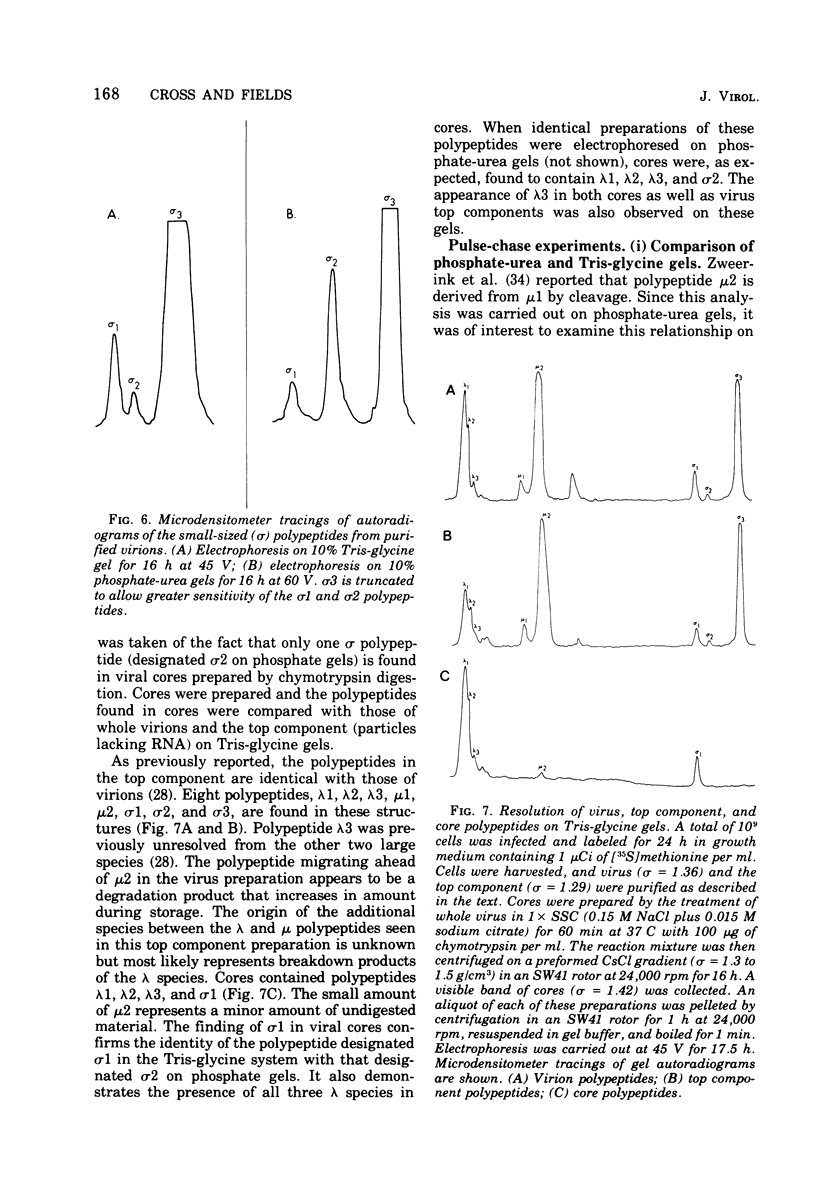
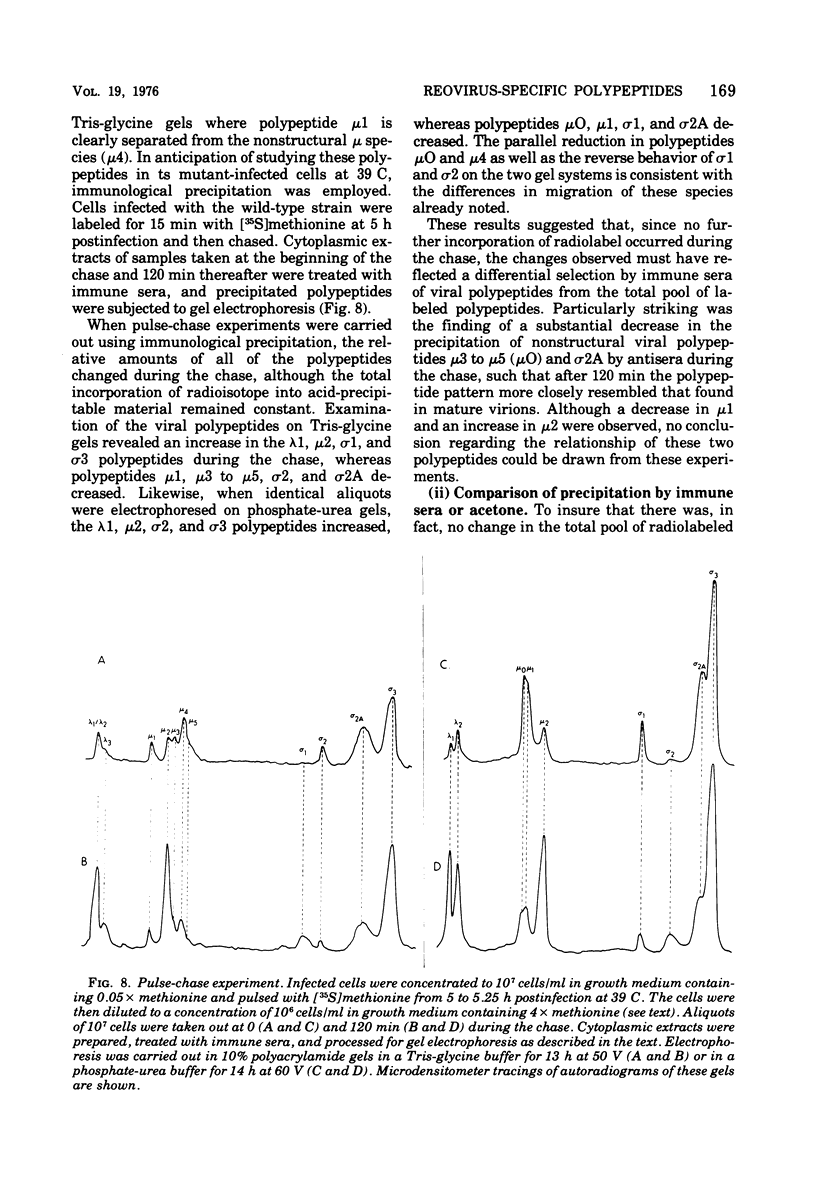
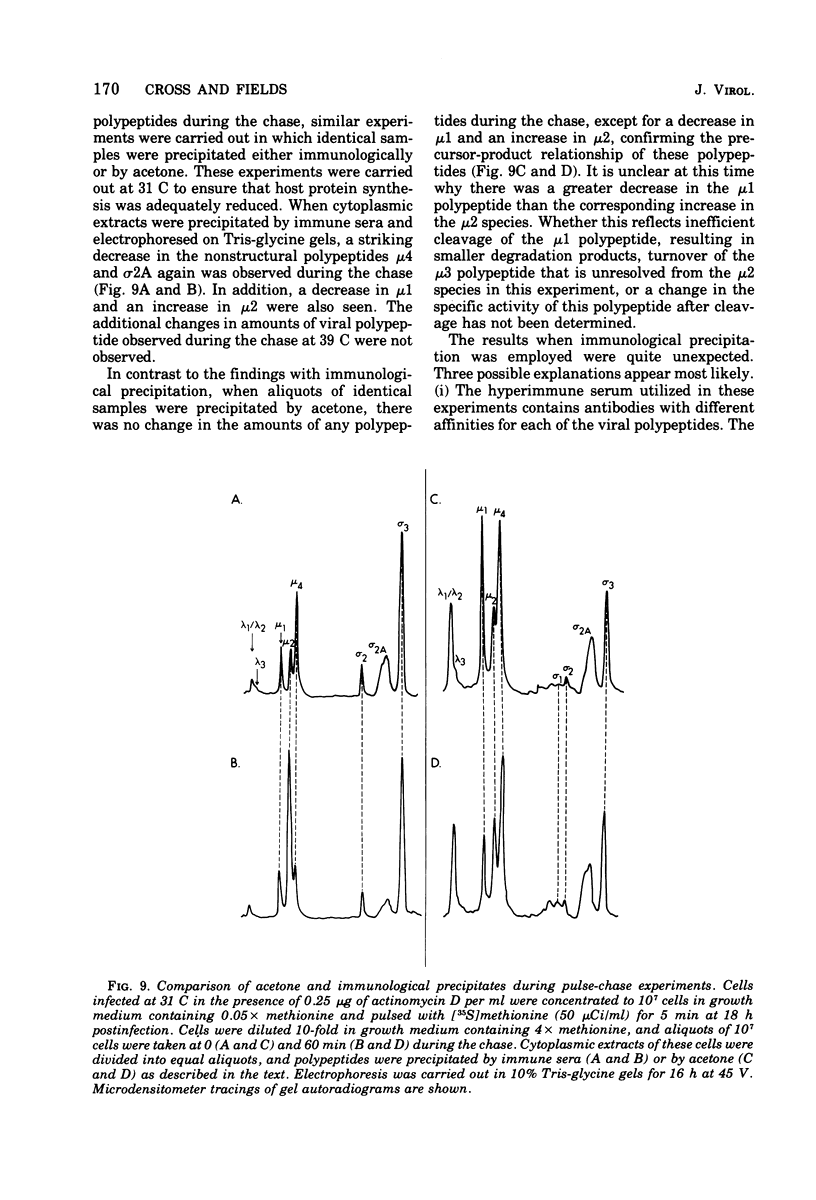
Abstract
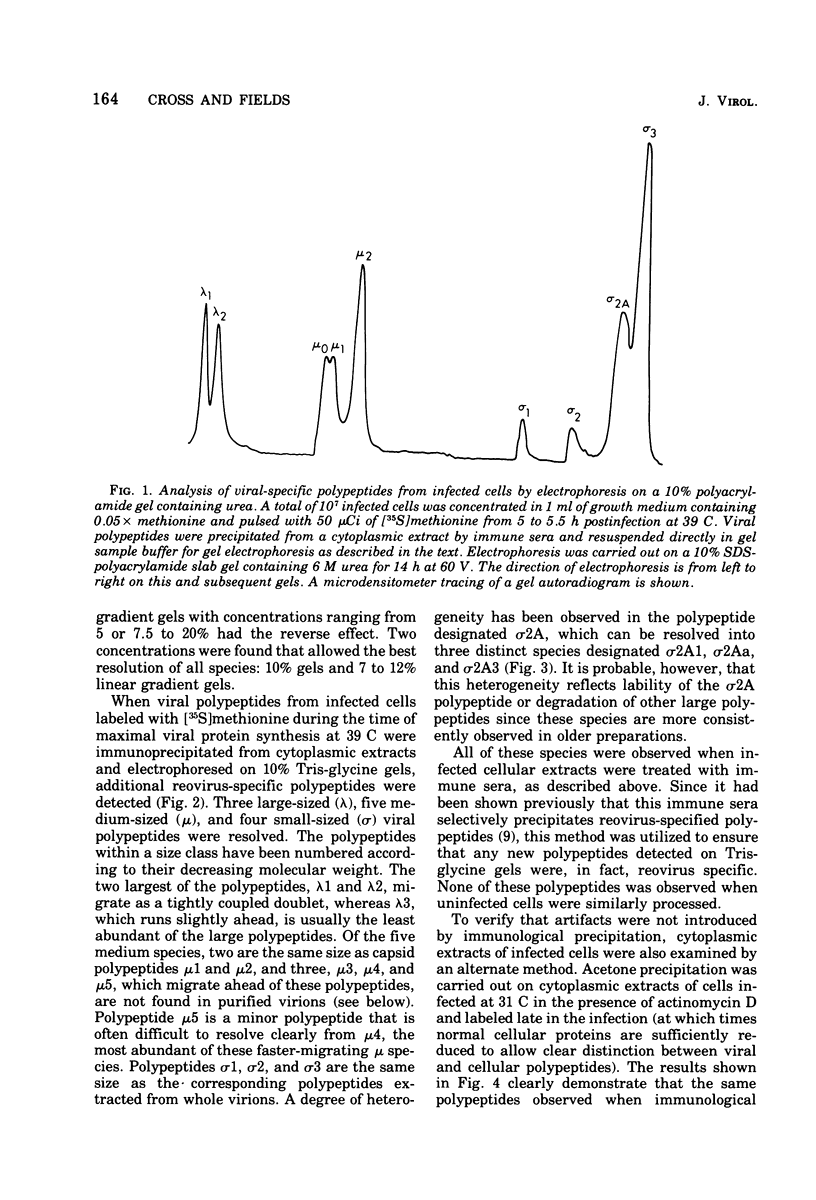
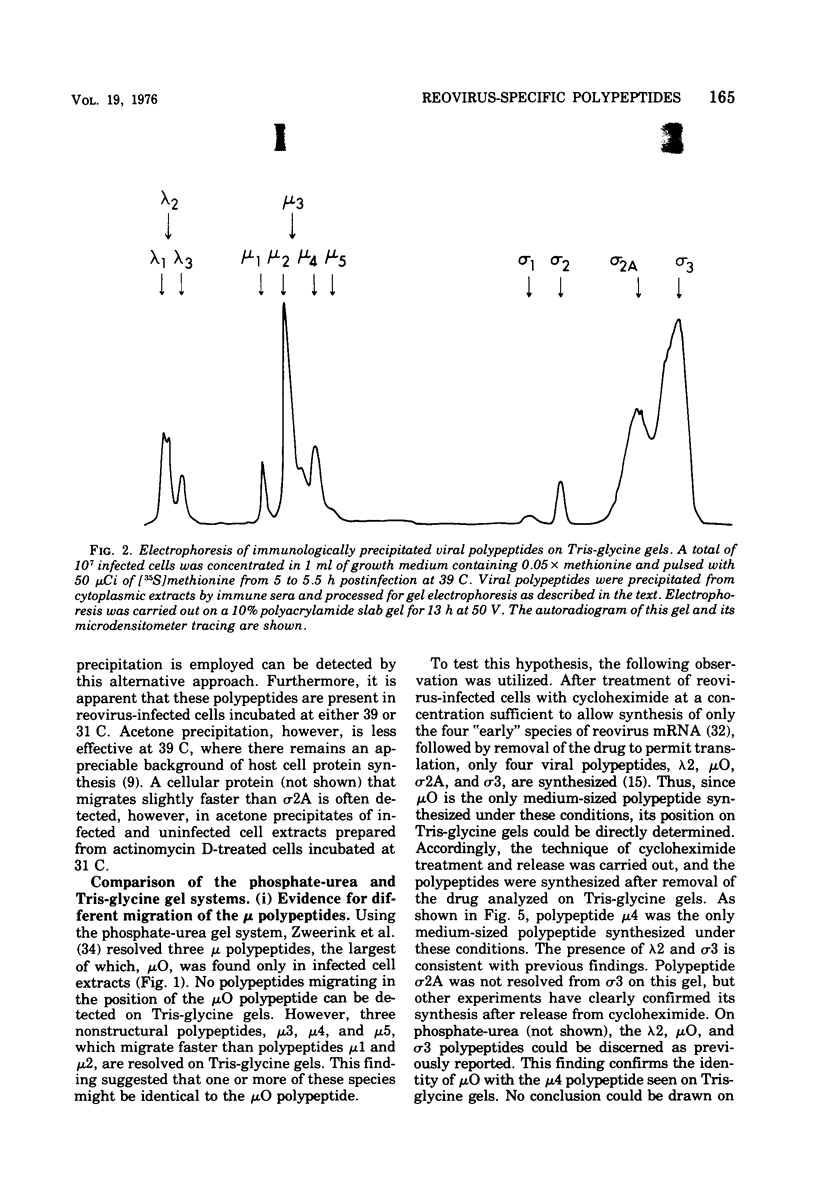
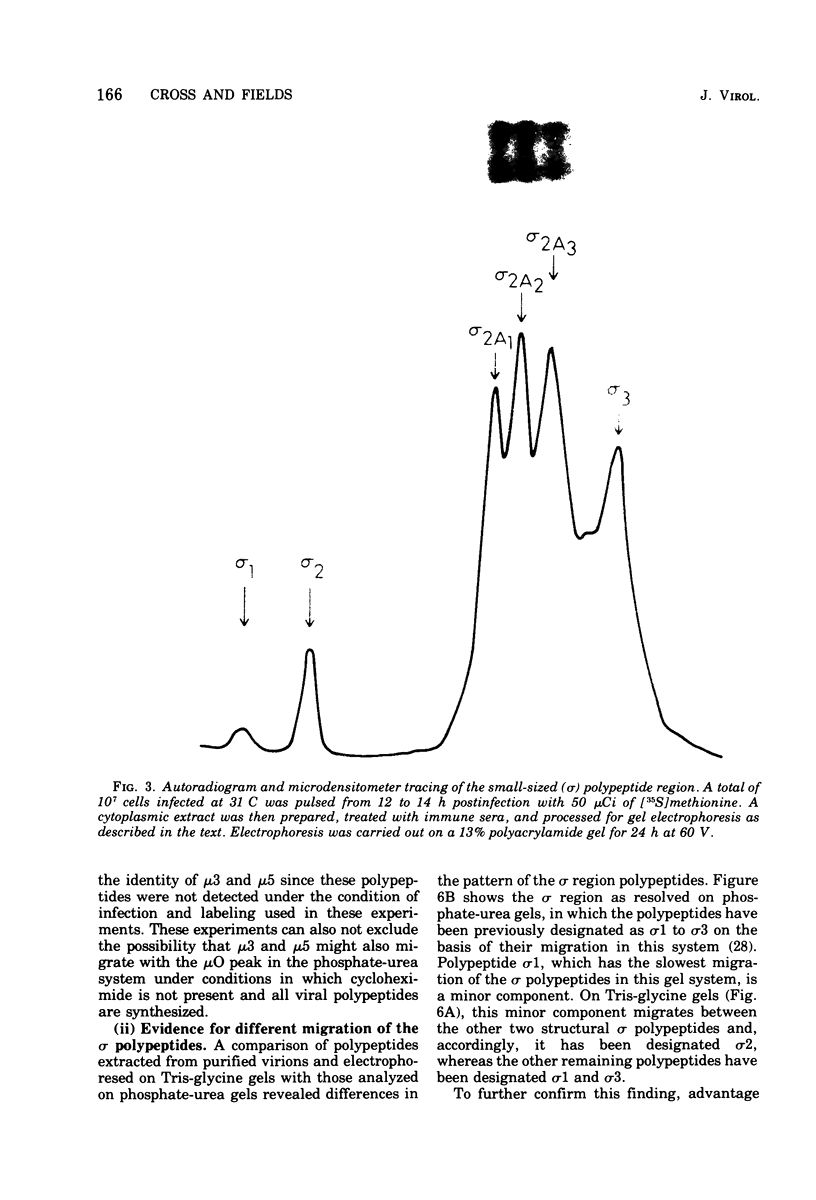
The electrophoretic analysis of reovirus-specific polypeptides in infected cells using a discontinuous gel system has allowed the resolution of additional viral-specific polypeptides, including one large-sized gamma3 and two (or possibly three) medium-sized (mu3, mu4, mu5(?)) species. The proteins designated mu0, sigma1, and sigma2 based on electrophoretic mobility in gel systems containing phosphate-urea correspond to mu4, sigma2, and sigma1, respectively, when analyzed in systems containing Tris-glycine. It is likely that protein modifications (phosphorylation and glycosylation) are responsible for at least some of these differences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: evidence for aberrant mu 1 and mu 2 polypeptide species. J Virol. 1976 Jul;19(1):174–179. doi: 10.1128/jvi.19.1.174-179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Laskov R., Scharff M. D. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral peptides. Virology. 1972 Oct;50(1):209–215. doi: 10.1016/0042-6822(72)90361-3. [DOI] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Lengyel P. Translation of in vitro synthesized reovirus messenger RNAs into proteins of the size of reovirus capsid proteins in a mouse L cell extract. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1816–1823. doi: 10.1016/0006-291x(72)90056-3. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Joklik W. K. Demonstration that the same strand of reovirus genome RNA is transcribed in vitro and in vivo. Virology. 1971 May;44(2):450–453. doi: 10.1016/0042-6822(71)90276-5. [DOI] [PubMed] [Google Scholar]
- Krystal G., Winn P., Millward S., Sakuma S. Evidence for phosphoproteins in reovirus. Virology. 1975 Apr;64(2):505–512. doi: 10.1016/0042-6822(75)90127-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Van Alstyne D., Berckmans R., Graham A. F. Synthesis of reovirus-specific polypeptides in cells pretreated with cycloheximide. J Virol. 1975 Sep;16(3):470–478. doi: 10.1128/jvi.16.3.470-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A. M., Bailey E. J., Tillotson J. R. Enterovirus hemagglutination: inhibition by several enzymes and sugars. J Immunol. 1965 Dec;95(6):1111–1115. [PubMed] [Google Scholar]
- Lerner A. M., Gelb L. D., Tillotson J. R., Carruthers M. M., Bailey E. J. Enterovirus hemagglutination: inhibition by aldoses and a possible mechanism. J Immunol. 1966 Apr;96(4):629–636. [PubMed] [Google Scholar]
- Levin D. H., Mendelsohn N., Schonberg M., Klett H., Silverstein S., Kapuler A. M., Acs G. Properties of RNA transcriptase in reovirus subviral particles. Proc Natl Acad Sci U S A. 1970 Jul;66(3):890–897. doi: 10.1073/pnas.66.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Millward S., Graham A. F. Control of transcription of the reovirus genome. Nucleic Acids Res. 1974 Mar;1(3):373–385. doi: 10.1093/nar/1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett D. M., Vanaman T. C., Joklik W. K. Studies on the amino and carboxyl terminal amino acid sequences of reovirus capsid polypeptides. Virology. 1973 Mar;52(1):174–186. doi: 10.1016/0042-6822(73)90407-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D. Identification of the vesicular stomatitis virus large protein as a unique viral protein. J Virol. 1973 Apr;11(4):520–526. doi: 10.1128/jvi.11.4.520-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson J. R., Lerner A. M. Effect of periodate oxidation on hemagglutinating and antibody-producing capacities of certain enteroviruses and reoviruses. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1143–1150. doi: 10.1073/pnas.56.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R., Banerjee A. K., LaFiandra A., Shatkin A. J. Reovirus-specific ribonucleic acid from polysomes of infected L cells. J Virol. 1972 Jan;9(1):61–69. doi: 10.1128/jvi.9.1.61-69.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., McDowell M. J., Joklik W. K. Essential and nonessential noncapsid reovirus proteins. Virology. 1971 Sep;45(3):716–723. doi: 10.1016/0042-6822(71)90185-1. [DOI] [PubMed] [Google Scholar]