Abstract
Objectives
Evidence indicates that decreased mucociliary clearance (MCC) is a major contributing feature to chronic rhinosinusitis. Tobacco-smoke exposure is thought to inhibit transepithelial Cl− secretion – a major determinant of airway surface liquid hydration and MCC. The objective of the current study was to evaluate the effects of acrolein exposure (a prominent tobacco smoke toxin) on vectorial Cl− transport through the major apical anion channel CFTR in sinonasal epithelium.
Study Design
In vitro investigation.
Methods
Primary murine nasal septal (MNSE, wild type and transgenic CFTR−/−) cultures were exposed to acrolein in Ussing chambers and effects on Cl− secretion investigated using pharmacologic manipulation. Cellular cAMP signaling and cytotoxicity were also investigated.
Results
Acrolein stimulated Cl− secretion (ΔISC – change in short-circuit current in µA/cm2) at concentrations similar to smoker’s airways (100 μM, 15.8 +/− 2.2 vs. 2.4 +/− 0.8(control); p<0.0001), suppressed forskolin-stimulated Cl− transport at 300 μM (13.3 +/− 1.2 vs. 19.9 +/− 1.0; p < 0.01}, and completely abolished all transport at 500 μM (−1.1+/− 1.6). Stimulated Cl− secretion was solely reliant upon the presence of CFTR (confirmed in transgenic CFTR−/− MNSE), but independent of cAMP signaling. Inhibition at higher concentrations was not secondary to cellular cytotoxicity.
Conclusions
The present study demonstrates that acrolein has complex, but pronounced interaction with the major apical Cl− transport mechanism that utilizes CFTR. Further investigations are required to determine acrolein’s impact as a tobacco smoke constituent on mucociliary transport.
Keywords: Tobacco, cigarette smoke, acrolein, sinusitis, CFTR, chloride, cAMP, LDH, electrophysiology, Ussing chamber
INTRODUCTION
Sinonasal respiratory epithelium maintains a highly regulated mucociliary apparatus that clears inhaled pathogens from the nose and paranasal sinuses. The airway surface liquid (ASL) is a crucial part of the mucociliary apparatus, and its composition is determined by the transepithelial vectorial transport of ions, such as chloride (Cl−) and sodium (Na+).1 One of the primary regulators of Cl− transport in sinonasal respiratory epithelium is the cystic fibrosis transmembrane conductance regulator (CFTR). Dysfunctional Cl− transport through acquired (e.g., toxic exposures, hypoxia) or genetic (i.e., cystic fibrosis) disease can lead to dehydration of the ASL, stasis of inspissated mucus, bacterial infection, and widespread chronic sinus disease.2–4
Cigarette smokers exhibit a higher rate of recurrent sinusitis and chronic rhinosinusitis (CRS) when compared to nonsmokers.5 Chronic smoking is known to contribute to immunosuppressive changes (decreased lipopolysaccharide-induced inflammatory cytokines and suppression of human monocyte-derived macrophage function) impairing host defenses and increasing susceptibility to infection.6 Cigarette smoke is a highly toxic substance that is composed of a complex mixture of over 5,000 chemicals, including several that have been identified as having high toxicity to respiratory cilia – specifically acrolein, formaldehyde, carbon monoxide, nicotine, cotinine, acetaldehyde, phenol, and potassium cyanide.3,6–9
Acrolein is one of the most abundant volatile, electrophilic compounds present in cigarette smoke and is known to elicit several different biochemical responses including transcription factor activation, oxidative injury, inflammation, alteration of immunologic function, ciliotoxicity, and cell death.10 Sarkar et al.11 demonstrated that one cigarette contains approximately 238–468ug acrolein, and that acrolein induces an inflammatory response through the rapid reduction of glutathione, protein sulfhydruls, and thiol-containing enzymes.
The effects of cigarette smoke on sinonasal epithelium are thought in part to be secondary to impairment of ciliary function and dehydration of the ASL.12–15 In a prior study by our group, cigarette smoke condensate (CSC), which contains the particulate and water-insoluble components of tobacco combustion, was shown to substantially decrease transepithelial transport of Cl−, and to strongly impair ciliary beat frequency.3 Others have reported suppression with the water soluble extract of cigarette smoke suggesting that a number of agents in tobacco could be responsible for reduction in mucociliary transport.16 While the inflammatory and immunologic effects of acrolein have been investigated previously, no reports to date have examined its impact on transepithelial Cl− secretion. The present study was designed to investigate the effects of acrolein on vectorial Cl− transport through CFTR, the major apical anion channel in sinonasal epithelium. Experiments were designed to test the hypothesis that acrolein reduces transepithelial CFTR-mediated Cl− secretion.
MATERIALS AND METHODS
Institutional review board and institutional animal care and use committee approval were obtained prior to the initiation of the study.
Tissue Culture
The culture technique for propagation of murine nasal septal epithelia (MNSE) at an air-liquid interface has been described previously.2,17–22 Briefly, tissue was harvested and grown on Costar 6.5-mm-diameter permeable filter supports (Corning Life Sciences, Lowell, MA) submerged in culture medium. The media was removed from the surface of the monolayers on day 4 and the cells fed via the basal chamber. MNSE cultures were genetically identical and derived from the C57/BL6 strain and from transgenic CFTR−/− knockout mice. Differentiation and ciliogenesis occurred in all cultures within 10 days to 14 days. Ussing chamber analysis was performed only when cell monolayers were fully differentiated with widespread ciliogenesis and transepithelial resistances (RT) > 500 Ω cm2. There were no significant differences between the RT and ISC of control and acrolein-exposed filters when the two groups were compared before the addition of acrolein, or pharmacologic agents.
Electrophysiology
Solutions and chemicals
The bath solution contained (mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose, with pH 7.3–7.4 when gassed in a mixture of 95% O2-5% CO2 at 37°C. Chemicals were obtained from Sigma (St. Louis, MO). Each solution was made as 1,000x stock and used at 1x in the Ussing chamber. All experiments were performed with low Cl− (6 mM) in the mucosal bath. Pharmacologic preparations were as follows: amiloride (100 µM); forskolin (20 µM); acrolein (1 µM to 500 µM); CFTRINH-172 (10 µM); amphotericin (50 µM). Amiloride blocks Na+ channels, ensuring that change in short-circuit current (ΔISC) from subsequent manipulations are secondary to effects on Cl− channel activity. Forskolin activates the cystic fibrosis transmembrane conductance regulator (CFTR) via a cyclic adenosine monophosphate (cAMP)-mediated mechanism. CFTRINH-172 is a very specific inhibitor of CFTR and was used to develop comparisons of the relative activity of CFTR in different systems. Acrolein is a water soluble component of cigarette smoke and, as such, was dissolved in water for all experiments.
Short circuit (Isc) measurements
Transwell inserts (Costar, Corning Life Sciences) were mounted in Ussing chambers to investigate ion transport using pharmacologic blockade and stimulation of plasma membrane ion channels. Monolayers were continuously short-circuited after fluid resistance compensation using automatic VCC 600 voltage clamps (Physiologic Instruments, San Diego, CA). Transwell filters were mounted in bath solution warmed to 37° C, and the solution gas-lifted with a 95%O2 / 5% CO2 mixture. The ISC was measured at 1 sample per second. A positive deflection in ISC is defined as the net movement of anion in the serosal to mucosal direction. All experiments were performed using ≥ 5 filters per condition.
Cellular cAMP Assay
Activation of CFTR anion transport requires protein kinase A (PKA)-dependent phosphorylation of the regulatory domain. An ELISA-based detection kit (Cayman Chemicals, Ann Arbor, MI) was used to measure stimulation of cellular cAMP by acrolein in MNSE cultures, as previously described.23
Assessment of Cell Viability
Membrane integrity of cells was quantified by detection of lactate dehydrogenase in the medium using an in vitro toxicology assay kit (Sigma, Catalog Number TOX7). Media was collected after cells were treated apically for 15 minutes with either acrolein (100µM, 300µM, 500µM) or PBS control. Absorbance was measured at a wavelength of 490 nm and subtracted from the background absorbance (690 nm) at analysis.
Insert summary
A total of 103 MNSE filters were used in the completion of these studies with n ≥ 4 per measured condition. MNSE filters were derived from genetically identical C57 mice or CFTR−/− transgenic mice as stated. Analyses were performed when cell monolayers were fully differentiated with widespread ciliogenesis and transepithelial resistances (Rt) > 500 Ω.cm2. There was no significant difference between the Rt and ISC of control and acrolein-exposed filters when groups were compared before the addition of pharmacologic agents.
Statistical Analyses
Statistical analyses were performed using two-tailed paired t test, unpaired t test, and analysis of variance, where applicable.
RESULTS
Activation and inhibition of CFTR-mediated Cl− transport is concentration dependent
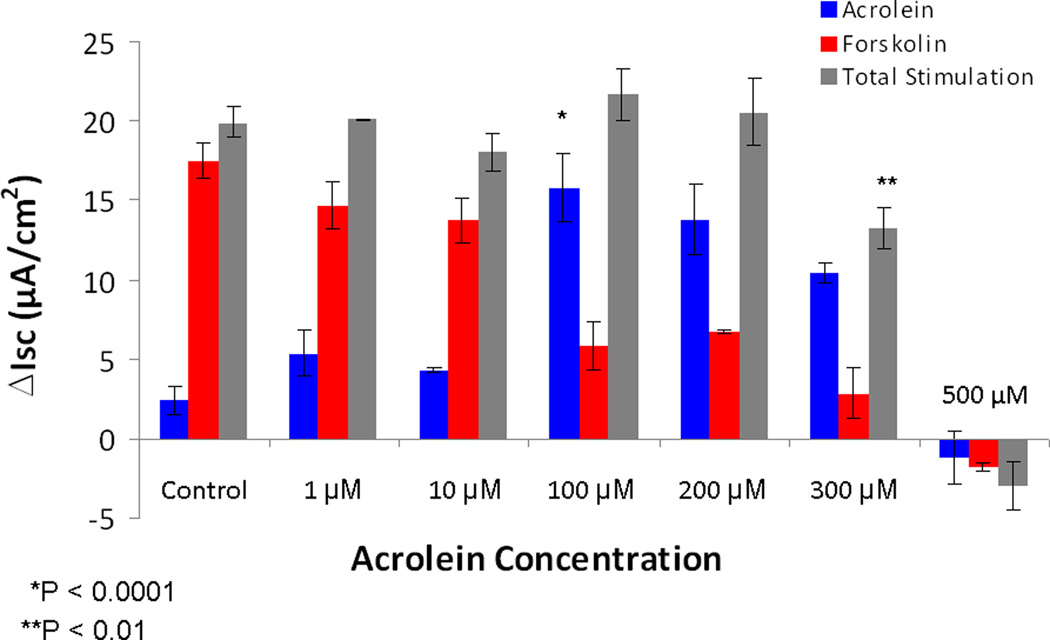
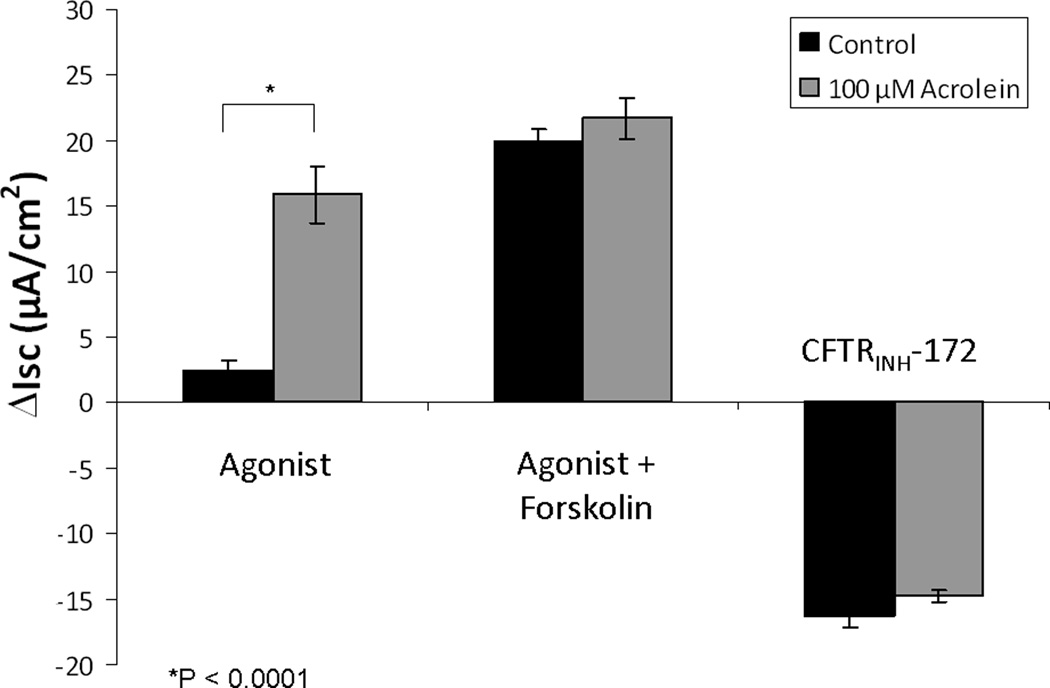
In dose-dependent studies, acrolein stimulated Cl− secretion (ΔISC – change in short-circuit current in µA/cm2) at a concentration expected in the setting of cigarette smoke inhalation in vivo (100 μM, 15.9 +/− 2.2 vs. 2.4 +/− 0.8(control); p<0.0001), but suppressed total apical Cl− secretion (acrolein + forskolin) at 300 μM (13.3 +/− 1.2 vs. 19.9 +/− 1.0; p < 0.01}, and completely abolished all transport at 500 μM (-1.1+/− 1.6). (Figure 1& 2) There were no significant differences in measurements of total stimulation (21.7 +/− 1.6 vs. 19.9 +/− 0.1; control) or CFTR inhibition with CFTRINH-172 (-14.8 +/− 0.5 vs. -16.3 +/− 0.9; control) with exposure to the 100 μM concentration (Figure 3). Stimulated Cl− secretion was solely reliant upon the presence of CFTR (confirmed in transgenic CFTR −/− MNSE, Figure 4).
Figure 1. Impact of acrolein on transepithelial Cl− secretion is concentration dependent.
Maximal ΔISC was noted at 100 µM (p < 0.0001), but decreased with increasing concentrations of compound. Total stimulation supplemented with forskolin was significantly inhibited at 300 µM (p<0.01) and completely abolished with exposure to 500 µM of acrolein.
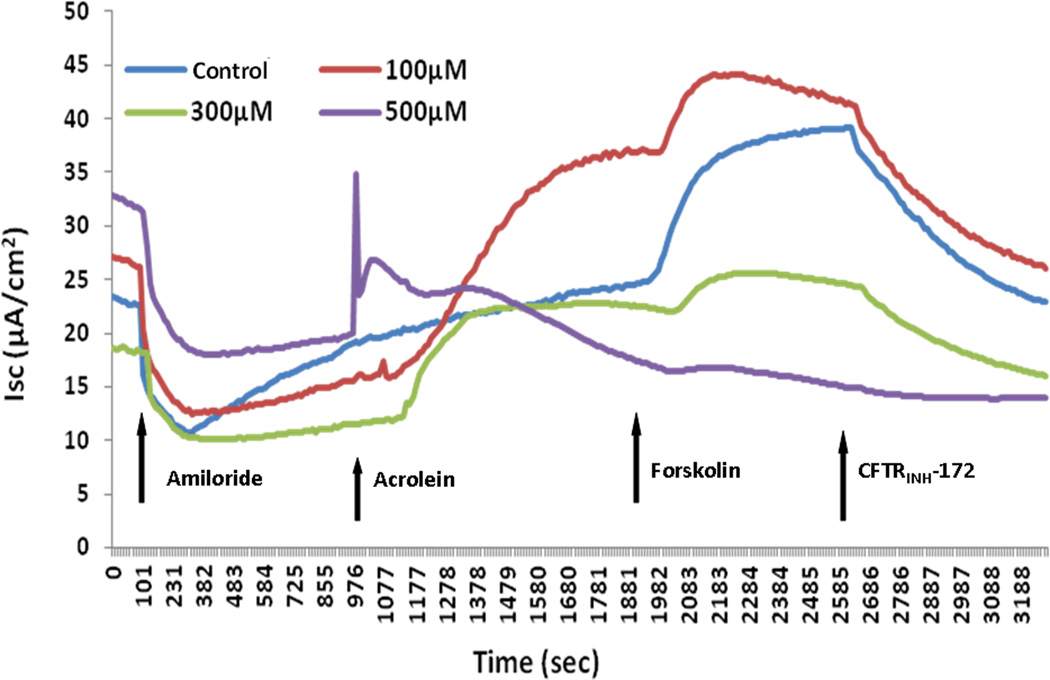
Figure 2. Representative Ussing chamber tracings of wild type MNSE cultures treated with acrolein.
Wild type MNSE cells grown on transwell permeable supports were mounted in Ussing chambers under short-circuit conditions and sequentially exposed to amiloride, acrolein, forskolin, and CFTRINH-172. A positive deflection indicates the net movement of an anion from the serosal to the mucosal direction. Note the paradoxical decrease in total ΔISC at higher concentrations.
Figure 3. Ussing chamber analysis of 100 µM acrolein exposure.
While maximal ΔISC was noted at this concentration (p < 0.0001), measurements of total stimulated (acrolein + forskolin) and CFTR inhibited (CFTRINH-172) ΔISC revealed no significant differences, suggesting near maximal stimulation of CFTR by acrolein alone.
Figure 4. Representative Ussing chamber tracings of transgenic CFTR−/− MNSE cultures treated with 100 µM acrolein.
No significant increase in ΔISC was measured signifying effects on apical Cl− transport are CFTR dependent.
Acrolein does not modulate CFTR through cAMP-mediated signal transduction
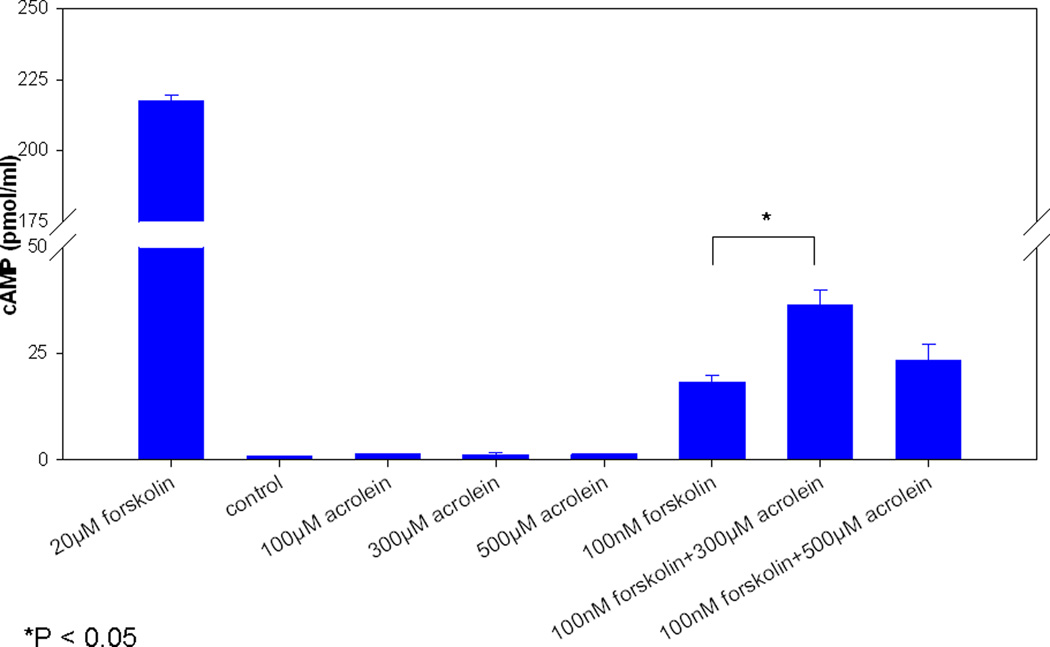
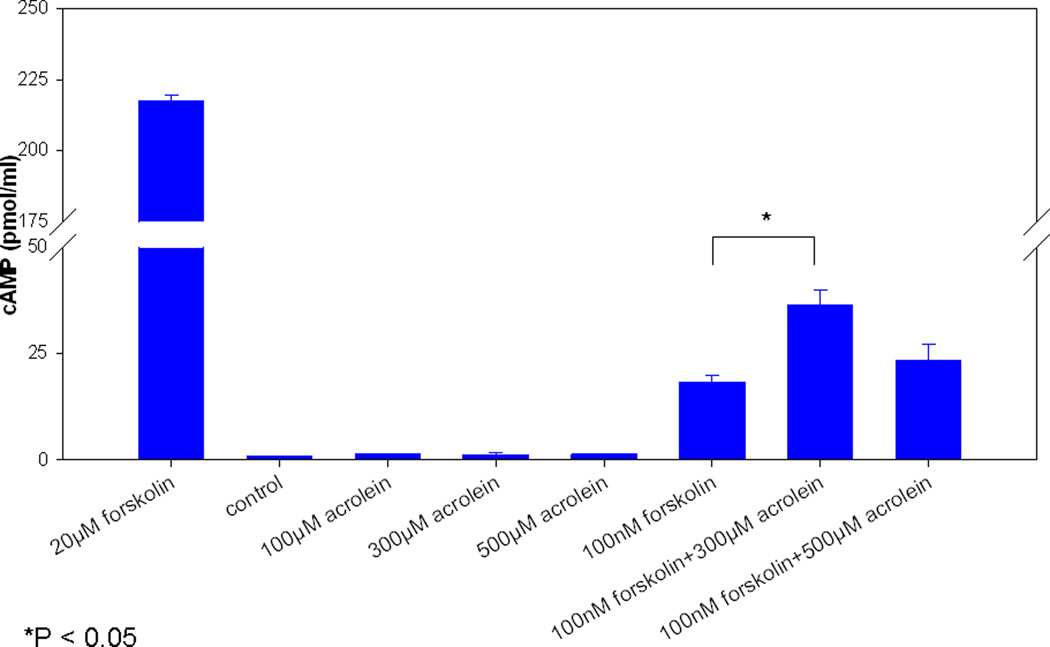
Modulation of CFTR related effects were independent of cAMP-signaling (expressed as pmol/ml) as there were no significant cAMP elevations (100 μM, 1.4 +/− 0.2; 300 μM, 1.3 +/− 0.6; 500 μM, 1.4 +/− 0.2) compared to controls [PBS (- control), 0.9 +/− 0.2 and forskolin (+ control), 217.6 +/− 2.1 (20 mM) and 18.4 +/− 1.5 (100 nM)]. (Figure 5) Importantly, pre-incubation with 300 or 500 μM acrolein did not inhibit cAMP stimulation by forskolin. In fact, administration of 100 nM forskolin after 300 μM acrolein resulted in a significant increase in cAMP over 100 nM forskolin alone (36.4 +/− 3.6 vs. 18.4 +/− 1.5, respectively).
Figure 5. Acrolein’s impact on CFTR is independent of cAMP stimulation.
No significant stimulation was demonstrated with graded concentrations of acrolein alone. Pre-incubation of cultures with higher doses of acrolein (300 and 500 µM) did not inhibit forskolin activation of adenylyl cyclase. A significant increase was seen over forskolin alone with the 300 µM concentration, but this is biologically insignificant.
CFTR-mediated effects are not reflective of acute cytotoxicity
Cell membrane integrity was not compromised by acute exposures to acrolein at any concentrations tested as judged by LDH release assay. The result is concordant with the lack of effect due to acrolein on tissue monolayer resistance in Ussing chamber (data not shown) which is often used as a proxy for cytotoxic drug effects. The negative impact of acrolein on CFTR dependent ISC measured at higher concentrations is therefore not secondary to cytotoxicity.
DISCUSSION
The present studies explored effects of the cigarette smoke toxin acrolein on transepithelial Cl− secretion and underlying cellular cAMP in a well established murine primary nasal cell culture model. Our prior investigations have shown that cigarette smoke condensate (CSC) inhibits major components of mucociliary transport, including transepithelial CFTR-mediated Cl− secretion, and ciliary beat in MNSE and human sinonasal epithelial (HSNE) cell cultures.3 Additionally, we demonstrated previously that CSC inhibits calcium activated chloride channel (CaCC) – mediated transepithelial Cl− secretion, and have confirmed this finding in transgenic CFTR−/− airway epithelial monolayers.14 These earlier findings lend support to the hypothesis that cigarette smoke suppresses apical Cl− secretion in respiratory epithelium and leads to ASL dehydration and dysfunctional MCC similar to cystic fibrosis airways. However, the specific components and the mechanism by which cigarette smoke suppresses Cl− secretion have not previously been determined.
At a dose (100 μM) that approximates the concentration found in the in vivo airways of tobacco smokers,10,24 acrolein significantly stimulated apical Cl− secretion. While cigarette smoke contains hundreds of toxins, acrolein by itself appears to promote hypersecretion at acute physiologic concentrations. Thus, inhibition of CFTR-mediated Cl- transport by water-soluble extract of cigarette smoke as previously reported is not mediated by this constituent. However, paradoxical inhibition of Cl− transport was demonstrated at higher test doses. Expression of CFTR is necessary to promote Cl− secretion through the pathway influenced by acrolein, since Ussing chamber tracings from transgenic CFTR−/− MNSE led to complete loss of this secretory mechanism. While CFTR-mediated pathways are stimulated, in part, by cAMP-dependent signaling, acrolein did not promote activation of adenylyl cyclase at any concentration. In addition, the suppression of forskolin-mediated activation of CFTR seen at higher concentrations of acrolein was not attributable to inhibitory effects on cellular cAMP. Importantly, no cytotoxic effects were demonstrated at the concentrations and exposures tested in the current investigations, indicating inhibitory effects at high concentrations are not due to cytotoxicity. While physiologic doses of acrolein led to an increase in CFTR-mediated Cl− transport, our data provide the first evidence of direct ion transport regulation by a cigarette smoke constituent. Paradoxical stimulation and inhibition have been demonstrated with other CFTR channel potentiating agents, such as flavonoids, in the absence of cAMP-mediated signaling and suggests that acrolein could interact with nucleotide-binding domains of CFTR in a similar fashion.2,14,21 Further studies, including patch clamp recordings, will be necessary to determine whether acrolein binds directly to CFTR or utilizes an alternative signaling mechanism to exert its effects on transepithelial Cl− transport.
Exposure to acrolein results in complex consequences for respiratory epithelial cells, including direct damage due to formation of adducts with proteins (disrupting cell structure or function) or nucleic acids (eliciting mutagenic or carcinogenic effects), and when effects such as depletion of antioxidants (e.g. glutathione) rendering a cell prone to damage from free radicals.25 Furthermore, expression of CFTR gene, protein, and function in response to cigarette smoke extract in vitro16,26 and our previous studies in MNSE and HSNE3,14 indicate deleterious consequences for mucociliary transport. While, the present findings establish that acute incubations with acrolein at physiologic concentrations suggest that hypersecretion would be more evident in the airways of individuals with acute exposure to this toxin, future investigations will examine prolonged exposures at physiologic concentrations and other tobacco smoke constituents for CFTR-related effects on mucociliary clearance.
CONCLUSION
The present study demonstrated that acrolein has complex, but significant interactions with CFTR, the major apical Cl− conductance pathway in sinonasal epithelium. The findings describe a means by which the effects of CFTR modulation by cigarette smoke can be better understood in the future.
Acknowledgments
Research Support: This research was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (1K08HL107142-01) and Flight Attendant’s Medical Research Institute Young Clinical Scientist Award (072218) to B.A.W.; and NIH/NIDDK (5P30DK072482-03) to E.J.S.
Footnotes
Conflict of Interest/Financial Disclosures: Bradford A. Woodworth, MD is a consultant for ArthroCare ENT and Gyrus ENT. Eric Sorscher, MD, and Bradford Woodworth, MD are inventors on a patent pending regarding the use of chloride secretagogues for therapy of sinus disease (35 U.S.C. n111(b) and 37 C.F.R n.53 (c) in the U.S. Patent and Trademark Office.
This manuscript was presented at the AAO-HNS annual meeting, San Francisco, September 12, 2011.
REFERENCES
- 1.Trout L, King M, Feng W, Inglis SK, Ballard ST. Inhibition of airway liquid secretion and its effect on the physical properties of airway mucus. Am J Physiol. 1998;274:L258–L263. doi: 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- 2.Alexander NS, Hatch N, Zhang Set al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope. 2011;121:1313–1319. doi: 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009 doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 4.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope. 2011;121:1929–1934. doi: 10.1002/lary.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieu JE, Feinstein AR. Confirmations and surprises in the association of tobacco use with sinusitis. Arch Otolaryngol Head Neck Surg. 2000;126:940–946. doi: 10.1001/archotol.126.8.940. [DOI] [PubMed] [Google Scholar]
- 6.Lee WK, Ramanathan M, Jr, Spannhake EW, Lane AP. The cigarette smoke component acrolein inhibits expression of the innate immune components IL-8 and human beta-defensin 2 by sinonasal epithelial cells. Am J Rhinol. 2007;21:658–663. doi: 10.2500/ajr.2007.21.3094. [DOI] [PubMed] [Google Scholar]
- 7.Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. Clin Otolaryngol. 1998;23:227–230. doi: 10.1046/j.1365-2273.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalhamn T, Rylander R. Ciliotoxicity of cigar and cigarette smoke. Arch Environ Health. 1970;20:252–253. doi: 10.1080/00039896.1970.10665581. [DOI] [PubMed] [Google Scholar]
- 9.Dalhamn T, Rylander R. Tar content and ciliotoxicity of cigarette smoke. Acta Pharmacol Toxicol (Copenh) 1967;25:369–372. doi: 10.1111/j.1600-0773.1967.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 10.Zemski Berry KA, Murphy RC. Characterization of acrolein-glycerophosphoethanolamine lipid adducts using electrospray mass spectrometry. Chem Res Toxicol. 2007;20:1342–1351. doi: 10.1021/tx700102n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar P, Hayes BE. Induction of COX-2 by acrolein in rat lung epithelial cells. Mol Cell Biochem. 2007;301:191–199. doi: 10.1007/s11010-007-9411-z. [DOI] [PubMed] [Google Scholar]
- 12.Moodie FM, Marwick JA, Anderson CS, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. Faseb J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 13.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Cremoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–634. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 14.Virgin FW, Azbell C, Schuster Det al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 16.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 17.Antunes MB, Woodworth BA, Bhargave G, et al. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques. 2007;43:195–196. doi: 10.2144/000112531. 198, 200 passim. [DOI] [PubMed] [Google Scholar]
- 18.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. Am J Rhinol. 2008;22:7–12. doi: 10.2500/ajr.2008.22.3125. [DOI] [PubMed] [Google Scholar]
- 19.Woodworth BA, Tamashiro E, Bhargave G, Cohen NA, Palmer JN. An in vitro model of Pseudomonas aeruginosa biofilms on viable airway epithelial cell monolayers. Am J Rhinol. 2008;22:234–238. doi: 10.2500/ajr.2008.22.3178. [DOI] [PubMed] [Google Scholar]
- 20.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120:1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]
- 21.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143:397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. Am J Rhinol Allergy. 2009;23:149–152. doi: 10.2500/ajra.2009.23.3285. [DOI] [PubMed] [Google Scholar]
- 23.Pyle L, Ehrhardt A, Nowotarski K, Rowe SM, Sorscher EJ. North American Cystic Fibrosis Foundation Annual Meeting. Denver, CO: 2006. The role of R-domain phosphorylation in cftr activation by potentiators. [Google Scholar]
- 24.Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- 25.O'Toole TE, Zheng YT, Hellmann J, Conklin DJ, Barski O, Bhatnagar A. Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages. Toxicol Appl Pharmacol. 2009;236:194–201. doi: 10.1016/j.taap.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantin AM, Hanrahan JW, Bilodeau Get al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]