Background: Nephrin tyrosine phosphorylation by Fyn is important for its function, but the mechanisms regulating this process are not well characterized.
Results: Nck promotes nephrin phosphorylation through activation of Fyn.
Conclusion: Nck appears to be involved in the regulation of nephrin phosphorylation.
Significance: We have identified an interaction that contributes to our understanding of the regulation of nephrin phosphorylation.
Keywords: Cell Signaling, Podocytes, Protein Phosphorylation, SH3 Domains, Tyrosine Protein Kinase (Tyrosine kinase), Nck, Nephrin
Abstract
The transmembrane protein nephrin is a key component of the kidney slit diaphragm that contributes to the morphology of podocyte foot processes through signaling to the underlying actin cytoskeleton. We have recently reported that tyrosine phosphorylation of the cytoplasmic tail of nephrin facilitates recruitment of Nck SH2/SH3 adaptor proteins and subsequent actin remodeling and that phosphorylation of the Nck binding sites on nephrin is decreased during podocyte injury. We now demonstrate that Nck directly modulates nephrin phosphorylation through formation of a signaling complex with the Src family kinase Fyn. The ability of Nck to enhance nephrin phosphorylation is compromised in the presence of a Src family kinase inhibitor and when the SH3 domains of Nck are mutated. Furthermore, induced loss of Nck expression in podocytes in vivo is associated with a rapid reduction in nephrin tyrosine phosphorylation. Our results suggest that Nck may facilitate dynamic signaling events at the slit diaphragm by promoting Fyn-dependent phosphorylation of nephrin, which may be important in the regulation of foot process morphology and response to podocyte injury.
Introduction
Podocytes are a specialized type of epithelial cell that comprise the outer layer of the blood filtration barrier of the kidney glomerulus (1). These cells have a unique three-dimensional structure formed by the extension of many small actin-rich foot processes, which interdigitate with one another to form a lattice-like structure. Adjacent foot processes are connected via a specialized intercellular junction known as the slit diaphragm.
One of the primary causes of many forms of podocyte damage is alteration in the integrity of the slit diaphragm concomitant with morphological changes to the actin cytoskeleton of the foot processes, resulting in a flattening, or effacement, of foot processes as well as a loss of slit diaphragms (2). Together, these perturbations can lead to the development of renal disease. A key component of the slit diaphragm is the transmembrane IgG family protein nephrin, which interacts with neighboring nephrin molecules to form both an external structural barrier through its IgG domains and an internal signaling hub using its cytoplasmic tail, which is connected to the podocyte actin cytoskeleton (3).
Several aspects of foot process architecture appear to be dependent on proper phosphorylation of podocyte proteins induced by Src family kinases (SFKs).3 Mice lacking both Fyn and Yes develop proteinuria and foot process effacement by 4 weeks of age (4), whereas Fyn knock-out mice present with altered foot process architecture (4, 5) and have been reported to develop proteinuria (5). Targets of phosphorylation by SFKs include a number of structural components of the slit diaphragm, such as nephrin (4) and the Neph1/2/3 family (6, 7) of IgG adhesion molecules, whose phosphorylated tyrosines serve to recruit downstream signaling proteins. Phosphorylated nephrin has been reported to interact with p85 (8–10), podocin (11, 12), Nck1/2 (13–15), and phospholipase C-γ (16), whereas phosphorylated Neph1 has been shown to interact with Grb2 (6, 7) and C-terminal Src kinase (Csk) (7).
Three of the six highly conserved tyrosine residues of the cytoplasmic tail of human nephrin, Tyr1176, Tyr1193, and Tyr1217, are contained within YDXV motifs. We (15, 17) and others (13, 14) have demonstrated that, when phosphorylated, each of these residues can recruit the Nck family of SH2/SH3 adaptor proteins. The single Nck SH2 domain binds the phosphorylated YDXV motifs, whereas the three tandem SH3 domains mediate interactions with downstream effectors of the actin cytoskeleton, thereby providing a link between nephrin and podocyte actin dynamics. SH2 domain-mediated recruitment of Nck to a phosphorylated substrate followed by SH3 domain recruitment of modulators of the actin cytoskeleton has also been demonstrated for many other Nck interacting partners (18). Additionally, we have also demonstrated an essential role for Nck adaptors in both the development of podocyte structure (15) as well as in the maintenance of foot process architecture in the adult mouse (19).
Both the amount and the significance of nephrin tyrosine phosphorylation at the slit diaphragm remain areas of ongoing investigation. There have been multiple reports demonstrating in vitro that nephrin can be phosphorylated by Fyn on more than one tyrosine residue (11, 14–16). Overall, it would appear that the conserved sites Tyr1193 and Tyr1217 are the strongest targets of Fyn kinase (15, 16). Additionally, phosphorylation on Tyr1193 and Tyr1217 in the healthy kidney has been demonstrated in vivo through multiple reports using independently generated phospho-specific nephrin antibodies (19–21). A separate study using mass spectroscopy reported phosphorylation on Tyr1193 in samples from normal rat kidneys (22).
Phosphorylation of nephrin on specific sites has also been examined during the development of renal disease, with decreases in nephrin phosphorylation on Tyr1217 reported in human patients with minimal change disease (20) as well as in the corresponding rat puromycin aminonucleoside model (19, 20) and the mouse LPS model of transient foot process effacement (21). However, despite changes in nephrin phosphorylation being associated with disease, the mechanisms regulating the dynamics of nephrin phosphorylation and their role in the development and maintenance of podocyte structure are currently not well understood.
In the present study, we have characterized a role for the Nck family of adaptor proteins in the regulation of nephrin phosphorylation. We demonstrate that both Nck1 and Nck2 increase nephrin phosphorylation in vitro and that this effect is dependent on functional SH3 domains of Nck. Further, we found that, when recruited to nephrin, Nck was able to interact with the kinase Fyn and that this interaction correlated with an increase in Fyn activity. Lastly, using a mouse model to perform a podocyte-specific deletion of both Nck1 and Nck2, we show that reduction of Nck in vivo is associated with rapid loss of nephrin phosphorylation. These data suggest an additional role for Nck adaptor proteins in cellular signaling that could contribute to the pathogenesis of kidney disease.
EXPERIMENTAL PROCEDURES
Plasmids
Constructs encoding human Myc-tagged nephrin, CD16-nephrin-GFP, and human FLAG-tagged Nck1 and Nck2 and mutants thereof have been previously described (15, 17). HA-Akt was provided by Jim Woodgett (Samuel Lunenfeld Research Institute (SLRI), Toronto, Ontario, Canada), pcDNA3-HA-Nck2 was a gift from Bruce Mayer (University of Connecticut, Farmington, CT), and pcDNA3-HA-Grb2 was provided by Tony Pawson (SLRI).
Antibodies
The following antibodies were obtained commercially: rabbit anti-GFP (ab290) (Abcam), mouse anti-GAPDH (G041) (Applied Biological Materials Inc.), mouse anti-Nck (BD Pharmingen), and guinea pig anti-nephrin (20R-NP002) (Fitzgerald Inc.). Rabbit anti-pSer473 Akt (4060), rabbit anti-pSer3-cofilin (3311), mouse anti-Myc 9B11 (2276), and rabbit anti-Tyr527 (active) Src (2107) were obtained from Cell Signaling. Mouse anti-CD16 (sc-19620), rabbit anti-pThr308 Akt (sc-16646), and rabbit anti-Fyn (sc-16) were obtained from Santa Cruz Biotechnology. Rabbit anti-cofilin (C8736), mouse anti-FLAG clone M2 (F3165), and anti-FLAG M2-HRP (A8592) were from Sigma-Aldrich. Mouse anti-HA clone 12CA5 and anti-pTyr clone 4G10 were provided by Sunnybrook Hybridoma Bank, Toronto, Ontario, Canada. Generation and testing of the following antibodies has previously been reported: rabbit anti-nephrin (11) and phospho-specific nephrin pTyr1193 and pTyr1217 (19).
Cell Culture
HEK293T cells were grown in Dulbecco's modified Eagle's medium-high glucose (HyClone) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were kept at 37 °C with 5% CO2. Transfections were performed using polyethyleneimine.
Cell Lysis, Immunoprecipitation, and Western Blotting
For phosphorylation analyses and co-immunoprecipitation experiments, cells expressing CD16-nephrin were starved in serum-free medium overnight and then stimulated for 10 min at 37 °C by using a 1 μg/ml dilution of anti-CD16 antibody. For inhibition of SFK activity, cells were treated with 10 μm PP2 (Sigma-Aldrich) for 3 h prior to CD16 stimulation as described above in the continued presence of PP2. Lysates were prepared from transfected cells by using phospholipase C lysis buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 10% glycerol, 1% Triton X-100, 15 mm MgCl2, 1 mm EGTA, 10 mm Na-PPi, 100 mm NaF) supplemented with fresh protease inhibitors. Immunoprecipitation procedures have been described previously (17).
For Western blotting, total lysates and immunoprecipitates were resolved on 8% SDS-PAGE gels followed by semidry transfer to PVDF membrane, blocked for 30 min in TBS with Tween containing either 5% nonfat milk powder or BSA, and incubated with the indicated primary antibody overnight at 4 °C. Detection was performed using ECL Western blotting substrate (Pierce). Blots were imaged using a ChemiDoc XRS+ (Bio-Rad) or exposed to film (Pierce). Values used for densitometry were obtained using ImageLab v2.0 analysis software (Bio-Rad).
Analysis of Nck Conditional Inducible Mutant Mice
Inducible podocyte-specific Nck2 knock-out mice on a Nck1 null background (referred to here as Nck1/2-cKO mice) containing the following transgenes (Podocin-rtTA; TetO-Cre; Nck2flx/flx; Nck1−/−) have been described previously (19).
We housed Nck1/2-cKO mice and their littermate controls (Nck2flx/flx; Nck1−/−) together and collectively treated them with doxycycline in their food (2,000 mg/kg) (TD.09751 Harlan Teklad; Madison, WI). Doxycycline was administered using this method for 1–2 weeks to generate animals with reduced Nck expression in podocytes. The duration of doxycycline treatment was determined to be the minimum length to allow sufficient reduction of Nck prior to development of proteinuria. Animal husbandry was approved by the University of Guelph Animal Care Committee and carried out in accordance with Canadian Council on Animal Care protocols.
Mice were euthanized with CO2 and immediately perfused with saline followed directly by removal of the kidneys. Either kidneys were processed for immunofluorescence as described below, or protein samples were prepared for Western blotting. For protein extraction, the renal cortex was separated and lysed (1 ml/0.4 g of tissue) in radioimmunoprecipitation assay buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 10% glycerol, 1% Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS, 1 mm EDTA), supplemented with fresh protease inhibitors via homogenization followed by sonication, and then centrifuged at 13,000 rpm for 20 min to remove any debris. Total protein samples were prepared from the resulting supernatant.
Immunofluorescence
Kidneys were flash-frozen in Cryomatrix (Thermo). 5-μm sections were cut onto Superfrost Plus slides (Fisher) on a cryostat (Shandon). Sections were fixed and permeabilized in acetone for 10 min at −20 °C, blocked in buffer containing 10% goat serum and PhosSTOP tablet (Roche Applied Science), and incubated with primary antibodies overnight at 4 °C (guinea pig anti-nephrin (1:100) and rabbit anti-pTyr1217 nephrin (1:50)). After washing, sections were incubated with secondary antibodies for 30 min at 25 °C (goat anti-guinea pig Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 594, both 1:400). Finally, slides were mounted using Prolong Gold antifade reagent with DAPI mounting medium (Invitrogen). Cryosection slides were visualized using a Leica M2 125 epifluorescence microscope with a 40× objective lens. Images were captured using Volocity and processed with Adobe Photoshop. The Alexa Fluor conjugated secondary antibodies obtained from Invitrogen for immunofluorescence were: goat anti-guinea pig 488 (A-11076) and goat anti-rabbit 594 (A11037).
Statistical Analysis
Experiments were performed a minimum of three times. Differences between two groups were analyzed using the Student's t test, whereas differences between three or more groups were compared using analysis of variance followed by Tukey's test with specific contrasts used for comparisons between multiple groups of means. Statistical significance was defined as p < 0.05. Values are reported as means ± S.E. Statistical analysis was performed using SAS version 9.2 (Cary, NC) for analysis of variance, whereas GraphPad Prism version 5.04 (San Diego, CA) was used for t tests. All graphs were prepared using GraphPad Prism.
RESULTS
Nck Augments Nephrin Tyrosine Phosphorylation
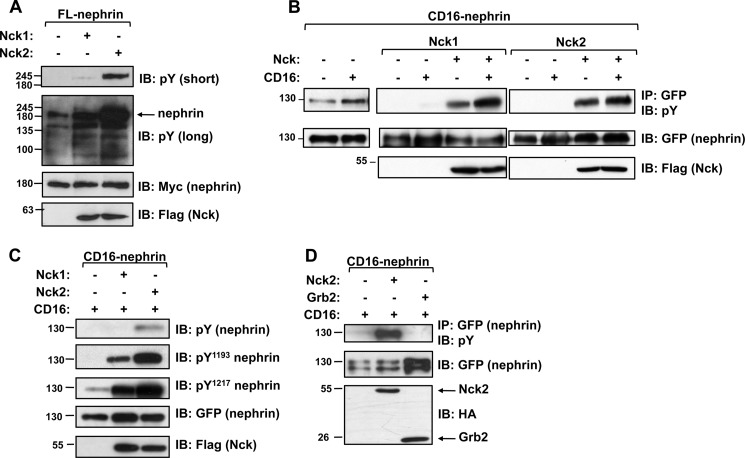
During our earlier analyses of interactions between nephrin and Nck (17), we noticed increased levels of nephrin tyrosine phosphorylation upon co-expression of Nck as compared with expression of nephrin alone. This prompted us to investigate whether Nck could be responsible for the difference in phosphorylation. To begin, we first confirmed that the presence of exogenous Nck1 or Nck2 could impact nephrin phosphorylation. When either Nck1 or Nck2 was co-expressed with full-length nephrin in HEK293T cells, we observed increased amounts of nephrin tyrosine phosphorylation (Fig. 1A). Similarly, using our chimeric CD16-nephrin construct containing the cytoplasmic tail of nephrin, which we have previously shown undergoes tyrosine phosphorylation following CD16-induced clustering (17), we found that both Nck1 and Nck2 increased the basal amount of nephrin tyrosine phosphorylation observed in the absence of CD16-induced clustering, which increased further following CD16-induced clustering (Fig. 1B).
FIGURE 1.
Nck increases nephrin tyrosine phosphorylation. A, HEK293T cells were transfected with full-length (FL) Myc-tagged nephrin and FLAG-tagged Nck1 or Nck2 as indicated, and lysates were immunoblotted (IB) for phosphotyrosine (pY), Myc, or FLAG. Co-expression of Nck1 or Nck2 increases overall nephrin phosphorylation as compared with the base-line phosphorylation seen in the long exposure. B, HEK293T cells were transfected with GFP-tagged CD16-nephrin and Nck1 or Nck2 and then stimulated with CD16 (+) or left untreated (−). CD16 clustering induces an increase in nephrin phosphorylation (left panel), which is enhanced in the presence of Nck1 or Nck2 and further increased after CD16 clustering (middle and right panels). Phosphorylation of CD16-nephrin alone can be seen after long pTyr exposure (left panel), whereas phosphorylation of CD16-nephrin co-expressed with Nck is visible in short pTyr exposures (middle and right panels). C, lysates from CD16-stimulated HEK293T cells expressing CD16-nephrin with or without Nck1 or Nck2 were immunoblotted as indicated to reveal increased levels of nephrin phosphorylation on residues Tyr1193 and Tyr1217 in the presence of Nck1 and Nck2. D, HEK293T cells were transfected with CD16-nephrin and HA-tagged Nck2 or Grb2 as indicated. CD16-induced hyperphosphorylation of nephrin is seen with Nck2 but not with Grb2.
We then used our phospho-specific antibodies against Tyr1193 and Tyr1217 (19) to determine whether the increased phosphorylation on nephrin was occurring on these important downstream signaling sites. We found that, similar to total phosphotyrosine levels, there was more nephrin phosphorylation with Nck1 and Nck2 at both Tyr1193 and Tyr1217 (Fig. 1C). Interestingly, the observed increase in total nephrin phosphorylation as well as site-specific phosphorylation was consistently larger with Nck2 as compared with Nck1. We also found that overall nephrin phosphorylation showed a dose-dependent increase with Nck expression (data not shown).
To verify that this change in phosphorylation was specific to Nck, we performed a similar experiment using the adaptor protein Grb2, which is also composed entirely of SH2 and SH3 domains. We observed no change in nephrin phosphorylation in the presence of Grb2 (Fig. 1D), suggesting that the enhancement seen with Nck reflects a unique function of the Nck family.
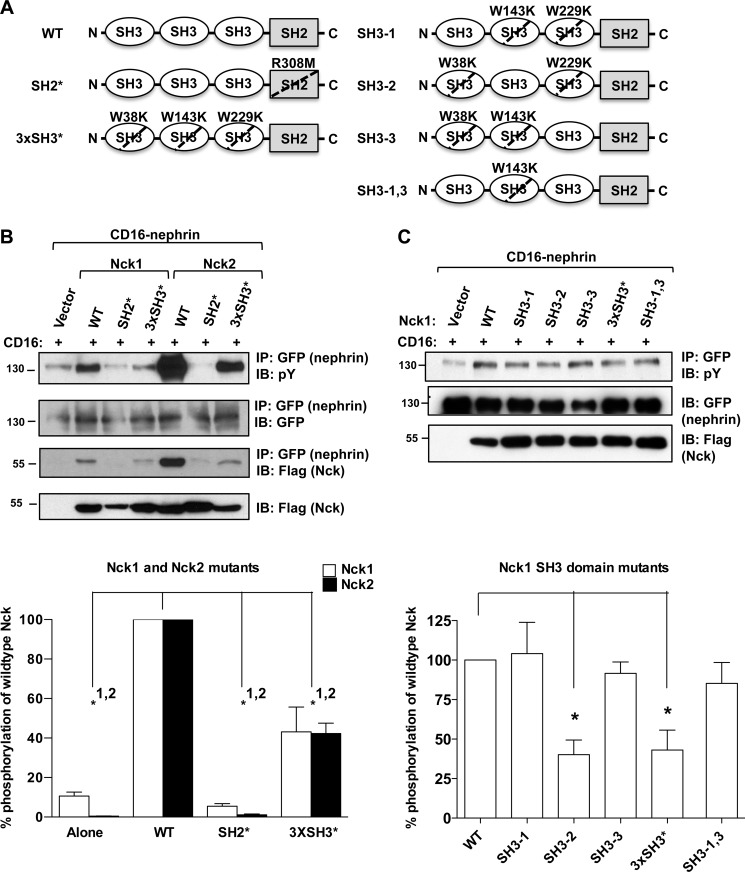
Nephrin Phosphorylation Requires the SH3 Domains of Nck
Nck1 and Nck2 are both composed of three similar SH3 domains followed by a single SH2 domain (Fig. 2A). To determine whether the SH3 domains contribute to increased nephrin phosphorylation, we employed mutant versions of Nck1 and Nck2, containing Trp-to-Lys substitutions in each of the SH3 domains (3×SH3*), rendering the domains unable to bind to their targets. We mutated all three SH3 domains as many Nck SH3 domain binding partners can bind to more than one Nck SH3 domain. As a control, we used Nck constructs with mutated SH2 domains (SH2*) containing an Arg-to-Met substitution, which are unable to interact with nephrin (14, 17).
FIGURE 2.
Increased nephrin phosphorylation is dependent on the SH3 domains of Nck. A, schematic illustrating the structure of Nck and the amino acid residues mutated to generate the SH2* and various SH3* mutant constructs in Nck1. The corresponding residues were mutated in Nck2. B, HEK293T cells were transfected with CD16-nephrin and various mutants of Nck1 or Nck2 as indicated. CD16-stimulated (+) lysates were examined for nephrin phosphorylation and Nck binding by Western blotting followed by densitometry to determine the amount of nephrin phosphorylation relative to wild-type Nck. With wild-type (WT) Nck1 (white) or Nck2 (black) normalized to 100%, the SH2 domain mutants (SH2*) do not cause an increase in CD16-nephrin phosphorylation for either Nck1 (5.4 ± 1.4%) or Nck2 (1.1 ± 0.43%) relative to the amount of phosphorylation observed with CD16-nephrin alone (Nck1: 10.6 ± 2.0%; Nck2: 0.47 ± 0.044%). Mutation of all SH3 domains (3×SH3*) leads to a decrease in nephrin phosphorylation to 43.1 ± 12.6% for Nck1 and 42.3 ± 5.3% for Nck2 of that observed with the wild-type Nck. The amount of nephrin phosphorylation correlates with the amount of Nck immunoprecipitated (IP) with nephrin. * indicates p < 0.05 as compared with wild type for Nck1 (indicated by 1) and Nck2 (indicated by 2). IB, immunoblotted; pY, phosphotyrosine. C, transfection of HEK293T cells with additional SH3 domain mutants of Nck1 and CD16-nephrin followed by CD16 stimulation (+) revealed that the first and third SH3 domains either alone (SH3-1, 104.1 ± 19.8%; SH3-3, 91.6 ± 7.2%) or in combination (SH3-1,3, 85.3% ± 13.2%) increase nephrin phosphorylation similarly to wild-type Nck1 (100%), whereas the second SH3 domain alone (SH3-2, 40.2 ± 9.3%) is equivalent to the 3×SH3* mutant (43.1 ± 12.6%). * indicates p < 0.05 as compared with wild type.
Interestingly, we observed that expression of mutant versions of Nck1 or Nck2 lacking functional SH3 domains with CD16-nephrin significantly impaired Nck-mediated nephrin tyrosine phosphorylation (Fig. 2B). We found that wild-type Nck1 produced an ∼10-fold increase in CD16-nephrin phosphorylation relative to control cells expressing vector alone (p < 0.05), whereas with the 3×SH3* mutant, only 43.1 ± 12.6% of the amount of phosphorylation seen with wild-type Nck1 was observed (p < 0.05). More strikingly, wild-type Nck2 increased CD16-nephrin phosphorylation ∼200-fold relative to CD16-nephrin alone (p < 0.05). The amount of CD16-nephrin phosphorylation observed with 3×SH3* mutant Nck2 was 42.3 ± 5.3% of the amount of phosphorylation observed with wild-type Nck2 (p < 0.05). Similar findings were obtained with full-length nephrin (supplemental Fig. S1).
We observed that mutation of all three SH3 domains in Nck1 or Nck2 produced a similar relative decrease in nephrin phosphorylation (∼40% of the amount seen with the corresponding wild-type Nck), suggesting that these domains perform equivalent functions in Nck1 and Nck2. To map the contributions of individual SH3 domains to nephrin phosphorylation, we expressed Nck1 mutants containing only a single functional SH3 domain (SH3-1, SH3-2, SH3-3) (as illustrated in Fig. 2A) along with CD16-nephrin in HEK293T cells, with wild-type and 3×SH3* Nck1 serving as controls. We noted a significant reduction in nephrin phosphorylation with the SH3-2 construct (40.2 ± 9.3%) as compared with wild-type Nck1 (100%) (p < 0.05), which was equivalent to the reduction seen with the full 3×SH3* mutant (43.1 ± 12.6%) (Fig. 2C). This effect was not seen with the SH3-1 (104.1 ± 19.8%) or SH3-3 (91.6 ± 7.2%) constructs or when only the second SH3 domain was mutated (SH3-1,3; 85.3% ± 13.2%) (Fig. 2C). These results indicate that both the first and the third SH3 domains of Nck can mediate binding to proteins that control nephrin tyrosine phosphorylation, whereas the second SH3 domain does not contribute appreciably to this process.
Similar to what we observed with Grb2 (Fig. 1D), neither of the SH2* mutants altered nephrin phosphorylation relative to the amount observed with CD16-nephrin alone (p > 0.05) (Fig. 2B). As previous findings have clearly established that the interaction between nephrin and Nck requires binding of the Nck SH2 domain to phosphorylated tyrosine residues on nephrin (13–15, 17), our results suggest that the increase in nephrin phosphorylation seen with wild-type Nck is dependent on SH2-mediated recruitment of Nck to nephrin and potentially via SH3-mediated association of a tyrosine kinase with Nck.
Hyperphosphorylation of Nephrin Correlates with Fyn Recruitment and Activation
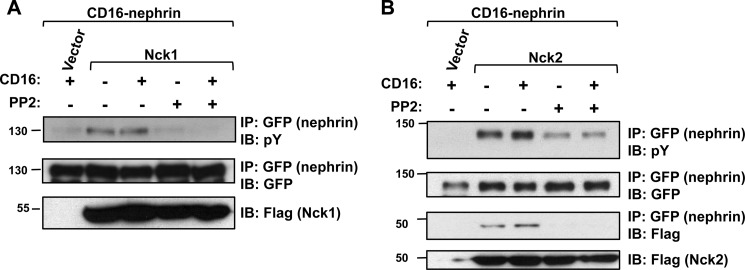
The SFK Fyn plays a central role in podocyte foot process architecture, and given its ability to phosphorylate nephrin both in vitro (16) and in vivo (4), we examined whether this tyrosine kinase might be responsible for the increase in nephrin phosphorylation mediated by Nck adaptor proteins. To test whether the increase in nephrin phosphorylation was a result of the action of SFKs, we blocked their activity using PP2, which is a SFK inhibitor. Treatment with PP2 suppressed the effect on Nck1-mediated CD16-nephrin phosphorylation, both in the presence and in the absence of CD16 clustering (Fig. 3A). Similarly, PP2 decreased the amount of CD16-nephrin phosphorylation observed with Nck2 (Fig. 3B). We also found that the interaction between nephrin and Nck induced by CD16 clustering was eliminated in cells treated with PP2 (Fig. 3B).
FIGURE 3.
Nck-induced hyperphosphorylation of nephrin requires SFK activity. A, HEK293T cells expressing CD16-nephrin and Nck1 were pretreated with 10 μm PP2 as indicated for 3 h prior to stimulation with CD16 (+) to induce CD16-nephrin phosphorylation or left unstimulated (−). PP2 inhibition of SFKs blocks Nck1-mediated nephrin phosphorylation. IP, co-immunoprecipitation; IB, immunoblot; pY, phosphotyrosine. B, identical experiment as in A, except that Nck2 was used instead of Nck1. Treatment with PP2 reduces Nck2-induced hyperphosphorylation of nephrin and the interaction between Nck2 and nephrin as observed by co-immunoprecipitation.
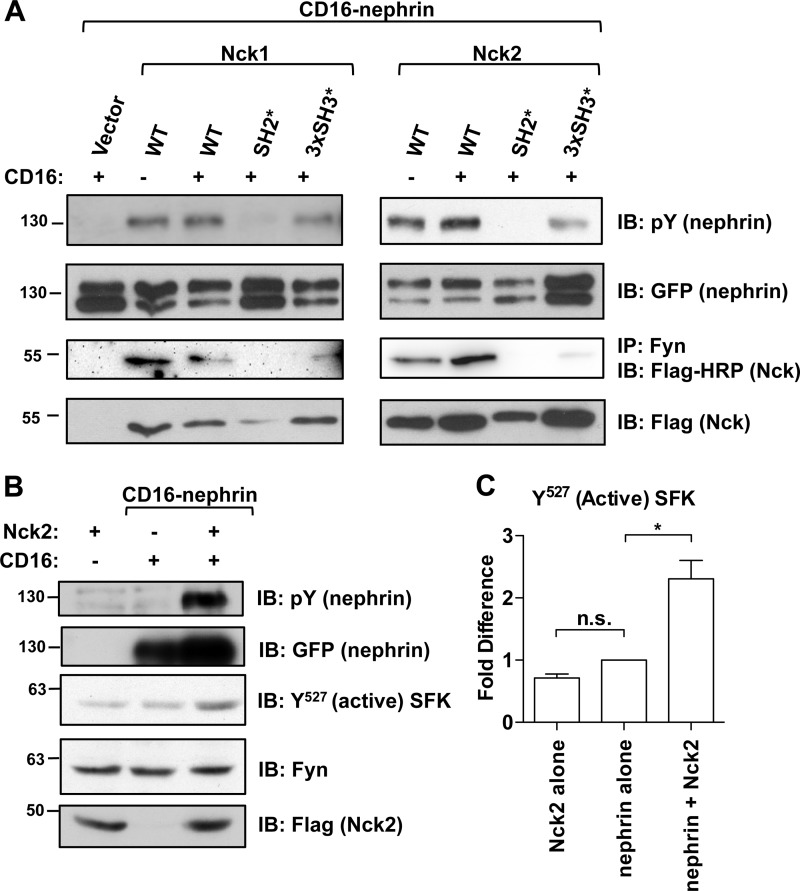
Having observed that SFK activity was required to increase nephrin phosphorylation in the presence of Nck (Fig. 3), we hypothesized that Nck and Fyn might form a signaling complex at the plasma membrane that affects nephrin phosphorylation. Using our CD16 clustering system, we expressed CD16-nephrin with wild type as well as the SH2* and 3×SH3* mutants of Nck1 and Nck2 and then examined binding between Fyn and Nck1 or Nck2 following clustering of CD16-nephrin (Fig. 4A). We observed that the binding of wild-type and mutant Nck to Fyn by co-immunoprecipitation correlated closely with the results we previously obtained with the same Nck constructs regarding total nephrin phosphorylation (Fig. 2B). Analogous to the effect of Nck on nephrin phosphorylation, we observed the strongest interaction of Fyn with wild-type Nck, with Nck2 showing a more robust interaction than Nck1. We were able to detect a very weak interaction between Fyn and both 3×SH3* Ncks, which correlates with 3×SH3* mutant Nck having a lesser effect on nephrin phosphorylation. We did not observe any binding of SH2* Nck to Fyn as anticipated; Fyn is localized at the plasma membrane, and as SH2* Nck cannot interact with nephrin (14, 17), it is not recruited to the plasma membrane and so remains spatially segregated from Fyn in the cytoplasm.
FIGURE 4.
Nck interacts with Fyn, which increases nephrin phosphorylation and Fyn activation. A, HEK293T cells were transfected with CD16-nephrin and Nck1 or Nck2 constructs as indicated and then stimulated with CD16 (+) or left untreated (−). Both Nck1 and Nck2 co-immunoprecipitate (IP) with endogenous Fyn; however, we observed that the interaction with Fyn was much stronger for Nck2 as compared with Nck1. The interaction between Fyn and Nck is very weak when all three SH3 domains are mutated and absent when the SH2 domain is mutated. The strength of these interactions correlates with the amount of nephrin phosphorylation seen in the lysates. IB, immunoblot; pY, phosphotyrosine. B, HEK293T cells were transfected with Nck2 alone as a control, CD16-nephrin alone, or CD16-nephrin and Nck2, and those containing CD16-nephrin were stimulated with CD16 (+). Lysates were immunoblotted for unphosphorylated Tyr527 Src, which recognizes activated SFKs, and then reprobed for Fyn. C, lysates were analyzed by densitometry to determine relative Fyn activity. Expression of Nck2 alone did not alter Fyn activity relative to CD16-nephrin alone. However, when Nck2 was co-expressed with CD16-nephrin, there was a 2.3 ± 0.30-fold increase in the amount of active Fyn relative to CD16-nephrin alone. * indicates p < 0.05 as compared with CD16-nephrin alone; n. s. indicates a nonsignificant difference.
We next investigated whether the presence of Nck might directly influence the activity of Fyn. To detect active Fyn, we used an antibody that recognizes the C-terminal SFK tyrosine Tyr527 only when it is dephosphorylated and therefore released from the inhibitory intramolecular interaction with its SH2 domain. We utilized this antibody to detect active Fyn and compared it with total Fyn to determine the level of Fyn activity in lysates from cells expressing Nck2 and/or CD16-nephrin (Fig. 4B). We observed a 2.3 ± 0.30-fold increase in active Fyn when CD16-nephrin was expressed with Nck2 as compared with CD16-nephrin alone (p < 0.05) (Fig. 4C). Notably, expression of Nck2 alone did not produce an increase in Fyn activity, ruling out a nephrin-independent effect of Nck2 on Fyn activity. Collectively, these data suggest that the mechanism by which Nck regulates nephrin phosphorylation occurs through an interaction with Fyn, likely involving the SH3 domains of Nck, which leads to increased Fyn activation.
Increased Nephrin Phosphorylation Correlates with Activation of Nephrin Signaling Pathways
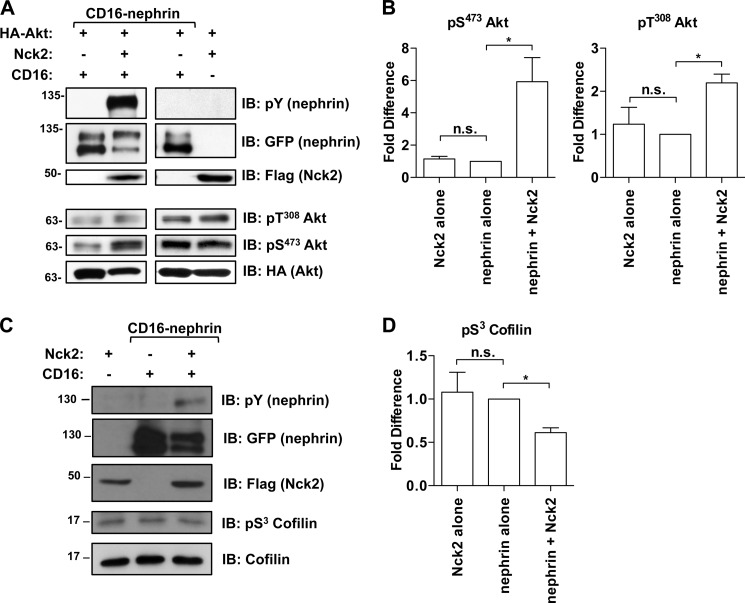
Because we observed a global increase in nephrin tyrosine phosphorylation in the presence of Nck, we next asked whether this increase translated into the activation of downstream nephrin signaling pathways. In addition to Nck, phosphorylated nephrin recruits p85 and activates PI3K-dependent signaling (9), which includes phosphorylation of Thr308 and Ser473 on Akt. To examine changes in the activation state of Akt, we co-expressed HA-tagged Akt with CD16-nephrin and/or Nck2 in HEK293T cells and monitored Akt phosphorylation by Western blotting of cell lysates. We observed increased phosphorylation on both sites on Akt only when CD16-nephrin and Nck2 were co-expressed (Fig. 5A). Specifically, Akt phosphorylation on Thr308 was increased 2.2 ± 0.21-fold (p < 0.05), whereas phosphorylation of Ser473 was increased 5.9 ± 1.5-fold (p < 0.05) with the addition of Nck2 as compared with CD16-nephrin alone (Fig. 5B). Phosphorylation on both sites was not appreciably altered when Nck2 was expressed in the absence of CD16-nephrin (Fig. 5A, right panel).
FIGURE 5.
Hyperphosphorylation of nephrin correlates with increased downstream signaling activity. A, HEK293T cells were transfected with HA-tagged Akt, CD16-nephrin and Nck2, as indicated. All cells expressing CD16-nephrin were stimulated with CD16 (+) to induce nephrin phosphorylation. Western blotting (IB) was performed on lysates for phosphorylated and total Akt. Nck2 positively influenced Akt activation on both sites in the presence but not the absence of CD16-nephrin. pY, phosphotyrosine; pS, phosphoserine; pT, phosphothreonine. B, densitometry showing that phosphorylation of Akt on Thr308 increased 2.2 ± 0.21-fold, whereas phosphorylation of Ser473 increased by 5.9 ± 1.5-fold with the addition of Nck2 to CD16-nephrin. Phosphorylation on these sites was not appreciably altered when Nck2 was expressed alone. C, HEK293T cells were transfected with Nck2 alone as a control, CD16-nephrin alone, or CD16-nephrin and Nck2, and those containing CD16-nephrin were stimulated with CD16 (+) to induce nephrin phosphorylation. Western blotting was performed on lysates for phosphorylated and total cofilin. D, densitometry showing that the presence of Nck2 decreased cofilin phosphorylation by 40% (0.61 ± 0.056) as compared with CD16-nephrin alone (1.0) and that expression of Nck2 alone did not alter cofilin activity. * indicates p < 0.05 as compared with CD16-nephrin alone in B and D; n. s. indicates a nonsignificant difference.
Another p85/PI3K signaling pathway that has recently been elucidated in the podocyte (23) involves cofilin-1, which when activated by slingshot, can promote actin rearrangement (24). We analyzed cofilin phosphorylation on Ser3, which reflects its inactive state, when CD16-nephrin and Nck2 were expressed alone or in combination (Fig. 5C). Quantitation of phosphorylated cofilin observed in each case revealed that the amount of phosphorylated cofilin observed when CD16-nephrin was clustered in the presence of Nck2 decreased by 40% (0.61 ± 0.056) as compared with the amount observed with CD16-nephrin and vector alone (1.0) (p < 0.05), whereas expression of Nck2 alone did not alter cofilin activity (Fig. 5D). The decrease in phosphorylated cofilin indicates an increase in cofilin activation downstream of CD16-nephrin in the presence of Nck2. Together these data demonstrate that the Nck2-mediated increase in nephrin phosphorylation alters nephrin signaling more broadly as it leads to the activation of two separate pathways downstream of the nephrin p85/PI3K axis.
Nephrin Phosphorylation Is Reduced in Vivo following Podocyte-specific Depletion of Nck
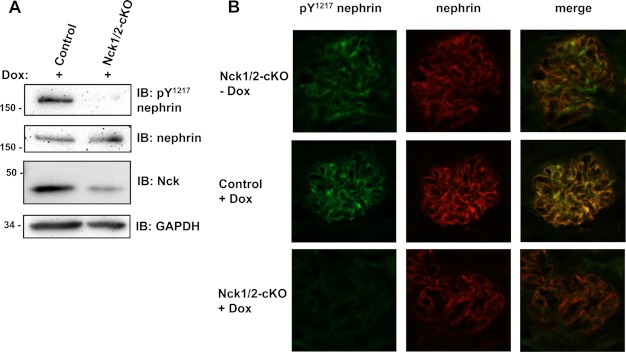
Our in vitro results clearly show that changes in Nck levels can alter nephrin phosphorylation. To validate these findings in an in vivo system, we took advantage of our existing mouse model system to conditionally modulate Nck levels. This system uses an inducible method (Podocin-rtTA/TetO-Cre) to genetically delete Nck2 specifically in podocytes of mice lacking Nck1 (19), referred to here as Nck1/2-cKO mice. Using this system, we have previously demonstrated that loss of both Nck1 and Nck2 in podocytes of adult mice via administration of doxycycline (Dox) leads to progressive renal failure in about 4–6 weeks (19). To avoid any complications related to the onset of renal disease, animals were induced for 1–2 weeks with lower levels of Dox, which allowed us to generate Nck1/2-cKO animals that had reduced, but not absent, levels of Nck2 in podocytes (Fig. 6A) and that did not develop renal disease (data not shown). Analysis of nephrin phosphorylation on Tyr1217 via immunoblotting of lysates prepared from the renal cortex of Nck1/2-cKO and control littermates revealed a striking decrease in phosphorylation upon removal of Nck expression in podocytes, which was not seen in control animals also exposed to Dox (Fig. 6A). Importantly, the levels of total nephrin expression remained unchanged (Fig. 6A). We further confirmed these observations using immunofluorescence on kidney cryosections obtained from Dox-treated animals. Nephrin phosphorylation on Tyr1217 was clearly seen in the glomeruli of Nck1/2-cKO and control animals prior to Dox exposure (Fig. 6B and data not shown). However, following treatment with Dox, glomeruli from Nck1/2-cKO animals showed reduced Tyr1217 phosphorylation as compared with control littermates, whereas levels of total nephrin remained comparable (Fig. 6B). These results support that the positive feedback loop that we have characterized in vitro, where Nck-based recruitment of Fyn contributes to nephrin phosphorylation, also occurs in vivo as induced deletion of Nck expression in podocytes leads to rapid loss of nephrin phosphorylation.
FIGURE 6.
Nephrin phosphorylation is reduced in vivo following podocyte-specific depletion of Nck2. A, Western blot analysis of kidney cortex lysates obtained from podocyte-specific Nck1/2 conditional knock-out mice (Nck1/2-cKO) and control littermates exposed to Dox for 1–2 weeks shows a decrease in phosphorylated (Tyr1217) nephrin but not total nephrin in Nck1/2-cKO animals. Reduced Nck expression is also only seen in Nck1/2-cKO animals, demonstrating the specificity of the Dox-induced gene excision. IB, immunoblot; pY, phosphotyrosine. B, dual immunofluorescence analysis of phosphorylated (pTyr1217) and total nephrin expression in glomeruli of Nck1/2-cKO mice and control littermates. Phosphorylated nephrin (green) and total nephrin (red) can be seen in Nck1/2-cKO animals prior to treatment with Dox (top panel). Upon Dox exposure, nephrin phosphorylation can still be readily observed in control animals (middle panel) but not in Nck1/2-cKO animals (bottom panel). Despite the decreased nephrin phosphorylation, total nephrin expression remains unchanged in Dox-treated Nck1/2-cKO animals.
DISCUSSION
We are interested in the mechanisms responsible for regulation of nephrin tyrosine phosphorylation by Fyn and related SFKs. In the present study, we observed that the Nck family of adaptors, which bind to phosphorylated nephrin, play a previously unappreciated role in modulating nephrin phosphorylation. We have shown that both Nck1 and Nck2 can increase nephrin phosphorylation in vitro, leading to engagement of downstream signaling pathways including activation of Akt and cofilin, and that reduced Nck expression in podocytes in vivo leads to rapid loss of nephrin phosphorylation. We have also provided evidence demonstrating that this enhanced phosphorylation of nephrin is likely due to Fyn activation as the effects can be blocked by inhibition of SFK activity. Furthermore, we found increased activation of Fyn in the presence of Nck as well as SH3 domain-mediated association of Nck with Fyn, thereby providing a potential mechanism for Nck-mediated spatial control of nephrin phosphorylation and subsequent signaling.
Differences between Nck1 and Nck2
Both Nck1 and Nck2 significantly increase nephrin phosphorylation, although the effect seems to be greater with Nck2. One explanation for increased phosphorylation with Nck2 could be that the SH2 domain of Nck2 has a higher affinity for the pTyr motifs of nephrin than Nck1; however, studies have determined that the SH2 domains of Nck1 and Nck2 have similar binding affinities to each of the three YDXV motifs of nephrin (17) as well as to other known pTyr sequences (25). Therefore, it seems more likely that the difference in the amount of nephrin phosphorylation between Nck1 and Nck2 should instead be attributed to variable recruitment of Fyn via the SH3 domains of Nck. Indeed, we found that the interaction with Fyn was much greater with Nck2 as compared with Nck1, which is in agreement with Nck2 inducing a larger amount of nephrin phosphorylation. In both cases, mutation of the SH3 domains dramatically reduced Fyn binding, suggesting that Nck1 and Nck2 interact with Fyn through a similar mechanism. Despite the lesser amount of binding between Nck1 and Fyn, it is enough to augment nephrin phosphorylation, consistent with previous observations that Nck SH3 domains can participate in weak interactions that are functionally relevant (26, 27). Furthermore, examination of podocytes from Nck2 knock-out mice did not reveal any abnormalities (15), suggesting that Nck1 can compensate for Nck2 in vivo.
Contributions of the SH3 Domains of Nck to Nephrin Phosphorylation
Although we observed increased nephrin phosphorylation with wild-type Nck, this effect was compromised when either the SH2 domain or all of the SH3 domains of Nck were mutated. However, the reduction in nephrin phosphorylation is likely due to two different mechanisms. Firstly, as the SH2 domain of Nck is the sole mediator of the interaction between phosphorylated nephrin and Nck (13, 14), mutation of the SH2 domain effectively creates a version of Nck that cannot interact with nephrin and remains spatially segregated from nephrin signaling at the plasma membrane. SH2* Nck therefore does not have any effect on nephrin phosphorylation (Fig. 2B), similar to what is seen with Grb2 (Fig. 1D).
Secondly, even when all three SH3 domains of Nck were mutated, this 3×SH3* Nck was still able to induce an increase in nephrin phosphorylation, although it was ∼40% of the amount generated with wild-type Nck. When we examined the role of individual SH3 domains, we found that if only the first or third SH3 domain was functional, the level of nephrin phosphorylation was similar to that seen with wild-type Nck, and that the combination of the two SH3 domains together (SH3-1,3 Nck) did not further enhance phosphorylation levels. This implies that there is no synergistic effect on nephrin phosphorylation with multiple Nck SH3 domains, consistent with our previous findings regarding actin polymerization (17). By contrast, the second SH3 domain of Nck alone was not able to augment nephrin phosphorylation, suggesting that it may be unable to bind to Fyn. Given that the second SH3 domain binds strongly to neuronal Wiskott-Aldrich syndrome protein (N-WASp) and is sufficient to induce full actin polymerization at sites of nephrin clustering (17), it is reasonable to conclude that the multiple SH3 domains on Nck could simultaneously play divergent roles in nephrin signaling.
Role of the SH3 Domains of Nck in Regulation of Fyn Kinase Activity
Nck has previously been implicated in the alteration of kinase activity (28–30), although the mechanism by which this occurs is not well understood. The first and second SH3 domains of Nck1 were found to activate Abl kinase (28) likely via binding of the second SH3 domain of Nck to a proline-rich region of Abl (29). More recently, interaction of Nck2 with Fyn downstream of VEGFR-2 has been shown to lead to Fyn activation (30), although no mechanism for the interaction was suggested. Consideration of potential interactions between Nck and Fyn in the context of our data suggests some possible mechanisms for how Nck could bind to Fyn and alter its activity. One possibility is that either the first or the third SH3 domain of Nck could bind to the SH2-kinase linker region known to engage the SH3 domain of Fyn (31). Binding of Nck to the linker region could release the Fyn SH3 domain and allow it to bind to an external ligand that can promote Fyn activation (32).
Alternatively, one of the SH3 domains of Nck could be engaging in a noncanonical interaction with either the SH3 or the SH2 domain of Fyn. This idea is not entirely without precedent given that noncanonical interactions with other modular domains have been reported for a number of SH2 and SH3 domains, including the Fyn SH2 (33) and SH3 (33, 34) domains, as well as the second (35) and third (36, 37) SH3 domains of Nck. Such an interaction would likely alter the conformation of the Fyn SH3 or SH2 domain, a process that has been found to result in Fyn activation through noncanonical binding of its SH3 domain to the SH2 domain of SAP (38). Additionally, noncanonical interactions can engage different surfaces than those used for proline-based ligands, which may explain why Trp-to-Lys SH3 domain mutations that eliminate canonical binding do not significantly disrupt noncanonical binding (33, 36). Indeed, the Trp-to-Lys mutations in the Nck SH3 domains still allow some increase in nephrin phosphorylation and binding to Fyn. Further investigation into these possibilities may aid in determining the exact nature of the complex interaction between Nck and Fyn.
Importance of Nephrin Signaling Pathways to Podocyte Function
Signaling pathways downstream of phosphorylated nephrin are required for proper organization of the podocyte actin cytoskeleton, which controls foot process architecture and ultimately podocyte function (3). In this work, we have uncovered a role for Nck in directly regulating Fyn-mediated phosphorylation of nephrin, which in turn potentiates activation of p85 signaling pathways known to influence podocyte cytoskeletal dynamics, namely Akt and cofilin. Disruption of these signaling pathways has been correlated with the development of renal disease. Decreased Akt phosphorylation on Ser473 has been reported during the onset of proteinuria in the rat puromycin aminonucleoside model (9). Furthermore, mice with a podocyte-specific knock-out of cofilin developed renal disease (10), and phosphorylation of cofilin, corresponding with inactivation, was increased in some forms of human glomerular disease including minimal change disease (39). Interestingly, decreased phosphorylation on the Nck binding sites Tyr1193 and Tyr1217 has been observed in the same animal models (19, 20) and human diseases (20) where decreased p85-based signaling has been reported (9, 39). Our findings thus suggest a model (see below) by which recruitment of Nck to nephrin contributes to the phosphorylation of additional tyrosine residues required for binding other key molecules. Such a global role for Nck in modulating nephrin phosphotyrosine-based signaling underscores the essential requirement for Nck expression in podocytes throughout life (15, 19).
Model for Nck-mediated Regulation of Nephrin Tyrosine Phosphorylation
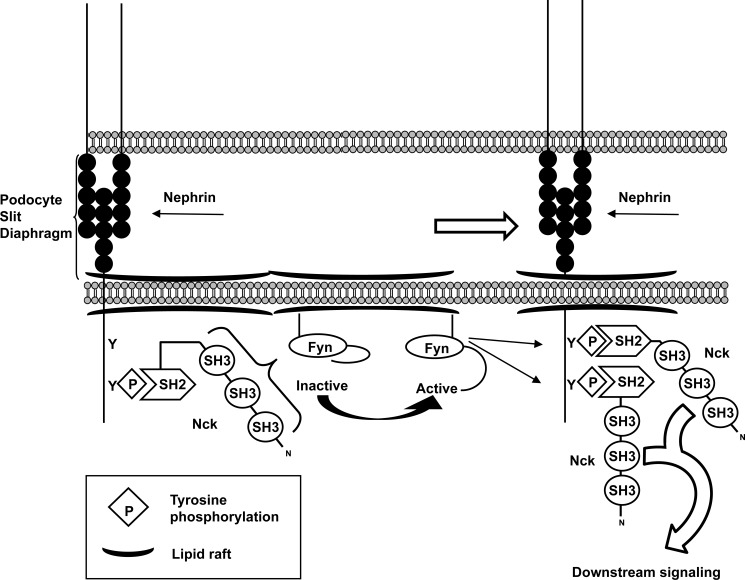
We have outlined a possible model of how Nck could be contributing to regulation of nephrin phosphorylation in the podocyte (Fig. 7). At the slit diaphragm, nephrin is recruited into lipid rafts, where it can be initially phosphorylated by Fyn, creating a binding site for the SH2 domain of Nck. Once Nck is recruited to phosphorylated nephrin, its SH3 domains appear to be able to promote activation of Fyn, through a still to be determined mechanism. This creates a positive feedback loop that allows for maximal phosphorylation of nephrin and recruitment of Nck as well as other binding partners, which together promote nephrin signaling.
FIGURE 7.
Hypothetical model of Nck-mediated Fyn activation and its role in nephrin signaling at the slit diaphragm. At the podocyte slit diaphragm, nephrin is recruited into lipid rafts where it is initially phosphorylated by Fyn. Nephrin phosphorylation allows for the recruitment of Nck via its SH2 domain, thereby allowing the SH3 domains of Nck to interact with Fyn at the cell membrane and in turn increase Fyn activation. This creates a positive feedback loop, wherein increased Fyn activity leads to the phosphorylation of adjacent nephrin molecules on multiple tyrosine residues. Additional Nck molecules as well as other binding partners can then be recruited to the newly phosphorylated nephrin complex, ultimately leading to the potentiation of downstream signaling pathways required for podocyte function.
This model is supported by observations that a fraction of nephrin is phosphorylated on Tyr1193 in the normal rat glomerulus (22), as well as evidence that nephrin, Fyn, and Nck2 are all present in lipid rafts isolated from mouse glomeruli (14). Further, our in vivo results show that reduction of Nck2 protein levels specifically in podocytes is followed by a decrease in nephrin phosphorylation, which occurs prior to the onset of renal disease known to be mediated by podocyte-specific loss of Nck (19). Together, this suggests that the pathway we have described may be actively occurring as part of normal podocyte dynamics.
In summary, our study demonstrates a role for Nck in the regulation of nephrin phosphorylation, which leads to the initiation of multiple downstream signals that coordinate efficient actin polymerization. This work supports the emerging hypothesis that in the podocyte, the engagement of signaling pathways downstream of phosphorylated nephrin is an important contributor to the proper maintenance of podocyte foot process architecture.
Acknowledgments
We are grateful to Melanie Wills for helpful discussions and assistance in preparation of Fig. 7 and to Mackenzie Smith for technical contributions.
This work was supported by grants from the Kidney Foundation of Canada (to N. J.).

This article contains supplemental Fig. S1.
- SFK
- Src family kinase
- Dox
- doxycycline
- Grb2
- growth-factor receptor binder 2
- Nck
- noncatalytic kinase
- rtTA
- reverse tetracycline trans-activator
- SH2
- Src homology domain 2
- SH3
- Src homology domain 3
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- p
- phospho.
REFERENCES
- 1. Pavenstädt H., Kriz W., Kretzler M. (2003) Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 [DOI] [PubMed] [Google Scholar]
- 2. Kriz W., Kretzler M., Provoost A. P., Shirato I. (1996) Stability and leakiness: opposing challenges to the glomerulus. Kidney Int. 49, 1570–1574 [DOI] [PubMed] [Google Scholar]
- 3. Welsh G. I., Saleem M. A. (2010) Nephrin—signature molecule of the glomerular podocyte? J. Pathol. 220, 328–337 [DOI] [PubMed] [Google Scholar]
- 4. Verma R., Wharram B., Kovari I., Kunkel R., Nihalani D., Wary K. K., Wiggins R. C., Killen P., Holzman L. B. (2003) Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J. Biol. Chem. 278, 20716–20723 [DOI] [PubMed] [Google Scholar]
- 5. Yu C. C., Yen T. S., Lowell C. A., DeFranco A. L. (2001) Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr. Biol. 11, 34–38 [DOI] [PubMed] [Google Scholar]
- 6. Garg P., Verma R., Nihalani D., Johnstone D. B., Holzman L. B. (2007) Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol. Cell Biol. 27, 8698–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harita Y., Kurihara H., Kosako H., Tezuka T., Sekine T., Igarashi T., Hattori S. (2008) Neph1, a component of the kidney slit diaphragm, is tyrosine phosphorylated by Src-family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J. Biol. Chem. 283, 9177–9186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huber T. B., Hartleben B., Kim J., Schmidts M., Schermer B., Keil A., Egger L., Lecha R. L., Borner C., Pavenstädt H., Shaw A. S., Walz G., Benzing T. (2003) Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell Biol. 23, 4917–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu J., Sun N., Aoudjit L., Li H., Kawachi H., Lemay S., Takano T. (2008) Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 73, 556–566 [DOI] [PubMed] [Google Scholar]
- 10. Garg P., Verma R., Cook L., Soofi A., Venkatareddy M., George B., Mizuno K., Gurniak C., Witke W., Holzman L. B. (2010) Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J. Biol. Chem. 285, 22676–22688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H., Lemay S., Aoudjit L., Kawachi H., Takano T. (2004) Src-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J. Am. Soc. Nephrol. 15, 3006–3015 [DOI] [PubMed] [Google Scholar]
- 12. Quack I., Rump L. C., Gerke P., Walther I., Vinke T., Vonend O., Grunwald T., Sellin L. (2006) β-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc. Natl. Acad. Sci. U.S.A. 103, 14110–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li H., Zhu J., Aoudjit L., Latreille M., Kawachi H., Larose L., Takano T. (2006) Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem. Biophys. Res. Commun. 349, 310–316 [DOI] [PubMed] [Google Scholar]
- 14. Verma R., Kovari I., Soofi A., Nihalani D., Patrie K., Holzman L. B. (2006) Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Invest. 116, 1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones N., Blasutig I. M., Eremina V., Ruston J. M., Bladt F., Li H., Huang H., Larose L., Li S. S. C., Takano T., Quaggin S. E., Pawson T. (2006) Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 [DOI] [PubMed] [Google Scholar]
- 16. Harita Y., Kurihara H., Kosako H., Tezuka T., Sekine T., Igarashi T., Ohsawa I., Ohta S., Hattori S. (2009) Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-γ1. J. Biol. Chem. 284, 8951–8962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blasutig I. M., New L. A., Thanabalasuriar A., Dayarathna T. K., Goudreault M., Quaggin S. E., Li S. S. C., Gruenheid S., Jones N., Pawson T. (2008) Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol. Cell Biol. 28, 2035–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lettau M., Pieper J., Janssen O. (2009) Nck adapter proteins: functional versatility in T cells. Cell Commun. Signal. 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones N., New L. A., Fortino M. A., Eremina V., Ruston J., Blasutig I. M., Aoudjit L., Zou Y., Liu X., Yu G. L., Takano T., Quaggin S. E., Pawson T. (2009) Nck proteins maintain the adult glomerular filtration barrier. J. Am. Soc. Nephrol. 20, 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchida K., Suzuki K., Iwamoto M., Kawachi H., Ohno M., Horita S., Nitta K. (2008) Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. 73, 926–932 [DOI] [PubMed] [Google Scholar]
- 21. Zhang S. Y., Kamal M., Dahan K., Pawlak A., Ory V., Desvaux D., Audard V., Candelier M., BenMohamed F., Matignon M., Christov C., Decrouy X., Bernard V., Mangiapan G., Lang P., Guellaën G., Ronco P., Sahali D. (2010) c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci. Signal. 3, ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y., Yoshida Y., Nameta M., Xu B., Taguchi I., Ikeda T., Fujinaka H., Magdeldin S., Mohamed S. M., Tsukaguchi H., Harita Y., Yaoita E., Yamamoto T. (2010) Glomerular proteins related to slit diaphragm and matrix adhesion in the foot processes are highly tyrosine phosphorylated in the normal rat kidney. Nephrol. Dial. Transplant. 25, 1785–1795 [DOI] [PubMed] [Google Scholar]
- 23. Berger K., Moeller M. J. (2011) Cofilin-1 in the podocyte: a molecular switch for actin dynamics. Int. Urol. Nephrol. 43, 273–275 [DOI] [PubMed] [Google Scholar]
- 24. Oser M., Condeelis J. (2009) The cofilin activity cycle in lamellipodia and invadopodia. J. Cell Biochem. 108, 1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frese S., Schubert W. D., Findeis A. C., Marquardt T., Roske Y. S., Stradal T. E. B., Heinz D. W. (2006) The phosphotyrosine peptide binding specificity of Nck1 and Nck2 Src homology 2 domains. J. Biol. Chem. 281, 18236–18245 [DOI] [PubMed] [Google Scholar]
- 26. Vaynberg J., Fukuda T., Chen K., Vinogradova O., Velyvis A., Tu Y., Ng L., Wu C., Qin J. (2005) Structure of an ultraweak protein-protein complex and its crucial role in regulation of cell morphology and motility. Molecular Cell 17, 513–523 [DOI] [PubMed] [Google Scholar]
- 27. Hake M. J., Choowongkomon K., Kostenko O., Carlin C. R., Sönnichsen F. D. (2008) Specificity determinants of a novel Nck interaction with the juxtamembrane domain of the epidermal growth factor receptor. Biochemistry 47, 3096–3108 [DOI] [PubMed] [Google Scholar]
- 28. Smith J. M., Katz S., Mayer B. J. (1999) Activation of the Abl tyrosine kinase in vivo by Src homology 3 domains from the Src homology 2/Src homology 3 adaptor Nck. J. Biol. Chem. 274, 27956–27962 [DOI] [PubMed] [Google Scholar]
- 29. Miyoshi-Akiyama T., Aleman L. M., Smith J. M., Adler C. E., Mayer B. J. (2001) Regulation of Cbl phosphorylation by the Abl tyrosine kinase and the Nck SH2/SH3 adaptor. Oncogene 20, 4058–4069 [DOI] [PubMed] [Google Scholar]
- 30. Lamalice L., Houle F., Huot J. (2006) Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J. Biol. Chem. 281, 34009–34020 [DOI] [PubMed] [Google Scholar]
- 31. Gonfloni S., Williams J. C., Hattula K., Weijland A., Wierenga R. K., Superti-Furga G. (1997) The role of the linker between the SH2 domain and catalytic domain in the regulation and function of Src. EMBO J. 16, 7261–7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solheim S. A., Torgersen K. M., Taskén K., Berge T. (2008) Regulation of FynT function by dual domain docking on PAG/Cbp. J. Biol. Chem. 283, 2773–2783 [DOI] [PubMed] [Google Scholar]
- 33. Panchamoorthy G., Fukazawa T., Stolz L., Payne G., Reedquist K., Shoelson S., Songyang Z., Cantley L., Walsh C., Band H. (1994) Physical and functional interactions between SH2 and SH3 domains of the Src family protein tyrosine kinase p59fyn. Mol. Cell Biol. 14, 6372–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan B., Lanyi A., Song H. K., Griesbach J., Simarro-Grande M., Poy F., Howie D., Sumegi J., Terhorst C., Eck M. J. (2003) SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 5, 155–160 [DOI] [PubMed] [Google Scholar]
- 35. Li C., Schibli D., Li S. S. (2009) The XLP syndrome protein SAP interacts with SH3 proteins to regulate T cell signaling and proliferation. Cell Signal. 21, 111–119 [DOI] [PubMed] [Google Scholar]
- 36. Tu Y., Li F., Wu C. (1998) Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol. Biol. Cell 9, 3367–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barda-Saad M., Shirasu N., Pauker M. H., Hassan N., Perl O., Balbo A., Yamaguchi H., Houtman J. C., Appella E., Schuck P., Samelson L. E. (2010) Cooperative interactions at the SLP-76 complex are critical for actin polymerization. EMBO J. 29, 2315–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simarro M., Lanyi A., Howie D., Poy F., Bruggeman J., Choi M., Sumegi J., Eck M. J., Terhorst C. (2004) SAP increases FynT kinase activity and is required for phosphorylation of SLAM and Ly9. Int. Immunol. 16, 727–736 [DOI] [PubMed] [Google Scholar]
- 39. Ashworth S., Teng B., Kaufeld J., Miller E., Tossidou I., Englert C., Bollig F., Staggs L., Roberts I. S., Park J. K., Haller H., Schiffer M. (2010) Cofilin-1 inactivation leads to proteinuria–studies in zebrafish, mice, and humans. PLoS One 5, e12626. [DOI] [PMC free article] [PubMed] [Google Scholar]