Abstract
The phospholipase A2 (PLA2) superfamily consists of 16 groups and many subgroups and constitutes a diverse set of enzymes that have a common catalytic activity due to convergent evolution. However, different PLA2 types have unique three-dimensional structures and catalytic residues as well as specific tissue localization and distinct biological functions. Understanding how the different PLA2 enzymes associate with phospholipid membranes, specific phospholipid substrate molecules, and inhibitors on a molecular basis has advanced in recent years due to the introduction of hydrogen/deuterium exchange mass spectrometry. Its theory, practical considerations, and application to understanding PLA2/membrane interactions are addressed.
Keywords: Enzyme Inhibitors, Isotope Exchange, Mass Spectrometry (MS), Membrane Lipids, Phospholipase A
Introduction
The phospholipase A2 (PLA2)2 superfamily consists of a diverse set of enzymes that catalyze hydrolysis of the sn-2 ester bond of phospholipids, thereby producing lysophospholipids and free fatty acids with important downstream signaling roles (1). Although the PLA2s have been formally classified into some 16 groups and numerous subgroups, each is more commonly thought of as being part of one of six main types based on a variety of sequence, structural, and other characteristics. The six main types are secreted (sPLA2), cytosolic (cPLA2), Ca2+-independent (iPLA2), platelet-activating factor acetylhydrolase (PAF-AH), lysosomal (LPLA2), and adipose (AdPLA) (2). It is well known that PLA2 enzymes are involved in the inflammatory process and various diseases, including atherosclerosis, diabetes, arthritis, and cancer (1, 3). A large body of evidence suggests that inhibiting the enzymatic activity of PLA2s has potential benefits for the treatment of specific diseases (4–6). Therefore, there has been considerable interest in understanding how the PLA2s interact with the lipid substrate, inhibitors, and membranes with the goal of developing effective new pharmaceutical agents.
Many PLA2s display a dramatic increase in activity when the phospholipid substrate is in an aggregated form (micelles, vesicles, liposomes, etc.) rather than in monomeric form. Most PLA2 enzymes have to bind to lipid/water interfaces and access the substrate from the lipid phase to complete a catalytic turnover. The concept of “surface-dilution kinetics” was successfully applied to explain these enzymes' kinetic characteristics (7). As part of surface-dilution considerations, the enzyme may undergo surface binding to membranes, whereby the enzyme either associates nonspecifically with the surface of the lipid aggregate or associates specially with a phospholipid(s) in the aggregate's surface (7). It is believed that PLA2s contain an interfacial surface distinct from their specific active site in terms of topology and function, which associates with phospholipid membranes.
Precisely how PLA2 enzymes associate with lipid membranes has long been a question in membrane protein enzymology. Many techniques have been applied to explore the interfacial surface, including some high resolution methods such as solution NMR and x-ray crystallography. Although these methods could provide structural information at an atomic level, the insolubility of the phospholipid substrate and its large aggregate form make it extremely difficult to study the interfacial surface. Moreover, some PLA2 enzymes exhibit oligomeric and/or allosteric properties when they are associated with a substrate or phospholipid membranes in solution. Success in studying membrane association has been achieved only using protein lipid FRET (8) and electron paramagnetic resonance spectroscopy (9). Certain limitations restrict the type of enzymes that can be studied using these methods because paramagnetic resonance spectroscopy requires the insertion of non-wild type amino acids, and protein/membrane FRET does not allow for localization of changes within the enzyme upon membrane association. A newer method, hydrogen/deuterium exchange mass spectrometry (DXMS), has been used successfully to investigate the dynamics of proteins in solution, as well as protein/ligand and protein/protein interactions, despite the size of the proteins and the complexity of their binding partners (10–15). DXMS works by monitoring the exchange of a protein's backbone amide hydrogen atoms with deuterium atoms, provided by incubating the protein in D2O. In recent years, DXMS has been used to study the structural dynamics and interactions of membrane proteins (16, 17) and membrane-associated proteins (18) such as various PLA2s (19–24). Although DXMS provides only medium to semi-high resolution, when combined with methods like x-ray crystallography, fluorescence spectrometry, mutagenesis, and quantum mechanics/molecular mechanics, it can provide useful information on protein/membrane, protein/inhibitor, and protein/substrate interactions.
In this minireview, we focus on the relatively recent application of DXMS in investigating membrane-associated PLA2 enzymes. We provide both a general description of how DXMS can be used to investigate protein/phospholipid membrane and protein/inhibitor interactions and examples of interfacial binding sites and/or interactions with specific inhibitors of specific PLA2 enzymes that have been identified.
Hydrogen/Deuterium Exchange Mass Spectrometry
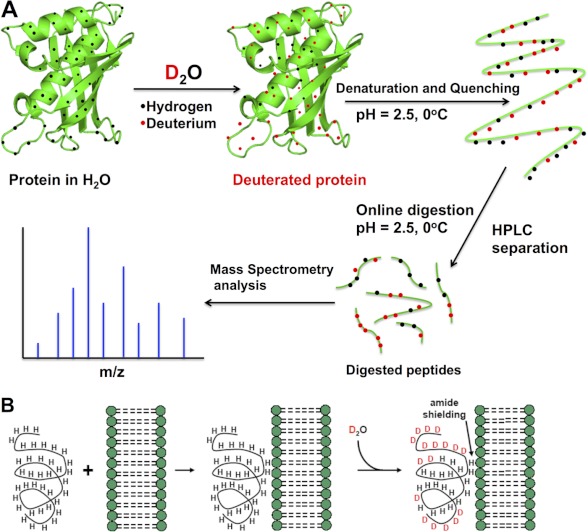
Hydrogen atoms in amino acid side chains and backbones are labile and are continuously exchanged with hydrogen atoms in the surrounding water. However, only the exchange rate of amide hydrogens can be conveniently monitored. The exchange rate of amide hydrogens in a protein varies greatly depending on their involvement in secondary structures as well as their access to the solvent. However, recent work has demonstrated that the involvement in hydrogen bonding networks, but not solvent accessibility, is the predominant factor in controlling hydrogen/deuterium (H/D) exchange rates (25). The H/D exchange rate of amide hydrogens is an acid-base catalyzed process with a calculated global minimum in the 2.5–4 pH range (26, 27), which is also highly dependent on temperature. When the protein is exposed to a D2O environment, deuterons incorporated into the protein can be detected by mass spectrometry, which means that the rate of H/D exchange can be measured. A generalized scheme for a DXMS experiment is outlined in Fig. 1A.
FIGURE 1.
Basis of H/D exchange. A, the protein of interest is incubated with deuterated water and labeled via exchangeable hydrogens. The exchange is then quenched to lock the deuterium atoms in position by lowering the pH of the protein-containing solution (which also denatures the protein) and lowering the solution temperature. The protein is then loaded onto an HPLC column that contains an immobilized protease that digests the protein into peptide segments. The reversed-phase column also separates these peptide segments according to their hydrophobicity. The HPLC eluent is directly connected to the mass spectrometer, where the mass analysis is carried out (the peptide segments may be further fragmented in the mass spectrometer for analysis). B, PLA2/lipid membrane interactions by DXMS. The peptide region of a protein associated with a membrane is less prone to H/D exchange (amide shielding), and this information can be used to infer this peptide region's interaction with a lipid membrane.
For a typical PLA2/membrane interaction experiment, DXMS experiments are carried out on the pure PLA2 enzyme alone and on the PLA2 enzyme in the presence of phospholipid membranes (lipid/protein ratio of 60 or higher) (Fig. 1B). It is important to note that H/D changes induced upon membrane binding can be caused by a variety of factors (oligomerization, allosteric conformational changes distant from the membrane-binding site, and direct membrane interactions). These H/D exchange differences must be interpreted in the context of previous biophysical and structural experiments. Detailed methodology is provided in the supplemental material, including typical data (supplemental Fig. S1), fragmentation schemes (supplemental Fig. S2), and a description of the kinetic parameters governing amide exchange. Further detail is provided in several recent excellent reviews (28–30).
Identifying the Interfacial Surfaces of PLA2 Enzymes
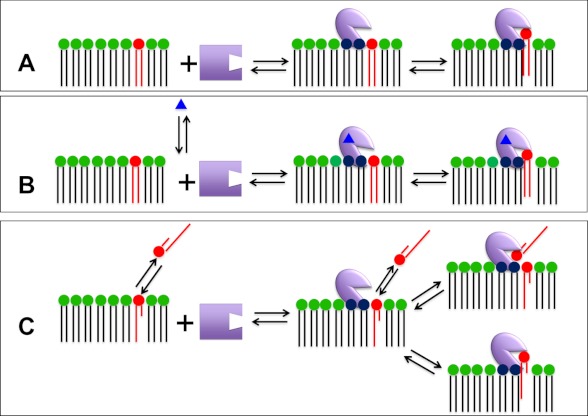
The crucial first step for a PLA2-catalyzed reaction is the association of the enzyme with membranes via its interfacial binding surface containing one or more “membrane interaction site(s).” Each PLA2 enzyme also contains a unique “catalytic site” that binds a substrate phospholipid for hydrolysis. Here, we consider membrane interaction sites to be typical allosteric sites such that when the PLA2 enzyme is associated with ligand (in this case, the membrane), the enzyme exists in a different conformational state as described by Changeux and co-workers (31, 32) in terms of R-to-T transitions as illustrated in Fig. 2A. In addition to membrane interaction site(s), PLA2s may contain one or more traditional “allosteric site(s)” that bind small molecules that regulate or activate the enzyme. We include the possibility that additional small molecule allosteric site(s) can affect the conformation of the membrane interaction sites(s) as illustrated in Fig. 2B. PLA2s are further complicated by the fact that the substrate molecules are aggregated to compose the membrane (or micelle, lipoprotein, or other aggregated form). However, for some phospholipids, the sn-2 fatty acid chain is an acetate or oxidized, and these phospholipids have a critical micelle concentration such that the phospholipid substrate molecules may exist as monomers or small aggregates in aqueous solution under physiological conditions, so there is the possibility that the membrane-associated enzyme binds substrate in the aqueous solution rather than in the membrane surface as illustrated in Fig. 2C. Note that although allosteric enzymes have generally been associated with oligomerization and sigmoid kinetics, we do not distinguish here whether the active enzyme is monomeric or oligomeric or if association with membranes changes the oligomerization state of the enzyme, although these are distinct possibilities.
FIGURE 2.
Possible binding modes for PLA2 via its membrane interaction site, another allosteric site, and its catalytic site. A, the enzyme associates with the membrane interface via its membrane interaction site, which is distinct from its catalytic site. Once associated with the membrane, a phospholipid substrate molecule is bound by the catalytic site. B, regulated enzymes bind traditional small molecule activators or regulators, referred to as “allosteric ligands” (blue triangles), which are bound to an allosteric site on the enzyme to facilitate optimal interfacial binding via the membrane interaction site. C, for PLA2s that act on water-soluble monomeric (or small aggregated) substrates, the enzyme associated with the interface through the membrane interaction site may, in principle, access a substrate phospholipid residing in either the aqueous or membrane phase.
For example, cPLA2 is generally considered to be a monomeric protein that binds membranes via more than one membrane interaction site and whose association and/or activity is regulated through one or more allosteric sites, including binding of Ca2+, ceramide 1-phosphate, and/or phosphatidylinositol 4,5-bisphosphate (33, 34), as typified by Fig. 2B. For the sPLA2s, if one considers the required Ca2+ as a regulator of membrane association or binding, then Ca2+ also constitutes an allosteric regulator, but in this case, the Ca2+ is located in the catalytic site, so it is hard to distinguish between the models depicted in Fig. 2 (A and B). iPLA2, which is regulated to some extent by ATP and/or indirectly by Ca2+ (35), is also depicted in Fig. 2B. PAF-AH/lipoprotein-associated PLA2 (Lp-PLA2) is depicted in Fig. 2C with high critical micelle concentration substrates.
Although numerous PLA2 crystal structures have been determined in the past decades, none of them have contained lipid substrates with normal fatty acyl chains or lipid membranes in the crystal lattice. DXMS is an ideal approach for studying PLA2/substrate/lipid membrane interactions because DXMS results can be obtained in the presence of large lipid aggregates and monomeric lipid substrates. DXMS has been applied to the study of the PLA2 superfamily of enzymes and has now been successfully used to identify the structural basis of four different types of PLA2/membrane interaction sites.
Using DXMS to study membrane interactions does have inherent limitations, and many of the technical hurdles have been summarized in an excellent review (36). One of these limitations is that DXMS experiments do not directly measure perturbations caused solely by side chain interactions and allow only for the determination of dynamic changes that affect the rate of H/D exchange of amide hydrogens. Many changes that are dominated by side chain interactions may not be detected. However, side chain interactions may rigidify the secondary structure, causing decreased amide H/D exchange (37).
Also, the different H/D exchange rates in the presence or absence of lipid vesicles may not be caused by a direct lipid interaction of the amides in that region, but by conformational changes induced by lipid binding or a combination. This includes both allosteric and oligomeric changes that may occur at the lipid surface. However, carefully controlled experiments on Group (G) IVA PLA2 were able to discriminate conformational changes upon substrate binding versus the interfacial surface (23). Care should be taken when examining the differences upon lipid binding to determine the role of direct binding versus conformational changes/aggregation distant from the lipid-binding surface.
GIA sPLA2
The interfacial enzymology of small, secreted, and highly disulfide-bonded sPLA2s has been widely studied. Several kinetic models, including surface dilution, scooting mode, and quasi-scooting mode, have been developed to explain the interfacial mechanism of sPLA2s (7, 38). Many sPLA2 structures, either from NMR or x-ray crystallography, have become available in the past decades, and these structures have provided us with useful information for understanding the interfacial binding surfaces. Unfortunately, none of the structures were solved in the presence of a normal lipid substrate. However, DXMS has allowed for the study of the protein dynamics of GIA sPLA2 in solution in the presence of divalent cations as well as lipid substrate (22). GIA sPLA2 is a 13-kDa protein with seven disulfide bonds, four α-helices, and three β-sheets. Interestingly, two of the core helices barely exhibit H/D exchange, but the other two helices exchange very rapidly (22). Four disulfide bonds are involved in the two core helical regions, which exhibit extremely slow H/D exchange rates. These disulfide bonds keep these helical regions extremely rigid, preventing the small fluctuations in secondary structure required for H/D exchange.
GIA sPLA2 requires Ca2+ to carry out its enzymatic activity. Structures of GIA PLA2 have been solved with either two Ca2+ ions present (39) or only one present (40) The DXMS experiments showed decreased H/D exchange in the second Ca2+-binding site upon exposure to either Ca2+ or Ba2+. This finding supports previous results showing two Ca2+-binding sites in the Naja naja atra GIA sPLA2 structure (41). When using dimyristoylphosphatidylcholine (DMPC) lipid vesicles to mimic lipid bilayers in DXMS experiments, the membrane interaction site residues were identified. The H/D levels at amide hydrogens in regions containing Tyr-3, Trp-61, Tyr-63, Phe-64, and Lys-6 decreased dramatically in the presence of DMPC lipid vesicles. The aromatic residues on the surface are believed to insert into the lipid phase, which benefits the hydrophobic interaction between the protein and lipid acyl tails. Lys-6 should contribute to electrostatic interactions with the polar headgroup of a lipid membrane surface. Regions containing several other hydrophobic residues Phe-5, Ile-9, and Trp-19 also demonstrated a decreased H/D level. These residues may also be involved in the interfacial binding to mediate the hydrophobic interactions.
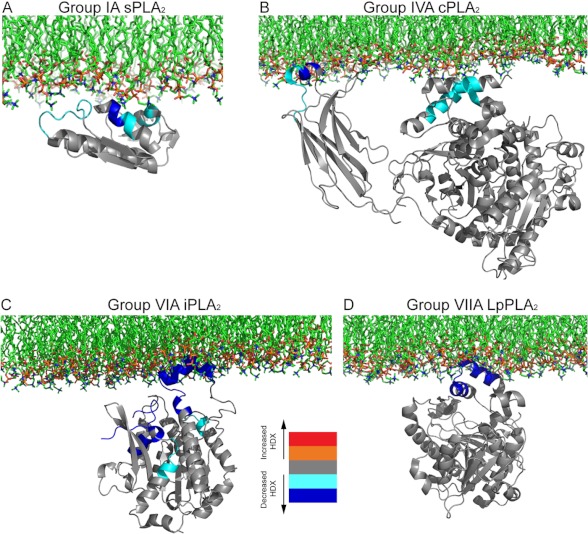
By mapping these decreases in the exchange rate onto the x-ray crystallographic structure, along with accounting for previous biochemical data, it was possible to generate a model for how this enzyme binds to the lipid surface (Fig. 3A). The aromatic amino acids, especially tryptophan, are thought to be the most potent contributors to interfacial binding. Indeed, tryptophan mutants have shown dramatically decreased binding affinity in human GV and GX sPLA2s (42, 43). Electrostatic interactions were also shown to contribute to the interfacial binding of other sPLA2s such as GIIA and GIB (44, 45). The decreases in H/D exchange are caused either by direct hydrogen bonding of the amide hydrogens to the lipid substrate or by side chain interactions rigidifying the secondary structure. Overall, the GIA sPLA2 DXMS study is consistent with the results obtained by other methods and with other sPLA2s but reveals more directly the lipid interface of the enzyme.
FIGURE 3.
Proposed schematic models of the interfacial binding surface of four different members of the PLA2 superfamily. Differences in H/D exchange (HDX) combined with biophysical experiments were employed to generate models of the monomers of the different PLA2 enzymes bound to membranes. A, GIA sPLA2 (Protein Data Bank code 1PSH), adapted from Ref. 22. B, GIVA cPLA2 (code 1CJY), adapted from Ref. 23. C, GVIA iPLA2 (homology model based on patatin crystal structure 1OXW), adapted from Ref. 19. D, GVIIA PAF-AH/Lp-PLA2 (code 3D5E), adapted from Ref. 24. Note that the images of structures are not to the same scale.
GIVA cPLA2
GIVA cPLA2 (21, 23) is a 85-kDa protein containing two domains linked by a flexible tether: a Ca2+-binding domain (C2) and a catalytic domain (15). GIVA cPLA2 has a distinct preference for substrates containing arachidonic acid at the sn-2 position (46). The crystal structure of GIVA cPLA2 shows that it contains a lid region in the catalytic domain that could block the direct insertion of a substrate molecule in the active site. From this structure, it was postulated that binding to the lipid surface might induce a conformational shift in this lid region, allowing phospholipid substrates to access the active site (15). DXMS on this enzyme was focused on determining differences between a single lipid substrate binding in the active site versus a bulk lipid surface binding.
DXMS experiments showed an increase in H/D exchange in regions directly underneath the lid region of GIVA PLA2 in the presence of both substrate vesicles and methyl arachidonyl fluorophosphonate (MAFP) alone. However, there was no further change in this region when comparing the MAFP-treated enzyme and the MAFP-treated enzyme plus vesicles. These experiments show that this conformational change can be caused by a single lipid molecule binding in the active site and does not require association with the lipid interface.
Comparing the DXMS data for the MAFP-treated enzyme with that for the MAFP-treated enzyme plus vesicles allowed the identification of regions that are responsible only for lipid surface binding. This allowed mapping of the interfacial binding surface of the enzyme, and by combining this work with previous biophysical studies (47–50) carried out on the C2 domain alone and full-length cPLA2 enzyme, this led to the creation of the model for membrane binding shown in Fig. 3B. Both the C2 and catalytic domains bind to phospholipid surfaces. Regions 35–39 and 96–98 on the C2 domain, which are very hydrophobic, can penetrate into the lipid phase. In contrast, regions 268–279 and 466–470 on the catalytic domain can mediate cPLA2 interfacial binding through electrostatic interactions with phospholipid headgroups.
GVIA iPLA2
The membrane interaction site of human GVIA-2 iPLA2β was also investigated using DXMS (19). GVIA-2 PLA2 (iPLA2β) contains seven ankyrin repeats and a catalytic domain connected by a linker region. There is no structural information available for this enzyme. The Protein Data Bank (PDB) contains only two homolog structures: human ankyrin-R, with 51% similarity to iPLA2 ankyrin repeat residues 88–474, and the lipase patatin, with 40% sequence similarity to iPLA2 catalytic domain residues 475–806. Based on this structural data, two homology models were developed, one for the iPLA2 ankyrin repeats and one for the catalytic domain (19). DXMS proved to be an effective approach to validate these homology models by allowing examination of the correlation between the H/D exchange rates and the predicted structure (51). GVIA-2 iPLA2 H/D data provided a good fit with the homology model (19). This homology model also showed a high correlation when validated by a recently developed program, DXCOREX, by which H/D exchange results were used to quantitatively assess the accuracy of tertiary protein structure models (52). The membrane interaction site was determined in an iPLA2/1-palmitoyl-2-arachidonoyl-sn-phosphatidylcholine lipid vesicle DXMS experiment in the presence of the inhibitor MAFP. The iPLA2/MAFP DXMS experiment was also performed to exclude the possibility that any H/D changes in the membrane interaction site residue(s) were induced by the MAFP inhibitor. H/D results indicated that only the catalytic domain residues interacted with the lipid membrane and that ankyrin repeat regions were not involved in the iPLA2/lipid association. Specifically, the hydrophobic region in the catalytic domain, including residues 708–730, was most likely to penetrate the membrane surface, and other regions, including residues 631–655, 658–664, and 773–778, were most likely to assist the iPLA2/membrane binding by electrostatic interactions with the charged headgroup of the phospholipids (19). These effects were mapped onto the catalytic domain homology model as shown in Fig. 3C. The catalytic residue Ser-519 was underneath the membrane penetration region 708–730, and the active site was relatively open to the solvent, which may explain why iPLA2 does not discriminate between substrates containing differing sn-2 acyl chains.
GVIIA PAF-AH PLA2
Although most PLA2 enzymes must first bind to the membrane and extract substrate phospholipid from the lipid phase, another type of PLA2 enzyme, PAF-AH, seems to utilize a different mechanism because the substrate PAF and PAF analogues are partially water-soluble as monomers or small aggregates. PAF-AH might bind the interface only through its membrane interaction site and take substrate from the aqueous phase, but it is not necessarily activated in the interface (Fig. 2C) (53). GVIIA PLA2 is a plasma PAF-AH, also known as Lp-PLA2 because it is found associated mainly with LDL and HDL in human plasma (54) and hydrolyzes oxidized phospholipids (55, 56).
Considering that both LDL and HDL have >30% surface monolayer phospholipids, it is reasonable that GVIIA PLA2 will associate with the lipid surface when it associates with lipoproteins and could bind substrate molecules from either the aqueous or lipid phase. Because GVIIA PLA2 has been shown to bind DMPC lipid vesicles tightly (53), DMPC lipid vesicles were used in GVIIA PLA2/membrane binding DXMS studies. Only one region in the enzyme, residues 113–120, demonstrated decreases in H/D exchange in the presence of DMPC lipid vesicles (24). Residues 113–120 are part of one surface of the α-helix, which is believed to be important for GVIIA PLA2/LDL association (57). This helix contains numerous hydrophobic residues, and within this helix, Trp-115 and Leu-116 are considered the most hydrophobic residues. When Trp-115 and Leu-116 are replaced with alanine residues, the mutant protein loses its membrane binding capacity, as detected by both fluorescence assay and DXMS (24). This result indicates that the GVIIA PLA2/lipid membrane interaction is due predominantly to hydrophobic interactions and that Trp-115 and Leu-116 are the major contributors. The proposed model is shown in Fig. 3D. Although GVIIA PLA2 may not be interfacially activated, it binds to the lipid membrane using a surface helix binding, which leaves its active site open to solvent and, in principle, could also allow access to substrates such as oxidized PC from the lipid phase. It is also important to note that regions that cannot be crystallized or are disordered using x-ray crystallography were all detected by DXMS (24, 58) and showed very rapid H/D exchange rates, indicating the structural flexibility of the residues within these regions. DXMS has also been used to provide some useful information on the structural basis of Lp-PLA2/HDL interactions (59).
DXMS Combined with Molecular Dynamics: Example of GIVA cPLA2 Inhibitors
Many inhibitors of GIVA cPLA2 have been developed, and although some of the inhibitors showed positive effects both in vitro and in vivo, none of them is currently in an advanced clinical trial due to side effects or bioavailability (1, 60). Understanding the location of inhibitor binding, as well as induced allosteric change, is important to design the next generation of inhibitors for therapeutic purposes. Membrane proteins, including the PLA2s, are inhibited by extremely lipophilic compounds, which can cause numerous solubility issues, making the use of high resolution methods such as x-ray extremely challenging. DXMS has been used alongside site-directed mutagenesis to locate the exact binding site of certain inhibitors (61, 62). The ability to locate the binding sites of inhibitors that cannot be co-crystallized with a protein of interest is an important advantage of the DXMS method.
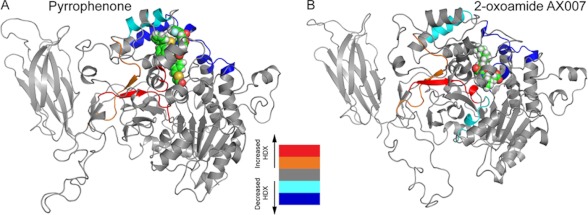
Two representative inhibitors from markedly different classes of molecules, pyrrophenone and a 2-oxoamide-derived inhibitor (AX007), were selected for GIVA cPLA2/inhibitor DXMS experiments (20). In the presence of pyrrophenone, DXMS in three regions of GIVA cPLA2 showed increased on-exchange rates (Fig. 4A). In the same regions, increased H/D exchange rates were also found in GIVA cPLA2/lipid substrate binding DXMS experiments, which is caused by the opening of the lid region upon substrate binding to the active site (23). It seems that inhibitor pyrrophenone binding also induced the same lid opening and increased the solvent accessibility in these regions. Five other regions demonstrated decreased H/D exchange rates in the presence of pyrrophenone. Molecular dynamics studies have confirmed that residues in these regions can form a hydrophobic pocket to accommodate the inhibitor. The combination of results from DXMS, molecular docking, and molecular dynamics has resulted in a model that elucidates the molecular basis of the pyrrophenone inhibition mechanism (20).
FIGURE 4.
Differences in deuterium exchange rates for GIVA cPLA2 upon binding to two lipophilic inhibitors. Time-dependent H/D exchange rates (HDX) in the presence and absence of the inhibitors pyrrophenone (A) and 2-oxoamide AX007 (B) are shown mapped onto the resulting molecular dynamics simulation of the structural model of GIVA cPLA2 (Protein Data Bank code 1CJY). The decreases/increases in the exchange rates are color-coded as shown in the legend (from Ref. 20).
The 2-oxoamide-derived inhibitor AX007 shared some binding sites with pyrrophenone, but not others. Molecular dynamics results indicated that both inhibitors have similar contacts in the shared regions (20). Not surprisingly, DXMS also exhibited some differences in the presence of AX007. Molecular dynamics indicated that interactions between Arg-200 and the carboxylic acid of the oxoamide and between the carbonyl of the 2-oxoamide and the oxyanion hole stabilize protein/inhibitor binding. Overall, the results suggest that the oxoamide inhibitor AX007 binds mainly to the active site, whereas pyrrophenone connects with the cap region near the interfacial binding surface.
Conclusions
DXMS has been an important tool for studying protein dynamics, conformation, and solvent accessibility. Recent advances in resolution, automation, and mass analysis capabilities have made this technique even more powerful and accessible to the scientific community. DXMS has been used to successfully define the interfacial binding surfaces of the four main types of PLA2s. Another type of PLA2 enzyme, adipose PLA, which has a transmembrane domain, undoubtedly interacts with membranes in still another unique way (63). Although the PLA2 superfamily has common catalytic activity due to convergent evolution, different structures and catalytic residues (including His-Asp, Ser-Asp, Ser-His-Asp, and Cys-His combinations) are engaged in distinct biological functions in the organism. Data obtained using DXMS are consistent and complementary to the data obtained with other biophysical and biochemical techniques. DXMS has also been shown to be an excellent method for identifying PLA2/inhibitor interaction mechanisms and is helpful in building a precise inhibitor-binding model.
DXMS could also be a powerful tool for studying other peripheral and integral membrane protein interactions with membranes and inhibitors and for elucidating conformational changes. However, no method is perfect. Little information can be obtained if the region of interest is located in extremely protected or disordered regions because it is extremely difficult to see H/D differences between very slow or very fast exchange rates. It is also hard to understand changes in the exchange rates without the availability of a three-dimensional structure. However, homology models coupled with DXMS data and computational modeling may be very useful to allow interpretation of H/D dynamic information (52). DXMS has to be used as a tool alongside other methods such as x-ray, NMR, mutagenesis, fluorescence, and molecular modeling. Together, these techniques allow for a much greater understanding of the dynamic nature of the proteins being studied.
Acknowledgments
We thank Drs. Yuan-hao Hsu, Sheng Li, and Tong Liu for longtime critical collaborations in our DXMS studies. We are appreciative of informative discussions with Professor Jean-Pierre Changeux (Pasteur Institute) on allostery in PLA2 enzymes. We thank Masada Disenhouse for editing this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM20501-37.
We dedicate this minireview to our longtime collaborator, the late Professor Virgil L. Woods, Jr., who contributed so much to the development of the DXMS technique over the years.

This article contains supplemental material, Figs. S1 and S2, Equations 1–6, and additional references.
- PLA2
- phospholipase A2
- sPLA2
- secreted PLA2
- cPLA2
- cytosolic PLA2
- iPLA2
- Ca2+-independent PLA2
- PAF-AH
- platelet-activating factor acetylhydrolase
- DXMS
- hydrogen/deuterium exchange mass spectrometry
- H/D
- hydrogen/deuterium
- Lp-PLA2
- lipoprotein-associated PLA2
- G
- Group
- DMPC
- dimyristoylphosphatidylcholine
- MAFP
- methyl arachidonyl fluorophosphonate
- C2
- Ca2+ binding domain.
REFERENCES
- 1. Dennis E. A., Cao J., Hsu Y. H., Magrioti V., Kokotos G. (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111, 6130–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaloske R. H., Dennis E. A. (2006) The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 1761, 1246–1259 [DOI] [PubMed] [Google Scholar]
- 3. Lambeau G., Gelb M. H. (2008) Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 77, 495–520 [DOI] [PubMed] [Google Scholar]
- 4. Scott K. F., Sajinovic M., Hein J., Nixdorf S., Galettis P., Liauw W., de Souza P., Dong Q., Graham G. G., Russell P. J. (2010) Emerging roles for phospholipase A2 enzymes in cancer. Biochimie 92, 601–610 [DOI] [PubMed] [Google Scholar]
- 5. Karabina S. A., Gora S., Atout R., Ninio E. (2010) Extracellular phospholipases in atherosclerosis. Biochimie 92, 594–600 [DOI] [PubMed] [Google Scholar]
- 6. The Lp-PLA2 Studies Collaboration, Thompson A., Gao P., Orfei L., Watson S., Di Angelantonio E., Kaptoge S., Ballantyne C., Cannon C. P., Criqui M., Cushman M., Hofman A., Packard C., Thompson S. G., Collins R., Danesh J. (2010) Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 375, 1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carman G. M., Deems R. A., Dennis E. A. (1995) Lipid signaling enzymes and surface dilution kinetics. J. Biol. Chem. 270, 18711–18714 [DOI] [PubMed] [Google Scholar]
- 8. Fernandes F., Loura L. M., Koehorst R., Spruijt R. B., Hemminga M. A., Fedorov A., Prieto M. (2004) Quantification of protein-lipid selectivity using FRET: application to the M13 major coat protein. Biophys. J. 87, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin Y., Nielsen R., Murray D., Hubbell W. L., Mailer C., Robinson B. H., Gelb M. H. (1998) Docking phospholipase A2 on membranes using electrostatic potential-modulated spin relaxation magnetic resonance. Science 279, 1925–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung K. Y., Rasmussen S. G., Liu T., Li S., DeVree B. T., Chae P. S., Calinski D., Kobilka B. K., Woods V. L., Jr., Sunahara R. K. (2011) Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. Nature 477, 611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimberlin C. R., Bornholdt Z. A., Li S., Woods V. L., Jr., MacRae I. J., Saphire E. O. (2010) Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc. Natl. Acad. Sci. U.S.A. 107, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Black B. E., Brock M. A., Bédard S., Woods V. L., Jr., Cleveland D. W. (2007) An epigenetic mark generated by the incorporation of CENP-A into centromeric nucleosomes. Proc. Natl. Acad. Sci. U.S.A. 104, 5008–5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Begley M. J., Taylor G. S., Brock M. A., Ghosh P., Woods V. L., Dixon J. E. (2006) Molecular basis for substrate recognition by MTMR2, a myotubularin family phosphoinositide phosphatase. Proc. Natl. Acad. Sci. U.S.A. 103, 927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Black B. E., Foltz D. R., Chakravarthy S., Luger K., Woods V. L., Jr., Cleveland D. W. (2004) Structural determinants for generating centromeric chromatin. Nature 430, 578–582 [DOI] [PubMed] [Google Scholar]
- 15. Dessen A., Tang J., Schmidt H., Stahl M., Clark J. D., Seehra J., Somers W. S. (1999) Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell 97, 349–360 [DOI] [PubMed] [Google Scholar]
- 16. Mehmood S., Domene C., Forest E., Jault J. M. (2012) Dynamics of a bacterial multidrug ABC transporter in the inward- and outward-facing conformations. Proc. Natl. Acad. Sci. U.S.A. 109, 10832–10836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. West G. M., Chien E. Y., Katritch V., Gatchalian J., Chalmers M. J., Stevens R. C., Griffin P. R. (2011) Ligand-dependent perturbation of the conformational ensemble for the GPCR β2 adrenergic receptor revealed by HDX. Structure 19, 1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burke J. E., Vadas O., Berndt A., Finegan T., Perisic O., Williams R. L. (2011) Dynamics of the phosphoinositide 3-kinase p110δ interaction with p85α and membranes reveals aspects of regulation distinct from p110α. Structure 19, 1127–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu Y. H., Burke J. E., Li S., Woods V. L., Jr., Dennis E. A. (2009) Localizing the membrane binding region of Group VIA Ca2+-independent phospholipase A2 using peptide amide hydrogen/deuterium exchange mass spectrometry. J. Biol. Chem. 284, 23652–23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burke J. E., Babakhani A., Gorfe A. A., Kokotos G., Li S., Woods V. L., Jr., McCammon J. A., Dennis E. A. (2009) Location of inhibitors bound to Group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry. J. Am. Chem. Soc. 131, 8083–8091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu Y. H., Burke J. E., Stephens D. L., Deems R. A., Li S., Asmus K. M., Woods V. L., Jr., Dennis E. A. (2008) Calcium binding rigidifies the C2 domain and the intradomain interaction of GIVA phospholipase A2 as revealed by hydrogen/deuterium exchange mass spectrometry. J. Biol. Chem. 283, 9820–9827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burke J. E., Karbarz M. J., Deems R. A., Li S., Woods V. L., Jr., Dennis E. A. (2008) Interaction of Group IA phospholipase A2 with metal ions and phospholipid vesicles probed with deuterium exchange mass spectrometry. Biochemistry 47, 6451–6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burke J. E., Hsu Y. H., Deems R. A., Li S., Woods V. L., Jr., Dennis E. A. (2008) A phospholipid substrate molecule residing in the membrane surface mediates opening of the lid region in Group IVA cytosolic phospholipase A2. J. Biol. Chem. 283, 31227–31236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao J., Hsu Y. H., Li S., Woods V. L., Dennis E. A. (2011) Lipoprotein-associated phospholipase A2 interacts with phospholipid vesicles via a surface-disposed hydrophobic α-helix. Biochemistry 50, 5314–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skinner J. J., Lim W. K., Bédard S., Black B. E., Englander S. W. (2012) Protein dynamics viewed by hydrogen exchange. Protein Sci. 21, 996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Connelly G. P., Bai Y., Jeng M. F., Englander S. W. (1993) Isotope effects in peptide group hydrogen exchange. Proteins 17, 87–92 [DOI] [PubMed] [Google Scholar]
- 27. Bai Y., Milne J. S., Mayne L., Englander S. W. (1994) Protein stability parameters measured by hydrogen exchange. Proteins 20, 4–14 [DOI] [PubMed] [Google Scholar]
- 28. Englander S. W., Kallenbach N. R. (1983) Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q. Rev. Biophys. 16, 521–655 [DOI] [PubMed] [Google Scholar]
- 29. Hoofnagle A. N., Resing K. A., Ahn N. G. (2003) Protein analysis by hydrogen exchange mass spectrometry. Annu. Rev. Biophys. Biomol. Struct. 32, 1–25 [DOI] [PubMed] [Google Scholar]
- 30. Hvidt A., Nielsen S. O. (1966) Hydrogen exchange in proteins. Adv. Protein Chem. 21, 287–386 [DOI] [PubMed] [Google Scholar]
- 31. Changeux J. P. (2010) Allosteric receptors: from electric organ to cognition. Annu. Rev. Pharmacol. Toxicol. 50, 1–38 [DOI] [PubMed] [Google Scholar]
- 32. Monod J., Wyman J., Changeux J.-P. (1965) On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 [DOI] [PubMed] [Google Scholar]
- 33. Mosior M., Six D. A., Dennis E. A. (1998) Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J. Biol. Chem. 273, 2184–2191 [DOI] [PubMed] [Google Scholar]
- 34. Stahelin R. V., Subramanian P., Vora M., Cho W., Chalfant C. E. (2007) Ceramide 1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J. Biol. Chem. 282, 20467–20474 [DOI] [PubMed] [Google Scholar]
- 35. Lio Y. C., Dennis E. A. (1998) Interfacial activation, lysophospholipase and transacylase activity of Group VI Ca2+-independent phospholipase A2. Biochim. Biophys. Acta 1392, 320–332 [DOI] [PubMed] [Google Scholar]
- 36. Percy A. J., Rey M., Burns K. M., Schriemer D. C. (2012) Probing protein interactions with hydrogen/deuterium exchange and mass spectrometry–a review. Anal. Chim. Acta 721, 7–21 [DOI] [PubMed] [Google Scholar]
- 37. Bai Y., Milne J. S., Mayne L., Englander S. W. (1993) Primary structure effects on peptide group hydrogen exchange. Proteins 17, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berg O. G., Gelb M. H., Tsai M. D., Jain M. K. (2001) Interfacial enzymology: the secreted phospholipase A2 paradigm. Chem. Rev. 101, 2613–2654 [DOI] [PubMed] [Google Scholar]
- 39. White S. P., Scott D. L., Otwinowski Z., Gelb M. H., Sigler P. B. (1990) Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science 250, 1560–1563 [DOI] [PubMed] [Google Scholar]
- 40. Fremont D. H., Anderson D. H., Wilson I. A., Dennis E. A., Xuong N. H. (1993) Crystal structure of phospholipase A2 from Indian cobra reveals a trimeric association. Proc. Natl. Acad. Sci. U.S.A. 90, 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott D. L., White S. P., Otwinowski Z., Yuan W., Gelb M. H., Sigler P. B. (1990) Interfacial catalysis: the mechanism of phospholipase A2. Science 250, 1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han S. K., Kim K. P., Koduri R., Bittova L., Munoz N. M., Leff A. R., Wilton D. C., Gelb M. H., Cho W. (1999) Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2. J. Biol. Chem. 274, 11881–11888 [DOI] [PubMed] [Google Scholar]
- 43. Bezzine S., Bollinger J. G., Singer A. G., Veatch S. L., Keller S. L., Gelb M. H. (2002) On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 277, 48523–48534 [DOI] [PubMed] [Google Scholar]
- 44. Winget J. M., Pan Y. H., Bahnson B. J. (2006) The interfacial binding surface of phospholipase A2s. Biochim. Biophys. Acta 1761, 1260–1269 [DOI] [PubMed] [Google Scholar]
- 45. Pan Y. H., Bahnson B. J. (2010) Structure of a premicellar complex of alkyl sulfates with the interfacial binding surfaces of four subunits of phospholipase A2. Biochim. Biophys. Acta 1804, 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burke J. E., Dennis E. A. (2009) Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 23, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nalefski E. A., McDonagh T., Somers W., Seehra J., Falke J. J., Clark J. D. (1998) Independent folding and ligand specificity of the C2 calcium-dependent lipid binding domain of cytosolic phospholipase A2. J. Biol. Chem. 273, 1365–1372 [DOI] [PubMed] [Google Scholar]
- 48. Perisic O., Paterson H. F., Mosedale G., Lara-González S., Williams R. L. (1999) Mapping the phospholipid-binding surface and translocation determinants of the C2 domain from cytosolic phospholipase A2. J. Biol. Chem. 274, 14979–14987 [DOI] [PubMed] [Google Scholar]
- 49. Malmberg N. J., Van Buskirk D. R., Falke J. J. (2003) Membrane-docking loops of the cPLA2 C2 domain: detailed structural analysis of the protein-membrane interface via site-directed spin-labeling. Biochemistry 42, 13227–13240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stahelin R. V., Rafter J. D., Das S., Cho W. (2003) The molecular basis of differential subcellular localization of C2 domains of protein kinase Cα and group IVa cytosolic phospholipase A2. J. Biol. Chem. 278, 12452–12460 [DOI] [PubMed] [Google Scholar]
- 51. Hamuro Y., Burns L., Canaves J., Hoffman R., Taylor S., Woods V. (2002) Domain organization of D-AKAP2 revealed by enhanced deuterium exchange-mass spectrometry (DXMS). J. Mol. Biol. 321, 703–714 [DOI] [PubMed] [Google Scholar]
- 52. Liu T., Pantazatos D., Li S., Hamuro Y., Hilser V. J., Woods V. L., Jr. (2012) Quantitative assessment of protein structural models by comparison of H/D exchange MS data with exchange behavior accurately predicted by DXCOREX. J. Am. Soc. Mass Spectrom. 23, 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Min J. H., Jain M. K., Wilder C., Paul L., Apitz-Castro R., Aspleaf D. C., Gelb M. H. (1999) Membrane-bound plasma platelet activating factor acetylhydrolase acts on substrate in the aqueous phase. Biochemistry 38, 12935–12942 [DOI] [PubMed] [Google Scholar]
- 54. Tjoelker L. W., Wilder C., Eberhardt C., Stafforini D. M., Dietsch G., Schimpf B., Hooper S., Le Trong H., Cousens L. S., Zimmerman G. A., Yamada Y., McIntyre T. M., Prescott S. M., Gray P. W. (1995) Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature 374, 549–553 [DOI] [PubMed] [Google Scholar]
- 55. Davis B., Koster G., Douet L. J., Scigelova M., Woffendin G., Ward J. M., Smith A., Humphries J., Burnand K. G., Macphee C. H., Postle A. D. (2008) Electrospray ionization mass spectrometry identifies substrates and products of lipoprotein-associated phospholipase A2 in oxidized human low density lipoprotein. J. Biol. Chem. 283, 6428–6437 [DOI] [PubMed] [Google Scholar]
- 56. Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., McIntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J., 2nd (2006) Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281, 4616–4623 [DOI] [PubMed] [Google Scholar]
- 57. Stafforini D. M., Tjoelker L. W., McCormick S. P., Vaitkus D., McIntyre T. M., Gray P. W., Young S. G., Prescott S. M. (1999) Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J. Biol. Chem. 274, 7018–7024 [DOI] [PubMed] [Google Scholar]
- 58. Samanta U., Bahnson B. J. (2008) Crystal structure of human plasma platelet-activating factor acetylhydrolase: structural implication to lipoprotein binding and catalysis. J. Biol. Chem. 283, 31617–31624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cao J., Hsu Y.-H., Li S., Woods V. L., Jr., Dennis E. A. (2012) J. Lipid Res. doi: 10.1194/jlr.M030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Magrioti V., Kokotos G. (2010) Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin. Ther. Pat. 20, 1–18 [DOI] [PubMed] [Google Scholar]
- 61. Brier S., Lemaire D., DeBonis S., Forest E., Kozielski F. (2006) Molecular dissection of the inhibitor binding pocket of mitotic kinesin Eg5 reveals mutants that confer resistance to antimitotic agents. J. Mol. Biol. 360, 360–376 [DOI] [PubMed] [Google Scholar]
- 62. Brier S., Lemaire D., DeBonis S., Kozielski F., Forest E. (2006) Use of hydrogen/deuterium exchange mass spectrometry and mutagenesis as a tool to identify the binding region of inhibitors targeting the human mitotic kinesin Eg5. Rapid Commun. Mass Spectrom. 20, 456–462 [DOI] [PubMed] [Google Scholar]
- 63. Pang X. Y., Cao J., Addington L., Lovell S., Battaile K. P., Zhang N., Rao J. L., Dennis E. A., Moise A. R. (2012) Structure/function relationships of adipose phospholipase A2 containing a Cys-His-His catalytic triad. J. Biol. Chem. 287, 35260–35274 [DOI] [PMC free article] [PubMed] [Google Scholar]