Background: Cystic fibrosis is characterized by defective autophagy and increased Burkholderia cenocepacia infection.
Results: The depletion of SQSTM1/p62 from ΔF508 macrophages improves bacterial clearance via autophagy.
Conclusion: p62 expression level determines the fate of B. cepacia infection in ΔF508 macrophages.
Significance: Our study reveals the role of p62 in diseases characterized by protein aggregates that compromise autophagy by consuming essential autophagy molecules.
Keywords: Autophagy, Bacterial Pathogenesis, Cystic Fibrosis, Innate Immunity, Trafficking, Burkholderia cenocepacia
Abstract
Cystic fibrosis is the most common inherited lethal disease in Caucasians. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), of which the cftr ΔF508 mutation is the most common. ΔF508 macrophages are intrinsically defective in autophagy because of the sequestration of essential autophagy molecules within unprocessed CFTR aggregates. Defective autophagy allows Burkholderia cenocepacia (B. cepacia) to survive and replicate in ΔF508 macrophages. Infection by B. cepacia poses a great risk to cystic fibrosis patients because it causes accelerated lung inflammation and, in some cases, a lethal necrotizing pneumonia. Autophagy is a cell survival mechanism whereby an autophagosome engulfs non-functional organelles and delivers them to the lysosome for degradation. The ubiquitin binding adaptor protein SQSTM1/p62 is required for the delivery of several ubiquitinated cargos to the autophagosome. In WT macrophages, p62 depletion and overexpression lead to increased and decreased bacterial intracellular survival, respectively. In contrast, depletion of p62 in ΔF508 macrophages results in decreased bacterial survival, whereas overexpression of p62 leads to increased B. cepacia intracellular growth. Interestingly, the depletion of p62 from ΔF508 macrophages results in the release of the autophagy molecule beclin1 (BECN1) from the mutant CFTR aggregates and allows its redistribution and recruitment to the B. cepacia vacuole, mediating the acquisition of the autophagy marker LC3 and bacterial clearance via autophagy. These data demonstrate that p62 differentially dictates the fate of B. cepacia infection in WT and ΔF508 macrophages.
Introduction
Cystic fibrosis (CF)3 is the most common inherited lethal disease among Caucasians, which is caused by mutations in the cftr gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). The most common CFTR mutation results in a deletion of phenylalanine at position 508 (ΔF508), which affects the processing of the CFTR protein in such way that it cannot reach the epithelial cell surface. This mutation results in an aggresome-prone protein that forms intracellular aggregates (1–4).
Autophagy is a conserved physiological process that eliminates non-functional organelles and recycles cytosolic components to generate nutrients during periods of stress or starvation (5, 6). Autophagy also targets cytosolic long-lived proteins and organelles for lysosomal degradation in eukaryotic cells and plays a role in innate immunity (7). Loss of autophagy in murine tissues is accompanied by accumulation of protein aggregates and disordered organelles, leading to life-threatening diseases (8). Autophagy plays a key role in protecting the cytosol from bacterial infection. The mechanisms of bacterial recognition by this pathway are starting to be elucidated. Some cellular cargos are marked for autophagy by acquiring adaptor proteins such as Calcoco2 (also known as NDP52) and neighbor of BRCA1 gene product (NBR1) (9–14). In addition, SQSTM1 (also known as p62) is required for targeting Salmonella enterica serovar Typhimurium (Salmonella typhimurium), intracytosolic Shigella, and Listeria to the autophagic pathway (9, 10).
The adaptor molecule p62 is a ubiquitously expressed cellular protein that is conserved in metazoa but not in plants or fungi (15, 16). The quantity of p62 is critical for cell viability and is strictly controlled (17). p62 has multiple protein-protein interaction domains, including the ubiquitin-associated domain for ubiquitinated cargo binding and the LC3 interaction region for binding LC3 (10). Accordingly, impaired autophagy is accompanied by accumulation of p62 followed by formation of aggregates containing p62 and ubiquitinated proteins. This accumulation occurs because of the nature of both self-oligomerization and ubiquitin binding of p62 (18, 19).
Burkholderia cenocepacia (B. cepacia) is an opportunistic Gram-negative bacterium that infects CF patients and leads to severe lung inflammation and lung tissue destruction. Occasionally, this infection results in a lethal necrotizing pneumonia (20–22). Unfortunately, B. cepacia is resistant to most known antibiotics and, thus, is nearly impossible to treat. B. cepacia adopts an extracellular or intracellular lifestyle (23, 24). This bacterium can survive within a variety of eukaryotic cells such as amoebae, epithelial cells, and macrophages (25–28).
We have demonstrated previously that in WT macrophages, the majority of B. cepacia-containing vacuoles slowly acquire the specific autophagy marker LC3 within 2 h of infection. Subsequently, these vacuoles fuse with the lysosomes, and the bacterium is degraded. In ΔF508 macrophages, B. cepacia-containing vacuoles do not acquire autophagosome markers and do not fuse with the lysosomes.
Here, we demonstrate that in WT macrophages, p62 is required for targeting B. cepacia to the autophagosome. Upon p62 down-regulation, bacterial growth increases, whereas the overexpression of p62 results in a significant decrease in B. cepacia replication. On the contrary, down-regulation of p62 in ΔF508 macrophages is associated with decreased bacterial growth, and p62 overexpression results in increased B. cepacia replication. p62 down-regulation in ΔF508 macrophages releases the trapped BECN1 from CFTR aggregates, allowing its recruitment to the B. cepacia vacuole. BECN1 acquired by the B. cepacia-containing vacuole subsequently attracts LC3, thereby mediating the fusion of the maturing autophagosome containing B. cepacia with the lysosome via the autophagic machinery. These data provide mechanistic insight on how B. cepacia persists in ΔF508 macrophages. This report also suggests that p62 may be an attractive drug target to improve B. cepacia clearance by autophagic machinery.
EXPERIMENTAL PROCEDURES
Bone Marrow-derived Macrophages
Animal experiments were performed according to protocols approved by the Animal Care and Use Committee of the Ohio State University College of Medicine. WT C57BL/6 were purchased from The Jackson Laboratory. ΔF508 mice on a C57BL/6 background were obtained from Case Western University and housed in the Ohio State University vivarium. Bone marrow-derived macrophages were isolated from the femurs of 6- to 12-week-old mice and were cultured in Iscove's modified Dulbecco's medium (Invitrogen, catalog no. 12440) containing 10% heat-inactivated FBS (Invitrogen, catalog no. 16000), 20% L cell-conditioned medium, 100 units/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, catalog no. 15140) at 37 °C in a humidified atmosphere containing 5% CO2. Macrophages were infected with B. cepacia K56-expressing m-RFP or the corresponding gentamicin-sensitive strain MHK1 at a multiplicity of infection of 10.
Bacterial Strains and Culture
B. cenocepacia strain K56-2 is a clinical isolate from a CF patient. The corresponding gentamicin-sensitive strain MHK1 was described previously (29). All bacterial strains were grown in Luria-Bertani broth at 37 °C overnight with high-amplitude shaking. To kill extracellular bacteria, Iscove's media (Invitrogen, catalog no. 12440) plus FBS (Invitrogen, catalog no. 16000) containing 50 μg/ml gentamicin (Invitrogen, catalog no. 3564) were added for 0.5 h, as described previously (29). To enumerate intracellular bacteria, infected macrophages were lysed with ice-cold PBS (Invitrogen, catalog no. 14190) at designated times. Recovered bacteria were quantified by plating serial dilutions on Luria-Bertani agar plates and counting colonies using the Acolyte Colony Counter, 5710/SYN.
Immunoblotting
Macrophages were stimulated with B. cepacia, and the culture supernatant was removed. Cells were lysed in lysis buffer solution supplemented with a protease inhibitor mixture (Roche Applied Science, catalog no. 10-519-978-001). The protein concentration was adjusted to 30 μg/ml. Proteins were separated on a sodium dodecyl sulfate 15% polyacrylamide gel and transferred to a PVDF membrane (Bio-Rad, catalog no. 1p62-0117). Membranes were immunoblotted for p62 (Sigma-Aldrich, catalog no. P0067), LC3 (Sigma-Aldrich, catalog no. L8918), Calreticulin (Stressgen, catalog no. SPA600), BECN1 (Abcam, catalog no. ab55878), NDP52 (Millipore, catalog no. MAB4386), NBR1 (Santa Cruz Biotechnology, Inc., SC-130380), and Actin (Abcam, catalog no. ab8299, and Atg7 (Sigma, A2856)). Protein bands were detected with secondary antibodies conjugated to horseradish peroxidase, followed by enhanced chemiluminescence reagents (Amersham Biosciences, ECL Western blotting detection reagents; GE Healthcare, catalog no. RPN2106).
siRNA Treatment and Plasmid Transfection
siRNA treatment was performed using siRNA against p62 (Dharmacon, catalog no. 18412) ACAGAUGCCAGAAUCGGAA, CUGCUCAGGAGGAGACGAU, GAACAGAUGGAGUCGGGAA, and CCAUGGGUUUCUCGGAUGA; siRNA against NDP52 (Dharmacon, catalog no. 76815) CAACACAGAGGGUCAAUAA, CAGAAGAGGACAUCCGGAU, CCAAGGAUGUGGAGCGCUA, and GAGUUGAGGUGUCCGUGUA; siRNA against NBR1 (Dharmacon, catalog no. 17966) GAAAUGGGAUUCUGCGACA, AGUCCGUGGAAGCGAGUAA, CAAGCAAAGCUGACGAUUU, and ACAGGAGGCAUUCGGGUUA; and siRNA against Atg7 (Dharmacon, catalog no. 49953) CAUCAUCUUUGAAGUGAAA, GCUAGAGACGUGACACAUA, AGCGAAAGCUGGUCAUCAA, and GGUCGUGUCUGUCAAGUGC. siRNA was nucleofected into primary macrophages 48 h before infection using a Lonza nucleofection kit and Amaxa equipment, as described previously (30, 31). Successful knockdown was confirmed by immunoblot analysis for each experiment. The DsRed-p62 plasmid was obtained from Addgene (32) and was nucleofected into primary macrophages using a Lonza nucleofection kit and Amaxa equipment. The plasmid was nucleofected 24 h before the infection. Successful p62 overexpression was confirmed by immunoblotting.
Real-time PCR
Total RNA was isolated from cells lysed in TRIzol (Invitrogen, catalog no. 15596-026) and then converted to cDNA. Gene expression was calculated as relative copy numbers, as described previously (30, 33). Briefly, Ct values of the p62 gene were subtracted from the average Ct of two housekeeping genes (GADPH and CAP1), and the resulting ΔCt was used in the following equation: RCN = (2−ΔCt) 100. The relative copy number of a gene is represented as the number of copies relative to the 100 copies of average housekeeping genes (30, 33).
Confocal Microscopy
Immunofluorescence experiments for colocalization with autophagy markers were performed as described previously (34, 6). Rabbit anti-LC3 (Abgent, catalog no. AP1805a), mouse anti-p62 (BD Biosciences, catalog no. 610832), FK2 mAb (Enzo Bioscience, catalog no. BML-PW8810), and rabbit anti-BECN1 (Abcam, catalog no. ab55878) were used, followed by fluorescent secondary antibodies (Molecular Probes, catalog no. A11008). Nuclei were stained with the nucleic acid dye DAPI (6, 35). Samples were analyzed with an Olympus Fluoview FV10i confocal microscope at the Ohio State University, Department of Microbial Infection and Immunity.
Statistical Analysis
All experiments were performed at least three times independently and yielded similar results. Comparisons of groups for statistical difference were conducted using Student's two-tailed t test. p ≤ 0.05 was considered significant.
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and Ohio State University. The Institutional Animal Care and Use Committee has approved our protocol number 2007A0070. All efforts were made to minimize suffering.
RESULTS
More B. cepacia Colocalized with p62 in WT Macrophages Than in ΔF508 Macrophages
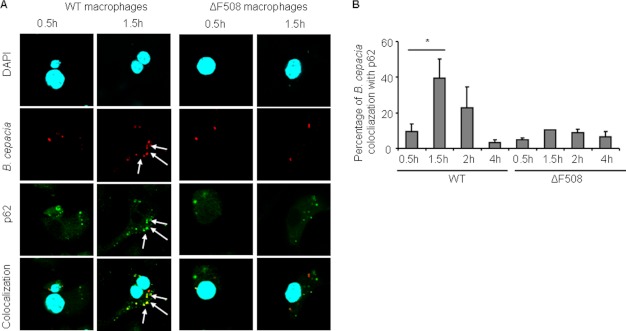
We demonstrated previously that B. cepacia is cleared by the autophagy machinery in WT macrophages but not in their ΔF508 counterparts. To determine why the B. cepacia vacuole is not efficiently recognized by the autophagy machinery in ΔF508 macrophages, we followed the trafficking within WT and ΔF508 macrophages. Recent studies showed that p62 is required for targeting S. typhimurium, Shigella, and Listeria to the autophagic pathway (9, 10). Therefore, we examined the colocalization of B. cepacia with p62 in WT and ΔF508 macrophages. The time course for infection was 0.5, 1.5, 2, and 4 h. In WT macrophages, a significant percentage of B. cepacia colocalized with p62 at 1.5 h post-infection. Colocalization then declined at later time points (Fig. 1, A and B). However, B. cepacia vacuoles in ΔF508 macrophages did not colocalize with p62 at any time point throughout infection (Fig. 1, A and B). Together, these data show that p62 labels the B. cepacia vacuole in WT but not in ΔF508 macrophages.
FIGURE 1.
More B. cepacia vacuoles colocalize with p62 in WT macrophages than in ΔF508 macrophages. A, confocal microscopy for WT and ΔF508 macrophages infected with B. cepacia-expressing m-RFP for 0.5 or 1.5 h. p62 stained green, whereas nuclei were stained with DAPI. B, the percentage of colocalization of B. cepacia with p62 was quantified at the indicated time points. More than 200 cells were scored. The white arrows indicate the sites of colocalization. Data in B are presented as means ± S.D. *, p < 0.05.
The B. cepacia Vacuole Efficiently Acquires Ubiquitin in ΔF508 Macrophages
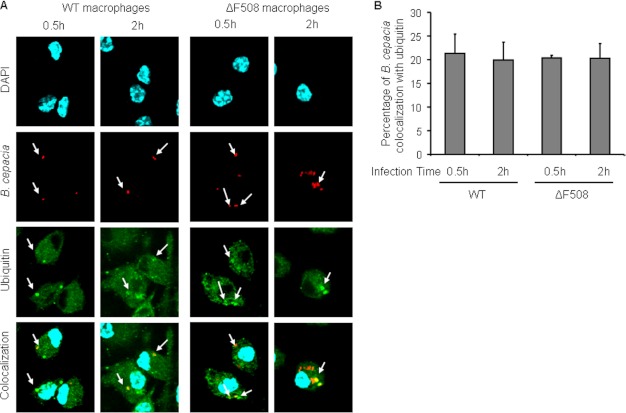
Autophagy recognizes cargo for uptake and degradation when it becomes ubiquitinated and bound to an autophagy adaptor molecule (10). The lack of p62 acquisition by the B. cepacia vacuole in ΔF508 macrophages could be due to defective ubiquitination of the B. cepacia-containing vacuole or because of lack of p62 expression in ΔF508 macrophages. To differentiate between these possibilities, we first infected WT and ΔF508 macrophages with B. cepacia-expressing m-RFP for 0.5 h or 2 h and examined the colocalization of B. cepacia with ubiquitin. There was no significant difference in the colocalization of B. cepacia with ubiquitin between WT and ΔF508 macrophages (Fig. 2, A and B). These data demonstrate that equivalent numbers of B. cepacia vacuoles acquired ubiquitin in WT and ΔF508 macrophages. Therefore, the lack of colocalization of B. cepacia with autophagosomes in ΔF508 macrophages is not due to the absence of ubiquitin around the B. cepacia vacuole.
FIGURE 2.
The ubiquitination of the B. cepacia vacuole is similar in WT and ΔF508 macrophages. A, confocal microscopy for WT and ΔF508 macrophages infected with m-RFP-expressing B. cepacia for 0.5 or 2 h. Ubiquitin stained green, and nuclei were stained with DAPI (the white arrow indicates the sites of colocalization). B, the percentage of colocalization of B. cepacia with ubiquitin was quantified. More than 200 cells were scored.
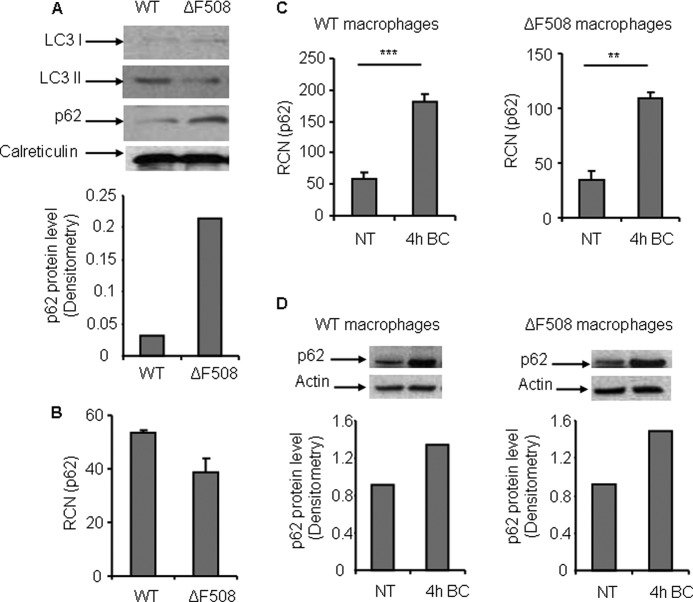
Next, to determine whether the failure of the autophagy machinery to target the B. cepacia vacuole is due to the lack of p62 expression in ΔF508 macrophages, we examined the level of p62 within WT and ΔF508 macrophages. Immunoblot analysis using an antibody against p62 revealed that murine macrophages harboring the ΔF508 mutation exhibited a higher level of p62 compared with WT macrophages (Fig. 3A). Quantitative PCR (q-PCR) was performed to determine whether the increase in p62 protein level in ΔF508 macrophages, compared with WT macrophages, is due to regulation of gene expression or accumulation of the p62 protein. There was no significant difference in the p62 mRNA level in both types of macrophages (Fig. 3B). Together, these data show that the increase in p62 level in ΔF508 macrophages is due to accumulation of the protein inside the cell, suggesting defective autophagy activity.
FIGURE 3.
Murine bone marrow-derived macrophages harboring the ΔF508 mutation have a higher level of p62 than WT macrophages. A, upper panel, immunoblot analysis of WT and ΔF508 macrophage lysates showing the expression level of LC3 (I/II) and p62, respectively. Lower panel, densitometry of the p62 protein level. B, q-PCR expression profile of p62 in WT and ΔF508 macrophages. C, q-PCR expression profile of p62 in WT and ΔF508 macrophages non-infected (NT) or infected with B. cepacia for 4 h (4h BC). **, p < 0.01; ***, p < 0.001. D, upper panel, immunoblot analysis for WT and ΔF508 macrophages using p62 antibody prior and at 4 h post-infection with B. cepacia. Lower panel, densitometry of the p62 protein level. Data in B and C are expressed as relative copy numbers (RCN) and shown as means ± S.D. of three independent experiments.
B. cepacia Infection Elevates p62 Expression within WT and ΔF508 Macrophages
p62 is well expressed in ΔF508 macrophages. However, B. cepacia infection down-regulates autophagy in both WT and ΔF508 macrophages. Thus, it is possible that B. cepacia infection is accompanied by depletion of p62 from ΔF508 macrophages upon infection. To examine this possibility, we examined the effect of B. cepacia on p62 expression upon infection in WT and ΔF508 macrophages by q-PCR and immunoblot analysis. At 4 h post infection, q-PCR analysis demonstrated increased expression of the p62 gene level in both WT and ΔF508 macrophages compared with non-infected macrophages (Fig. 3C). Similarly, immunoblotting showed a higher p62 level in both types of macrophages (Fig. 3D). Together, these data show that B. cepacia infection increases the expression level of p62 in WT and ΔF508 macrophages.
Overexpression of p62 Conversely Affects B. cepacia Replication in WT and ΔF508 Macrophages
To determine the role of p62 in B. cepacia replication in WT and ΔF508 macrophages, we examined B. cepacia survival in the presence of ectopically expressed p62. WT and ΔF508 macrophages were nucleofected with p62 plasmid or vector control and after 24 h, cells were infected with B. cepacia for 2, 4, and 6 h (Fig. 4G). In WT macrophages harboring the p62 plasmid, recovered B. cepacia CFUs decreased at 6 h post-infection compared with the cells harboring the vector alone (Fig. 4A). Confocal microscopy revealed significantly less bacterial accumulation upon overexpression of p62 (Fig. 4, B and C). In contrast, ΔF508 macrophages harboring the p62 plasmid allowed significantly increased B. cepacia accumulation after 6 h post-infection (Fig. 4D). Confocal microscopy confirmed increased bacterial accumulation (Fig. 4, E and F). Together, these results demonstrate that the availability of p62 differentially determines the fate of B. cepacia in WT and ΔF508 macrophages.
FIGURE 4.
p62 overexpression decreases growth of B. cepacia in WT macrophages, whereas increases bacterial growth in ΔF508 macrophages. A and D, WT and ΔF508 macrophages were nucleofected with the p62 plasmid or vector control 24 h prior to infection and then infected with B. cepacia for 2, 4, and 6 h. CFUs were enumerated. B and E, confocal microscopy for WT and ΔF508 macrophages overexpressing p62 at 0.5 or 2 h post-infection. The white arrows indicate B. cepacia stained with DAPI. C and F, both types of macrophages overexpressing p62 were infected for 0.5, 2, and 4 h, and the number of bacteria/100 cells was quantified. More than 200 cells were scored. G, immunoblot analysis for WT macrophages after 24-h nucleofection with the DsRed-p62 plasmid showing the overexpression of p62. Data are representative of three different experiments and presented as the means ± S.D. A, C, D, and F, *, p < 0.05; **, p < 0.01; significant differences from the vector at the respective time points.
Down-regulation of p62 Decreases the Growth of B. cepacia in ΔF508 Macrophages
To determine whether p62 targets B. cepacia vacuoles to autophagosomes for degradation, we nucleofected WT and ΔF508 macrophages with p62 siRNA or scrambled siRNA (Fig. 5G). After 48 h, cells were infected with B. cepacia for 2, 4, and 6 h. In WT macrophages, B. cepacia CFUs significantly increased upon down-regulation of p62 (Fig. 5A). In addition, confocal microscopic analysis demonstrated significantly increased bacterial numbers at 2 h post-infection (Fig. 5, B and C). In contrast, ΔF508 macrophages showed decreased B. cepacia CFUs upon down-regulation of p62 (Fig. 5D). Furthermore, confocal microscopy revealed significantly low bacterial accumulation 2 h after B. cepacia infection upon down-regulation of p62 (Fig. 5, E and F). Therefore, these data demonstrate that p62 controls B. cepacia infection in WT macrophages but not in ΔF508 macrophages. The details of this differential role are not clear.
FIGURE 5.
Down-regulation of p62 results in increased growth of B. cepacia in WT murine macrophages, whereas in ΔF508 macrophages it leads to decreased growth. A and D, WT and ΔF508 macrophages were nucleofected with siRNA against p62 (siRNA-p62) or control siRNA (siRNA-CT) 48 h prior to infection and then infected with B. cepacia for 2, 4, and 6 h. CFUs were enumerated. B and E, confocal microscopy of p62-depleted WT and ΔF508 macrophages infected with B. cepacia for 0.5 or 2 h. The white arrows show B. cepacia stained with DAPI. C and F, both types of macrophages were infected for 0.5 and 2 h after depletion of p62. The number of bacteria/100 cells was quantified. More than 200 cells were scored. G, upper panel, immunoblot analysis for WT and ΔF508 macrophages after 48-h nucleofection with siRNA against p62 (si-p62) or control siRNA (si-CT). Lower panel, densitometry of the p62 protein level. Data are representative of three different experiments and presented as the means ± S.D. A, C, D, and F, *, p < 0.05; **, p < 0.01; ***, p < 0.001; significant differences from the siRNA-CT at the respective time points.
Decreased p62 Expression Promotes LC3 Acquisition by B. cepacia Vacuole in ΔF508 Macrophages
LC3 is the main marker for autophagosomes. The conversion of LC3-I to LC3-II denotes autophagy stimulation and autophagosome formation (7, 36). We have demonstrated previously that B. cepacia colocalization with LC3 is markedly decreased in ΔF508 macrophages compared with WT macrophages (37, 38). To determine the underlying mechanism, WT and ΔF508 macrophages were nucleofected with either siRNA against p62 to down-regulate p62 or with scrambled siRNA, and after 48 h, nucleofected macrophages were infected with B. cepacia-expressing m-RFP for 0.5 and 2 h. Confocal microscopy showed that in WT macrophages, B. cepacia colocalization with LC3 decreased significantly when p62 was down-regulated compared with the siRNA control-treated cells (Fig. 6, A and C). In contrast, ΔF508 macrophages allowed significantly more B. cepacia colocalization with LC3 after the down-regulation of p62 compared with the siRNA control-treated cells (Fig. 6, B and D). Together, these data suggest that p62 is required for the delivery of B. cepacia to the autophagosomes in WT macrophages, a role that is compromised for unknown reasons in ΔF508 macrophages.
FIGURE 6.
Down-regulation of p62 decreases B. cepacia colocalization with LC3 in WT macrophages but increases the colocalization in ΔF508 macrophages. A and B, confocal microscopy for WT macrophages and ΔF508 macrophages infected with B. cepacia-expressing m-RFP for 0.5 or 2 h. LC3 stained green, whereas nuclei were stained with DAPI. The percentage of colocalization of B. cepacia with LC3 at the indicated time points was scored in both WT and ΔF508 macrophages, respectively. C and D, more than 200 cells were scored. Data are presented as means ± S.D. *, p < 0.05; **, p < 0.01.
Depletion of p62 Liberates BECN1, Allowing Its Redistribution and Recruitment by the B. cepacia Vacuole in ΔF508 Macrophages
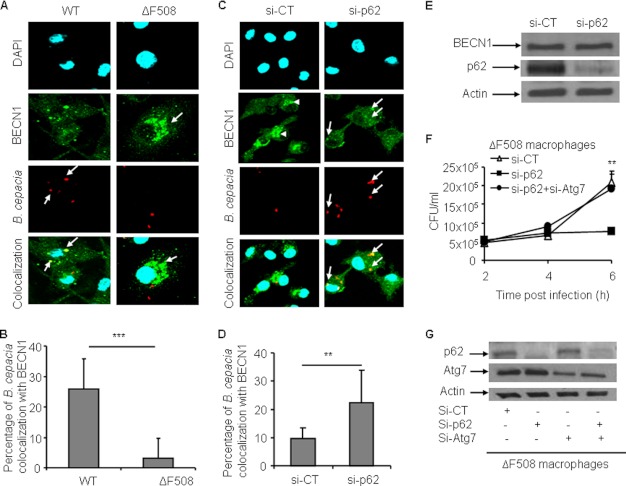
A growing body of evidence indicates that BECN1 is sequestered within the mutant CFTR aggresomes (1, 2). BECN1/Atg6 is a member of the class III PI3K complex and is essential for the early stages of autophagosome formation (5, 39). Thus, its unavailability leads to defective autophagic activity (1, 2). Mutant CFTR aggregates sequester autophagy molecules such as BECN1, depleting them from their storage areas, leading to defective autophagy. We examined the colocalization of B. cepacia with BECN1 in WT and ΔF508 macrophages. Confocal microscopy showed that in WT macrophages, high numbers of B. cepacia colocalized with BECN1 compared with ΔF508 macrophages (Fig. 7, A and B, arrows). In WT macrophages, BECN1 was distributed throughout the cytosol, whereas in ΔF508 macrophages, BECN1 was condensed in patches (Fig. 7 A, arrowheads).
FIGURE 7.
Colocalization of B. cepacia with BECN1 is increased in ΔF508 macrophages upon depletion of p62. A, confocal microscopy for WT and ΔF508 macrophages infected with B. cepacia-expressing m-RFP for 2 h. BECN1 stained green, and nuclei were stained with DAPI. The white arrows indicate B. cepacia, whereas the arrowheads indicate BECN1 aggregates. B, the percentage of colocalization of B. cepacia with BECN1 was scored by examining more than 400 cells. C, confocal microscopy for ΔF508 macrophages nucleofected with siRNA against p62 (Si-p62) or scrambled siRNA control (si-CT) 48 h prior to infection. Nucleofected macrophages were infected with B. cepacia-expressing m-RFP for 2 h. BECN1 stained green, whereas nuclei were stained with DAPI. The arrows indicate B. cepacia, whereas the arrowheads indicate BECN1 aggregates. D, the percentage of colocalization of B. cepacia with BECN1 at the assigned time point was scored. More than 400 bacteria were scored. E, immunoblot for ΔF508 macrophages nucleofected with siRNA against p62 or control siRNA for 48 h. Antibodies against p62 and BECN1 were used. F, ΔF508 macrophages were nucleofected with siRNA against p62 or control siRNA or siRNA against p62 and Atg7 together (si-p62+si-Atg7) for 48 h and then infected with B. cepacia for 2, 4, and 6 h. CFUs were enumerated. G, immunoblot analysis for ΔF508 macrophages nucleofected with control siRNA, siRNA against p62, siRNA against Atg7 (si-Atg7), or siRNA against p62 and Atg7 together for 48 h. Antibodies specific to p62 And Atg7 were used to detect the down-regulation. Data in B, D, and F are presented as means ± S.D. of three different experiments. B and D, **, p < 0.01; ***, p < 0.001; significant differences between both types of macrophages at the designated time point.
Because the sequestration of BECN1 in CFTR aggregates requires p62 (1, 2), we examined the effect of p62 depletion on BECN1 distribution within the cytosol and around the B. cepacia vacuole inside ΔF508 macrophages. ΔF508 macrophages nucleofected with p62 siRNA showed significantly more colocalization of B. cepacia with BECN1 compared with the siRNA control (Fig. 7, C, arrows, and D). Additionally, within the ΔF508 macrophages nucleofected with siRNA against p62, BECN1 was redistributed within the cytosol with the disappearance of BECN1-containing patches (Fig. 7C) after down-regulation of p62 (B). Notably, our immunoblot analysis using antibody specific to BECN1 showed equal amounts of the total BECN1 in the ΔF508 macrophages before and after p62 depletion (Fig. 7E). Together, these data show that depletion of p62 from ΔF508 macrophages allows the redistribution of BECN1 throughout the cell and increases its availability for the B. cepacia-containing vacuole.
Together, these data suggest that depletion of p62 from ΔF508 macrophages mediates B. cepacia clearance via recuperated autophagy. To confirm this conclusion, ΔF508 macrophages were depleted of p62 and Atg7 (an essential autophagy molecule) (40) to disrupt the autophagy machinery and then infected with B. cepacia. Depletion of p62 alone from ΔF508 macrophages improved B. cepacia clearance, yet concomitant depletion of Atg7 hindered bacterial clearance (Fig. 7, F and G). Thus, improved bacterial clearance upon depletion of p62 from ΔF508 macrophages is mediated by autophagy.
NBR1 and NDP52 Contribute to the Delivery of B. cepacia to Autophagosomes after Down-regulation of p62 in ΔF508 Macrophages
Thus, down-regulation of p62 in ΔF508 macrophages improves B. cepacia clearance by restoring autophagy activity. However, what targets the B. cepacia vacuole for autophagy in the absence of adequate amounts of p62 is unknown. Recently, it has been shown that p62 and NDP52 act cooperatively to drive efficient antibacterial autophagy of Salmonella, Shigella, and Listeria (9, 41). Furthermore, it was revealed that NBR1 and p62 mark ubiquitinated cargo for autophagy (11, 42). To determine whether NDP52 or NBR1 contribute to the delivery of B. cepacia to autophagosomes, we nucleofected WT and ΔF508 macrophages with siRNA against NDP52 or NBR1 prior to infection with B. cepacia. Our results showed that in WT macrophages, down-regulation of NDP52 did not affect B. cepacia recovered CFUs, whereas that of NBR1 resulted in a significant increase in B. cepacia growth. In ΔF508 macrophages, the down-regulation of either NDP52 or NBR1 resulted in an increase in B. cepacia growth. This result demonstrates that both NDP52 and NBR1 facilitate the delivery of B. cepacia to autophagosomes in ΔF508 macrophages. In WT macrophages, however, only NBR1 contributes to the delivery of B. cepacia to autophagosomes (Fig. 8, A, B, and D). To determine whether NDP52 and NBR1 mark the B. cepacia vacuole for autophagy uptake in p62-depleted ΔF508 macrophages, we nucleofected ΔF508 macrophages with siRNA against p62 alone or in combination with either NDP52 or NBR1. Simultaneous down-regulation of p62 and NDP52 or p62 and NBR1 in ΔF508 macrophages resulted in a significant increase in B. cepacia growth (Fig. 8, C and E). Together, our data suggest that both NDP52 and NBR1 contribute to labeling the B. cepacia vacuole for autophagy uptake when p62 is unavailable.
FIGURE 8.
Depletion of p62 in ΔF508 macrophages improves clearance of B. cepacia by autophagosomes via NDP52 and NBR1. A, B, and C, WT (A) and ΔF508 (B and C) macrophages were nucleofected with siRNA against NDP52 (si-NDP52), NBR1 (si-NBR1), or control siRNA (si-CT) (A and B). C, ΔF508 macrophages nucleofected with siRNA against p62 (si-p62), si-p62+si-NDP52, or si-p62+si-NBR1. Macrophages in A, B, and C were then infected with B. cepacia for 2, 4, and 6 h. CFUs were quantified. D and E, Western blot analysis of macrophages treated as in A and B, respectively, with specific antibodies to NDP52, NBR1, p62, or actin. Data in A, B, and C are presented as the means ± S.D. A, C, D, and E, *, p < 0.05); **, p < 0.01; ***, p < 0.001; significant differences at the respective time points.
DISCUSSION
Human and mouse CF macrophages and airway epithelial cells exhibit impaired autophagy that is associated with formation of aggregates, including p62 and ubiquitinated mutant CFTR protein. This is due to both the self-oligomerization and ubiquitin-binding nature of p62 (1, 2, 10, 37, 38). Similar aggregates have been identified in various neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and cancer (1, 2, 43, 44). Impaired turnover of p62 is a major cause of the pathogenic changes seen in the autophagy-deficient mice, as the loss of Atg7 in mouse livers results in severe p62 accumulation. Loss of p62 greatly attenuates liver injury resulting from autophagy deficiency (18).
Here, we found that ΔF508 macrophages express more p62 than WT macrophages. This could be due to p62 accumulation because of reduced recycling in ΔF508 macrophages as a consequence of compromised autophagosome formation and maturation. Alternatively, the accumulation of p62 could stimulate the formation of more ΔF508 CFTR aggregates. This latter possibility agrees with the observation that depletion of p62 from ΔF508 macrophages improves autophagy and decreases the BECN1-positive aggregates. Also, a previous study using CF epithelial cells showed that p62 promotes aggresome accumulation of misfolded or modified proteins (43, 45). Recently, it has reported that reducing the levels of p62 can rescue ΔF508-CFTR trafficking to the plasma membrane of CF airway epithelial cells (1, 2, 46).
The presence of intracellular bacteria such as B. cepacia increases the level of p62 expression in both WT and ΔF508 macrophages. It is possible that p62 overexpression upon infection worsens the biology of ΔF508 macrophages, providing an explanation for the deterioration of lung function and innate immune responses in the infected CF lung. There are several mechanisms by which B. cepacia may lead to the accumulation of p62. It is plausible that B. cepacia increases p62 accumulation by inhibiting autophagy in ΔF508 macrophages, as we have published previously (37, 38). Notably, B. cepacia infection increases p62 mRNA. Regardless of the mechanism of p62 accumulation, the p62 aggregates sequester essential autophagy molecules such as BECN1, making them unavailable for efficient autophagosome formation (48).
The sequestration of BECN1 occurs via transglutaminase 2 (TG2)-mediated cross-linking in aggresomes because the BECN1 protein sequence contains QP and QXXP motifs, which are specific target sites for TG2 activity (48), and TG2 is an autophagy inhibitor in pancreatic adenocarcinoma cells (49). Increased reactive oxygen species in CF epithelia sustain high TG2 levels through TG2 SUMOylation (48). Thus, BECN1, and not all autophagy molecules, is specifically recruited to aggresomes in CF cells.
Examining the sequential acquisition of autophagy molecules by the B. cepacia vacuole revealed that although ubiquitination is efficient in both WT and ΔF508 macrophages, BECN1 acquisition is defective only in ΔF508 macrophages. BECN1, also known as autophagy-related gene product 6 (Atg6), and its binding partner class III PI3K (also named Vps34) are required for the initiation of the autophagosome formation (47). Thus, supplementation of p62 alone to ΔF508 macrophages will not improve the targeting of B. cepacia to autophagosomes. This conclusion is supported by the overexpression experiment of p62 in ΔF508 macrophages, which actually leads to more bacterial growth. Therefore, to correct the trafficking defect of B. cepacia in ΔF508 macrophages, “free” BECN1 is required, which is achieved by depletion of p62.
p62 targets several pathogens, such as S. typhimurium, Shigella, and Listeria to the autophagosome (9, 10). Similarly, p62 associates with the B. cepacia vacuole in WT macrophages. However, depletion of p62 from ΔF508 macrophages promotes B. cepacia uptake by autophagosomes and decreases the bacterial burden. It is possible that another adaptor molecule, such as NBR1, compensates for the loss of p62. The structure of NBR1 resembles that of p62. It can bind both LC3 and ubiquitinated proteins through the LC3 interaction region and ubiquitin-associated domain, respectively (11, 14). NDP52 is another cargo marker that drives certain bacteria to the autophagy machinery (9, 41). In this study, we found that NDP52 facilitates autophagy uptake of B. cepacia in ΔF508 macrophages but not in WT cells. NBR1, however, appears to contribute to the delivery of B. cepacia to the autophagy machinery in both WT and ΔF508 macrophages. To our knowledge, this is the first demonstration of a role for NBR1 in bacterial targeting by autophagy.
We showed previously (37) that autophagy stimulation by rapamycin can overcome the down-regulating effect of B. cepacia on the ATG genes and can control the B. cepacia infection in the ΔF508 mouse model both in vivo and in vitro. In this work, we demonstrate that p62 depletion from ΔF508 mouse macrophages is another approach to improve autophagic control on B. cepacia infection.
Together, these data provide a molecular framework to better understand the emerging complexity of diseases related to autophagic defect such as CF and the ability of macrophages to defend against the bacterial infection. This study also indentifies p62 as a promising drug target for improving B. cepacia clearance in CF macrophages.
This work was supported by a doctoral fellowship from the Egyptian Bureau of Education (to B. A. A.). This work was supported, in whole or in part, by the National Institutes of Health (Grant R01HL094586 (to A. O. A.)) and a Cystic Fibrosis Canada grant (to M. A. V).
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane regulator
- q-PCR
- quantitative PCR
- CFU
- colony-forming unit
- RFP
- red fluorescent protein.
REFERENCES
- 1. Luciani A., Villella V. R., Esposito S., Brunetti-Pierri N., Medina D., Settembre C., Gavina M., Pulze L., Giardino I., Pettoello-Mantovani M., D'Apolito M., Guido S., Masliah E., Spencer B., Quaratino S., Raia V., Ballabio A., Maiuri L. (2010) Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 12, 863–875 [DOI] [PubMed] [Google Scholar]
- 2. Luciani A., Villella V. R., Esposito S., Brunetti-Pierri N., Medina D. L., Settembre C., Gavina M., Raia V., Ballabio A., Maiuri L. (2011) Cystic fibrosis. A disorder with defective autophagy. Autophagy 7, 104–106 [DOI] [PubMed] [Google Scholar]
- 3. Knorre A., Wagner M., Schaefer H. E., Colledge W. H., Pahl H. L. (2002) ΔF508-CFTR causes constitutive NF-κB activation through an ER-overload response in cystic fibrosis lungs. Biol. Chem. 383, 271–282 [DOI] [PubMed] [Google Scholar]
- 4. Witko-Sarsat V., Sermet-Gaudelus I., Lenoir G., Descamps-Latscha B. (1999) Inflammation and CFTR. Might neutrophils be the key in cystic fibrosis? Mediators Inflamm. 8, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deretic V. (2010) Autophagy in infection. Curr. Opin. Cell Biol. 22, 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amer A. O., Byrne B. G., Swanson M. S. (2005) Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy 1, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Z., Klionsky D. J. (2010) Mammalian autophagy. Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizushima N., Levine B. (2010) Autophagy in mammalian development and differentiation. Nat. Cell Biol. 12, 823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mostowy S., Sancho-Shimizu V., Hamon M. A., Simeone R., Brosch R., Johansen T., Cossart P. (2011) p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 286, 26987–26995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Y. T., Shahnazari S., Brech A., Lamark T., Johansen T., Brumell J. H. (2009) The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 183, 5909–5916 [DOI] [PubMed] [Google Scholar]
- 11. Lamark T., Kirkin V., Dikic I., Johansen T. (2009) NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle 8, 1986–1990 [DOI] [PubMed] [Google Scholar]
- 12. Kirkin V., Lamark T., Johansen T., Dikic I. (2009) NBR1 cooperates with p62 in selective autophagy of ubiquitinated targets. Autophagy 5, 732–733 [DOI] [PubMed] [Google Scholar]
- 13. Kirkin V., Lamark T., Sou Y. S., Bjørkøy G., Nunn J. L., Bruun J. A., Shvets E., McEwan D. G., Clausen T. H., Wild P., Bilusic I., Theurillat J. P., Øvervatn A., Ishii T., Elazar Z., Komatsu M., Dikic I., Johansen T. (2009) A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505–516 [DOI] [PubMed] [Google Scholar]
- 14. Kirkin V., McEwan D. G., Novak I., Dikic I. (2009) A role for ubiquitin in selective autophagy. Mol. Cell 34, 259–269 [DOI] [PubMed] [Google Scholar]
- 15. Hocking L. J., Lucas G. J., Daroszewska A., Mangion J., Olavesen M., Cundy T., Nicholson G. C., Ward L., Bennett S. T., Wuyts W., Van Hul W., Ralston S. H. (2002) Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget's disease. Hum. Mol. Genet. 11, 2735–2739 [DOI] [PubMed] [Google Scholar]
- 16. Laurin N., Brown J. P., Morissette J., Raymond V. (2002) Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am. J. Hum. Genet. 70, 1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Komatsu M., Ichimura Y. (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 584, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 18. Komatsu M., Waguri S., Koike M., Sou Y. S., Ueno T., Hara T., Mizushima N., Iwata J., Ezaki J., Murata S., Hamazaki J., Nishito Y., Iemura S., Natsume T., Yanagawa T., Uwayama J., Warabi E., Yoshida H., Ishii T., Kobayashi A., Yamamoto M., Yue Z., Uchiyama Y., Kominami E., Tanaka K. (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131, 1149–1163 [DOI] [PubMed] [Google Scholar]
- 19. Nezis I. P., Simonsen A., Sagona A. P., Finley K., Gaumer S., Contamine D., Rusten T. E., Stenmark H., Brech A. (2008) Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J. Cell Biol. 180, 1065–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kotrange S., Kopp B., Akhter A., Abdelaziz D., Abu Khweek A., Caution K., Abdulrahman B., Wewers M. D., McCoy K., Marsh C., Loutet S. A., Ortega X., Valvano M. A., Amer A. O. (2011) Burkholderia cenocepacia O polysaccharide chain contributes to caspase-1-dependent IL-1β production in macrophages. J. Leukocyte Biol. 89, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orvedahl A., Levine B. (2009) Eating the enemy within. Autophagy in infectious diseases. Cell Death Differ. 16, 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakagawa I., Amano A., Mizushima N., Yamamoto A., Yamaguchi H., Kamimoto T., Nara A., Funao J., Nakata M., Tsuda K., Hamada S., Yoshimori T. (2004) Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 [DOI] [PubMed] [Google Scholar]
- 23. Loutet S. A., Valvano M. A. (2010) A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 78, 4088–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saldías M. S., Valvano M. A. (2009) Interactions of Burkholderia cenocepacia and other Burkholderia cepacia complex bacteria with epithelial and phagocytic cells. Microbiology 155, 2809–2817 [DOI] [PubMed] [Google Scholar]
- 25. Birmingham C. L., Smith A. C., Bakowski M. A., Yoshimori T., Brumell J. H. (2006) Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281, 11374–11383 [DOI] [PubMed] [Google Scholar]
- 26. McCloskey M., McCaughan J., Redmond A. O., Elborn J. S. (2001) Clinical outcome after acquisition of Burkholderia cepacia in patients with cystic fibrosis. Ir. J. Med. Sci. 170, 28–31 [DOI] [PubMed] [Google Scholar]
- 27. Tolman J. S., Valvano M. A. (2012) Global changes in gene expression by the opportunistic pathogen Burkholderia cenocepacia in response to internalization by murine macrophages. BMC Genomics 13, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosales-Reyes R., Skeldon A. M., Aubert D. F., Valvano M. A. (2012) The Type VI secretion system of Burkholderia cenocepacia affects multiple Rho family GTPases disrupting the actin cytoskeleton and the assembly of NADPH oxidase complex in macrophages. Cell. Microbiol. 14, 255–273 [DOI] [PubMed] [Google Scholar]
- 29. Hamad M. A., Skeldon A. M., Valvano M. A. (2010) Construction of aminoglycoside-sensitive Burkholderia cenocepacia strains for use in studies of intracellular bacteria with the gentamicin protection assay. Appl. Environ. Microbiol. 76, 3170–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gavrilin M. A., Bouakl I. J., Knatz N. L., Duncan M. D., Hall M. W., Gunn J. S., Wewers M. D. (2006) Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc. Natl. Acad. Sci. U.S.A. 103, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gavrilin M. A., Mitra S., Seshadri S., Nateri J., Berhe F., Hall M. W., Wewers M. D. (2009) Pyrin critical to macrophage IL-1β response to Francisella challenge. J. Immunol. 182, 7982–7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan W., Tang Z., Chen D., Moughon D., Ding X., Chen S., Zhu M., Zhong Q. (2010) Keap1 facilitates p62-mediated ubiquitin aggregate clearance via autophagy. Autophagy 6, 416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fahy R. J., Exline M. C., Gavrilin M. A., Bhatt N. Y., Besecker B. Y., Sarkar A., Hollyfield J. L., Duncan M. D., Nagaraja H. N., Knatz N. L., Hall M., Wewers M. D. (2008) Inflammasome mRNA expression in human monocytes during early septic shock. Am. J. Respir. Crit. Care Med. 177, 983–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amer A., Franchi L., Kanneganti T. D., Body-Malapel M., Ozören N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223 [DOI] [PubMed] [Google Scholar]
- 35. Akhter A., Gavrilin M. A., Frantz L., Washington S., Ditty C., Limoli D., Day C., Sarkar A., Newland C., Butchar J., Marsh C. B., Wewers M. D., Tridandapani S., Kanneganti T. D., Amer A. O. (2009) Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5, e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang C. W., Klionsky D. J. (2003) The molecular mechanism of autophagy. Mol. Med. 9, 65–76 [PMC free article] [PubMed] [Google Scholar]
- 37. Abdulrahman B. A., Khweek A. A., Akhter A., Caution K., Kotrange S., Abdelaziz D. H., Newland C., Rosales-Reyes R., Kopp B., McCoy K., Montione R., Schlesinger L. S., Gavrilin M. A., Wewers M. D., Valvano M. A., Amer A. O. (2011) Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy 7, 1359–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devenish R. J. (2011) Autophagy and the evasion of host defense: a new variation on the theme for Burkholderia cepacia? Autophagy 7, 1269–1270 [DOI] [PubMed] [Google Scholar]
- 39. Vergne I., Deretic V. (2010) The role of PI3P phosphatases in the regulation of autophagy. FEBS Lett. 584, 1313–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang J., Randall M. S., Loyd M. R., Dorsey F. C., Kundu M., Cleveland J. L., Ney P. A. (2009) Mitochondrial clearance is regulated by Atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 114, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cemma M., Kim P. K., Brumell J. H. (2011) The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 7, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johansen T., Lamark T. (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathew R., Karp C. M., Beaudoin B., Vuong N., Chen G., Chen H. Y., Bray K., Reddy A., Bhanot G., Gelinas C., Dipaola R. S., Karantza-Wadsworth V., White E. (2009) Autophagy suppresses tumorigenesis through elimination of p62. Cell 137, 1062–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuusisto E., Salminen A., Alafuzoff I. (2001) Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12, 2085–2090 [DOI] [PubMed] [Google Scholar]
- 45. Moscat J., Diaz-Meco M. T. (2009) p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137, 1001–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luciani A., Villella V. R., Esposito S., Gavina M., Russo I., Silano M., Guido S., Pettoello-Mantovani M., Carnuccio R., Scholte B., De Matteis A., Maiuri M. C., Raia V., Luini A., Kroemer G., Maiuri L. (2012) Targeting autophagy as a novel strategy for facilitating the therapeutic action of potentiators on ΔF508 cystic fibrosis transmembrane conductance regulator. Autophagy 8, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erlich S., Mizrachy L., Segev O., Lindenboim L., Zmira O., Adi-Harel S., Hirsch J. A., Stein R., Pinkas-Kramarski R. (2007) Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy 3, 561–568 [DOI] [PubMed] [Google Scholar]
- 48. Lorand L., Graham R. M. (2003) Transglutaminases. Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4, 140–156 [DOI] [PubMed] [Google Scholar]
- 49. Akar U., Ozpolat B., Mehta K., Fok J., Kondo Y., Lopez-Berestein G. (2007) Tissue transglutaminase inhibits autophagy in pancreatic cancer cells. Mol. Cancer Res. 5, 241–249 [DOI] [PubMed] [Google Scholar]
- 50. Nezis I. P., Papassideri I. (2008) Monitoring autophagy in insect eggs. Methods Enzymol. 451, 669–683 [DOI] [PubMed] [Google Scholar]